Abstract
Orexin/hypocretin (orx/hcrt) neurons are thought to ensure that reward-seeking is accompanied by alertness, but the underlying circuit organization is unclear. Reports of differential regulation of lateral versus medial orx/hcrt cells produced a hypothesis of ‘efferent dichotomy’, in which lateral orx/hcrt cells innervate the ventral tegmental area (VTA) and control reward, while medial orx/hcrt cells innervate locus coeruleus (LC) and control arousal. Two distinct types of orx/hcrt cells also emerged from analysis of intrinsic and input-driven single-cell electrical activity. To examine the projections of these emerging orx/hcrt subtypes to LC and VTA, we injected retrograde tracer into these regions in the mouse brain in vivo, and then examined the properties of tracer-containing orx/hcrt cells in hypothalamic slices. VTA- and LC-projecting orx/hcrt cells were found across the entire orx/hcrt field, including the zona incerta, perifornical area, dorsomedial/anterior and lateral hypothalamus. Within these areas, orx/hcrt cells had similar probabilities of projecting to VTA or LC. Examination of lateral versus medial sections revealed that VTA and LC received inputs from both lateral and medial orx/hcrt cells, but, unexpectedly, lateral orx/hcrt cells were more likely to project to LC than medial orx/hcrt cells. Finally, patch-clamp recordings revealed that VTA and LC received projections from both electrical classes of orx/hcrt cells, which had similar likelihoods of projecting to VTA or LC. Contrary to previous predictions, our data suggest that medial and lateral orx/hcrt cells, and the different electrical and morphological subclasses of orx/hcrt cells identified to date, send projections to both LC and VTA.
Keywords: hypocretin, hypothalamus, mouse, orexin
Introduction
Effective goal-oriented behaviour requires coordination between brain areas controlling different functions. Such coordination is presumably achieved by widely projecting neurons that provide concurrent inputs to diverse brain areas. A recently discovered network of this kind is the orexin/hypocretin (orx/hcrt) circuit (de Lecea et al., 1998; Sakurai et al., 1998). Orx/hcrt peptides are neurotransmitters made by neurons whose cell bodies are located exclusively in the hypothalamus, but project widely throughout the brain (de Lecea et al., 1998; Sakurai et al., 1998). Arousal and reward-related brain centres receive dense inputs from orx/hcrt cells (Peyron et al., 1998). Such inputs are presumably of critical importance for brain state control, as emphasized by findings that loss of orx/hcrt cells leads to narcolepsy (Chemelli et al., 1999; Lin et al., 1999; Thannickal et al., 2000) and impairs the translation of reduced food availability into appropriate food-seeking behaviour (Yamanaka et al., 2003), while selective optical stimulation of orx/hcrt neurons causes awakening (Adamantidis et al., 2007). Thus, when the orx/hcrt system is activated by physiological stimuli such as fasting (Sakurai et al., 1998; Cai et al., 1999), it is thought to concurrently engage alertness and reward-seeking, thereby optimizing the success of goal-oriented behaviour (Burdakov & Alexopoulos, 2005; Sakurai, 2007).
Although the importance of the orx/hcrt system for arousal and reward is well defined, the circuit basis for this ‘multitasking’ is unclear. A recently proposed hypothesis is that arousal- and reward-regulating orx/hcrt cells are two topographically distinct neuronal populations (Harris & Aston-Jones, 2006). Specifically, medial orx/hcrt cells were hypothesized to control arousal while lateral orx/hcrt cells were proposed to control reward, in part through projections to locus coeruleus (LC) and ventral tegmental area (VTA), respectively (Harris & Aston-Jones, 2006). Although orx/hcrt signalling in LC and VTA is indeed important for arousal and reward (Hagan et al., 1999; Borgland et al., 2006), how the orx/hcrt system is topographically arranged in relation to projections to these areas has not been comprehensively examined. Apart from perifornical, dorsomedial and lateral hypothalamus, orx/hcrt cells are also found in zona incerta and anterior hypothalamus (Nambu et al., 1999), but where these cells project is unknown. Further heterogeneity in the orx/hcrt system is suggested by recent studies showing that orx/hcrt cells form two biophysically distinct subgroups, which encode different aspects of extracellular glucose concentration (Williams et al., 2008), display different voltage-gated currents and synaptic inputs (Schöne et al., 2011), and are differentially affected in Huntington’s disease (Williams et al., 2011). However, projection targets of these two orx/hcrt cell types have not been studied.
Thus it is currently unknown whether subgroups of mouse orx/hcrt neurons project to similar or different targets, and hence whether they are functionally overlapping or distinct in relation to arousal and reward. To obtain information directly relevant to this question, here we combined retrograde tracing, transgenic cell tagging, confocal microscopy and brain-slice electrophysiology to define the electrical properties and hypothalamic locations of mouse orx/hcrt neurons projecting to the LC and VTA.
Materials and methods
Retrograde tracing
All animal procedures were performed in accordance with the Animals (Scientific Procedures) Act, 1986 (UK), with appropriate institutional approvals and permits from the UK Government (Home Office). Transgenic mice that selectively express enhanced green fluorescent protein (eGFP) in orx/hcrt cells were used in all the experiments; this animal model is validated and described in detail in our previous studies (Burdakov et al., 2006). Six and seven mice were used for stereotaxic injections into the VTA and LC, respectively. The animals were 4–6 weeks old at the time of surgery. The retrograde tracer consisted of latex beads labelled with rhodamine (Red RetroBeads IX, Lumafluor, Durham, NC, USA). These beads are taken by nerve endings and transported back to the soma; no anterograde labelling or labelling of fibres of passage is known to occur with this tracer (Katz et al., 1984). For technical reasons, each animal was injected only once into either LC or VTA; thus we did not examine whether the same cells project to both areas. Injections were performed as described by Cetin et al. (2006) with a borosilicate glass pipette (1.0 mm OD, 0.6 mm ID, Narishige GD-1) pulled with a Zeitz DMZ horizontal puller to obtain a long (about 2 cm), narrow tip, which was then slightly cut with small surgical scissors. The pipette was filled with paraffin oil (Sigma, St Louis, MO, USA) and attached to thin tubing connected to a 10-μL Hamilton syringe mounted on a two-way infusion pump (Harvard Apparatus, Edenbridge, UK). Tubing and syringe were also filled with paraffin oil so that no air was left in the system. The pipette was mounted on the stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA), and its tip was dipped into a drop (4–8 μL) of tracer (diluted 1:3 in 0.9% NaCl) placed on a lid cut out from a 0.5-mL Eppendorf tube. Gentle suction was applied with the pump to load the tracer into the pipette.
Mice were anaesthetized with ketamine/medetomidine (75 mg/kg, 1 mg/kg i.p., in 0.9% NaCl), followed by a single dose of the analgesic carprofen (5 mg/kg s.c.). Once anaesthesia was achieved (as tested by a lack of paw withdrawal reflex upon pinching) the animals were placed on the stereotaxic frame, the hair on the dorsal surface of the head was trimmed with scissors, a drop of eye lubricant (Lubrithal) was applied to each eye to prevent dryness of the cornea, and the scalp was cut along the midline (about 1 cm long) with a scalpel to expose bregma and lambda. With the aid of the pipette tip and a dissection microscope, the head was levelled so that bregma and lambda were at the same horizontal plane. The coordinates of interest on the x- (medio-lateral) and y- (rostro-caudal) axes were then identified on the surface of the skull, and a small hole was drilled (with an Ideal Micro-Drill; round burr 0.6 mm) to gain access to the surface of the brain. The meninges were carefully torn with a fine needle, and then the pipette was lowered through the hole until its tip reached the desired z coordinate. The injection coordinates were (lateral/caudal/ventral relative to Bregma) for VTA: 0.45 mm/3.28 mm/4.45 mm; for LC: 0.83 mm/5.45 mm/3.8 mm, based on the atlas of Paxinos & Franklin (2001). The tracer was injected to deliver a volume of ∼50 nL. The infusion pump was then switched off and the pipette was left in place to let the tissue settle for 10 min before withdrawal. Finally, the borders of the scalp incision were brought together and glued with tissue adhesive (Vetbond 3M), the animals were removed from the frame and placed under a heat lamp, atipamezole (1 mg/kg s.c.) was given to help reverse the effects of anaesthesia, and NaCl 0.9% (about 10 μL/g) was also injected s.c. to help replace fluids lost during surgery. The animals were usually awake and walking within 1 h post-surgery. It has been previously shown that after 48 h, retrograde transport remains unchanged even after 10 weeks’ survival time (Katz et al., 1984); thus, animals were allowed to recover for at least 4 days before they were used for the electrophysiology and histology experiments described below. Typically, 80% of the injected region was filled with tracer (range 60–100%).
Validation of injection site specificity
In every animal used for analysis, the precision of injection was confirmed by direct examination of the full (three-dimensional) series of 200- to 300-μm-thick parasagittal brain slices covering the entire volume of the VTA or LC (typical examples of individual slices are shown in Fig. 1A and B). In each slice, we obtained composite confocal images consisting of both reflected (rhodamine fluorescence) and transmitted light (for anatomical reference), as described below. Note that both VTA and LC have clearly defined anatomical boundaries (see Supporting Information Fig. S2), and that with our transmitted light detector we could clearly see the neuronal cell bodies in our fixed tissue. For this reason, and because we targeted anatomical regions rather than specific cell populations within these regions, counterstaining was not necessary to detect region boundaries. The careful validation of injection sites that we performed was important because orx/hcrt fibres are present in areas adjacent to the VTA and LC (Peyron et al., 1998), and here we rejected from further analysis all brains where the tracer was found to ‘spill-over’ beyond the VTA or LC boundaries.
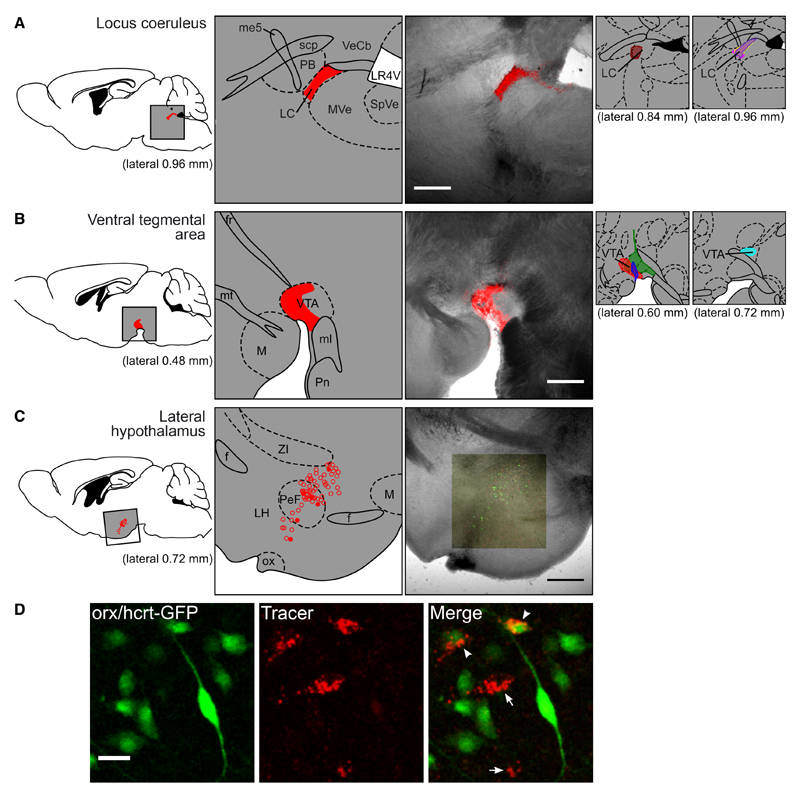
Fig. 1. Analysis of projection-defined orx/hcrt neurons.
(A, B) Typical examples of brain slices showing selective delivery of Retrobeads to LC (A) or VTA (B). Each image (third column) is a z-projection of a two-channel confocal series set to detect fluorescence (red, tracer) and transmitted light (black-and-white). The labelled diagram of the area (second column) and the reference figure (first column) are adapted from Paxinos & Franklin (2001). The smaller diagrams to the right show the anatomical placing of the tracer in the remaining animals used in these experiments. Here, the brain slice from each animal which contained the largest volume of tracer was plotted on the corresponding figure from the Mouse Brain Atlas, using one colour per animal. (C) A typical example image of orx/hcrt cells 6 days after injection of retrograde tracer into the LC. The map (middle) was created by superimposing the confocal image (right) to the best-matching figure from Paxinos & Franklin (2001), then marking eGFP cells without tracer (open circles) and eGFP cells with tracer (filled circles). (D) An example of high-magnification analysis of a group of orx/hcrt cells (green) 8 days after injection of retrograde tracer (red) into the LC. The image to the right shows cells that took up tracer and expressed eGFP (arrowheads) and cells that took up tracer but did not express eGFP (arrows). Scale bar: 500 μm (A–C), 25 μm (D). f, fornix; fr, fasciculus retroflexus; LC, locus coeruleus; LH, lateral hypothalamus; LR4V, lateral recess of the 4th ventricle; M, mammillary nucleus; me5, mesencephalic trigeminal tract; ml, medial lemniscus; mt, mammillothalamic tract; MVe, medial vestibular nucleus; ox, optic chiasm; PB, parabrachial nucleus; PeF, perifornical area; Pn, pontine nuclei; scp, superior cerebellar peduncle; SpVe, spinal vestibular nucleus; VeCb, vestibulocerebellar nucleus; VTA, ventral tegmental area; ZI, zona incerta.
Electrophysiology and imaging
Parasagittal hypothalamic brain slices 250 μm thick were prepared as in our previous studies (Gonzalez et al., 2008). Living orx/hcrt cells were identified with an Olympus BX51WI microscope equipped with epifluorescence, a mercury lamp, and appropriate filters for visualizing rhodamine and eGFP. During electrophysiological recordings, extracellular solution was artificial cerebrospinal fluid (composition in mm: NaCl 125, KCl 2.5, MgCl2 2, NaH2PO4 1.2, NaHCO3 21, CaCl2 2, glucose 1) oxygenated with 5% CO2/95% O2. Whole-cell current-clamp recordings were made from tracer- and eGFP-containing cells at 35 °C, as in our previous studies (Gonzalez et al., 2008). Patch pipettes were filled with a solution containing (in mm): K-gluconate 120, HEPES 10, KCl 10, EGTA 1, MgCl2 2, K2ATP 4 and Na2ATP 1. Data were acquired using Patchmaster software (Heka, Pfalz, Germany), analysed using custom-written software in Python (http://www.python.org) and plotted with Matplotlib (http://www.mat-plotlib.sourceforge.net). Type H and Type D orexin cells were electrophysiologically distinguished by the nature of post-inhibitory rebound potential (hyperpolarized and depolarized respectively, after a 1-s current injection that brought the cells to −80 mV; see Fig. 4B), following criteria in Williams et al. (2008) and Schöne et al. (2011). After recording, brain slices were fixed overnight in paraformaldehyde [4% in phosphate-buffered saline (PBS)] at 4 °C, rinsed in PBS three times for 10 min at room temperature, and mounted onto gelatin-coated slides in Vectashield Mounting Medium (Vector Laboratories, Peterborough, UK). The slides were stored at 4 °C and protected from light until analysis. Images were acquired with an upright confocal microscope (Olympus BX61WI with Fluoview 1000). Rhodamine and eGFP were excited at 559 and 488 nm, respectively, and fluorescence collected at 570–670 and 500–545 nm, respectively. Optimal separation of fluorescence signals was achieved using a sequential scanning mode. We analysed the entire extent of the orx/hcrt field, which in our mouse model stretched from 0.48 to 1.08 mm latero-medially, and from Bregma from −0.75 to −2.25 mm rostro-caudally.
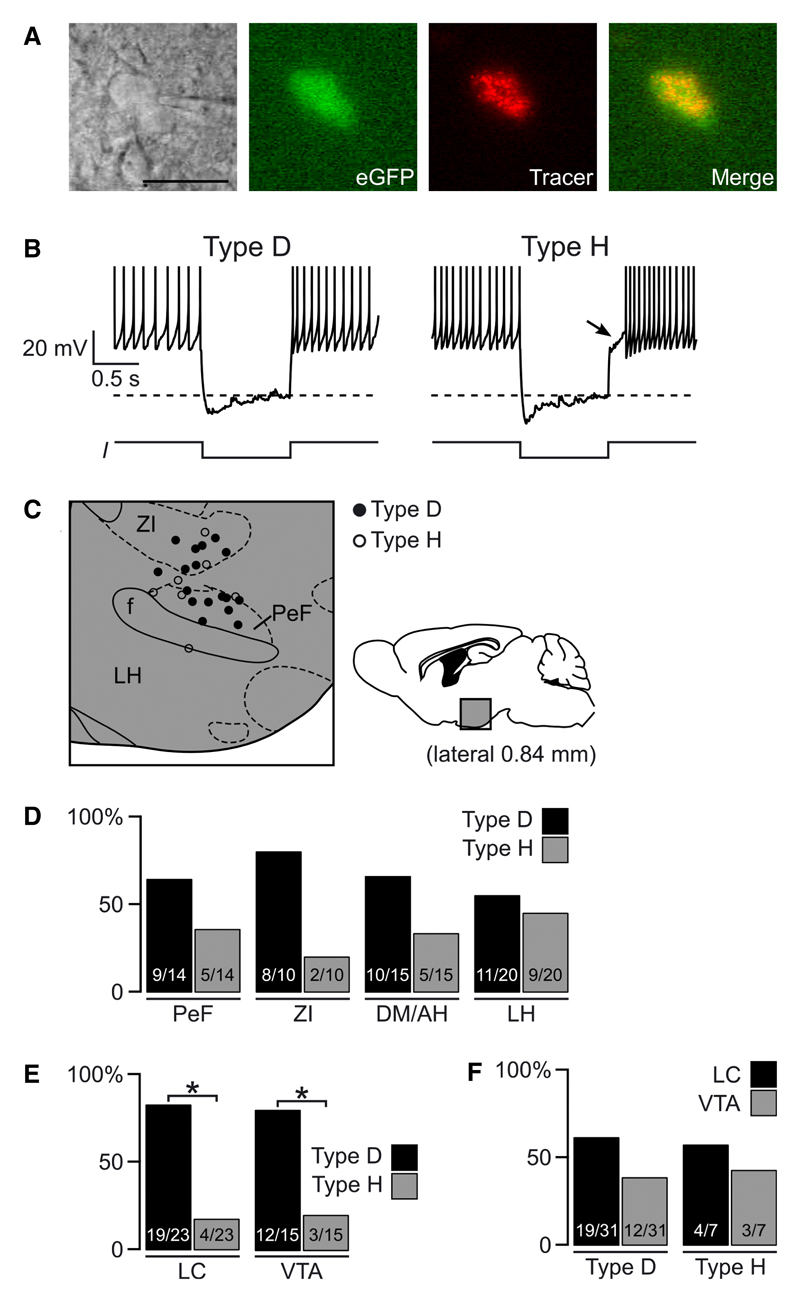
Fig. 4. Intrinsic electrical properties of orx/hcrt cells innervating LC or VTA.
(A) Example of a tracer-containing eGFP cell during whole-cell recording. Scale bar = 30 μm. (B) Examples of the two electrical subtypes of orx/hcrt cells; note delayed repolarization (arrow) after a current (I) injection (shown below each trace) that hyperpolarizes the membrane to −80 mV (dotted line). Action potentials are truncated at 0 mV. (C) Representative example of topographic distribution of H (○) and D (●) cells in a sagittal section 0.84 mm from midline (quantified for different hypothalamic regions in D). (D) Proportion of H and D orx/hcrt cells in different hypothalamic regions. Figures inside bars are number of cells of each type/total number of orx/hcrt cells in that hypothalamic region. (E) Proportion of Type H and Type D orx/hcrt cells that project to LC or VTA. In each case, the population of orx/hcrt cells was composed of significantly more D than H cells (*P < 0.05, exact binomial test). Numbers inside bars are orx/hcrt cells of each type/total number of orx/hcrt cells that project to the area indicated. (F) Proportion of orx/hcrt cells that project to LC or VTA grouped by cell type. Like D cells, H cells projected to LC and VTA in a statistically equal proportion (P > 0.2, exact binomial test). Figures inside bars are number of LC- or VTA-projecting orx/hcrt cells/number of orx/hcrt cells of the type indicated.
Cell counts
For cell counts, we took three-dimensional (z-stack) confocal images of the orx/hcrt field in each parasagittal slice with a 10× water-immersion objective; both tracer beads and eGFP-containing cells were clearly visible at this magnification (e.g. see Fig. 1D). At this magnification, one z-stack per brain slice was enough to include the full extent of the orx/hcrt field so it was not necessary to overlap images along the x- or y-axes. Note that when counting tracer-containing and tracer-negative orx/hcrt-eGFP cells, we had the full confocal z-series available, and could thus navigate up and down the z-axis to remove any ambiguity in assigning tracer to GFP cells and in distinguishing between cells. Any artefacts related to cell overlap or cell size were therefore highly unlikely. Regardless, the impact of such artefacts on our results would be minimal, because we examined the ratios of (tracer + GFP cells)/(total GFP cells), i.e. the proportion of co-localized cells would be unchanged even if the same cell was counted twice. No formal stereology was necessary for these reasons.
To assign cells to anatomical structures accurately, the location of each fluorescent cell (first examined for green and red fluorescence at 10× magnification) was marked (using ImageJ, http://rsb.info.nih.gov/ij/) and then superimposed on lower magnification (5× objective) transmitted light images. The latter were obtained as the confocal laser traverses the tissue onto a photomultiplier detector, creating images that resemble those obtained with differential interference contrast microscopy. In these images the boundaries between different anatomical structures are clearly discernible (see Fig. 1A–C, right column), thus making counterstaining unnecessary and allowing us to overlay these unambiguously with matching diagrams from the anatomical atlas (Paxinos & Franklin, 2001) (see Supporting Information Fig. S1).
Statistics
For analysis of the topographical distribution of fluorescent cells, tracer-positive and tracer-negative eGFP cells were grouped into anatomical regions in the lateral hypothalamic area (Fig. 2) or into ‘medial’ and ‘lateral’ locations with respect to the fornix (Fig. 3). Because of the categorical nature of the data thus grouped, statistical analyses were performed using a two-sided Fisher’s exact test for count data. As is appropriate for this test, in all cases we used raw cell counts of mutually exclusive groups (tracer-positive eGFP cells and tracer-negative eGFP cells); the relative (percentage) values were only used for presentation purposes and not for statistical analysis. For analysis of hypothalamic distribution and VTA/LC connection likelihood of Type H and Type D orx/hcrt cells (Fig. 4D–F), we used a two-sided exact binomial test with the null hypothesis that half of orx/hcrt cells were Type H and half were Type D. Statistical analysis was performed with R software version 2.13.1 (http://www.r-project.org), and differences were considered significant at P < 0.05.
Fig. 2. Sagittal topography of orx/hcrt cells innervating LC or VTA.
(A) Typical examples of sagittal diagrams showing orx/hcrt cells found at around 0.84 mm from the midline. Open circles denote orx/hcrt-eGFP cells without tracer and filled circles are orx/hcrt-eGFP cells which contained tracer after injections into LC or VTA (as labelled above the sketches). Similar maps were created for each parasagittal section in the lateral range 0.48–1.08 mm (Paxinos & Franklin, 2001, figs 105–110). (B) Proportion of orx/hcrt-eGFP cells that contained tracer after it was injected into the LC. The cells were grouped into four distinct hypothalamic regions. No statistically significant differences between regions were found (P > 0.05, Fisher’s exact test). Figures inside bars are number of eGFP cells with tracer/total number of eGFP cells. (C) Proportion of orx/hcrt-eGFP cells that contained tracer after it was injected into the VTA, grouped into four hypothalamic regions. No statistically significant differences between regions were found (P > 0.05, Fisher’s exact test). The number of orx/hcrt cells projecting to LC versus VTA in the corresponding regions shown in B and C were also not significantly different (P > 0.05 for each region compared, Fisher’s exact test). Numbers inside bars are eGFP cells with tracer/total number of eGFP cells. ZI, zona incerta; PeF, perifornical area; DM/AH, dorsomedial/anterior hypothalamus; LH, lateral hypothalamus.
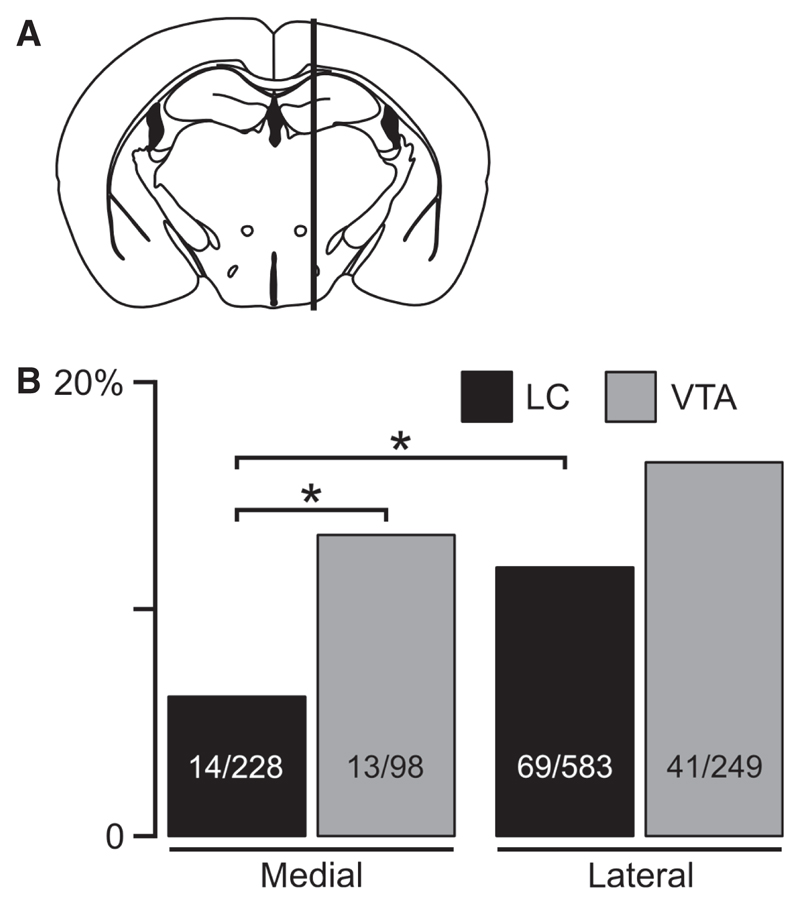
Fig. 3. Medio-lateral distribution of orx/hcrt cells innervating LC or VTA.
(A) The hypothalamus was divided into lateral and medial regions by a vertical line through the fornix (following convention in previous studies, see Results). Orx/hcrt cells that were mapped medial or lateral to this line were grouped together. (B) Proportion of orx/hcrt cells that project to LC or VTA in lateral and medial parts of the lateral hypothalamic area. Only significant differences are indicated (*P < 0.05, Fisher’s exact test). Numbers inside bars are eGFP cells with tracer/total number of eGFP cells.
Results
Identifying orx/hcrt neurons that project to LC or VTA
To study the properties of projection-defined orx/hcrt neurons, we stereotaxically injected fluorescent retrograde tracer beads into brains of mice expressing eGFP under the control of the human prepro-orexin promoter (see Methods). In this mouse line, eGFP is a highly selective and reliable marker of orx/hcrt cells (Yamanaka et al., 2003; Burdakov et al., 2006). Using the injection conditions described in the Methods, we achieved tracer delivery well-localized to LC (Fig. 1A) or, in a different set of animals, to VTA (Fig. 1B). Four days after injection, we observed tracer accumulation in cell bodies in the tuberal hypothalamus (Fig. 1C). We were able to clearly resolve which orx/hcrt cells (eGFP cells) contained the tracer, both in formaldehyde-fixed tissue (Fig. 1D) and in acutely isolated living brain slices (see Fig. 4A).
Sagittal topography of orx/hcrt cells that project to VTA or LC
To quantify the number of orx/hcrt cells that project to VTA and LC, we injected tracer into either VTA or LC, and then counted tracer-labelled and unlabelled orx/hcrt-eGFP cells in a series of sagittal sections of lateromedial span 0.48–1.08 mm (Fig. 2A; cell counts are given in the figures). We performed this analysis separately in the four hypothalamic regions where orx/hcrt cells were found: zona incerta (ZI), perifornical area (PeF), dorsomedial/anterior hypothalamus (DM/AH) and lateral hypothalamus (LH) (see Methods). We found LC-projecting orx/hcrt cells in all hypothalamic regions where orx/hcrt cells are distributed (Fig. 2B), with no significant differences in the number of LC-projecting orx/hcrt cells in the four regions examined (P = 0.208, Fisher’s exact test, see Methods). We found a similar ‘spread-out’ distribution for orx/hcrt cells projecting to VTA (Fig. 2C), with no significant differences in the number of orx/hcrt cells that project to VTA in the four regions examined (P = 0.170, Fisher’s exact test). Within each of the four regions examined, the probabilities of orx/hcrt cells projecting to LC or to VTA were also not significantly different (PeF, P = 0.080; ZI, P = 0.055; DM/AH, P = 0.226; LH, P = 0.865; Fisher’s exact test).
Coronal topography of orx/hcrt cells that project to VTA or LC
The above data suggest that orx/hcrt cells that project to VTA or LC are spread out across ZI, PeF, DM/AH and LH. For comparison with other published data, we also ran a similar analysis that, instead of the formal anatomical subdivisions analysed above, was based on grouping GFP cells into a medial (plates 0.48, 0.60 and 0.72 mm, Paxinos & Franklin, 2001) and a lateral (plates 0.84, 0.96 and 1.08 mm, Paxinos & Franklin, 2001) group, according to their distance from the midline in parasagittal slices. This grouping was equivalent to that which would have been obtained with a vertical line through the fornix in a coronal slice, and was designed to be consistent with Fadel et al. (2002) and Williams et al. (2008); see Fig. 3A (the rostro-caudal range we examined was bregma –0.75 to –2.25 mm). Within the medial region, orx/hcrt cells were more likely to project to VTA than to LC (Fig. 3B, P = 0.047, Fisher’s exact test). Within the lateral region, the number of VTA-projecting orx/hcrt cells was not significantly different from LC-projecting orx/hcrt cells (Fig. 3B, P = 0.075). When the two regions were compared, orx/hcrt cells in the lateral region were more likely to project to the LC than those in the medial region (P = 0.015, Fisher’s exact test), whereas medial and lateral orx/hcrt cells were equally likely to project to the VTA (P = 0.776; Fig. 3B). This suggests that lateral orx/hcrt cells may provide a denser projection to the LC than medial orx/hcrt cells, which is unexpected considering the current models of orx/hcrt system organization (see Discussion).
Electrical properties of orx/hcrt cells that project to VTA or LC
To determine whether VTA- and LC-projecting orx/hcrt cells correspond to the two previously described electrical subtypes of orx/hcrt cells (Williams et al., 2008, 2011; Schöne et al., 2011), we performed whole-cell current-clamp recordings from tracer-containing orx/hcrt-eGFP cells in acute living brain slices (Fig. 4A). We used a simple current-clamp protocol involving a 1-s hyperpolarization to −80 mV (Fig. 4B) to classify orx/hcrt as Type H cells (hyperpolarized post-inhibitory rebound) or Type D cells (depolarized post-inhibitory rebound), following previously described criteria (Williams et al., 2008; Schöne et al., 2011). In sagittal sections, Type D cells were generally more prevalent than Type H cells in ZI, PeF and DM/AH, but the two cell types were about equally prevalent in LH (Fig. 4D). This is consistent with previous analysis in coronal sections (Williams et al., 2008). Overall, we succeeded in obtaining high-quality current-clamp recordings from 38 neurons that contained both tracer beads and hcrt/orx-eGFP (Fig. 4A), of which 31 were Type D and seven were Type H cells, in agreement with the relative prevalence of the two cell types described previously (Williams et al., 2008). When the tracer was injected into the VTA or LC, both Type H and Type D orx/hcrt cells became labelled in the hypothalamus, suggesting that projections from the two cell types converge on both VTA and LC. When we grouped these cells by electrical fingerprint and by projection target, we found that both VTA and LC were more likely to receive inputs from Type D than Type H orx/hcrt cells (Fig. 4E; LC, P = 0.003; VTA, P = 0.035, exact binomial test). This suggests that the two cell types project to the VTA and LC in proportion to their relative numbers in the hypothalamus (Type D > Type H, Fig. 4D). Indeed, when we analysed the projections of each electrical class separately, we found similar likelihoods of VTA or LC projection within each cell class (Fig. 4F; Type D, P = 0.281; Type H, P = 1, exact binomial test).
Discussion
Accumulating data suggest that orx/hcrt neurons fall into distinct subpopulations with regard to intrinsic electrical and synaptic properties (Schöne et al., 2011), glucose-sensing (Williams et al., 2008), susceptibility to neurodegeneration (Williams et al., 2011), topographic distribution of neural inputs (Yoshida et al., 2006) and regulation by different drugs and behavioural paradigms (Fadel et al., 2002; Harris et al., 2005). In this study, we aimed to determine whether such emerging orx/hcrt subgroups diverge or converge in their output projections to the LC and VTA. Although orx/hcrt neurons are likely to regulate reward and arousal by projecting to multiple brain areas (Peyron et al., 1998), here we focused on LC and VTA because (1) these two areas have been specifically proposed to receive projections from topographically distinct orx/hcrt cells (Harris & Aston-Jones, 2006), and (2) orx/hcrt actions in the LC and VTA have been directly shown to regulate arousal and reward (Hagan et al., 1999; Borgland et al., 2006).
Examination of neural inputs to orx/hcrt neurons in the rat revealed that orx/hcrt cells in the medial and perifornical areas of the orx/hcrt field are preferentially innervated by hypothalamic regions, whereas more lateral orx/hcrt are preferentially innervated by the brainstem and reward-related nuclei (Yoshida et al., 2006). Together with findings on differential regulation of lateral versus medial orx/hcrt cells by inputs related to arousal and reward, this led to the ‘divergent projection’ hypothesis: a topographic segregation of orx/hcrt neurons into lateral and medial groups projecting to reward- and arousal-related areas, respectively (Harris & Aston-Jones, 2006; Fig. 2). At least in relation to projections to the VTA and LC, our analysis of the entire extent of the orx/hcrt field in the mouse (Fig. 2) supports an alternative model, that arousal- and reward-regulating orx/hcrt cells are not topographically segregated into subregions, but instead are spread throughout the orx/hcrt field. It is noteworthy that our data in the mouse do not contradict the existing data in the rat. For example, Espana et al. (2005) reported no major difference in the topographic distribution of LC-injected retrograde label in medial and lateral orx/hcrt-immunoreactive cells. In turn, Fadel et al. (2002) examined topographic properties of VTA-projecting orx/hcrt cells in the rat, and found that they are present in both LH and PeF. In addition, our new study for the first time compared the likelihoods of topographically distinct orx/hcrt clusters of projecting to LC versus VTA. Unexpectedly, and contrary to hitherto proposed models, we found that in the medial part of the orx/hcrt field, a greater proportion of orx/hcrt cells project to VTA than to LC (Fig. 3B).
Although our results do not rule out the possible existence of topographic clusters of orx/hcrt neurons projecting to areas other than VTA or LC (Peyron et al., 1998), they do show that, contrary to the ‘divergent projection’ hypothesis, there is no absolute topographic separation of ‘arousal’- and ‘reward’-regulating orx/hcrt cells in the mouse. It is thus possible that previous reports of differential regulation of medial versus lateral orx/hcrt cells (reviewed by Harris & Aston-Jones, 2006) could be due to the documented topographic differences in regulatory inputs to the orx/hcrt cells (Yoshida et al., 2006), rather than differences in orx/hcrt efferent projections. We propose that convergent projections from different orx/hcrt populations to LC and VTA may strengthen the temporal association between arousal and reward-seeking, which is essential to optimize the success of goal-oriented behaviours.
Our new results also reveal similar likelihoods of projecting to LC or VTA for the two distinct electrical subtypes of orx/hcrt neurons that were described previously, but whose projection targets were hitherto unknown (Schöne et al., 2011). Previous work implies that, of these two electrical orx/hcrt subtypes, Type D cells serve as ‘adaptive’ glucose sensors, while Type H cells have more sustained responses to glucose, which may encode absolute magnitude and relative changes of glucose levels, respectively (Williams et al., 2008). Furthermore, in a mouse model of Huntington’s disease, Type H cells also selectively lose membrane area and receive abnormally increased glutamate drive, while Type D cells receive a reduced glutamate input (Williams et al., 2011). In relation to the new findings reported here, this may suggest that the orx/hcrt system conveys a complex representation of glucose homeostasis to both arousal- and reward-regulating areas, and that neurode-generative changes in Type H or Type D orx/hcrt cells are likely to impact on both arousal and reward.
In summary, this study represents the first description of projection targets of the recently discovered subclasses of mouse orx/hcrt cells. These new data offer new interpretations for previous studies in the field, suggesting that the functional and anatomical subdivisions in the orx/hcrt system discovered so far control both LC and VTA, and thus at least partially overlap in the regulation of arousal and reward. This clarification may help us to understand the role of the orx/hcrt system in normal brain function and neurological disorders.
Supporting Information
Additional supporting information may be found in the online version of this article:
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset by Wiley-Blackwell. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Acknowledgements
This work was funded by the European Research Council (FP7).
Abbreviations
- DM/AH
dorsomedial/anterior hypothalamus
- eGFP
enhanced green fluorescent protein
- LC
locus coeruleus
- LH
lateral hypothalamus
- orx/hcrt
orexin/hypocretin
- PeF
perifornical area
- VTA
ventral tegmental area
- ZI
zona incerta
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Alexopoulos H. Metabolic state signalling through central hypocretin/orexin neurons. J Cell Mol Med. 2005;9:795–803. doi: 10.1111/j.1582-4934.2005.tb00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O’Kelly I, Gerasimenko O, Fugger L, Verkhratsky A. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Cai XJ, Widdowson PS, Harrold J, Wilson S, Buckingham RE, Arch JR, Tadayyon M, Clapham JC, Wilding J, Williams G. Hypothalamic orexin expression: modulation by blood glucose and feeding. Diabetes. 1999;48:2132–2137. doi: 10.2337/diabetes.48.11.2132. [DOI] [PubMed] [Google Scholar]
- Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nat Protoc. 2006;1:3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Espana RA, Reis KM, Valentino RJ, Berridge CW. Organization of hypocretin/orexin efferents to locus coeruleus and basal forebrain arousal-related structures. J Comp Neurol. 2005;481:160–178. doi: 10.1002/cne.20369. [DOI] [PubMed] [Google Scholar]
- Fadel J, Bubser M, Deutch AY. Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci. 2002;22:6742–6746. doi: 10.1523/JNEUROSCI.22-15-06742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Metabolism-independent sugar sensing in central orexin neurons. Diabetes. 2008;57:2569–2576. doi: 10.2337/db08-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Katz LC, Burkhalter A, Dreyer WJ. Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature. 1984;310:498–500. doi: 10.1038/310498a0. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999;827:243–260. doi: 10.1016/s0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2001. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schöne C, Venner A, Knowles D, Karnani MM, Burdakov D. Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol. 2011;589:2767–2779. doi: 10.1113/jphysiol.2011.208637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Alexopoulos H, Jensen LT, Fugger L, Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proc Natl Acad Sci USA. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RH, Morton AJ, Burdakov D. Paradoxical function of orexin/hypocretin circuits in a mouse model of Huntington’s disease. Neurobiol Dis. 2011;42:438–455. doi: 10.1016/j.nbd.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, ichi Yagami K, Sugiyama F, Goto K, Yanagisawa M, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.