In October 16th issue of Nature (1986), Louis Kunkel and his associates of Harvard Medical School announced the isolation of the gene the defect of which results in Duchenne muscular dystrophy and showed that its mRNA is as large as 16 kb.1) This suggests that the gene product has a huge molecular weight of more than 500,000. The possible candidate proteins, hitherto known, are nebulin (MW, 700 kDa) and connectin (also called titin; MW, 3000 kDa) in skeletal muscle, as first suggested by Orkin.2)
Nebulin was isolated in a denatured form and its localization at N2 line in a sarcomere was shown by Wang,3) but its physiological role in muscle function has remained obscure. Connectin is an elastic filamentous protein linking myosin filaments to Z lines in a sarcomere.4)
Wood and members of Columbia University reported in January 8 issue of New England Journal of Medicine that nebulin is largely deficient in skeletal muscles of 30 Duchenne muscular dystrophy patients examined by an SDS (sodium dodecyl sulfate) gel electrophoresis.5) Thus it has been suggested that nebulin may be the product of Duchenne muscular dystrophy (DMD) gene. We have also studied this problem, and reached a conclusion different from the above authors using immunoblot technique. The present communication describes that nebulin and also connectin tend to be more or less degraded in DMD muscles, yet the both proteins are evidently expressed in DMD muscles.
Materials and methods
Human biopsied muscles stored at -80°C were homogenized in 10 val. of an SDS solution (10% SDS, 50 mM dithiothreitol,10 mM EDTA and 0.1 M Tris-HC1p, H 8.0) and heated for 2 min at 90°C. After insoluble debris was removed by centrifugation, the supernatant was subjected to an SDS gel electrophoresis according to Fairbanks et al.6) using 2-12% polyacrylamide gradient gels. Sometimes, Laemmli's7) or Weber-Osborn's8) system was used. The protein bands were transferred to a nitrocellulose sheet by the method of Towbin et al.9) The sheets were treated with anti-chicken nebulin or anti-chicken connectin antiserum raised in rabbits. The protein bands were detected by alkaline phosphatase-labeled goat anti-rabbit IgG antibodies.
Results
Three groups of human skeletal muscles were examined for nebulin and connectin by immunoblot techniques: normal (a, 6 months; b, 1 year and one month; c, 2 years and 2 months), preclinical muscular dystrophy (a, 1 year and 5 months; b, 2 years and 2 months) and symptomatic muscular dystrophy (a, 2 years and 9 months; b, 6 years; c, 8 years). The results are summarized in Fig. 1. In normal skeletal muscle, both nebulin and connectin bands were clearly recognized in Coomassie Blue stained gels (left lane in each panel 1) and immunoblots identified their presence (middle (connectin) and right (nebulin) lanes). Note that there were 400 kDa fragments of connectin reacted with anti-connectin. In preclinical (panel 2) and symptomatic muscular dystrophy (panel 3) muscles, there were seen several bands around nebulin (each left lane). However, from the immunoblot (each right lane), it was possible to identify the nebulin band in all the cases. It is likely that other bands were derived either from connectin or from nebulin. Due to weak reactivity with the antiserum, it was not possible to identify each of them. It appeared that the reactivities with anti-nebulin and with anti-connectin were weaker in muscular dystrophy (panels 2 and 3) than in control muscle (panel 1).
Fig. 1.
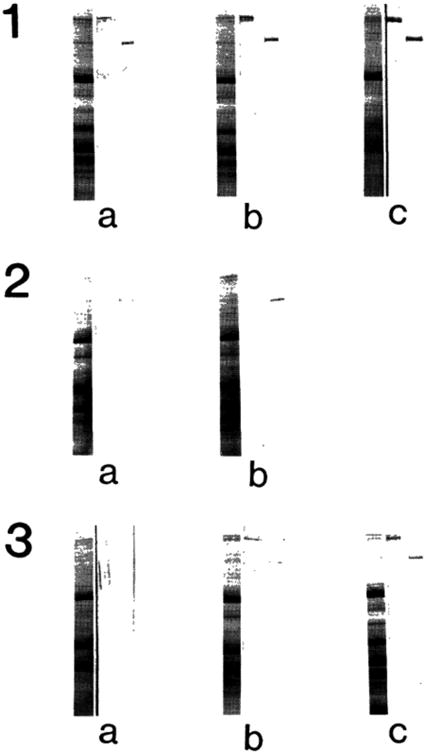
Detection of nebulin and connectin in an SDS extract of the biopsied muscle of Duchenne muscular dystrophy. Panel 1, normal; Panel 2, preclinical stage; Panel 3, symptomatic stage. Left lane of each panel, Coomassie Brilliant Blue stained protein bands; Middle lane, immunoblot treated with anti-connectin serum; Right lane
An SDS gel electrophoresis pattern clearly shows that nebulin band was much less distinct in Duchenne dystrophy muscle than in control, as shown in Fig. 2. It is also evident that α-connectin was less and β-connectin was more in dystrophic muscle than in control.
Fig. 2.
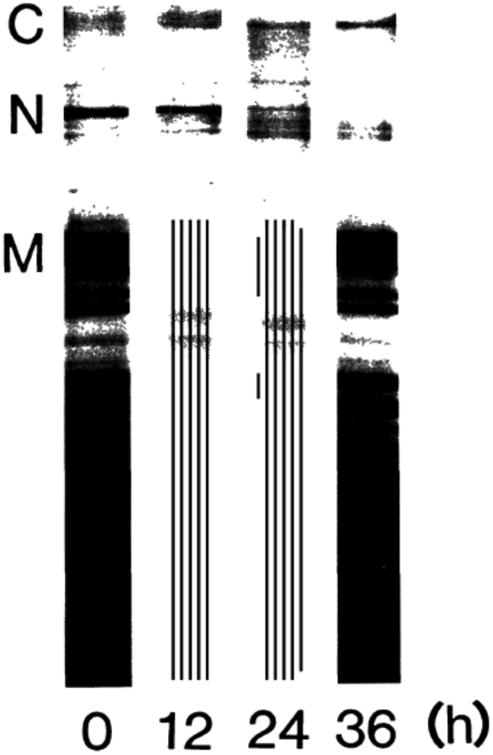
SDS gel electrophoresis patterns of Duchenne dystrophy muscle. Weber-Osborn method.8) N, normal; D, dystrophy.Cα, α-connectin; Cβ, β-connectin; n, nebulin; M, myosin heavy chain.
Our work strongly suggests that both nebulin and connectin are expressed in Duchenne muscular dystrophy. However, both proteins tend to be degraded into smaller polypeptides. For the latter, we could follow the degradation process of both proteins, when freshly excised chicken breast muscles were stored at 0°C. As shown in Fig. 3, there were not appreciable changes up to 12 hr, but at 24 hr both connectin and nebulin were considerably degraded as seen in dystrophy muscle and at 36 hr nebulin disappeared completely. These degradations were apparently due to the hydrolytic actions of endogenous proteases.
Fig. 3.
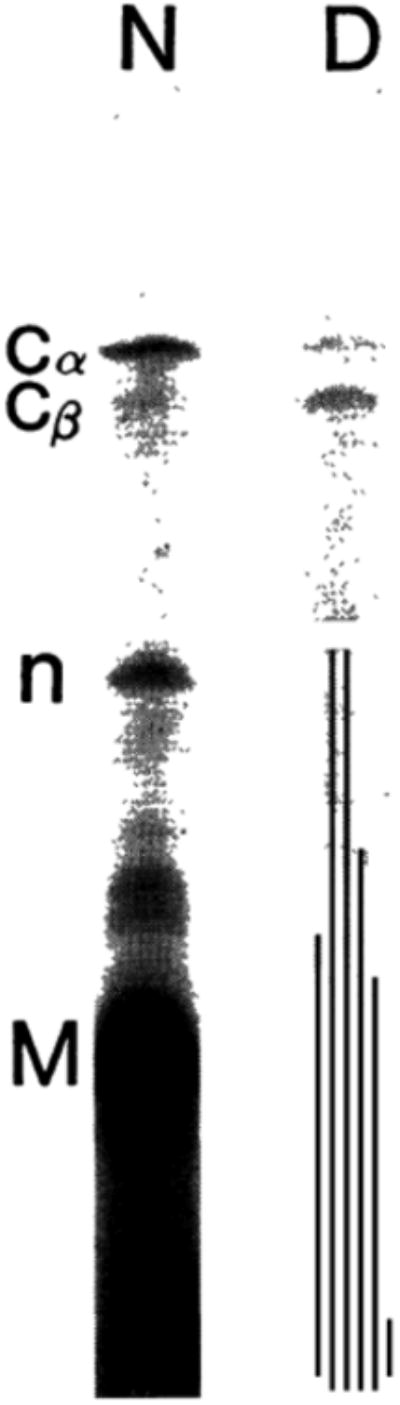
Degradation of nebulin and connectin in chicken breast muscle during storage at 0°C. Stored at 0°C for hours shown under each lane. Laemmli method.7) C, connectin; N, nebulin; M, myosin heavy chain.
Discussion
Wood et al.5) showed that nebulin band was absent or extremely faint in SDS gel electrophoresis patterns of Duchenne dystrophy muscles. However, on closer examinations, there were several additional bands below nebulin suggesting its degradation. This is in good agreement in the present results, although the presence of nebulin in dystrophy muscle was evident from the immunoblot technique in this investigation.
It is well known that proteinases, especially calcium activated neutral proteinase, are activated in Duchenne dystrophy muscle (see Ref. 10) for a review). The calcium activated proteinase quickly hydrolyzes nebulin in myofibrils.11) Therefore, it is likely that nebulin is degraded by the action of this enzyme in dystrophy muscle.
In conclusion, it is unlikely that nebulin is the product of Duchenne muscular dystrophy gene.
Acknowledgments
We wish to express our cordial thanks to Prof. Y.Nonomura of the University of Tokyo for his helpful advice and suggestions. Acknowledgements are also due to Prof. S. Ebashi, M. J. A., for his warm encouragements. This work was supported by Grant nos. 86-01 and 02 from the National Center of Neurology and Psychiatry (NCNP) of the Ministry of Health and Welfare, Japan and a grant-in-aid from the Muscular Dystrophy Association, Inc.
References
- 1.Monaco AP, et al. Nature. 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 2.Orkin SH. Cell. 1986;47:845–850. doi: 10.1016/0092-8674(86)90799-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Williamson CL. Proc Natl Acad Sci US. 1980;77:3254–3258. doi: 10.1073/pnas.77.6.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maruyama K. Int Rev Cytol. 1986;104:81–114. doi: 10.1016/s0074-7696(08)61924-5. [DOI] [PubMed] [Google Scholar]
- 5.Wood DS, et al. New Eng J Med. 1987;316:107–108. [PubMed] [Google Scholar]
- 6.Fairbanks G, Steck TL, Wallach DFH. Biochemistry. 1971;0:2607–2617. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- 7.Laemmli UK. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Weber K, Osborn M. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 9.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci US. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obinata T, et al. Muscle & Nerve. 1981;4:456–488. doi: 10.1002/mus.880040604. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama K, et al. J Biochem. 1981;89:711–715. doi: 10.1093/oxfordjournals.jbchem.a133250. [DOI] [PubMed] [Google Scholar]


