Abstract
It was suggested half a century ago that electrical impulses from the lateral hypothalamic area stimulate breathing. It is now emerging that these effects may be mediated, at least in part, by neurons containing orexin neuropeptides (also known as hypocretins). These cells promote wakefulness and consciousness, and their loss results in narcolepsy. Recent data also show that orexin neurons directly project to respiratory centres in the brainstem, which express orexin receptors, and where injection of orexin stimulates breathing. Because orexin neurons receive inputs that signal metabolic, sleep/wake and emotional states, it is tempting to speculate that they may regulate breathing according to these parameters. Knockout of the orexin gene in mice reduces CO2-induced increases in breathing by ~50% and increases the frequency of spontaneous sleep apneas. The relationship between orexins and breathing may be bidirectional: the rate of breathing controls acid and CO2 levels, and these signals alter the electrical activity of orexin neurons in vitro. Overall, these findings suggest that orexins are important for the regulation of breathing and may potentially play a role in the pathophysiology and medical treatment of respiratory disorders.
The brains of higher animals perform a staggering array of tasks at the same time, posing a fundamental control problem: how does the brain arrange different actions to generate meaningful behaviour? For example, how does it generate a heightened state of alertness when needed, such as during hunger, but reduce wakefulness when it may not be translated into efficient outcomes, for example during darkness? The hypothalamus, an almond-sized area of the brain in humans, has long been recognised as a fundamental orchestrator of diverse behaviours and adaptive responses (Refs 1, 2). This neuron-dense structure integrates a wide range of peripheral and sensory signals, while interfacing with a multitude of circuits with more specialised functions. Recently, hypothalamic cells containing orexins (also known as hypocretins; HCRTs) have emerged as critical and multifaceted players in hypothalamic behavioural co-ordination. In this article, we review recent data suggesting that orexin neurons are important for a successful partnership between arousal states and changes in ventilation.
Orexins: arousal signals from the lateral hypothalamic area
Orexins are a pair of peptide neurotransmitters (orexin-A and -B) produced from the same precursor (Refs 3, 4). In the mammalian brain, orexin-containing neurons (orexin neurons) are thought to be located solely within the hypothalamus. While only a few thousand orexin neurons are found in this restricted location, they project to virtually the entire brain, except the cerebellum (Refs 3, 5, 6). The orexin peptides act on specific G-protein-coupled receptors (OX1R/HCRTR1 and OX2R/HCRTR2), resulting primarily in electrical excitation of the receptor-expressing cell (Refs 6, 7). The two receptors have different specificities for the orexins (OX2R is nonselective whereas OX1R prefers orexin-A) and different distributions in the central nervous system (CNS) (Ref. 6), alluding to the so far unexplored possibility of distinct physiological roles for each orexin.
Orexins play crucial roles in the maintenance of wakefulness, energy homeostasis, and reward-seeking behaviour (Refs 8, 9, 10, 11). Abnormalities in orexin signalling – produced by deletion of orexin peptides, orexin neurons or OX2Rs – produce narcolepsy (Refs 12, 13, 14, 15, 16, 17), a disease characterised by irresistible attacks of sleep, cataplexy, and hypnagogic hallucinations. Although orexins stimulate feeding, selective orexin cell ablation leads to obesity due to reduced energy expenditure (Ref. 17). Furthermore, mice lacking orexins fail to exhibit normal foraging-like activity during food shortage (Ref. 18).
Neural pathways involved in the effects of orexins on wakefulness and feeding have been recently reviewed in detail (Ref. 6). Briefly, orexin neurons send excitatory inputs to all key components of the classical reticular activating system, as well as directly to attention-promoting regions of the cortex. Orexin cell activity, when monitored using microdialysis, FOS expression, or electrophysiology, appears to be maximal during active wakefulness and minimal during slow-wave sleep (Refs 19, 20, 21, 22). Interestingly, stimuli potentially associated with danger, such as sudden loud noises, cause rapid firing in orexin cells (Ref. 22), suggesting that the orexin system may enhance arousal in fight-or-flight situations. Overall, evidence points towards orexin as a key stabiliser of the ‘wake’ position in the sleep–wake flip–flop switch (Ref. 23).
The regulation of orexin cell firing is a subject of ongoing research. Known stimulators of orexin cell activity include the hunger hormone ghrelin, while inhibitors include the satiety signals leptin and glucose (Ref. 18). Transmitters of other ‘arousal’ systems, such as noradrenaline and serotonin, also inhibit orexin cell firing (Refs 24, 25), presumably acting as a negative feedback system and possibly allowing orexin cells to take over the maintenance of wakefulness when the activity of aminergic systems wanes (Ref. 26).
The lateral hypothalamic area and breathing: historical links
The involvement of the lateral hypothalamus in both cognitive arousal and breathing was recognised many decades ago. In the 1920s, the Viennese neurologist Constantin von Economo performed post-mortem studies of brain lesions of patients with encephalitis lethargica, and predicted that the lateral hypothalamus is the source of a critical wake-promoting signal, now thought to come from orexin neurons (reviewed in detail in Ref. 23). In relation to breathing, a key prediction of a stimulatory role for the lateral hypothalamus was made by Redgate and Gellhorn in the 1950s. These authors used high-frequency currents to produce localised lesions in the lateral hypothalamus of lightly anaesthetised cats, while monitoring the rate and depth of respiration (Ref. 27). The lesions resulted in an immediate decrease in the rate and/or depth of respiration, and these effects increased with the size and number of lateral hypothalamic lesions (Ref. 27). Chemical inhibition of lateral hypothalamic activity, caused by injection of barbiturates, also reduced respiratory activity (Ref. 27). On the basis of these data, Redgate and Gellhorn concluded that ‘impulses from the lateral hypothalamus exert a tonic facilitatory action on the respiratory centre’ (Ref. 27). This is supported by recent data revealing an anatomical ‘hotspot’ for breathing stimulation very close to the hypothalamic area where orexin neurons are found (Ref. 28).
Anatomical links between orexin neurons and breathing centres
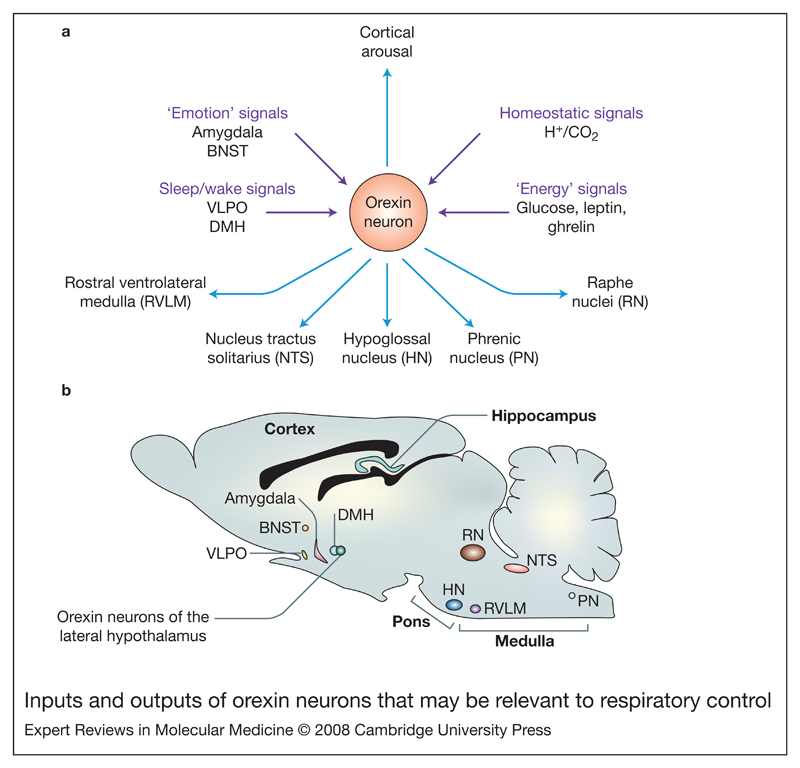
Apart from heightening cognitive arousal, orexins have also been found to stimulate the sympathetic system, increasing heart rate and blood pressure (Ref. 29), possibly generating a defence or stress-type behavioural response. Do orexins also stimulate breathing as part of their orchestration of the ‘fight-or-flight’ response? The cardiovascular effects of orexin were observed when it was applied to the rostral ventrolateral medulla (RVLM) (Refs 30, 31, 32, 33), an area that is also critical for respiratory rhythmogenesis (Refs 34, 35). Immunohistochemical evidence documented orexin-A-immunoreactive nerve fibres in close proximity of RVLM sympathoexcitatory neurons projecting to the spinal cord (Ref. 32), and within the pre-Bötzinger region (part of the respiratory rhythm generator), the nucleus tractus solitarius (area containing inspiratory cells responsive to sensory afferents), and the hypoglossal and phrenic nuclei (areas that control swallowing and diaphragm movements) (Refs 36, 37, 38, 39). These studies were complemented by demonstration of OXR1 expression within the pre-Bötzinger region of the RVLM and on phrenic motor neurons (Ref. 39). Indirect pathways by which orexins may modulate respiration were also found: for example, there are orexinergic projections to the raphe nuclei in the brainstem that regulate respiratory long-term facilitation (Ref. 40). Thus, a number of anatomical pathways through which orexin neurons may modulate respiration are now known. The relative importance of these pathways in the stimulation of breathing remains to be determined. The percentage of orexin neurons directly projecting to the pre-Bötzinger region and the phrenic nucleus appears to be rather small: 0.5% and 2.9%, respectively (Ref. 39). This may imply either that only a small subset of orexin cells regulates breathing or that the majority of orexin neurons control breathing through indirect projections. The inputs and outputs of orexin neurons that may be relevant to breathing are summarised in Figure 1.
Figure 1. Inputs and outputs of orexin neurons that may be relevant to respiratory control.
(a) Orexin neurons in the lateral hypothalamus send projections both ‘upwards’, to arousal-regulating regions such as the thalamus and the cortex (among other areas – see Ref. 5), and ‘downwards’ to brain stem nuclei directly or indirectly involved in respiratory control: the raphe nuclei, nucleus tractus solitarius (Refs 76, 77), the rostral ventrolateral medulla (Refs 32, 39) and the phrenic and hypoglossal nuclei (Refs 36, 37, 39). In turn, orexin neurons receive anatomical inputs from the amygdala and bed nucleus of stria terminalis (BNST), the dorsomedial hypothalamic nucleus (DMH), GABAergic neurons in the ventrolateral preoptic area (VLPO), and many other hypothalamic and extrahypothalamic areas (see Refs 52, 53). Orexin cells are also able to act as sensors of body energy levels, and acid and CO2 (see Fig. 2). (b) Saggital mouse brain section indicating the location of input and output nuclei mentioned in part a. Abbreviation: GABA, gamma-aminobutyric acid.
Effects of stimulation of orexin receptors in breathing centres
Central administration of orexin-A in mice increases the tidal volume (the amount of air inhaled or exhaled during normal ventilation) at concentrations previously shown to induce other orexin-stimulated physiological effects (Refs 39, 41). This appears to be an orexin-specific effect, localised selectively to actions on the pre-Bötzinger complex and spinal cord (Ref. 39). Secondary increases in respiratory frequency and blood pressure do occur but these may be due to later indirect mechanisms (Ref. 41). In addition to the expression of OX1R in breathing centres (Ref. 39), involvement of OX2R is also likely, since in some experiments orexin-B elicits greater stimulation of ventilation than orexin-A (Ref. 42), and OX2R mRNA has been found on hypoglossal motoneurons (Ref. 36).
Effects of genetic knockout of orexins on breathing
Further evidence linking the orexin system and breathing comes from mice with genetic deletion of the gene encoding prepro-orexin, the peptide precursor for both orexin-A and orexin-B. These animals lack orexins and develop narcolepsy–cataplexy (Ref. 13). In a series of recent papers, Kuwaki and colleagues identified several clear abnormalities in the respiration of orexin-knockout mice. Increases in breathing caused by increased atmospheric CO2 levels (hypercapnia) were reduced by ~50% in the orexin knockouts (Ref. 43). Chemical stimulation of the lateral hypothalamus resulted in weaker respiratory increases in orexin-knockout mice than in wild-type mice (Ref. 44). The orexin-knockout mice also displayed a markedly increased frequency of spontaneous sleep apneas (Ref. 43). Interestingly, basal ventilation remained similar in orexin-knockout and wild-type mice, irrespective of whether they were asleep or awake (Ref. 45), suggesting that either the role of orexin in basal respiration is compensated for by other systems in the knockouts, or orexins do not set the basal breathing tone but selectively stimulate breathing during stressful situations. A full description of known respiratory abnormalities in orexin-knockout mice is given in Ref. 45.
Effects of breathing-related signals on orexin neurons
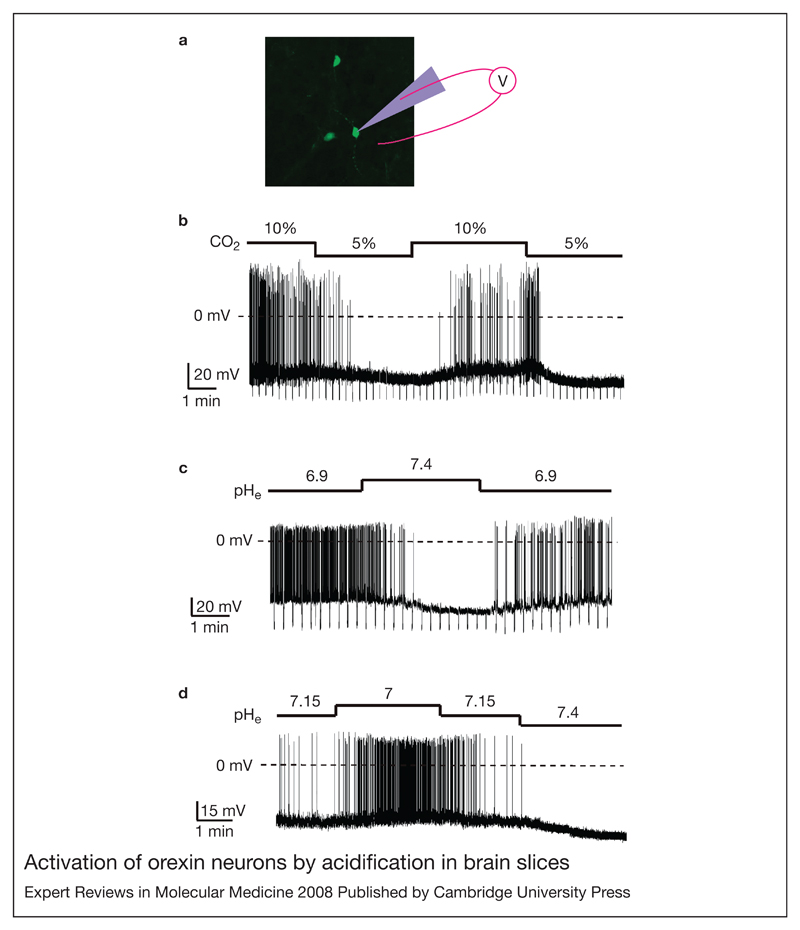
As mentioned above, orexins do not seem to contribute to basal respiration, but become increasingly important for appropriate respiratory stimulation during hypercapnia (Ref. 43). This raises the question of how orexin neurons ‘know’ when to become involved in respiration. One theoretical possibility is that they can directly sense homeostatic parameters controlled by breathing. A key chemical variable controlled by breathing is the extracellular levels of H+ and CO2, which in the body are inextricably linked by the reaction (Ref. 46). Falling pH (or rising CO2) is a powerful stimulus for both respiration and behavioural arousal (Refs 47, 48, 49). Patch-clamp recordings of the membrane potential of orexin neurons in mouse brain slices showed that the firing rate of these cells is exquisitely sensitive to the ambient levels of H+ and CO2 (Fig. 2). Physiological acidosis (e.g. pH = 7) depolarises orexin neurons and increases their electrical activity, while alkalosis (e.g. pH = 7.4) causes hyperpolarisation and an inhibition of orexin cell firing (Fig. 2). These effects appear to be mediated, at least in part, by acid-induced inhibition of background K+ currents in the membrane of orexin cells (Ref. 50). Although these currents display some functional properties of TASK channels (Ref. 50), their molecular identity remains to be determined.
Figure 2. Activation of orexin neurons by acidification in brain slices.
(a) Orexin neurons (green), identified in a mouse brain slice by transgenic green fluorescent protein expression, are shown with a cartoon of a patch-clamp pipette used to measure their membrane potential (full details in Ref. 50). (b) CO2 effects on firing, membrane potential, and membrane resistance of an orexin neuron. (c) pH effects on firing, membrane potential, and membrane resistance of an orexin neuron. (d) Effects of small extracellular pH changes on firing and membrane potential of an orexin neuron. Parts b–d are reprinted from Ref. 50 [Williams, R.H. et al. (2007) Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A 104, 10685–10690 (© 2007 by The National Academy of Sciences of the USA)]. Since acid and CO2 levels in the brain are controlled by breathing, modulation of orexin neurons by these signals may communicate breathing state to many brain areas through the widespread projections of orexin cells.
Because even small changes in orexin cell firing (e.g. 5 Hz) can stimulate wakefulness (Ref. 51), it is tempting to speculate that breathing can regulate states of consciousness by controlling the firing rate of orexin neurons via pH. This theory remains to be examined, for example by comparing breathing and pH-induced changes in cognitive arousal in control and orexin-knockout animals. It also remains to be determined whether different subsets of orexin neurons (Ref. 10) are differentially sensitive to acid and CO2; indeed, our preliminary experiments suggest that some orexin neurons are considerably more sensitive to changes in pH than others (D.Burdakov, unpublished). While many aspects require further investigation, we would like to propose that orexin neurons are part of the central pH-sensing network that also includes the classical pH-sensing neurons in the brainstem (Refs 47, 48, 49). It seems likely that orexin neurons also integrate other signals relevant to breathing; for example, they receive afferents from the amygdala and the bed nucleus of stria terminalis (Refs 52, 53), which may be important for regulation of breathing during emotional stimuli.
Clinical implications
Blunted hypercapnic respiratory response in orexin-knockout mice can be partially restored by administration of orexin, and, conversely, the orexin antagonist SB-334867 (selective for OX1R) reduces the hypercapnic response in wild-type mice (Ref. 42). These results indicate that respiration can be effectively altered by drugs targeting the orexin system, potentially suggesting new strategies for treating respiratory disorders.
Sleep apnoeas
Sleep apnoea is a condition characterised by temporary breathing interruptions during sleep, which causes the sufferer to awake gasping for breath. Some patients suffering from sleep apnoea/hypopnoea have increased orexin-A levels in plasma, suggesting the orexin system may be involved in the awakening mechanism of these patients (Ref. 54). However, the development of sleep apnoea may itself be a side effect of a defect in the orexin system. For example, there are numerous studies indicating that orexin levels are reduced in patients suffering from sleep apnoea (Refs 55, 56, 57, 58, 59). Similarly, patients with Guillain–Barré syndrome, who display respiratory paralysis, have significantly reduced orexin concentration in their cerebrospinal fluid (Ref. 60). Potentially, orexin agonists may be helpful in maintaining normal respiration in these cases of orexin deficiency at doses that do not detrimentally affect sleep–wake patterns.
Obesity hypoventilation syndrome and chronic obstructive pulmonary disease
Obesity is the most prominent risk factor for development of sleep apnoea (Refs 61, 62). Obesity hypoventilation syndrome (also known as Pickwickian syndrome) produces a characteristic phenotype comparable to sleep apnoea (Ref. 63). In these patients, weaker responses of respiratory centres to ventilatory stimuli may contribute to sleep apnea (Ref. 64), although other causes are also likely to play a role (Ref. 65). Furthermore, diabetes mellitus and hyperglycaemia also appear to promote sleep apnoea (Ref. 66), and are a powerful predictor of impaired breathing during sleep in both animals and humans (Refs 67, 68). Because the activity of orexin neurons is potently suppressed by physiological concentrations of glucose (Refs 18, 69), and modulates breathing (Refs 39, 41), hyperglycaemia may potentially cause a dysfunction in orexin signalling and lead to respiratory abnormalities (Refs 39, 41). Another breathing disorder potentially linked to orexins is chronic obstructive pulmonary disease (COPD), also known as chronic obstructive airway disease (COAD), which is associated with reduced plasma orexin levels (Ref. 70).
Hyperventilation
Hyperventilation has numerous causes, including stress, and results in the alkalinisation of the blood and brain interstitial fluid due to excessive exhalation of CO2. Low CO2 levels cause the blood vessels of the brain to constrict, resulting in reduced blood flow to the brain, light-headedness, and fainting (Refs 71, 72). Considering the in vitro data demonstrating a suppression of orexin cell activity by alkalosis (Fig. 2), it is tempting to speculate that loss of orexin cell activity contributes to loss of consciousness caused by hyperventilation. Fainting and reduced breathing would cause CO2 levels to rise, perhaps reactivating orexin neurons and reinitiating arousal, with concomitant stimulation of sympathetic outflow to increase heart rate and respiration (Refs 41, 73, 74, 75). It is not yet known whether hyperventilation may be caused by an increased activity of orexin neurons, but the possibility that orexin cells increase their firing in stressful situations (Ref. 22) is consistent with this theory.
Overview
Orexin neurons are emerging as a neurophysiological link between breathing and arousal states. They are connected to both the central respiratory nuclei and arousal regions, and may serve to match ventilation to changes in states of consciousness. Although demonstrated only in vitro so far, the chemosensing ability of these cells (Fig. 2) may play a role in the generation of appropriate responses to hypercapnic insults. This could potentially explain the weakened hypercapnic chemoreflex in orexin-knockout mice, which can be partly mimicked in wild-type mice by blocking orexin receptors. By sensing breathing-controlled variables such as pH, orexin neurons may communicate the respiratory status to higher brain areas, potentially providing a bidirectional control circuit linking breathing and overall brain state. It remains to be determined how the breathing-related functions of the orexin system coexist with its other attributes such as glucose sensing (Ref. 18) and stimulation of appetite (Ref. 4). It would also be important to address whether imbalances in energy or sleep homeostasis could have a knock-on effect on the ability of orexin cells to regulate respiration.
Further reading, resources and contacts.
References
Nishino, S. and Sakurai, T. (2006) The Orexin/Hypocretin System: Physiology and Pathophysiology (Contemporary Clinical Neuroscience) (1st edn), Humana Press Inc., USA
The book centres around effects of orexin loss as well as discussing techniques and experimental methods for orexin research.
de Lecea, L. and Sutcliffe, J.G. (2005) Hypocretins: Integrators of Physiological Signals (1st edn), Springer-Verlag, New York Inc., USA
Simerly, R.B. (2004) Anatomical substrates of hypothalamic integration. In The Rat Nervous System (3rd edn) (Paxinos, G., ed.), pp. 335-368, Elsevier, USA
This excellent chapter provides background on the hypothalamus and details its anatomical organisation.
Websites
Patient-support websites on sleep-related disorders and narcolepsy can be found at:
http://www.sleepfoundation.org
The BrainMaps.org website is a very useful site that allows users to search maps of the brain by species:
Research-group websites of interest:
http://www.scripps.edu/mb/delecea/index.htm (Luis de Lecea)
http://www.hhmi.org/research/investigators/mignot.html (Emmanuel Mignot)
http://www.hhmi.org/research/investigators/yanagisawa.html (Masashi Yanagisawa)
Acknowledgements and funding
We thank the peer reviewers for thorough and constructive comments on the manuscript, and the BBSRC and the Royal Society for support.
References
- 1.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 2.Morton GJ, et al. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 3.de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 5.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 7.Burdakov D. Electrical signaling in central orexin/hypocretin circuits: tuning arousal and appetite to fit the environment. Neuroscientist. 2004;10:286–291. doi: 10.1177/1073858404263597. [DOI] [PubMed] [Google Scholar]
- 8.Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5(Suppl):1071–1075. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- 9.Willie JT, et al. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–458. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 10.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 11.de Lecea L, et al. Addiction and arousal: alternative roles of hypothalamic peptides. J Neurosci. 2006;26:10372–10375. doi: 10.1523/JNEUROSCI.3118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 13.Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 14.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 16.Nishino S, et al. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 17.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 18.Yamanaka A, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 19.Estabrooke IV, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyashchenko LI, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, et al. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka A, et al. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003b;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- 26.Zeitzer JM, et al. Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23:3555–3560. doi: 10.1523/JNEUROSCI.23-08-03555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redgate ES, Gellhorn E. Respiratory activity and the hypothalamus. Am J Physiol. 1958;193:189–194. doi: 10.1152/ajplegacy.1958.193.1.189. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M, McAllen RM. Functional topography of the dorsomedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R477–486. doi: 10.1152/ajpregu.00633.2007. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, et al. Multiple components of the defense response depend on orexin: evidence from orexin knockout mice and orexin neuron-ablated mice. Auton Neurosci. 2006;126–127:139–145. doi: 10.1016/j.autneu.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 30.Samson WK, et al. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999;831:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- 31.Shirasaka T, et al. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- 32.Machado BH, et al. Pressor response to microinjection of orexin/hypocretin into rostral ventrolateral medulla of awake rats. Regul Pept. 2002;104:75–81. doi: 10.1016/s0167-0115(01)00351-2. [DOI] [PubMed] [Google Scholar]
- 33.Chen CT, et al. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R692–697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- 34.Feldman JL, et al. Neurogenesis of respiratory rhythm and pattern: emerging concepts. Am J Physiol. 1990;259:R879–886. doi: 10.1152/ajpregu.1990.259.5.R879. [DOI] [PubMed] [Google Scholar]
- 35.Hilaire G, Bou C, Monteau R. Rostral ventrolateral medulla and respiratory rhythmogenesis in mice. Neurosci Lett. 1997;224:13–16. doi: 10.1016/s0304-3940(97)13458-9. [DOI] [PubMed] [Google Scholar]
- 36.Volgin DV, Saghir M, Kubin L. Developmental changes in the orexin 2 receptor mRNA in hypoglossal motoneurons. Neuroreport. 2002;13:433–436. doi: 10.1097/00001756-200203250-00014. [DOI] [PubMed] [Google Scholar]
- 37.Fung SJ, et al. Hypocretin (orexin) input to trigeminal and hypoglossal motoneurons in the cat: a double-labeling immunohistochemical study. Brain Res. 2001;903:257–262. doi: 10.1016/s0006-8993(01)02318-6. [DOI] [PubMed] [Google Scholar]
- 38.Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: a double-virus tracing study. Neuroscience. 2003;118:853–866. doi: 10.1016/s0306-4522(02)00997-1. [DOI] [PubMed] [Google Scholar]
- 39.Young JK, et al. Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol. 2005;98:1387–1395. doi: 10.1152/japplphysiol.00914.2004. [DOI] [PubMed] [Google Scholar]
- 40.Terada J, et al. Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol. 2008;104:499–507. doi: 10.1152/japplphysiol.00919.2007. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. doi: 10.1016/j.neulet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 42.Deng BS, et al. Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol. 2007;103:1772–1779. doi: 10.1152/japplphysiol.00075.2007. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura A, et al. Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol. 2007;102:241–248. doi: 10.1152/japplphysiol.00679.2006. [DOI] [PubMed] [Google Scholar]
- 44.Kayaba Y, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–593. doi: 10.1152/ajpregu.00671.2002. [DOI] [PubMed] [Google Scholar]
- 45.Kuwaki T. Orexinergic modulation of breathing across vigilance states. Respir Physiol Neurobiol. 2008 Mar 30; doi: 10.1016/j.resp.2008.03.011. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 47.Nattie EE. Central chemosensitivity, sleep, and wakefulness. Respir Physiol. 2001;129:257–268. doi: 10.1016/s0034-5687(01)00295-x. [DOI] [PubMed] [Google Scholar]
- 48.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO(2) and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol. 2004;287:C1493–526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- 49.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 50.Williams RH, et al. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–10690. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adamantidis AR, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakurai T, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida K, et al. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Igarashi N, et al. Plasma orexin-A levels in obstructive sleep apnea-hypopnea syndrome. Chest. 2003;124:1381–1385. doi: 10.1378/chest.124.4.1381. [DOI] [PubMed] [Google Scholar]
- 55.Busquets X, et al. Decreased plasma levels of orexin-A in sleep apnea. Respiration. 2004;71:575–579. doi: 10.1159/000081757. [DOI] [PubMed] [Google Scholar]
- 56.Kanbayashi T, et al. CSF hypocretin measures in patients with obstructive sleep apnea. J Sleep Res. 2003;12:339–341. doi: 10.1046/j.0962-1105.2003.00373.x. [DOI] [PubMed] [Google Scholar]
- 57.Nishijima T, et al. Plasma orexin-A-like immunoreactivity in patients with sleep apnea hypopnea syndrome. Peptides. 2003;24:407–411. doi: 10.1016/s0196-9781(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 58.Sakurai S, et al. Clinical significance of daytime plasma orexin-A-like immunoreactivity concentrations in patients with obstructive sleep apnea hypopnea syndrome. Respiration. 2004;71:380–384. doi: 10.1159/000079643. [DOI] [PubMed] [Google Scholar]
- 59.Sakurai S, et al. Plasma orexin-A levels in obstructive sleep apnea-hypopnea syndrome. Chest. 2004;125:1963. doi: 10.1378/chest.125.5.1963. author reply 1963-1964. [DOI] [PubMed] [Google Scholar]
- 60.Ripley B, et al. CSF hypocretin/orexin levels in narcolepsy and other neurological conditions. Neurology. 2001;57:2253–2258. doi: 10.1212/wnl.57.12.2253. [DOI] [PubMed] [Google Scholar]
- 61.Manzella D, et al. Soluble leptin receptor and insulin resistance as determinant of sleep apnea. Int J Obes Relat Metab Disord. 2002;26:370–375. doi: 10.1038/sj.ijo.0801939. [DOI] [PubMed] [Google Scholar]
- 62.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 63.Lavie P. Who was the first to use the term Pickwickian in connection with sleepy patients? History of sleep apnoea syndrome. Sleep Med Rev. 2008;12:5–17. doi: 10.1016/j.smrv.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Auchincloss JH, Jr, Cook E, Renzetti AD. Clinical and physiological aspects of a case of obesity, polycythemia and alveolar hypoventilation. J Clin Invest. 1955;34:1537–1545. doi: 10.1172/JCI103206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rapoport DM, et al. Hypercapnia in the obstructive sleep apnea syndrome. A reevaluation of the “Pickwickian syndrome”. Chest. 1986;89:627–635. doi: 10.1378/chest.89.5.627. [DOI] [PubMed] [Google Scholar]
- 66.Villa MP, et al. Sleep apnoea in children with diabetes mellitus: effect of glycaemic control. Diabetologia. 2000;43:696–702. doi: 10.1007/s001250051365. [DOI] [PubMed] [Google Scholar]
- 67.Polotsky VY, et al. The impact of insulin-dependent diabetes on ventilatory control in the mouse. Am J Respir Crit Care Med. 2001;163:624–632. doi: 10.1164/ajrccm.163.3.2007120. [DOI] [PubMed] [Google Scholar]
- 68.Punjabi NM, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 69.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsumura T, et al. Plasma orexin-A levels and body composition in COPD. Chest. 2003;123:1060–1065. doi: 10.1378/chest.123.4.1060. [DOI] [PubMed] [Google Scholar]
- 71.Lagi A, et al. Cerebral vasoconstriction in vasovagal syncope: any link with symptoms? A transcranial Doppler study. Circulation. 2001;104:2694–2698. doi: 10.1161/hc6172.099397. [DOI] [PubMed] [Google Scholar]
- 72.Norcliffe-Kaufmann LJ, Kaufmann H, Hainsworth R. Enhanced vascular responses to hypocapnia in neurally mediated syncope. Ann Neurol. 2008;63:288–294. doi: 10.1002/ana.21205. [DOI] [PubMed] [Google Scholar]
- 73.Antunes VR, et al. Orexins/hypocretins excite rat sympathetic preganglionic neurons in vivo and in vitro. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1801–1807. doi: 10.1152/ajpregu.2001.281.6.R1801. [DOI] [PubMed] [Google Scholar]
- 74.Dergacheva O, et al. Hypocretin-1 (orexin-A) facilitates inhibitory and diminishes excitatory synaptic pathways to cardiac vagal neurons in the nucleus ambiguus. J Pharmacol Exp Ther. 2005;314:1322–1327. doi: 10.1124/jpet.105.086421. [DOI] [PubMed] [Google Scholar]
- 75.Geerling JC, Mettenleiter TC, Loewy AD. Orexin neurons project to diverse sympathetic outflow systems. Neuroscience. 2003;122:541–550. doi: 10.1016/j.neuroscience.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Smith BN, et al. Selective enhancement of excitatory synaptic activity in the rat nucleus tractus solitarius by hypocretin 2. Neuroscience. 2002;115:707–714. doi: 10.1016/s0306-4522(02)00488-8. [DOI] [PubMed] [Google Scholar]
- 77.Smith PM, Connolly BC, Ferguson AV. Microinjection of orexin into the rat nucleus tractus solitarius causes increases in blood pressure. Brain Res. 2002;950:261–267. doi: 10.1016/s0006-8993(02)03048-2. [DOI] [PubMed] [Google Scholar]