Abstract
Glucose-inhibited neurones are an integral part of neurocircuits regulating cognitive arousal, body weight and vital adaptive behaviours. Their firing is directly suppressed by extracellular glucose through poorly understood signalling cascades culminating in opening of post-synaptic K+ or possibly Cl− channels. In mammalian brains, two groups of glucose-inhibited neurones are best understood at present: neurones of the hypothalamic arcuate nucleus (ARC) that express peptide transmitters NPY and agouti-related peptide (AgRP) and neurones of the lateral hypothalamus (LH) that express peptide transmitters orexins/hypocretins. The activity of ARC NPY/AgRP neurones promotes food intake and suppresses energy expenditure, and their destruction causes a severe reduction in food intake and body weight. The physiological actions of ARC NPY/AgRP cells are mediated by projections to numerous hypothalamic areas, as well as extrahypothalamic sites such as the thalamus and ventral tegmental area. Orexin/hypocretin neurones of the LH are critical for normal wakefulness, energy expenditure and reward-seeking, and their destruction causes narcolepsy. Orexin actions are mediated by highly widespread central projections to virtually all brain areas except the cerebellum, including monosynaptic innervation of the cerebral cortex and autonomic pre-ganglionic neurones. There, orexins act on two specific G-protein-coupled receptors generally linked to neuronal excitation. In addition to sensing physiological changes in sugar levels, the firing of both NPY/AgRP and orexin neurones is inhibited by the ‘satiety’ hormone leptin and stimulated by the ‘hunger’ hormone ghrelin. Glucose-inhibited neurones are thus well placed to coordinate diverse brain states and behaviours based on energy levels.
Keywords: appetite, glucose, hypocretin, hypothalamus, orexin, sleep
Discovery of hypothalamic glucose-inhibited neurones
Animal survival depends on constantly adjusting behaviour to body energy resources. In mammals, the hypothalamus is central for this process. Hypothalamic neurones sense diverse information relevant to body energy status and translate it into coordinated changes in brain state, energy expenditure and behaviour. One of the earliest clues for how the hypothalamus measures body energy levels was provided by the discovery of ‘glucose-sensing’ neurones about half a century ago. These cells exhibit specialized excitatory (glucose-excited neurones) or inhibitory (glucose-inhibited neurones) firing responses to changes in extracellular glucose concentration (Anand et al. 1964, Oomura et al. 1969). Glucose-inhibited neurones were first called ‘glucose-sensitive’, but because this term is confusing, giving no indication about the nature of the sensitivity, we adopt the clearer name ‘glucose-inhibited’ in this article. Apart from several regions of the hypothalamus, glucose-inhibited neurones are also found in the brainstem, but this article primarily focuses on hypothalamic glucose-inhibited neurones.
The first indication of the existence of hypothalamic glucose-inhibited neurones was provided by in vivo experiments of Anand and co-workers in the 1960s, that tried to correlate blood glucose levels with firing activity in putative ‘hunger’ and ‘satiety’ regions of the hypothalamus. Using steel microelectrodes, these authors recorded the electrical activity of lateral and ventromedial hypothalamic regions in anaesthetized dogs and unanaesthetized, Flaxedil-immobolized, cats, and found that spike firing of lateral hypothalamic neurones was significantly decreased by intravenous infusion of glucose (Anand et al. 1964). A couple of years later, Oomura et al. (1969) were making in vivo recordings of spike activity of hypothalamic neurones in the rat, and found that injection of glucose into the extracellular space around the site of recording reversibly suppressed spike firing in approx. 20% of lateral hypothalamic neurones. In contrast, the firing of cortical and thalamic neurones could not be modulated by direct application of glucose (Oomura et al. 1969). In the rat lateral hypothalamus (LH), very similar percentages of glucose-inhibited neurones appear to be found in 2-day old or 100-day-old animals, suggesting that glucose-inhibited neurones become functionally established very early on (Shibata et al. 1982), which is consistent with their emerging critical roles in brain function.
Here we will briefly discuss the mechanisms of glucose-induced inhibition of hypothalamic neurones, and then focus in more detail on neurochemical fingerprints and the physiological significance of glucose-inhibited neurones of the LH and hypothalamic arcuate nucleus (ARC). It should be emphasized that extracellular levels of glucose in the brain are generally lower than those in the blood, and the normal range of brain glucose is thought to be 1–2.5 mm (Routh 2002). A possible exception is the ARC, which may ‘see’ higher glucose levels, closer to those in the blood, as it is located next to a blood–brain barrier ‘window’ in the median eminence (Fioramonti et al. 2004). On the other hand, a glucose concentration of 0 mm is completely unphysiological (it would lead to coma), causes widespread neuronal silencing not specific to glucosensing neurones (Mobbs et al. 2001) and thus is not a good stimulus for studying glucose-sensing cells. Nevertheless, some authors do use 0 mm glucose as a stimulus to identify glucosensing neurones, perhaps as an homage to some classical studies on brain glucosensing (Minami et al. 1986). Throughout the review, we will highlight the concentrations of glucose used in various experiments, as it may give an indication of the physiological relevance of the findings.
Mechanisms of glucose-induced inhibition
Attempts to determine how glucose-inhibited neurones operate commenced soon after the discovery of these cells. Oomura et al. (1974) performed intracellular recordings of rat LH neurones in vivo, and found that extracellular application of the Na+/K+ ATPase blocker ouabain reversed the inhibitory effects of extracellularly injected glucose on the firing of these cells. Ouabain injection around the recorded neurone also prevented subsequent application of glucose from causing inhibition of firing. Based on these results, and also on their observation that glucose inhibition is abolished by the metabolic poison azide, Oomura et al. (1974) suggested that glucose stimulates the Na+/K+ ATPase pump, and the resulting hyperpolarizing current, caused by electrogenic exchange action of the pump (3Na+ per 2K+), inhibits the cell. However, the ouabain inhibition of glucose action was readily reversible (Oomura et al. 1974), whereas ouabain inhibition of the pump is thought to be irreversible. It is therefore possible that the ouabain effects were due to indirect phenomena, for example a discharge of excitatory transmitter stores. The contribution of Na+/K+ ATPase to glucose-induced inhibition remains unresolved to this day.
Another idea that has been put forward to explain glucose-induced inhibition is glucose-induced activation of post-synaptic Cl− channels, possibly belonging to the CFTR family. This idea comes from in vitro whole-cell patch-clamp recordings from glucose-inhibited neurones of the mediobasal hypothalamus. Song et al. (2001) reported that, in rat ventromedial hypothalamic neurones in vitro, switching between 0.1 and 2.5 mm extracellular glucose induced membrane hyperpolarization and activated a current displaying a reversal potential of about −50 mV, close to that of chloride in their solutions (−57 mV). Subsequently, Fioramonti et al. (2007), working on glucose-inhibited neurones of the mouse arcuate nucleus reported that switching between 0.5 and 5 mm glucose activated a membrane current with a reversal potential of either −80 or −64 mV (value depending on intracellular [Cl−]), when the Cl− equilibrium potential was −74 or −57 mV respectively. The proximity of the apparent reversal potentials of glucose-activated current to the predicted Cl− equilibrium potential led to the Cl− channel hypothesis of glucose inhibition (Song et al. 2001, Routh 2002, Fioramonti et al. 2007).
However, some technical issues remain to be satisfied to validate this theory. For example, the whole-cell current–voltage relationships on which the above-mentioned reversal potentials are based were measured using pipette solutions containing low [Cl−] and were taken from membrane responses to hyperpolarizing current pulses in the current-clamp mode (Song et al. 2001, Fioramonti et al. 2007). Low [Cl−] pipette solutions can potentially introduce a substantial ‘junction potential’ error to measurements of reversal potentials (Barry & Lynch 1991), whereas the current-clamp mode leaves the membrane potential unprotected from distortions such as the H-current-mediated ‘sag’. While the Cl− theory of glucose-induced inhibition in the mediobasal hypothalamus remains to be examined using voltage-clamp recordings with high [Cl−] pipette solutions, there is some possible pharmacological support for the involvement of the CFTR Cl− channel. Fioramonti et al. (2007) mentioned that after treatment with gemfibrozil (a CFTR channel blocker in other tissues; Chen et al. 2004), glucose-inhibited neurones could no longer respond to glucose. However, this finding is presently difficult to interpret because gemfibrozil by itself appeared to activate a hyperpolarizing current in the same cells (Fioramonti et al. 2007), and can act on targets unrelated to CFTR (Walsh & Wang 1996, Sanguino et al. 2003).
So far, the Cl− theory has been examined most directly in orexin/hypocretin-containing neurones of the LH, whose physiological roles are discussed below. In these cells, biophysical elimination of all inhibitory Cl− currents leaves glucose-induced inhibition completely unaffected, while measurement of membrane current–voltage relationship using high [Cl−] pipette solutions shows that glucose activates a current selective for K+ ions (Burdakov et al. 2006). Large post-synaptic K+ currents are triggered by extracellular [glucose] switches from 0.2 to 5 mm and from 1 to 2.5 mm, suggesting that this mechanism is physiologically relevant (Burdakov et al. 2006). Thus in orexin neurones at least, the final effectors in glucose-induced inhibition are K+ currents. Not much is currently known about how changes in extracellular glucose levels control these channels.
NPY/AgRP neurones: glucose-inhibited cells regulating feeding and body weight
While the mechanism(s) of glucose-induced inhibition remains elusive, much concrete information appeared recently about the neurochemical and physiological identities of glucose-inhibited neurones of the ARC and LH areas of the hypothalamus. In the ARC, glucose-inhibited neurones were found to co-express NPY and agouti-related peptide (AgRP), peptide transmitters that stimulate feeding and weight gain. When Muroya et al. (1999) measured cytosolic [Ca2+] levels in neurones isolated from rat ARC, they found that 94% of the neurones that were stimulated by lowering extracellular glucose from 10 to 1 mm contained NPY immunoreactivity. Presumably these were NPY/AgRP neurones as in the ARC ~100% of NPY neurones contain AgRP (Hahn et al. 1998). Subsequently, Fioramonti et al. (2007) examined electrophysiological glucose sensitivity of ARC NPY/AgRP neurones in brain slices of transgenic mice selectively expressing GFP in NPY neurones. They found that switching extracellular glucose between 0.5 and 5 mm reversibly hyperpolarized and inhibited 40% of ARC NPY neurones through a direct, post-synaptic action.
Agouti-related peptide is expressed uniquely in NPY/AgRP neurones of the ARC, allowing the projections of ARC NPY/AgRP neurones to be determined by looking at the distribution of AgRP-immunoreactive nerve terminals. This was done by Broberger et al. (1998b), who found that NPY/AgRP cells project to many hypothalamic areas as well as extra-hypothalamic sites such as the amygdala, thalamus and ventral tegmental area. The NPY/AgRP cells are thus anatomically well set to coordinate the emotional, rewarding and metabolic aspects of feeding. Indeed, destruction of NPY/AgRP neurones through activation of transgenically targeted diphtheria toxin receptor in adult mice leads to pronounced reduction in food intake and body weight, illustrating that these cells are essential for driving feeding (Luquet et al. 2005). Thus their inhibition by glucose may act to suppress appetite. The extent to which NPY/AgRP neurones contribute to glucose-induced inhibition of feeding is unclear. When Luquet et al. (2007) destroyed NPY/AgRP neurones in neonatal mice, they found that the feeding responses to glucoprivation were unaffected. However, the same group previously showed that, in contrast to what happens upon elimination of NPY/AgRP cells in adult mice, neonatal destruction of NPY/AgRP neurones has minimal effects on feeding in general, which illustrates that compensatory mechanisms readily develop upon neonatal destruction of these cells (Luquet et al. 2005). Thus it is still possible that glucose-induced inhibition of NPY/AgRP neurones has profound effects on feeding when the brain is allowed to develop normally, a theory that can presumably be tested in mice whose NPY/AgRP cells are ablated in adulthood.
Orexin/hypocretin neurones: glucose-inhibited cells orchestrating cognitive arousal
Orexins/hypocretins are peptide transmitters that in mammalian brains appear to be expressed exclusively in a subpopulation of LH neurones (de Lecea et al. 1998, Sakurai et al. 1998). The first evidence that LH orexin neurones might be glucose-inhibited was indirect, coming from in vivo experiments showing that systemic hypoglycaemia activates orexin neurones (Sakurai et al. 1998, Moriguchi et al. 1999, Cai et al. 2001). Muroya et al. (2001) subsequently provided more direct support for their glucose-inhibited nature by showing that lowering extracellular glucose levels from 8.3 to 2.8 mm around isolated rat orexin neurones increases their cytosolic Ca2+ levels. Definitive evidence that LH orexin neurones act as electrical glucose sensors came a couple of years later, when Yamanaka et al. (2003) found that in 80–100% of isolated mouse orexin neurones, the firing was stimulated by lowering [glucose] from 10 mm to 5 or 0 mm, and inhibited by increasing glucose from 10 mm to 15 or 30 mm. We subsequently found that in mouse brain slices 90–100% of orexin neurones are inhibited by increasing glucose from 0.2 to 5 mm (Burdakov et al. 2005). Smaller concentrations of glucose are also effective, for example, increasing extracellular glucose from 1 to 2.5 mm evokes pronounced and reversible inhibition of mouse orexin neurones (Burdakov et al. 2006). In terms of their sensitivity to sugar, LH orexin neurones thus appear well placed to translate small daily variations in brain glucose levels into changes in brain activity.
The glucose sensitivity of orexin neurones is likely to have specific physiological implications because the behavioural consequences of orexin release are well defined, and even small (5 Hz) changes in orexin cell firing can lead to behavioural responses (Adamantidis et al. 2007). Soon after their discovery by de Lecea et al. (1998) and Sakurai et al. (1998), it was realized that orexins are essential for stable wakefulness and consciousness in a variety of mammalian species including humans (Chemelli et al. 1999, Lin et al. 1999, Peyron et al. 2000, Thannickal et al. 2000, Hara et al. 2001). Loss of orexins, orexin neurones or orexin type 2 receptor results in narcolepsy, a neurological disorder where wakefulness is frequently interrupted by irresistible attacks of sleep and unconsciousness and night-time sleep is also fragmented (reviewed in Mignot et al. 2002). Orexins are thought to stabilize wakefulness through excitatory projections to all classical ‘ascending arousal systems’ as well as directly to the cortex (reviewed in Sakurai 2007). Recent evidence also indicates that orexins may have a similar excitatory action on brain stress pathways, such as the neurones expressing the corticotropin-releasing factor (Sakamoto et al. 2004). Centrally injected orexin evokes anxiety-like behaviour (Suzuki et al. 2005), induces a long-lasting brain reward deficit and can trigger drug-seeking relapse (Boutrel et al. 2005). Emotional stress elevates orexin levels (Reyes et al. 2003), and an orexin antagonist can prevent stressful stimuli from inducing cocaine-seeking behaviour (Boutrel et al. 2005). The reward-modulating actions of orexins are likely to be mediated, at least in part, by dopaminergic neurones of the ventral tegmental area (Borgland et al. 2006) that are innervated by orexin fibres and excited by orexin (Korotkova et al. 2003). Last but not least, orexin neurones also appear to stimulate food seeking and food intake (Sakurai et al. 1998). Direct excitatory actions of orexins on appetite-promoting ARC NPY/AgRP neurones (van den Top et al. 2004) and indirect inhibitory effects of orexins on anorexigenic ARC POMC neurones (Ma et al. 2007) are proposed to contribute to appetite-stimulating effects of orexins, which are apparent when orexin is injected directly into the ARC (Muroya et al. 2004). However, the net effect of orexins on energy balance appears to be negative – loss of orexin neurones causes obesity rather than leanness, which is likely due to decreased energy expenditure and locomotor activity (Hara et al. 2001). Apart from orexin, LH orexin neurones also appear to use glutamate and dynorphin as transmitters (Collin et al. 2003, Rosin et al. 2003), which may be co-released with orexin to modulate targets in a complex and target-specific manner (Li & van den Pol 2006).
In just 10 years, LH orexin neurones thus emerged as a wide-projecting neural system essential for appropriate temporal coordination of cognitive arousal and reward-seeking behaviour. Glucose-induced inhibition of orexin neurones is thus hypothesized to have widespread behavioural implications, for example stimulation of sleep and lowering of stress levels (discussed in Burdakov 2007), but these speculations remain to be addressed by direct in vivo experiments.
Interactions of glucose-inhibited cells with each other and other signals
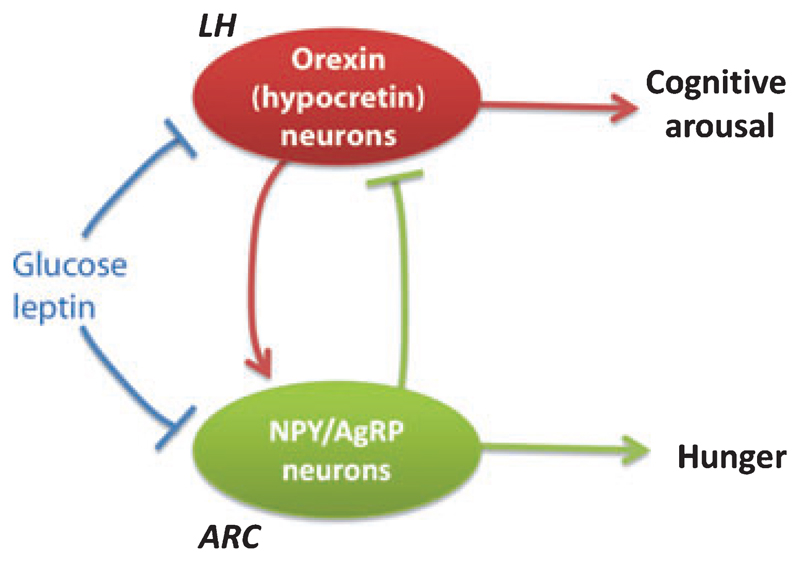
Recent evidence suggests that different groups of glucose-inhibited neurones of the hypothalamus are anatomically connected. Both cell bodies and dendritic processes of LH orexin neurones are in close apposition with NPY-containing nerve terminals that appear to come from the ARC as they co-express AgRP (Broberger et al. 1998a). In turn, ARC NPY/AgRP cells are surrounded by nerve terminals containing orexin (Muroya et al. 2004). Exogenously applied orexin stimulates ARC NPY/AgRP neurones in both Ca2+ imaging and patch-clamp electrophysiology assays (Muroya et al. 2004, van den Top et al. 2004). Positive signals from orexin to NPY cells seem to have a simple behavioural rationale of ensuring that states of high alertness and energy expenditure are associated with feelings of hunger (Fig. 1). Much more difficult to rationalize is the observation that exogenously applied NPY robustly inhibits orexin neurones (Fu et al. 2004), which would imply a decrease in orexin cell activity when ARC NPY neurones are active (Fig. 1). The functional importance of this negative feedback loop is likely to remain unclear until quantitative knowledge becomes available about how much orexin and NPY are released in the ARC and LH, respectively, under different behavioural circumstances.
Figure 1.
Schematic model of hypothalamic glucose-inhibited neurones in the context of brain function. Arrows indicate activation, t-bars indicate inhibition.
Early models of hypothalamic function saw the mediobasal and lateral hypothalamic areas as behaviourally opposing centres of perhaps equal importance, interacting through reciprocal inhibitory connections (Oomura et al. 1964). Later, as the ARC became increasingly well characterized, it started to be called a ‘first-order’ centre that collects peripheral metabolic information and communicates it to ‘second-order’ centres such as the LH (Schwartz et al. 2000). However, subsequent experiments on the LH made it clear that, with regard to metabolic sensing at least, the first-order/second-order model is inaccurate. Similar to neurones of the ARC, the cells in the LH also directly sense circulating indicators of body energy status, i.e. they are also ‘first order’ with regard to capturing metabolic information. This is well exemplified by glucose-inhibited neurones of the ARC and the LH. Apart from glucose, both types of neurones directly sense the ‘satiety’ hormone leptin and the ‘hunger’ hormone ghrelin. ARC NPY/AgRP neurones are directly depolarized and excited by ghrelin, and are hyperpolarized by leptin (Cowley et al. 2003, van den Top et al. 2004). LH orexin neurones exhibit similar direct sensing responses – when mechanically isolated from surrounding circuits, they are electrically inhibited by leptin and excited by ghrelin (Yamanaka et al. 2003). Thus glucose-inhibited neurones of the ARC and LH appear to process several key inputs in a parallel, rather than sequential, manner. The logic of this arrangement (Fig. 1) will probably only become clear when functional interactions with other neighbouring circuits, such as those controlling temporal organization of behaviour (Saper et al. 2005), become fully mapped and characterized.
Other glucose-inhibited neurones
Although this review focuses on the hypothalamic ARC and LH areas, several other glucose-inhibited neurones exist and are likely to be important players in behavioural and metabolic coordination. In particular, they are found in brainstem areas that play a key role in body energy homeostasis, for example by controlling exocrine pancreatic function (Ritter et al. 1981, Balfour et al. 2006). The neurochemical fingerprints and projections of these glucose-inhibited cells are currently not understood. In the hypothalamus, many glucose-inhibited neurones are found in the ventromedial nucleus (VMN), the classical ‘satiety centre’. Recent experiments by Routh and coworkers suggest that the sensitivity of the VMN glucose-inhibited neurones can be modulated by systemic hyperglycaemia (Canabal et al. 2007) and hypoglycaemia (Song & Routh 2006), potentially highlighting an important role of these cells in disease states. However, the neurochemistry and projections of VMN glucose-inhibited cells remain a challenge to elucidate due to the paucity of molecular markers specific to this brain region.
Overview and perspectives
In the past few years, glucose-inhibited neurones ceased to be a mere electrophysiological curiosity. It is now clear that these cells are fundamental players in a number of behaviourally vital brain circuits, and their loss can lead to profound detriment of mental and physical well-being, as seen in narcolepsy and anorexia. As well as glucose, they sense many other key chemical features of the internal environment (Yamanaka et al. 2003, van den Top et al. 2004, Williams et al. 2007), and communicate this information to the rest of the brain through widespread projection fields. However, we still do not know the specific behavioural impact of glucose sensing in these cells. Ongoing research into the molecular mechanisms of glucose-induced inhibition would provide tools necessary to answer this question.
Acknowledgments
Work in the authors’ laboratory is supported by BBSRC and Diabetes UK.
Footnotes
Conflict of interest
There is no conflict of interest for this study.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand BK, Chhina GS, Sharma KN, Dua S, Singh B. Activity of single neurons in the hypothalamic feeding centers: effect of glucose. Am J Physiol. 1964;207:1146–1154. doi: 10.1152/ajplegacy.1964.207.5.1146. [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, Trapp S. Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol. 2006;570:469–484. doi: 10.1113/jphysiol.2005.098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. doi: 10.1007/BF01870526. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998a;402:460–474. [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci USA. 1998b;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D. K(+) channels stimulated by glucose: a new energy-sensing pathway. Pflugers Arch. 2007;454:19–27. doi: 10.1007/s00424-006-0189-8. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, Williams G. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50:105–112. doi: 10.2337/diabetes.50.1.105. [DOI] [PubMed] [Google Scholar]
- Canabal DD, Potian JG, Duran RG, McArdle JJ, Routh VH. Hyperglycemia impairs glucose and insulin regulation of nitric oxide production in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R592–R600. doi: 10.1152/ajpregu.00207.2007. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu LL, Ye LL, McGuckin C, Tamowski S, Scowen P, Tian H, Murray K, Hatton WJ, Duan D. Targeted inactivation of cystic fibrosis transmembrane conductance regulator chloride channel gene prevents ischemic preconditioning in isolated mouse heart. Circulation. 2004;110:700–704. doi: 10.1161/01.CIR.0000138110.84758.BB. [DOI] [PubMed] [Google Scholar]
- Collin M, Backberg M, Ovesjo ML, Fisone G, Edwards RH, Fujiyama F, Meister B. Plasma membrane and vesicular glutamate transporter mRNAs/proteins in hypothalamic neurons that regulate body weight. Eur J Neurosci. 2003;18:1265–1278. doi: 10.1046/j.1460-9568.2003.02840.x. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Lorsignol A, Taupignon A, Penicaud L. A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes. 2004;53:2767–2775. doi: 10.2337/diabetes.53.11.2767. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- Fu LY, Acuna-Goycolea C, van den Pol AN. Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci. 1998;1:271–272. doi: 10.1038/1082. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, et al. The hypocretins: hypothalamus-specific peptides with neuro-excitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides. 2007;28:214–225. doi: 10.1016/j.peptides.2006.08.036. [DOI] [PubMed] [Google Scholar]
- Ma X, Zubcevic L, Bruning JC, Ashcroft FM, Burdakov D. Electrical inhibition of identified anorexigenic POMC neurons by orexin/hypocretin. J Neurosci. 2007;27:1529–1533. doi: 10.1523/JNEUROSCI.3583-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5(Suppl.):1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- Minami T, Oomura Y, Sugimori M. Electrophysiological properties and glucose responsiveness of guinea-pig ventromedial hypothalamic neurones in vitro. J Physiol. 1986;380:127–143. doi: 10.1113/jphysiol.1986.sp016276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs CV, Kow LM, Yang XJ. Brain glucose-sensing mechanisms: ubiquitous silencing by aglycemia vs. hypothalamic neuroendocrine responses. Am J Physiol Endocrinol Metab. 2001;281:E649–E654. doi: 10.1152/ajpendo.2001.281.4.E649. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett. 1999;264:101–104. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci Lett. 1999;264:113–116. doi: 10.1016/s0304-3940(99)00185-8. [DOI] [PubMed] [Google Scholar]
- Muroya S, Uramura K, Sakurai T, Takigawa M, Yada T. Lowering glucose concentrations increases cytosolic Ca2+ in orexin neurons of the rat lateral hypothalamus. Neurosci Lett. 2001;309:165–168. doi: 10.1016/s0304-3940(01)02053-5. [DOI] [PubMed] [Google Scholar]
- Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M, Sakurai T, et al. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524–1534. doi: 10.1111/j.1460-9568.2004.03255.x. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal activities of the ventromedial and lateral hypothalamic areas of cats. Science. 1964;143:484–485. doi: 10.1126/science.143.3605.484. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222:282–284. doi: 10.1038/222282a0. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Walker JR, DeCino C, Hogenesch JB, Sawchenko PE. Categorically distinct acute stressors elicit dissimilar transcriptional profiles in the paraventricular nucleus of the hypothalamus. J Neurosci. 2003;23:5607–5616. doi: 10.1523/JNEUROSCI.23-13-05607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science. 1981;213:451–452. doi: 10.1126/science.6264602. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Routh VH. Glucose-sensing neurons: are they physiologically relevant? Physiol Behav. 2002;76:403–413. doi: 10.1016/s0031-9384(02)00761-8. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 2004;118:183–191. doi: 10.1016/j.regpep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuiat of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sanguino E, Ramon M, Roglans N, Alegret M, Sanchez RM, Vazquez-Carrera M, Laguna JC. Gemfibrozil increases the specific binding of rat-cortex nuclear extracts to a PPRE probe. Life Sci. 2003;73:2927–2937. doi: 10.1016/j.lfs.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Shibata S, Oomura Y, Kita H. Ontogenesis of glucose sensitivity in the rat lateral hypothalamus: a brain slice study. Brain Res. 1982;281:114–117. doi: 10.1016/0165-3806(82)90120-1. [DOI] [PubMed] [Google Scholar]
- Song Z, Routh VH. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1283–R1287. doi: 10.1152/ajpregu.00148.2006. [DOI] [PubMed] [Google Scholar]
- Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH. Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Walsh KB, Wang C. Effect of chloride channel blockers on the cardiac CFTR chloride and L-type calcium currents. Cardiovasc Res. 1996;32:391–399. doi: 10.1016/0008-6363(96)00075-2. [DOI] [PubMed] [Google Scholar]
- Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]