Abstract
Drug-reward cues trigger motivational circuitry, a response linked to drug-seeking in animals and in humans. Adverse life events have been reported to increase sensitivity to drug rewards and to bolster drug reward signaling. Therefore, we hypothesized that cocaine-dependent individuals with prior emotional, physical and sexual abuse might have a heightened mesolimbic brain response to cues for drug reward in a new brief-cue probe. Cocaine-dependent human individuals (N = 68) were stabilized in an inpatient setting and then completed an event-related blood-oxygen-level dependent functional magnetic resonance imaging task featuring 500-ms evocative (cocaine, sexual, aversive) and comparator (neutral) cues. Responses to three questions about emotional, physical and sexual abuse from the Addiction Severity Index were used to divide the patients into subgroups (history of Abuse [n = 40] versus No Abuse [n = 28]). When subjects were grouped by the historical presence or absence of emotional, physical or sexual abuse, the Abuse group showed a heightened midbrain, thalamic, caudate, and caudal orbitofrontal cortex response to cocaine cues; a similar result was found in other evocative cues, as well. These findings are the first reported for a 500-ms cocaine-cue probe, and they highlight the ability of very brief evocative cues to activate the brain’s motivational circuitry. Although all participants had severe cocaine use disorders, individuals reporting prior abuse had a heightened mesolimbic response to evocative cues. To our knowledge, this is the first study in humans linking a history of abuse to a brain vulnerability (heightened mesolimbic response to drug cues) previously shown to contribute to drug-seeking.
Keywords: Abuse, addiction, cocaine, fMRI, limbic, stress
INTRODUCTION
Individuals with substance-use disorders face a multitude of problems, including issues related to health, social and family relations, occupation, criminal justice and homelessness (National Institute on Drug Abuse 2007). Among these issues is an overrepresentation of prior physical and sexual abuse, with rates up to 50 percent in some samples (Ouimette et al. 2000; Rice et al. 2001; Rosen et al. 2002; Pirard et al. 2005; Charney, Palacios-Boix, & Gill 2007). In one study, when emotional abuse was assessed, as well, 59 percent of people with addiction reported at least one type of abuse (Rice et al. 2001). The abuse rates in these studies were captured by questions from the Addiction Severity Index (ASI) (McLellan et al. 1992), which fortunately are similar in content and structure to other brief questionnaires commonly used to measure abuse (Gil-Rivas et al. 1997; Felitti et al. 1998).
Abuse can be experienced as an acute or chronic stressor (Hyman, Paliwal, & Sinha 2007; Sinha 2008). There is a well-established connection between psychological stress and addiction (Koob and Volkow, 2010). Importantly, just as there is an overlap in the prevalence of stress and addiction (E. Goeders 2003; Sinha 2008; See & Waters 2010; Bossert et al. 2013) there is also an overlap of stress and reward signaling in the brain (D’Angio et al. 1987; Sorg & Kalivas 1991; Rougé-Pont et al. 1993). We hypothesize that even within an addicted population, the presence or lack of abuse in one’s history may create brain differences in response to external, motivationally significant stimuli.
Indeed, studies have shown that abuse is associated with modulated dopamine signaling in mesolimbic brain regions (Pruessner et al. 2004; Dillon et al. 2009), comprised of areas that process drug and non-drug rewards. Extensive research has linked stress and reward signaling to drug seeking in animals (Belin et al, 2013; Saal et al, 2003; Wise, 2004) and to relapse in humans (Fatseas et al., 2011; McHugh et al., 2004; Stewart, 2000).
Researchers have posited that a history of abuse might be associated with stress-related problems like relapse (Hyman et al., 2007), but how that might be reflected at the level of the brain has only recently begun to be addressed (Elton et al., 2015). Our own lab (Childress et al. 1999; Franklin et al. 2007; Langleben et al. 2008; Wetherill et al. 2014; Young et al. 2014) and others (Chase et al. 2011; Kühn & Gallinat 2011) have shown that drug cues activate motivational circuits. The brain findings are robust, but there is intriguing individual variability (Jasinska et al. 2014)—and it could reflect historical variables, such as abuse. Thus, we set out to investigate whether lifetime abuse would be reflected in the response of mesolimbic circuitry to cocaine cues. Given the interaction of stress and reward circuitry, we predicted that individuals with cocaine-use disorders and a history of abuse might have an enhanced response to cocaine cues, as compared to their non-abused counterparts.
Finally, previous work in the lab has often focused on the first half of the task to minimize the contribution of ‘carryover arousal’ as the task progresses. However, as highlighted by research on fear and anxiety (Plichta et al. 2014), the temporal dynamics of brain responding can reveal pathology (Swartz et al. 2013). For example, individuals with greater anxiety (Hare et al. 2008) tend to have a persistent brain response to repeated emotional stimuli. Similarly, individuals with autism have a more persistent response even to repeated neutral stimuli than controls (Swartz et al. 2013). Potentially relevant to the present study, individuals with posttraumatic stress disorder show a non-reducing brain response to repeated emotionally significant stimuli (Hendler, Rotshtein, & Hadar 2001). Adopting this approach for the current study, we explicitly examined both the first and second half of the task in order to fully characterize the response profiles for the Abuse and NoAbuse phenotypes.
METHODS
Participants
Sixty-eight treatment-seeking, cocaine-dependent participants from successive cohorts at our center were included in the current analyses. They met standard eligibility for imaging studies, criteria described previously (Wetherill et al. 2014; Young et al. 2014). Briefly, they reported cocaine use on at least 8 of the last 30 days, and they were available for a 7–10 inpatient stay. Participants reported smoking as the primary route of cocaine administration. Exclusion criteria included: contraindications for functional magnetic resonance imaging (fMRI), use of medications that might affect dopamine transmission, a history of psychosis, seizures, or other organic brain syndrome, clinically significant cardiovascular, hematologic, hepatic, renal, neurological, or endocrine abnormalities, or a history of head trauma or injury. The Mini International Neuropsychiatric Interview was used to screen for psychiatric disorders (Sheehan et al. 1998). Other than cocaine dependence, participants with current Axis I psychiatric diagnoses were generally excluded, with the following exceptions: nicotine dependence, marijuana dependence and alcohol dependence not requiring detoxification. Individuals with current depression linked solely to periods of cocaine use/cessation were not excluded.
Study design
The basic features of our study design have been described previously (Childress et al. 2008). Briefly, participants were stabilized in a controlled, inpatient setting for 3–5 days to minimize the contribution of either cocaine intoxication or cessation symptoms. After stabilization, subjects participated in a scanning session that included a ‘fast’ event-related fMRI task (Fig. 1) with 24 novel 500-ms target cues in four categories (cocaine, sexual, aversive, neutral). Although, the current study is the first to examine a 500-ms duration, it is otherwise modeled closely after our prior event-related cocaine studies (Childress et al. 2008; Young et al. 2014). The cocaine cues (e.g. images of smoked cocaine, paraphernalia, etc.) and neutral cues (household or office objects; outdoor scenes) were from laboratory archives. The sexual and aversive cues were selected from the top quartile (e.g. ‘most pleasant’ and ‘most unpleasant’, respectively) of the International Affective Picture System (Lang et al. 1999). The targets were presented in a quasi-random order (no more than two of a kind in sequence), with an average 1500-ms interstimulus interval (gray screen with cross-hair). Twenty-four unique cues in each category were presented once and then repeated, for a total of 48 presentations per category. Thus, the task entailed two halves with 24 presentations of each cue category.
Figure 1.
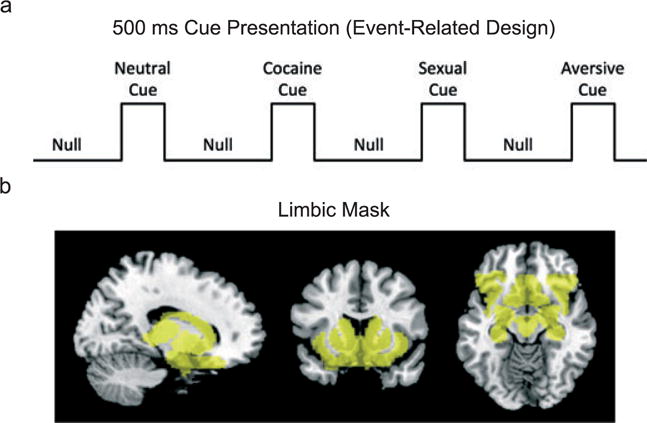
Task design and Regions of Interest (ROI) mask. a) The event-related fMRI task included presentation of visual cues (cocaine, sexual, aversive and neutral) for 500 ms preceded by an intertrial stimulus interval of 1500 ms consisting of a gray screen and a fixation cross. For the first half, 24 of each cue were presented in a pseudo-random order, and for the second half, the same 24 cues were repeated but in a different order. b) The ROI mask (semi-transparent yellow) shown at the sagittal, coronal and horizontal (coordinates, 14, 17,−14) captured midbrain, thalamus, caudate, putamen, ventral striatum, amygdala, hippocampus, lateral and caudal orbitofrontal cortex, and anterior insula, as well as interconnecting areas
Abuse versus NoAbuse subgroups
Participants were subdivided into two groups based on three items from the ASI probing whether individuals experienced physical, sexual or emotional abuse at any point in their lifetime perpetrated by family members (e.g. mother, father, siblings partner/spouse, other family) or friends (e.g. close friends, neighbors, co-workers). Using the ASI allowed us to extract abuse information from more than ten years of data collection from cocaine patients. Previous studies have used ASI as a measure for abuse (Ouimette et al. 2000; Rosen et al. 2002; Pirard et al. 2005; Charney et al. 2007). The ASI has been found to have better selectivity than sensitivity compared to other measures (Najavits et al. 1998; Langeland, Draijer, & van den Brink 2003), and the ASI probes are similar to other studies examining abuse (Gil-Rivas et al. 1997; Felitti et al. 1998): ‘Did any of these people (family members, friends, etc) abuse you (1) physically (cause you physical harm)?; (2) sexually (force sexual advances or sexual acts)?; or (3) emotionally (make you feel bad through harsh words)?’. If participants reported abuse at any point in their lives, in any of the three categories (physical, sexual or emotional abuse), that participant was included in the ‘Abuse’ group. If an individual reported no abuse in all of the three categories, that participant was included in the ‘NoAbuse’ group.
fMRI acquisition
As described previously (Childress et al. 2008; Young et al. 2014), a Siemens 3 T scanner was used for acquisition of blood-oxygen-level dependent images. For normalization and coregistration purposes, a 5-min high-resolution 3-Dimensional T1-weighted (MPRAGE) structural scan was acquired with the following parameters: repetition time (TR) 1620 ms; echo time (TE) 3.87 ms; 160 slices; slice thickness 1 mm; matrix 192 × 256; flip angle 15°. Functional images were acquired via a T2*-weighted single-shot gradient-echo, planar-imaging sequence with the following parameters: TR 2000 ms; TE 30 ms; 33 interleaved slices; slice thickness 3 mm without any gap between adjacent slices; FOV 192 mm; matrix 64 × 64; flip angle 80°.
Data analysis
FMRI data were preprocessed using Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK) batch mode scripts modified from ASLtbx. The processing steps were the same as in our previous study (Young et al. 2014), including slice timing correction, motion correction, temporal filtering, spatial smoothing and registration to the Montreal Neurological Institute (MNI) standard brain. The motion time courses were further removed from fMRI data using simple regression. Spatial smoothing was performed with an 8-mm3 full-width half-maximum Gaussian kernel.
Statistical analysis was performed with a general linear model using a canonical hemodynamic response function with both the first (time) and the second (dispersion) derivatives. Following the previously described design (see study design section above), we defined six first-level contrasts to assess the cue effects: cocaine versus neutral (first and second half), sex versus neutral (first and second half) and aversive versus neutral (first and second half).
Based on several studies reporting cue reactivity in limbic regions, we limit our main results to a priori regions of interest or ROIs (e.g. ventral tegmental area, amygdala, ventral striatum, midbrain, caudal orbitofrontal cortex [cOFC]), as well as other addiction-relevant regions, such as the insula (Naqvi & Bechara 2010), dorsal striatum (Everitt & Robbins 2013) and thalamus (Asensio et al. 2010). Regions of interest were identified using xjview (http://www.alivelearn.net/xjview8/) and FSL (Functional MRI of the Brain [FMRIB] Software Library, Oxford Centre for FMIRB). The ROI mask was created by first identifying anatomical regions (cOFC, striatum [caudate, putamen, ventral striatum], midbrain (VTA), anterior insula, amygdala and hippocampus) with fslview. Then, anatomical regions were thresholded, made into binary maps and added together into one mask with fslmaths.
We calculated a group by cue (2 × 3) ANOVA with GLM Flex Fast 2 (http://mrtools.mgh.harvard.edu) for each half of the task and examined the results of functional ROIs based on significant main effects of group for each half. Clusters from both of the ANOVA were considered significant at p < 0.01, corrected using Monte-Carlo (AFNI, 3dClustSim; http://afni.nimh.nih.gov). Parameter estimates (beta weights) for the functional clusters were extracted using Marsbar (http://marsbar.sourceforge.net); they were then imported into MATLAB (The Mathworks, Inc., Natick, Massachusetts, USA) for generating F values and for plotting.
For our primary analysis examining the Abuse versus NoAbuse groups on cue responding, independent t-tests were conducted for each cue type (for each half of the task). T-tests were considered significant at p < 0.01, corrected using Monte-Carlo (AFNI, 3dClustSim software; http://afni.nimh.nih.gov). Our results and discussions focus on regions overlapping our ROI mask (Fig. 1); however, we also present cluster-corrected whole brain results.
RESULTS
Demographics and clinical results
Participants were cocaine-dependent males averaging 44.4 years of age and 17.2 years of cocaine use. Most were African–American (89.9 percent) with an average of 12.5 years of education. Participants averaged 17.6 years of alcohol use; 42 percent used marijuana and 3 percent used heroin.
Prevalence of lifetime abuse
Forty individuals (59 percent) reported a history of any type of abuse (Table 1). Of these 40 individuals in the Abuse group, 36 reported emotional abuse, 20 reported physical abuse and 12 reported sexual abuse, and more than half reported two or more types of abuse (Table 1). The reports of abuse in the successive cohorts from our center were similar to previous reports (Ouimette et al. 2000; Pirard et al. 2005; Charney et al. 2007), including a sample with more than a thousand individuals with substance-use disorders (Rice et al. 2001). This suggests that our cohort was representative of a larger whole and underscores the ability of the ASI to capture the relevant phenotype (lifetime abuse).
Table 1.
Demographics and reported abuse.
| Demographics Gender Race |
No abuse (N = 28) 100% male (28) 89% black (25) |
Abuse (N = 40) 100% male (40) 93% black (37) |
P value* — p = 0.69 |
|---|---|---|---|
| Age | 45.2 | 43.2 | p = 0.12 |
| Education | 12.8 years | 12.3 years | p = 0.34 |
| Cocaine use | 18.8 years | 16.2 years | p = 0.22 |
| Alcohol use | 17.1 years | 17.6 years | p = 0.89 |
| Cannabis use | 37% (10) | 45% (18) | p = 0.41 |
| Heroin use | 0% | 5% (2) | — |
| History of abuse | ALL participants (n = 68) | ||
| Any abuse | 59% (40) | ||
| Emotional | 53% (36) | ||
| Physical | 29% (20) | ||
| Sexual | 18% (12) | ||
| Two or more | 35% (24) | ||
Note: When p values could not be calculated, it is indicated by a ‘—’.
Imaging results
Abuse versus No Abuse ANOVA results
The 2 × 3 ANOVA revealed a main effect of group (F (1,66) = 7.36, p = 0.009) in a thalamus cluster (that extended to midbrain and caudate) in the first half of the task. In the second half, there was a main effect of group (F (1,66) = 10.53, p = 0.002) in a cOFC cluster (that extended to the medial temporal lobe). Overall, the Abuse group had a greater response to the evocative cues, compared to the NoAbuse group (not shown). There was also a main effect of cues in a large interconnected thalamus/caudate cluster (F (2,66) = 9.75, p = 0.001) in the first half of the task (not shown) but no significant interactions survived cluster correction.
Abuse versus NoAbuse: drug cues
Overall, participants (N = 68) exhibited a robust and widespread mesolimbic response to drug cues. However, this pattern was more evident in the Abuse group (N = 40) than the NoAbuse group (N = 28, Fig. 2) and occurred in both halves of the task. Independent t-tests, corrected at the ROI mask level (p < 0.01, k > 147), revealed that the Abuse group had a greater response to drug cues in a thalamus/caudate/midbrain cluster (peak t = 2.98) during the first half of the task (Fig. 3). The Abuse group also had a greater response to drug cues in a cluster that included the cOFC (peak t = 4.74) in the second half of the task (Fig. 3). For purposes of demonstrating that the NoAbuse group had a differential response to drug (versus neutral) cues, we also present images from the NoAbuse group in the Supporting Information (Supplemental Fig. 1).
Figure 2.
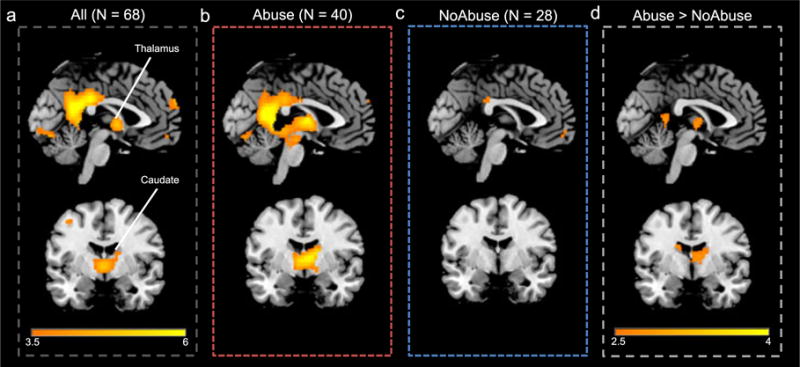
Example images showing brain response (threshold: 3.5 < t < 6, coordinates: x =−2, y =−4) to drug (versus neutral) cues for the entire sample, and for the Abuse and NoAbuse subgroups (for the first half of the task). a) The overall group (N = 68) responded to drug cues in several nodes of the ROI mask striatum, midbrain and thalamus. b) This overall pattern was evident in individuals history of abuse (N = 40), c) but was lacking* in individuals without a history of abuse (N = 28). d) Comparing Abuse versus NoAbuse (threshold p < 0.01), the participants with a history of abuse had a greater response to drug cues in the first the thalamus and the caudate. *Also see Figure S1 for the pattern of responding in the NoAbuse group at a reduced threshold
Figure 3.
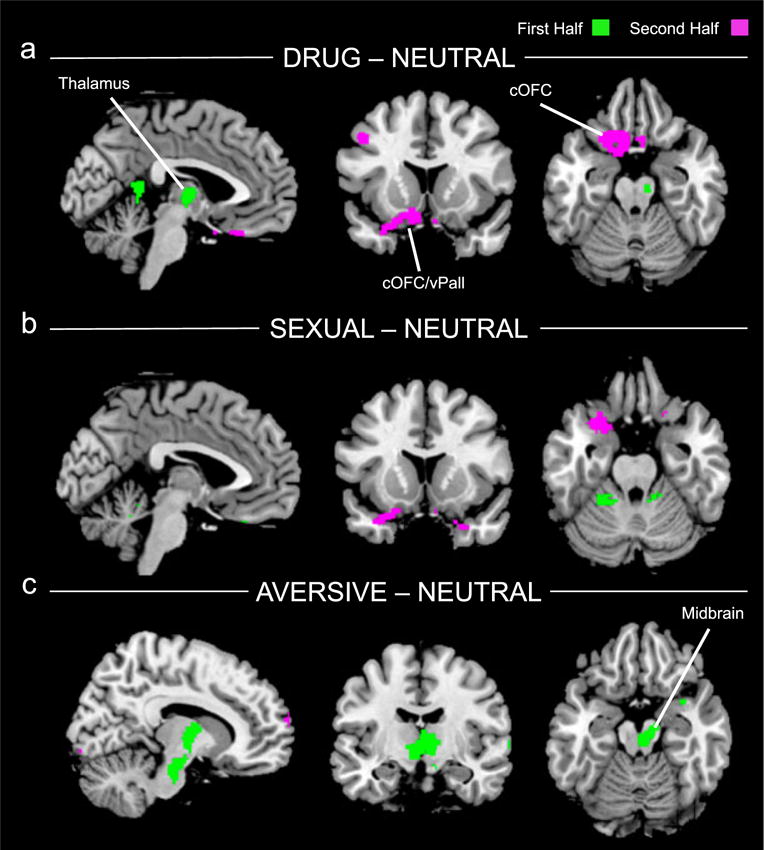
Abuse > NoAbuse contrasts for each of the cue categories as compared to neutral (threshold, p < 0.01, uncorrected for display) for both first (green) and second (violet) halves of task. a) Individuals with a history of Abuse had a greater response to cocaine cues than NoAbuse in both the first and second halves of the task. This difference was significant in a thalamus/caudate cluster in the first half (midbrain cluster did not survive correction) and in a caudal orbitofrontal cortex (cOFC) cluster in the second half that extended into the medial temporal lobe just ventral to the amygdala. b) Individuals with a history of Abuse had a greater response to sexual cues than NoAbuse only in the second half of the task. This difference was significant in a cOFC cluster that extended into the medial temporal lobe just ventral to the amygdala. c) Individuals with a history of Abuse had a greater response to aversive cues than NoAbuse only in the first half of the task. This difference was significant in a cluster that included the thalamus, caudate and midbrain
Clusters found with whole brain correction (k > 265) outside of the ROI mask for drug cues are reported in Table 2.
Table 2.
A priori mask and whole brain results: Abuse > NoAbuse at p < 0.01, cluster-corrected.
| Regions | Cluster Size | T value | P value |
Coordinates
|
||
|---|---|---|---|---|---|---|
| (A priori mask) | (k > 147) | (uncorrected) | x | y | z | |
| Drug1 – Neutral1 | ||||||
| Thalamus/caudate | 342 | 2.98 | 0.002 | 2 | −8 | 8 |
| Aversive1 – Neutral1 | ||||||
| Midbrain | 235 | 3.39 | 0.001 | 4 | −24 | −20 |
| Thalamus/caudate | 361 | 2.84 | 0.002 | 6 | −10 | 2 |
| Drug2 – Neutral2 | ||||||
| Caudal OFC/Amyg (Left) | 487 | 4.74 | <0.001 | −20 | 16 | −22 |
| Sexual2 – Neutral2 | ||||||
| Caudal OFC/Amyg (Left) | 168 | 3.47 | <0.001 | −22 | 12 | −26 |
|
| ||||||
| Regions | Cluster size | T value | P value |
Coordinates
|
||
| (Outside the mask) | (k > 265) | (uncorrected) | x | y | z | |
|
| ||||||
| Drug1 – Neutral1 | ||||||
| PCC/Precuneus | 274 | 3.10 | 0.001 | 2 | −46 | 10 |
| Sexual1 – Neutral1 | ||||||
| Cerebellar (V,VI, Left) | 370 | 2.90 | 0.003 | −22 | −46 | −20 |
| Drug2 – Neutral2 | ||||||
| Sup. Occipital (Left) | 756 | 3.69 | <0.001 | −50 | −58 | 46 |
| dlPFC (Right) | 272 | 3.26 | 0.001 | 40 | 36 | 32 |
| Sexual2 – Neutral2 | ||||||
| dlPFC (Right) | 409 | 3.26 | 0.001 | 44 | 32 | 34 |
Abuse versus NoAbuse: comparator cues
For the comparator cues, the differences between Abuse and NoAbuse were limited to one half of the task or the other. In response to sexual cues differences between Abuse and NoAbuse groups were evident only in the second half of the task in a left cOFC (peak t = 3.47, Fig. 3b). Finally, for the aversive cues, differences between Abuse and NoAbuse were found only in the first half of the task in a thalamic/midbrain cluster (peak t = 3.39, Fig. 3c).
Clusters found with whole brain correction (k > 265) outside of the ROI mask for sexual and aversive cues are reported in Table 2.
Abuse versus NoAbuse: temporal pattern
To examine the general temporal patterns across the task, we collapsed across the three cue categories and plotted responding for the two significant clusters from the main effect of group (Abuse versus NoAbuse) in the whole-brain analysis. As shown in Fig. 4, the impact of the Abuse phenotype was expressed differently for the two clusters. For the cOFC cluster, responding was similar between the two groups in the first half but diverged in the second half of the task, with sustained responding in the Abuse group but decreased responding in the NoAbuse group (Fig. 4). For the thalamus/caudate cluster, the Abuse group showed a large initial response, but responding was low and similar for the two groups in the second half of the task (Fig. 4).
Figure 4.
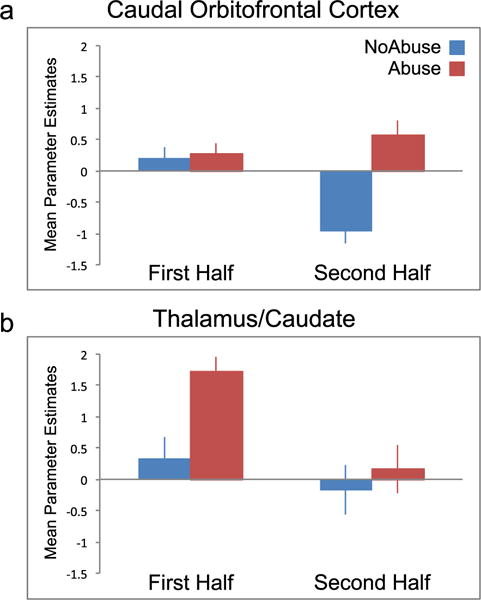
Mean (± SEM) parameter estimates (beta weights) of functional clusters from the main effect of group (Abuse versus NoAbuse), collapsed across cues (drug, sex, aversive) and plotted for first half and second halves of the task. a) For the cOFC cluster, NoAbuse (blue) and Abuse (red) only differed in the second half of the task, reflecting a sustained response in the Abuse group. b) For the thalamus/caudate cluster, NoAbuse and Abuse groups differed only in the first half of the task, evidenced by a large initial response in the Abuse group
DISCUSSION
Mesolimbic brain response to 500-ms evocative cues differed between individuals with cocaine-use disorders, depending upon abuse history. As predicted, individuals with cocaine-use disorders and a history of abuse had a greater mesolimbic response to cocaine cues compared to those without lifetime abuse. These differential neurobiological results were evident in two main clusters, one that included the thalamus, midbrain and caudate and another that included the left caudal OFC and left parahippocampus. These results are novel in two ways. First, they are the first to show a response to brief 500-ms cues, a response that is similar to previous studies with different cue lengths (Childress et al. 1999, 2008; Volkow et al. 2006). Second, though a recent study found a link between abuse and cortical activation (Elton et al., 2015) the present results are the first to show that lifetime abuse is associated with a heightened mesolimbic response to cocaine cues in a human population with substance-use disorders.
Prior studies have mostly looked at magnitude as a marker of vulnerability between patients and controls. The current study allowed us to look at temporal patterns of responding as related to abuse history. Our examination of the temporal patterns in the task showed that the Abuse phenotype could be expressed in more than one way, depending on brain region. In the thalamus/caudate cluster, the response pattern was initially large but reducing; this ‘early’ response in cue tasks may reflect clinically-relevant vulnerability. In the cOFC, the response pattern was sustained, consistent with reports of non-reducing responses to repeated stimuli in other pathologies (Hendler et al. 2001; Hare et al. 2008; Swartz et al. 2013; Plichta et al. 2014). Ongoing and prospective studies will allow for the examination of whether response magnitude, temporal dynamics or both have greater clinical relevance.
Previous research has reported the effect of prior abuse on brain activity to negative stimuli (Dannlowski et al. 2012); however, that lifetime abuse was linked to differential response to appetitive cues may seem counter-intuitive. The impact of adverse events on reward signaling has several dimensions. Pre-clinical and clinical studies have found that stress increases drug-seeking and drug-taking behaviors (Sinha 2001) and produces an increase in dopamine signaling (Louilot, Le Moal, & Simon 1986; Sorg & Kalivas 1991; Rougé-Pont et al. 1993; Pruessner et al. 2004). Studies of significant stress, like prior abuse, have revealed reduced mesolimbic brain-region volume (Weniger et al. 2008; Dannlowski et al. 2012; Van Dam et al. 2014) as well as blunted mesolimbic response to cues linked with monetary rewards (Dillon et al. 2009; Elman et al. 2009; Mehta et al. 2009; Goff et al. 2013). In contrast, prior abuse was associated with increased mesolimbic response to a stimulant (amphetamine) and emotional stimuli in non-addicted males (Dannlowski et al. 2012; Oswald et al. 2014). At this stage of the literature, there are several differences across studies that may account for differences in results: (1) the nature of the populations (e.g. adult cocaine users versus adolescents or non-addicted adults); (2) cues (e.g. evocative versus reward anticipation); (3) a potentially non-linear relationship of severity of stress and brain response (e.g. linear versus inverted U); and (4) anatomical regions (e.g. heightened VTA and amygdala versus blunted ventral striatum). With these several differences, future studies will be needed to disentangle the nature and direction of the effects of prior trauma on brain responding to various probes.
To our knowledge, this is the first study showing that addicted individuals with prior abuse have a greater limbic response to cues signaling their preferred drug. Further, this enhanced reactivity extended to both the appetitive and aversive domains. This might occur because, from an evolutionary perspective, the promise of reward or the threat of danger may take on increased salience for an individual who has survived danger but whose future may be uncertain or short-lived. From another perspective, the heightened mesolimbic response to cues in a subset of our clinical population also has parallels to findings in the pre-clinical literature on incentive salience (Robinson & Berridge 1993). A subgroup of individuals (‘sign-trackers’) in the general population are more responsive to signals for reward (Flagel et al. 2010) even without prior stress; these results have recently been extended to the aversive domain (Morrow, Maren, & Robinson 2011; Morrow et al. 2015). These two viewpoints (evolutionary and incentive salience) may actually be complementary, in that a subgroup of individuals could be inherently more cue-reactive, but a history of stress or abuse may enhance or even create this vulnerability (Lomanowska et al. 2011).
As with any initial finding, there are limitations that can guide further research. For ethical reasons, human studies of abuse necessarily rely upon self-report; future pre-clinical studies can determine how various stressor parameters (e.g. type, frequency and developmental stage) impact the learned response to drug cues. A basic issue with new findings is generalizability: the present study investigated older males with a chronic cocaine-use history. Future studies can determine generalizability of the current abuse findings to different clinical populations (differing in age, gender, type of addiction), to other brain-behavioral probes (e.g. for inhibition). The study was limited to self-report of lifetime abuse using the ASI as a measure. The ASI captured both childhood and adult abuse, but did not specify when specific abuses occurred. Even without temporal sensitivity, this simple tool clearly separated clinical phenotypes (e.g. Abuse versus NoAbuse) with measurable brain differences. As the study was retrospective, we were limited to available tools already collected (e.g. no measures related directly to stress, such as cortisol), but future research will utilize multiple item abuse probes (e.g. Child Trauma Questionnaire [CTQ]), which will allow for a comparison of abuse reported from the ASI versus the CTQ and to obtain information about abuse occurring before the age of 15. Finally, our imaging cohort did not include participants with any DSM IV Axis 1 comorbid mood or anxiety disorders. Thus, future studies may include or even explicitly study the impact of these diagnoses (Hart & Rubia 2012), especially because psychopathology is often more severe in populations with lifetime abuse (Rice et al. 2001; Pirard et al. 2005; Charney et al. 2007). Worth noting, inclusion of individuals with common comorbidities (e.g. depression, anxiety, posttraumatic stress disorder) would not necessarily be expected to undermine our results, and could even enhance them.
Physical, sexual and emotional abuse affects millions of people every year and is highly correlated with addiction (Felitti et al. 1998). As discussed, abuse can have dramatic effects on the brain (for a review, see [Hart & Rubia 2012]). Research on the effects of abuse on addiction trajectory and outcome has been mixed (Gil-Rivas et al. 1997; Pirard et al. 2005; Charney et al. 2007), but a recent study suggests that abuse predicts relapse (Van Dam et al. 2014). Thus, identifying brain systems affected by abuse may not only help us understand the harsh impacts of the past, but could also guide targeted interventions to help ‘reset’ these systems thus improving the odds of recovery for our future patients.
Supplementary Material
Supplemental Figure 1. Individuals without a history of abuse and their response to Drug minus Neutral (threshold 2 < t < 5, first half). At this reduced threshold (compared to Figure 2), the NoAbuse group had activity within some of the ROI mask regions, such as the striatum and anterior insula.
Acknowledgments
We thank the clinical staff (nurses, intake and support personnel) at the Center for Studies of Addiction, University of Pennsylvania Perelman School of Medicine, and the imaging support staff at the Center for Functional Neuroimaging Department of Radiology, University of Pennsylvania Perelman School of Medicine and the imaging component of the VAVISN for MIRECC at the Department of Veteran’s Medical Affairs, Philadelphia, PA.
FUNDING AND DISCLOSURE
Support for the research was provided by RO1-P50—the Commonwealth of Pennsylvania (CURE), Addiction Center of Excellence: Brain Mechanisms of Relapse and Recovery; NIH/NIDA U54 Cocaine Cooperative Medication Development Center (DA039002); NIH/NIDA T32 Translational Addiction Research Fellowship Program (T32DA028874).
Footnotes
AUTHOR CONTRIBUTIONS
PR, AT and ARC contributed to study concept. PR performed all second-level analyses. KJ performed all first-level analyses. ARC, ZM, KJ and ZW assisted with data analysis. ARC and PR were responsible for interpretation of findings. ARC, TF and DL were responsible for study design. JS, MG, KK and ZM assisted with data collection. PR and ARC drafted the manuscript. TR and RW provided critical assessment of manuscript revision; COB and KY edited the manuscript for accuracy. All authors critically reviewed content and approved final version for publication.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- Asensio S, Romero MJ, Romero FJ, Wong C, Alia-Klein N, Tomasi D, Wang G-J, Telang F, Volkow ND, Goldstein RZ. Striatal dopamine D2 receptor availability predicts the thalamic and medial prefrontal responses to reward in cocaine abusers three years later. Synapse. 2010;64:397–402. doi: 10.1002/syn.20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Murray JE, Everitt BJ. Addiction: failure of control over maladaptive incentive habits. Current Opinion in Neurobiology. 2013;23:564–572. doi: 10.1016/j.conb.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DA, Palacios-Boix J, Gill KJ. Sexual abuse and the outcome of addiction treatment. Am J Addict. 2007;16:93–100. doi: 10.1080/10550490601184225. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O’Brien CP. Prelude to passion: limbic activation by “unseen” drug and sexual cues. PLoS One. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angio M, Serrano A, Rivy JP, Scatton B. Tail-pinch stress increases extracellular DOPAC levels (as measured by in vivo voltammetry) in the rat nucleus accumbens but not frontal cortex: antagonism by diazepam and zolpidem. Brain Res. 1987;409:169–174. doi: 10.1016/0006-8993(87)90755-4. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Holmes AJ, Birk JL, Brooks N, Lyons-Ruth K, Pizzagalli DA. Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biol Psychiatry. 2009;66:206–213. doi: 10.1016/j.biopsych.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Smitherman S, Young J, Kilts CD. Effects of childhood maltreatment on the neural correlates of stress- and drug cue-induced cocaine craving. Addict Biol. 2015;20:820–831. doi: 10.1111/adb.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci Biobehav Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Fatseas M, Denis C, Massida Z, Verger M, Franques-Rénéric P, Auriacombe M. Cue-Induced Reactivity, Cortisol Response and Substance Use Outcome in Treated Heroin Dependent Individuals. Biological Psychiatry. 2011;70:720–727. doi: 10.1016/j.biopsych.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O’Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Gil-Rivas V, Fiorentine R, Douglas Anglin M, Taylor E. Sexual and physical abuse: do they compromise drug treatment outcomes? J Subst Abuse Treat. 1997;14:351–358. doi: 10.1016/s0740-5472(97)84631-4. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003;13:435–441. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler T, Rotshtein P, Hadar U. Emotion–perception interplay in the visual cortex: “the eyes follow the heart.”. Cell Mol Neurobiol. 2001;21:733–752. doi: 10.1023/a:1015156222101. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Sinha R. Childhood maltreatment, perceived stress, and stress-related coping in recently abstinent cocaine dependent adults. Psychol Addict Behav. 2007;21:233–238. doi: 10.1037/0893-164X.21.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, et al. International affective picture system (IAPS): instruction manual and affective ratings. Cent Res Psychophysiol Univ Fla 1999 [Google Scholar]
- Langeland W, Draijer N, van den Brink W. Assessment of lifetime physical and sexual abuse in treated alcoholics: Validity of the Addiction Severity Index. Addict Behav. 2003;28:871–881. doi: 10.1016/s0306-4603(01)00297-0. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O’Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain fMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Lomanowska AM, Lovic V, Rankine MJ, Mooney SJ, Robinson TE, Kraemer GW. Inadequate early social experience increases the incentive salience of reward-related cues in adulthood. Behav Brain Res. 2011;220:91–99. doi: 10.1016/j.bbr.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Louilot A, Le Moal M, Simon H. Differential reactivity of dopaminergic neurons in the nucleus accumbens in response to different behavioral situations. An in vivo voltammetric study in free moving rats. Brain Res. 1986;397:395–400. doi: 10.1016/0006-8993(86)90646-3. [DOI] [PubMed] [Google Scholar]
- Mchugh MJ, Demers CH, Salmeron BJ, Devous MDS, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Front Psychiatry. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mehta MA, Gore-Langton E, Golembo N, Colvert E, Williams SCR, Sonuga-Barke E. Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. J Cogn Neurosci. 2009;22:2316–2325. doi: 10.1162/jocn.2009.21394. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–243. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Saunders BT, Maren S, Robinson TE. Sign-tracking to an appetitive cue predicts incubation of conditioned fear in rats. Behav Brain Res. 2015;276:59–66. doi: 10.1016/j.bbr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits LM, Weiss RD, Reif S, Gastfriend DR, Siqueland L, Barber JP, Butler SF, Thase M, Blaine J. The Addiction Severity Index as a screen for trauma and posttraumatic stress disorder. J Stud Alcohol. 1998;59:56–62. doi: 10.15288/jsa.1998.59.56. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Drugs, brains, and behavior: the science of addiction. 2007:1–36. [Google Scholar]
- Oswald LM, Wand GS, Kuwabara H, Wong DF, Zhu S, Brasic JR. History of childhood adversity is positively associated with ventral striatal dopamine responses to amphetamine. Psychopharmacology (Berl) 2014;231:2417–2433. doi: 10.1007/s00213-013-3407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette PC, Kimerling R, Shaw J, Moos RH. Physical and sexual abuse among women and men with substance use disorders. Alcohol Treat Q. 2000;18:7–17. [Google Scholar]
- Pirard S, Sharon E, Kang SK, Angarita GA, Gastfriend DR. Prevalence of physical and sexual abuse among substance abuse patients and impact on treatment outcomes. Drug Alcohol Depend. 2005;78:57–64. doi: 10.1016/j.drugalcdep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Grimm O, Morgen K, Mier D, Sauer C, Haddad L, Tost H, Esslinger C, Kirsch P, Schwarz AJ, Meyer-Lindenberg A. Amygdala habituation: a reliable fMRI phenotype. Neuroimage. 2014;103:383–390. doi: 10.1016/j.neuroimage.2014.09.059. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C, Mohr CD, Del Boca FK, Mattson ME, Young L, Brady K, Nickless C. Self-reports of physical, sexual and emotional abuse in an alcoholism treatment sample. J Stud Alcohol. 2001;62:114–123. doi: 10.15288/jsa.2001.62.114. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rosen CS, Ouimette PC, Sheikh JI, Gregg JA, Moos RH. Physical and sexual abuse history and addiction treatment outcomes. J Stud Alcohol. 2002;63:683–687. doi: 10.15288/jsa.2002.63.683. [DOI] [PubMed] [Google Scholar]
- Rougé-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res. 1993;602:169–174. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- See RE, Waters RP. Pharmacologically-induced stress: a cross-species probe for translational research in drug addiction and relapse. Am J Transl Res. 2010;3:81–89. [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2013;52:84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam NT, Rando K, Potenza MN, Tuit K, Sinha R. Childhood maltreatment, altered limbic neurobiology, and substance use relapse severity via trauma-specific reductions in limbic gray matter volume. JAMA Psychiatry. 2014;71:917–925. doi: 10.1001/jamapsychiatry.2014.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci Off J Soc Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Lange C, Sachsse U, Irle E. Amygdala and hippocampal volumes and cognition in adult survivors of childhood abuse with dissociative disorders. Acta Psychiatr Scand. 2008;118:281–290. doi: 10.1111/j.1600-0447.2008.01246.x. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, O’Brien CP, Franklin TR. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology (Berl) 2014;231:1397–1407. doi: 10.1007/s00213-013-3342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Young KA, Franklin TR, Roberts DCS, Jagannathan K, Suh JJ, Wetherill RR, Wang Z, Kampman KM, O’Brien CP, Childress AR. Nipping cue reactivity in the bud: baclofen prevents limbic activation elicited by subliminal drug cues. J Neurosci. 2014;34:5038–5043. doi: 10.1523/JNEUROSCI.4977-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Individuals without a history of abuse and their response to Drug minus Neutral (threshold 2 < t < 5, first half). At this reduced threshold (compared to Figure 2), the NoAbuse group had activity within some of the ROI mask regions, such as the striatum and anterior insula.


