Abstract
The purinergic receptor P2X ligand-gated ion channel 7 (P2X7 receptor) is a promising imaging target to detect neuroinflammation. Herein, we report development of a potent iodinated labeled radiotracer for P2X7 receptor, [123I]TZ6019. The radiosynthesis of [123I]TZ6019 was accomplished by allylic-tin precursor iodination using [123I]NaI with good radiochemical yield of 85% and high radiochemical purity of > 99%. Human embryonic kidney 293 (HEK-293) cell line stably transfected with the human P2X7 receptor was used to characterize the binding affinity of TZ6019 in fluorescence, radioactive competitive, and saturation binding assays. A radioligand competitive assay with [123I]TZ6019 demonstrated that the nonradioactive compound TZ6019 has an IC50 value of 9.49 ± 1.4 nM, and the known P2X7 receptor compound GSK1482160 has an IC50 value of 4.30 ± 0.86 nM, consistent with previous reports. The radioligand saturation binding assay and competitive assay revealed that [123I]TZ6019 specifically bound to the human P2X7 receptor with high affinity (Ki = 6.3 ± 0.9 nM). In vitro autoradiography quantification with brain slices collected from 9-month old P301S tau transgenic mice along with wild type controls, revealed higher binding of [123I]TZ6019 (35% increase) in the brain of P301S transgenic mice (n = 3, p = 0.04) compared to wild type controls. The immunofluorescence microscopy confirmed that expression of P2X7 receptor was colocalized with astrocytes in the tauopathy P301S transgenic mice. [123I]TZ6019 has specific binding for P2X7 receptor and has great potential to be a radiotracer for screening new compounds and quantifying expression of P2X7 receptor in neuroinflammation related diseases.
Keywords: Neuroinflammation, P2X7 receptor, P301S, I-123
1. Introduction
Neuroinflammation plays an important role in progression of many neurodegenerative diseases including Alzheimer disease (AD) and Parkinson disease (PD) (Bhattacharya and Biber, 2016; Monif et al., 2010). Elevated purinergic receptors expression has shown close association with neuroinflammation due to the large release of adenosine/uridine derivatives from the damage site (Bhattacharya et al., 2013; Boumechache et al., 2009). Purinergic receptors are adenosine-triphosphate (ATP) gated non-selective ion channels which are expressed on the surface of many different cell types (Able et al., 2011; Burnstock, 2009a, b). Among these purinergic receptors, P2X7 receptor has been implicated in microglial activation, astrogliosis, or phagocytosis at sites of injury (Bartlett et al., 2014; Bhattacharya and Biber, 2016; Franke and Illes, 2014; Grygorowicz et al., 2016), and release of pro-inflammatory cytokines (Bianco et al., 2006; Collo et al., 1997; Ferrari et al., 2006). Upregulation of extracellular ATP in neuroinflammatory conditions significantly increases expression of P2X7 receptor. The P2X7 receptor knockout mice display a significantly reduced neuroinflammatory response (Basso et al., 2009; Monif et al., 2009). Therefore, P2X7 receptor represents a novel molecular target for imaging neuroinflammation. A potent P2X7 receptor antagonist, GSK1482160, has recently been labeled with carbon-11 (half-life 20.4 min) for positron emission tomography (PET) imaging of neuroinflammation in an animal model (Gao et al., 2015; Han et al., 2017; Ory et al., 2016). However, this radioligand showed tight binding to human P2X7 receptor, but weak binding to rodents P2X7 receptor (Gao et al., 2015; Ory et al., 2016). And the human applications of this tracer remain to be explored. New potent ligands specifically targeting both human and rodent P2X7 receptor, and capable to be translated for clinic are still demanding. Screening new analogues for P2X7 ligand development requires a reliable assay platform for P2X7 receptor. For the in vitro assays, labeled probes with longer half-life radioisotopes facilitate implementation of these assays. Previously two tritium labeled ligands ([3H]A804598 and [3H]JNJ54232334) have been reported for P2X7 receptor (Donnelly-Roberts et al., 2009; Lord et al., 2015), but they have limited availability. Herein we report the radiosynthesis of the Iodine-123 (half-life 13.3 h) labeled radiotracer [123I]TZ6019 and initial characterization of [123I]TZ6019 for P2X7 receptor. The radiosynthesis of [123I]TZ6019 was accomplished by allylic-tin precursor iodination. The binding properties of [123I]TZ6019 were determined using P2X7 receptor expressed HEK-293 living cells, and in vitro autoradiography studies with [123I]TZ6019 were performed with postmortem brain tissue from P301S transgenic mice, a tauopathy mouse model. Our results showed that [123I]TZ6019 is a potent and specific radiotracer for P2X7 receptor, and the binding of P2X7 receptor was associated with the neuroinflammation in the tauopathy mouse model.
2. Materials and Methods
2.1 Chemistry
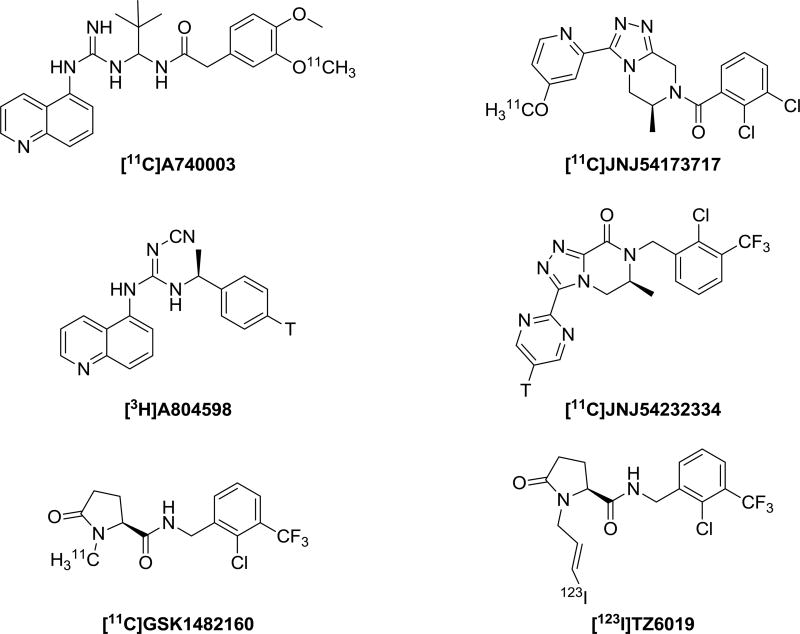
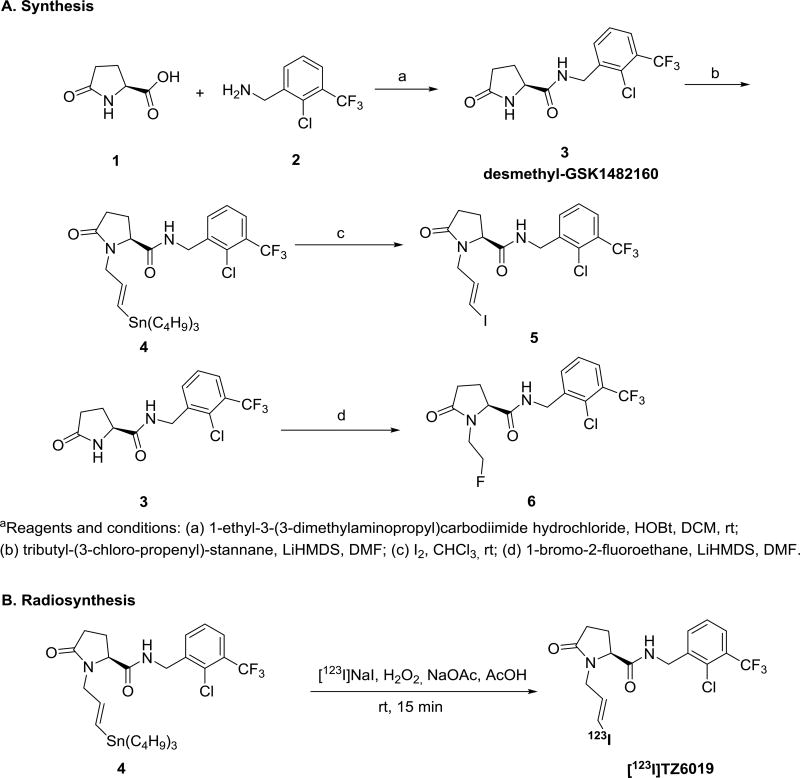
The structures of several known P2X7 receptor radioligands including [123I]TZ6019 are summarized in Fig. 1. Compound GSK1482160 was synthesized by following the reported procedure (Gao et al., 2015; Han et al., 2017). The standard compound 5 (TZ6019) was also synthesized from demethyl-GSK1482160, depicted in Scheme 1A. All reagents and chemicals were obtained from standard commercial sources and used without further purification, unless otherwise stated. The organic reaction was carried out under inert nitrogen and moisture-free conditions with a dry solvent. Thin layer chromatography (TLC) was used to monitor the reaction with pre-coated glass plates of silica gel 60 F254 from EMD Chemicals Inc. (Gibbstown, NJ). The product of each step was purified by silica gel 40–63 µm from SiliCycle Inc. (Quebec City, Quebec, Canada). 1H and 13C spectra were recorded at 400 MHz on a Varian Mercury-VX spectrometer with CDCl3 as solvents and tetramethylsilane (TMS) as the internal standard. The chemical shifts were reported in δ (ppm) values relative to CHCl3 (δ 7.26 ppm for 1H NMR and δ 77.2 ppm for 13C NMR), multiplicities were indicated by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and br (broad). High resolution mass spectrum (HRMS) was performed on a Waters ZQ 4000 single quadrupole mass spectrometer equipped with an electrospray ionization (ESI) LC−MS interface.
Fig. 1.
Chemical structures of radioligands for P2X7 receptor.
Scheme 1.
Synthesis of target compounds 5, 6 and radiolabeling of [123I]TZ6019
aReagents and conditions: (a) 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, HOBt, DCM, rt; (b) tributyl-(3-chloro-propenyl)-stannane, LiHMDS, DMF; (c) I2, CHCl3, rt; (d) 1-bromo-2-fluoroethane, LiHMDS, DMF.
2.1.1 (E)-N-(2-chloro-3-(trifluoromethyl)benzyl)-5-oxo-1-(3-(tributylstannyl)allyl)pyrrolidine-2-carbox-amide (4)
At 0 °C, 3 ml Lithium bis(trimethylsilyl)amide (LiHMDS, 1 M in tetrahydrofuran, THF) was added into an oven-dried 100 ml round bottle flask with a solution of compound 3 (600 mg, 1.8 mmol, 1.0 equiv.) in 10 ml dimethylformamide (DMF) (Gao et al., 2015; Han et al., 2017). Then 2.0 equiv. of tributyl-(3-chloro-propenyl)-stannane was added into the flask at 0 °C. The mixture was stirred overnight at room temperature, then extracted with ethyl acetate (EtOAc). The organic solution was dried using Mg2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column using hexane/EtOAc (1/1, v/v) to yield product 4 as a yellow solid (320 mg, 27% yield). 1H NMR (400 MHz,CDCl3): δ 7.66 (d, J = 7.9 Hz, 1H), 7.57 (d, J = 7.5 Hz, 1H), 7.35 (t, J = 7.8 Hz, 1H), 6.41 (t, J = 5.5 Hz, 1H), 6.02 (d, J = 19.0 Hz, 1H), 5.67 – 5.79 (m, 1H), 4.58 (d, J = 6.0 Hz, 2H), 4.48 – 4.56 (m, 1H), 4.03 (dd, J = 8.8, 3.4 Hz, 1H), 3.26 (dd, J = 15.3, 7.3 Hz, 1H), 2.44 – 2.56 (m, 1H), 2.24 – 2.42 (m, 2H), 1.99 – 2.11 (m, 1H), 1.36 – 1.54 (m, 6H), 1.20 – 1.32 (m, 6H), 0.77 – 0.94 (m, 15H); 13C NMR (100 MHz, CDCl3): δ 175.5, 171.5, 140.5, 137.5, 134.1, 133.8, 131.6, 129.2 (q, JC−F = 28.0 Hz), 127.2 (q, JC−F = 5.4 Hz), 126.9, 122.7 (q, JC−F = 272 Hz), 60.5, 47.6, 41.7, 29.65, 29.0 (JC-Sn = 20.7 Hz), 29.6, 27.2 (JC-Sn = 54.7 Hz), 23.5, 13.7, 9.4 (JC-Sn = 345 Hz). HRMS (ESI) calculated for C28H42ClF3N2O2Sn [M + H]+ 651.1982 revealed [M + H]+ 651.1978.
2.1.2 (S,E)-N-(2-chloro-3-(trifluoromethyl)benzyl)-1-(3-iodoallyl)-5-oxopyrrolidine-2-carboxamide (5)
A solution of iodine in chloroform (0.1 M, 2 ml) was added dropwise to a solution of 4 (130 mg, 0.2 mmol) in dry chloroform (10 ml). The reaction was stirred at room temperature for 20 h. The solution then was diluted with chloroform (15 ml), washed with saturated Na2S2O3 (6 × 25 ml) and then saturated aqueous NaCl solution. The combination of organic layers were dried over anhydrous sodium sulfate, concentrated under reduced pressure. The residue was purified by flash silica gel chromatography using hexane/EtOAc (1/4, v/v) as the eluent to yield the final product 5 (TZ6019) in a brown solid (86 mg, 88% yield). 1H NMR (400 MHz, CDCl3): δ 7.62 (d, J = 7.7 Hz, 1H), 7.53 (d, J = 7.5 Hz, 1H), 7.32 (t, J = 7.8 Hz, 1H), 6.67 (t, J = 5.5 Hz, 1H), 6.42 – 6.24 (m, 1H), 6.18 (d, J = 14.7 Hz, 1H), 4.64 – 4.44 (m, 2H), 4.14 (dd, J = 14.9, 5.2 Hz, 1H), 3.99 (dd, J = 8.5, 3.6 Hz, 1H), 3.31 (dd, J = 15.3, 7.9 Hz, 1H), 2.55 – 2.35 (m, 1H), 2.33 – 2.18 (m, 2H), 1.92 – 2.06 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 175.4, 171.1, 139.2, 137.5, 133.9 (q, JC−F = 1.6 Hz), 129.2 (q, JC−F = 32 Hz), 127.3 (q, JC−F = 5.4 Hz), 127.0, 122.7 (q, JC−F = 272 Hz), 80.6, 60.7, 46.0, 41.8, 29.5, 23.5. HRMS (ESI) calcd for C16H15ClF3IN2O2 [M + H]+ 487.6651 revealed [M + H]+ 487.6656.
2.1.3 (S)-N-(2-chloro-3-(trifluoromethyl)benzyl)-1-(2-fluoroethyl)-5-oxopyrrolidine-2-carboxamide (6)
Compound 6 (TZ5038) was prepared similarly to compound 4. Compound 6 white solid (360 mg, 54% yield) was derived from compound 3 (600 mg, 1.8 mmol, 1.0 equiv.) and 1-bromo-2-fluoroethane (2.0 equiv.) 1H NMR (400 MHz, CDCl3): δ 7.64 (d, J = 8 Hz, 1H), 7.57 (d, J = 7.2 Hz, 1H), 7.34 (t, J = 7.6 Hz, 1H), 4.59 (d, J = 6 Hz, 2H), 4.39 – 4.53 (m, 1H), 4.19 – 4.24 (m, 1H), 3.74 – 3.90 (m, 1H), 3.14 – 3.29 (m, 1H), 2.43 – 2.56 (m, 1H), 2.26 – 2.37 (m, 2H), 1.97 – 2.06 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 176.0, 171.4, 137.6, 133.6, 131.6, 129.1 (q, JC−F = 30.8 Hz), 127.1 (q, JC−F = 5.4 Hz), 126.9, 122.7 (q, JC−F = 272 Hz), 82.8 (d, JC−F = 166.7 Hz), 62.3 (d, JC−F = 2.1 Hz), 42.5 (d, JC−F = 19.4 Hz), 41.7, 29.3, 28.8. HRMS (ESI) calculated for C15H15ClF4N2O2 [M + H]+ 367.0831 revealed [M + H]+ 367.0836.
2.2 Radiochemistry
The radiosynthesis of [123I]TZ6019 was depicted in Scheme 1B. Briefly, 111 MBq [123I]NaI (Nordion International Inc., Ottawa, Canada) was added to a mixture of tin-precursor 4 (300 to 400 µg) in 30 µl acetonitrile (ACN) and 50 µl of a 5% (w/v) solution of sodium acetate in glacial acetic acid, followed by the addition of 50 µl of an oxidant solution consisting of a 2:l ratio (v/v) of acetic acid/H2O2 (30%). After the mixture was stirred at room temperature for 15 min, the residue was injected into a reversed-phase semi-preparative high-performance liquid chromatography (HPLC) column (250×9.6 mm, 5 µm) equipped with a 1 ml injection loop for purification. The system was operated at a flow rate of 2 ml/min with ACN/0.1M ammonium formate (AMF) (50/50, v/v, pH = 4.5) as the mobile phase. The retention times of free iodine and the desired product were 4 and 17 min, respectively. Under these conditions, product was collected in a flask containing sterile water (50 ml). The diluted aqueous solution was passed through C18 Sep-Pak Plus (WAT020515, Waters, Milford, MA), and washed with water (20 ml). Final radioactive product [123I]TZ6019 was eluted using EtOH (0.6 ml).
2.3 Radioligand binding assay
2.3.1 HEK-293-hP2X7 receptor cell culture
HEK-293 cells stably transfected with the human P2X7 receptor (HEK-293-hP2X7R) were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum, antibiotics (50 Units/ml penicillin and 50 µg/ml streptomycin) and 300 µg/mL Geneticin (Invitrogen Inc., Carlsbad, CA). Cells were grown in a 75 ml cell culture flask with a humidified atmosphere of 5% CO2 at 37 °C. Confluent HEK293-hP2X7R cells were washed twice with cold PBS, then FACS (Fluorescence Activation Cell Sorting) and western blot experiments confirmed expression of P2X7 receptor were followed as our previous publication (Han et al., 2017).
2.3.2 In vitro fluorescence assay for measurement uptake of ethidium bromide
Activation of P2X7 receptor-associated pore formation was observed using a fluorescence plate reader by measuring the cellular uptake of ethidium bromide dye in HEK-293-P2X7R cells, as reported (Chessell et al., 1997; Michel et al., 2006; Park et al., 2016). For the actual assay, ~2 ×105 human HEK293-hP2X7R cells per well were placed in 96-well poly-lysine coated black microplate (Corning Inc., Kennebunk, ME) overnight. The phosphate-buffered saline (PBS) consisting of 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4 was used for washing HEK293-P2X7 cells in the growth medium and was removed before performing the assay. Each well then was replaced with 90 µL of assay solution consisting of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (10 mM), N-methyl-D-glucamine (5 mM), KCl (5.6 mM), D-glucose (10 mM), and CaCl2 (0.5 mM) (pH 7.4) and supplemented with 280 mM sucrose. Ten µl of 60 µM BzATP (final 6 µM), and of 10 µl of 10 × of the synthesized compound at serial diluted concentration (final concentration 0.3 nM – 3 µM) was added to each well. After incubation for 15 min, ethidium bromide uptake was detected by measuring the fluorescence with a VictoR3 plate reader (Perkin Elmer Inc., Waltham, MA), using an excitation wavelength of 540 nm and emission wavelength of 615 nm. The results were expressed as relative to the maximum accumulation of ethidium bromide when stimulated with BzATP only, and the IC50 values were determined using nonlinear regression by 3-parameter inhibitor-effect equation in Prism (GraphPad Software, Inc., San Diego, CA).
2.3.3 Radioligand saturation binding assay
For saturation binding studies, ~2 ×105 human HEK293-hP2X7R cells per well were placed in regular 96-well poly-lysine coated microplate (Bioworld Inc., Dublin, OH) overnight. Culture medium was removed on the second day, and cells were washed three times with 250 µl/well ice-cold assay buffer that was 50 mM Tris–HCl pH 7.4 with 0.1% BSA. Then 90 µl/well of assay buffer with serial diluted [123I]TZ6019, and 10 µl of assay buffer only (for total binding) or 10 µl of assay buffer with 100 µM final concentration of cold GSK1482160 were added (for nonspecific binding). The final concentration of [123I]TZ6019 ranged from 0.2 – 400 nM, calculated based on the stock radioactivity concentration and the theoretic specific activity (34.7 GBq/µmol). Triplicate wells were made for each concentration in the assay. Wells were incubated for 1 h on ice in a shaking water bath. Binding activities were terminated by rapid washing of the plate 5 times with 200 µl/well of ice-cold assay buffer. After the last wash, 100 µl/well of 0.5% sodium dodecyl sulfate (SDS) was added. After 1–2 min clean cell lysate from the 96-well microplate were transferred to a scintillation 96-well microplate (PerkinElmer Life and Analytical Sciences Inc., Shelton, CT), and 200 µl of Bio-Safe II complete counting cocktail (RPI research products Inc., Mount Prospect, IL) were added to each sample. Radioactivity of each sample was counted on a Wallac 1450 MicroBeta TriLux liquid scintillation counter (PerkinElmer, Boston, MA). Each sample was counted 30 s at the energy window of 5–1024 kev, and radioactivity of all samples (cpm) were decay corrected (half-life of 13.3 h) to the time when the first well was counted. Data analysis was accomplished using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). The specifically bound counts (cpm) from the saturation binding experiments were calculated by subtracting the non-specific binding from the total binding. The binding assay data were curve-fit to a one-site binding (hyperbola) equation to derive the dissociation constant of the radioligand (Kd) and number of binding sites (Bmax, fmol/ mg), in which the cpm and cell number were normalized to fmol/mg as described for the online internal Prism calibration procedures: http://www.graphpad.com/quickcalcs/radcalch).
2.3.4 Radioligand competition binding assay using [123I]TZ6019
In radioligand competition binding studies, ~2 × 105 HEK293-hP2X7R cells were placed in 96-well poly-lysine coated microplate (Bioworld Inc., Dublin, OH) overnight. Culture medium was removed on the second day, and cells were washed three times with 250 µl/well ice-cold assay buffer (50 mM Tris–HCl pH 7.4 with 0.1% BSA). Then 80 µl/well of assay buffer, 10 µl of assay buffer with serial diluted cold GSK1482160 or TZ6019, final concentration in the range of 0.1 nM – 3 µM), and 10 µl of assay buffer with fixed concentration of [123I]TZ6019 (final 9.7 nM) were added. Wells without adding the testing compound were considered as the maximum bound, and wells adding 100 µM of GSK1482160 were considered as the non-specific bound. Triplicate wells were made for each concentration in the assay. The rest of the procedure was as same as the saturation binding. The specific bound counts (the nonspecific bound counts subtracted from the total observed counts) (cpm) were expressed as a percentage of the maximal bound, and then these binding data were curve-fit to a 3-parameter inhibitor-effect equation to derive the potency (IC50) of the test compound in Prism (GraphPad Software, Inc, San Diego, CA). The equilibrium dissociation constant (Ki) of the test compound was calculated by the Cheng–Prusoff equation (Cheng and Prusoff, 1973): Ki = IC50/(1 + L*/Kd), Where L* is the [123I]TZ6019 concentration used and Kd was set to the mean value from saturation binding assays.
2.3.5 Radioligand competition binding assay using [3H]A804598
Radioligand [3H]A804598 was purchased from Moravek Inc. (Brea, CA USA). For all experiments, ~2×105 HEK293-hP2X7R cells were placed in 96-well poly-lysine coated microplate (Bioworld Inc., Dublin, OH) overnight. Followed the same procedure as the [123I]TZ6019 based radioligand competition assay. Nonspecific binding was determined in the presence of GSK1482160 (100 µM). All assays were carried out at 4°C incubations by the adding of 80 µl of assay buffer to the cell, 10 µl of assay buffer with fixed concentration of [3H]A804598 (2 nM based on radioactivity concentration and specific activity 1.33 GBq/µmol), and 10 µl of assay buffer with serial diluted compounds including GSK1482160 and TZ6019. The final concentration ranged from 0.1 nM – 3 µM. The binding–effect data were curve-fit to a 3-parameter inhibitor-effect equation to derive the potency (IC50) of the test compound using Prism (GraphPad Software, Inc., San Diego, CA).
2.4 Mutant tau P301S mouse model
All rodent experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals under protocols approved by Washington University's Animal Studies Committee. P301S transgenic mice expressing the human tau P301S mutant form of human microtubule-associated protein tau (MAPT) under the direction of the mouse prion protein promoter (Prnp) and resulting in 5-fold greater human tau expression over endogenous mouse tau protein (Yoshiyama et al., 2007). Breeding pairs were purchased from the Jackson Laboratory, and resulting progenies were maintained on a B6C3 background to generate both P301S transgenic and wild type littermate controls. Mice had access to food and water ad libitum and were housed on a 12-hour light/dark cycle. Nine-month-old P301S mutant mice, along with their wild type littermates, were applied for this study.
2.5 In vitro autoradiography studies of P301S mouse brain
After saline perfusion and euthanasia of the mice, brain tissue was quickly collected and each mouse brain was cut sagittal into two parts along the midline. One half-brain was directly frozen in liquid nitrogen for post-mortem autoradiography studies; the other half was drop-fixed in 4% formaldehyde at 4°C for use in immunofluorescence microscopy studies. For autoradiography studies, 20 µm slices from P301S mice and wild type control mice were incubated with 2.8 MBq/mL [123I]TZ6019 on ice for 60 min, following by a 15-min pre-incubation in assay buffer (50 mM Tris–HCl buffer, pH 7.4, 0.1% BSA, and 1 mM EDTA). Following this incubation, sections were washed 2 times in assay buffer at 4 °C and rinsed once using distilled water at 4°C to remove buffer salts. Then the slides were air-dried and exposed to the storage phosphor screen (Fuji Photo Film Inc., Tokyo, Japan) in an imaging cassette for 2 – 4 hr in 4°C at dark. The distribution of radioactivity was visualized by a Fuji Bio-Imaging Analyzer FLA-7000 (GE healthcare Life Sciences Inc., Pittsburgh, PA). Photo-stimulated luminescence was quantified in one representative brain section using Multi Gauge v3.0 software (Fuji Photo Film Inc., Tokyo, Japan). Data were background-corrected and expressed as photo-stimulated luminescence signals per square millimeter (PSL/mm2). For statistical comparison, the student t-test were applied using MS Excel and p < 0.05 was considered statistically significant.
2.6 Immunofluorescence (IF) microscopy
The fixed brain tissue was cut into 50 µm slices, stored free-floating in cryoprotectant solution (10% ethylene glycol, 30 mM phosphate buffer, 15% sucrose) and kept at 4°C until use. Slices were blocked at room temperature for 1 h in 5% normal horse serum (NHS) prior to incubation with the primary antibodies: P2X7 receptor (rabbit anti-P2X7 receptor polyclonal antibody, 1:50, Alomone labs Inc., Jerusalem, Israel), and GFAP (chicken anti-GFAP polyclonal antibody, 1:1000, Abcam Inc, Cambridge, MA) in 5% NHS with 1× TBS 0.1% Triton X-100 overnight at 4°C. Sections were incubated for 1 h at room temperature with fluorescent-conjugated secondary antibodies (1:1000 anti-chicken DyLight 488 and 1:1000 anti-rabbit Alex 594, ThermoFisher Scientific Inc., Waltham, MA) and counter stained with DAPI for 5 min. Sections were then mounted onto slides, and cover slipped using Fluoromount G mounting solution (Southern Biotech Inc., Birmingham, AL). The 60× confocal microscope images were collected from a Nikon A1Rsi scanning confocal microscope system in the Washington University Center for Cellular Imaging. All images were taken with the same fluorescent settings and subsequently adjusted equally for brightness and contrast to ensure accurate pathologic quantification.
3. Results
3.1 Chemistry
The precursor desmethyl-GSK1482160 was synthesized by following standard methods (Gao et al., 2015; Han et al., 2017) (Scheme 1A). The condensation of L-pyroglutamic acid with amine under 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDAC) and 1-hydrobenzotrizole (HOBt) produced product 3 in 70% yield. Then reaction of desmethyl-GSK1482160 3 with tributyl-(3-chloro-propenyl)-stannane in the presence of LiHMDS gave the radiolabeling precursor 4. Iodination of precursor in chloroform afforded the standard reference 5 (TZ6019) in good yield (65%). The fluoroethyl modified compound 6 (TZ5038) was produced from 3 and 1-bromo-2-fluoroethane in similar procedure. The structure of compound 4, 5, 6 were confirmed by NMR spectroscopy and LC-MS.
3.2 Radiochemistry
The one-step iodine-123 radiolabeling of the tin precursor 4 proceeded smoothly at room temperature. Under the optimized conditions (Scheme 1B), the product was obtained in high yield (85 ± 5%, n = 8, decay corrected to the end of synthesis), excellent radiochemical purity (> 99%), and theoretical specific activity 34 GBq/µmol.
3.3 Fluorescence assay
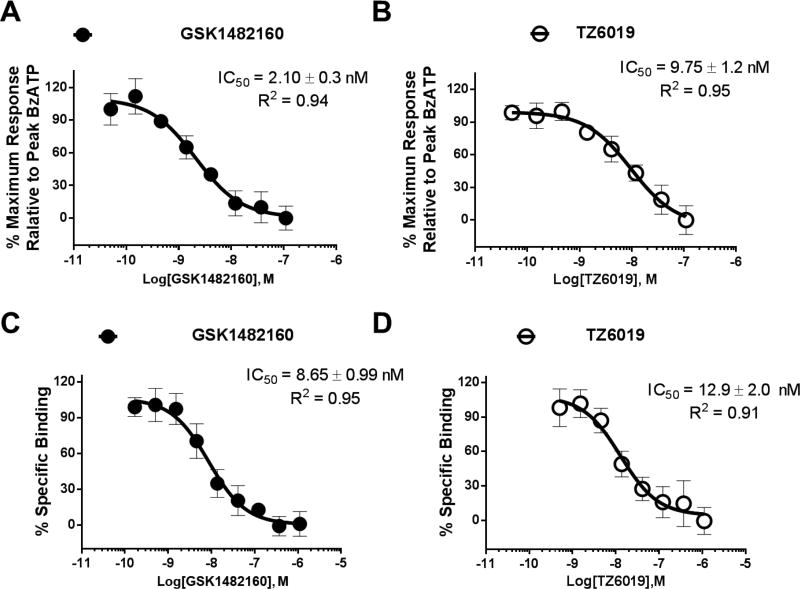
A fluorescence assay based on the ethidium bromide measurement of the pore permeation was used to assess the binding potency of the newly synthesized iodinated compounds and other known P2X7 compounds. The known P2X7 compound GSK1482160 used as an internal control had an IC50 value of 2.10 ± 0.30 nM, consistent with previous reports (Gao et al., 2015; Han et al., 2017). The fluoroethyl modified compound 6, TZ5038 (9.93 ± 2.1 nM) showed slightly higher IC50 value compared to GSK1482160 (Fig. 2, Table 1). The assay revealed that iodinated compounds TZ6019 had 5-fold (9.75 ± 1.2 vs 2.10 ± 0.3 nM) lower binding potency than compound GSK1482160 (Scheme 1, Table 1), but similar binding potency to the fluoroethyl substituted compound TZ5038, suggesting that extending the alkyl chain of pyrrolidine may interfere with the P2X7 receptor binding but the binding potency was still in nanomolar range.
Fig. 2.
Characterization of compound TZ6019 binding to HEK-293 cells expressed recombinant human P2X7 receptor. Representative binding curves from fluorescence assay for GSK1482160 (filled circles) (A) and TZ6019 (open circles) (B), and from [3H]A804598 based radioactive competitive binding curve for GSK1482160 (filled circles) (C) and TZ6019 (open circles) (D). The reported IC50 values (nM) were based on three independent assays with mean ± S.D..
Table 1.
Summary of the P2X7 receptor binding potency (IC50 (nM), mean± S.D., n = 3) for 3 ligands obtained from different assays using HEK-293 transfected P2X7 receptor cells.
| Compounds | Ethidium bromide Fluorescence assay IC50 (nM) |
[123I]TZ6019 Competition assay IC50 (nM) |
[3H]A804598 Competition assay IC50 (nM) |
|---|---|---|---|
| GSK1482160 | 2.10 ± 0.3 | 4.30 ± 0.9 | 8.65 ± 1.0 |
| TZ6019 (compound 5) | 9.75 ± 1.2 | 9.49 ± 1.4 | 12.9 ± 2.0 |
| TZ5038 (compound 6) | 9.93 ± 2.1 | 8.10 ± 1.3 | 11.90 ± 2.2 |
3.4 Saturation binding [123I]TZ6019
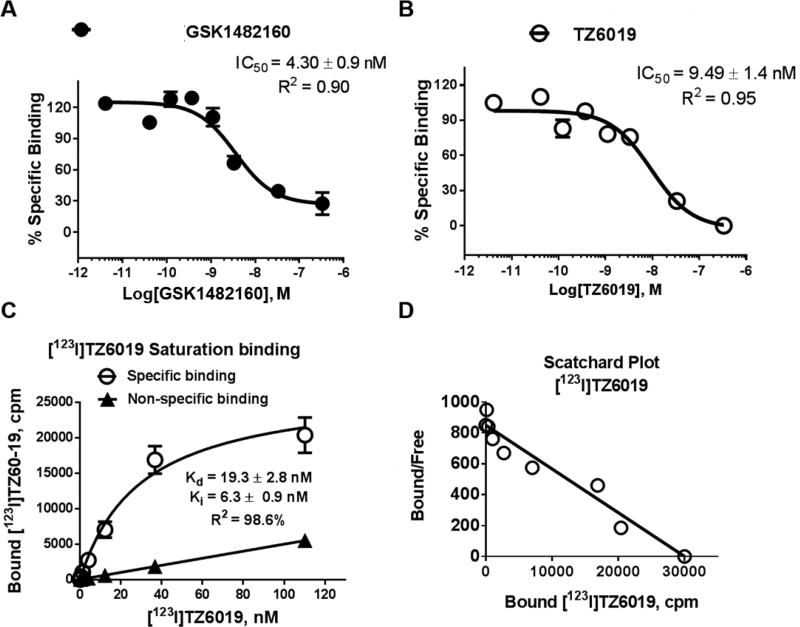
At the radioligand saturating binding assays, non-specific binding of [123I]TZ6019 remained constant in 2–5×105 cells of HEK-hP2X7R, suggesting that the non-specific binding to the cell is minimal (Fig.3.). The specific binding curve showed a typical saturation binding curve, with nanomolar range (Kd = 19.3 ± 2.8 nM) of binding affinity (Fig. 3). The low dissociation rate permits the washing of the plate for the separation of free from bound radioligand (Han et al., 2017; Letavic et al., 2013; Territo et al., 2016), while the association rate supports 60 min incubations for attaining steady-state binding. The saturation binding data indicated that specific binding represented approximately 40% of total binding. The [123I]TZ6019-based competition binding assay revealed that the IC50 value is 9.75 ± 1.2 nM and the calculated Ki value based on Cheng–Prusoff equation was 6.30 ± 0.9 nM. Therefore [123I]TZ6019 binds to the recombinant human P2X7 receptor with high affinity. These results were consistent with our fluorescence ethidium bromide assay result. The specific binding data fit a straight line on a Scatchard plot, indicating that one-site binding model is a good model for [123I]TZ6019 binding to human P2X7 receptor. Receptor density (Bmax) as determined by saturation binding with [123I]TZ6019 was 262 ±10 fmol/mg, which were consistent with the carbon-11 labeled GSK1482160 measurement from previous studies (Han et al., 2017; Territo et al., 2016).
Fig. 3.
Characterization of [123I]TZ6019 binding to HEK-293 cells expressed recombinant human P2X7 receptor. [123I]TZ6019 based radioligand competitive binding curve for GSK1482160 (A) and TZ6019 (B). C. Saturation binding curve in the range of 0.2 – 115 nM of [123I]TZ6019. The curve fitted Kd is 19.3 ± 2.8 nM (n = 3) and Bmax is 262 fmol/mg (n = 3). The calculated Ki is 6.3 ± 0.9 nM (n = 3) based on Cheng-Prusoff equation. The reported IC50 or Kd values (nM) were based on three independent assays with mean ± S.D.. D. The specific binding data is fitted a straight line in Scatchard plot.
3.5 Competition binding potency
To validate the suitability of using radioligand [123I]TZ6019 to screen P2X7 compounds, the competition binding assays applying radioligands [123I]TZ6019 and [3H]A804598 were carried out (Fig 2C, 2D, Fig 3A, 3B) and compared. The known P2X7 compound (GSK1482160) showed stronger binding (IC50 value of 4.3 ± 0.9 nM) in the [123I]TZ6019 based radioactive competitive assay, while the values were 8.65 ± 1.0 nM using [3H]A804598. The iodinated substituted analog TZ6019 (compound 5), and fluoroethane substituted analog TZ5038 (compound 6) showed similar trends in stronger binding in [I123]TZ6019 based radioactive competitive assay (Table 1). Among these assays, radioactive competitive assay based [123I]TZ6019 showed systematic stronger binding than using [3H]A804598 (Table 1). The radioactive competitive assay results using [123I]TZ6019 showed results consistent with the fluorescence assay.
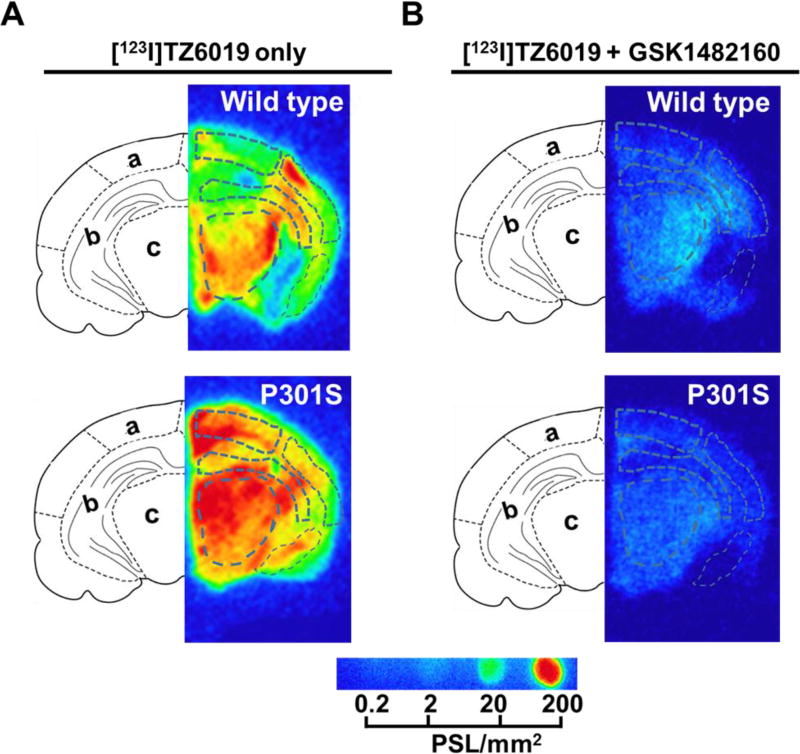
3.6 In vitro autoradiography of [123I]TZ6019 in brain tissue of P301S mice
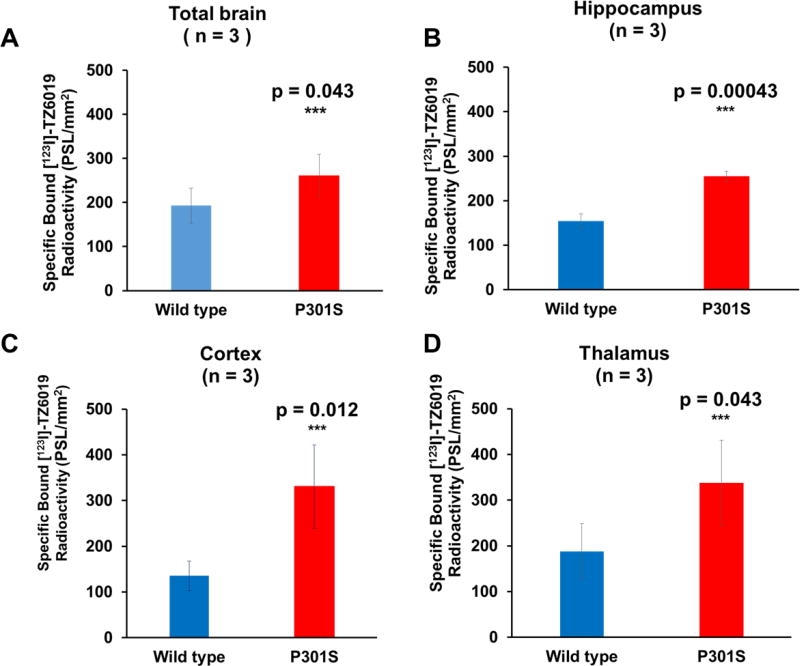
To further evaluate the iodinated radioligand [123I]TZ6019, in vitro autoradiography studies of [123I]TZ6019 were performed to detect the expression of P2X7 receptor in the brain of a mouse model of tauopathy. P2X7 receptor expression was quantified using [123I]TZ6019 was significantly elevated in P301S mice (Fig. 4, Table 2). Different brain regions including cortex, hippocampus, thalamus and total brain were quantified and compared to wild type (Fig. 5, Table 2). In hippocampus, P2X7 receptor was highly expressed in the P301S mice (n = 3, 255.05 ± 10.90 PSL/mm2), which was 1.7-fold higher than the wild type mice group (154.18 ± 16.14 PSL/mm2, n = 3, P = 0.00043). Specific binding in cortex (n = 3) was 330.53 ± 91.08 PSL/mm2 for P301S mice which was 2.4-fold higher than in wild type mice (135.23 ± 32.55 PSL/mm2; P = 0.012) (Fig. 4, 5, Table 2). The expression of P2X7 receptor in thalamus was also significantly higher in P301S mice than wild type mice (P = 0.039, n =3), 337.98 ± 93.45 PSL/mm2 for P301S and 187.41 ± 61.02 PSL/mm2. Finally the expression of P2X7 receptor in total brain was significantly higher in P301S mice than wild type mice (P = 0.043, n = 3), 260.86 ± 48.93 PSL/mm2 for P301S and 193.05 ± 39.92 PSL/mm2. An overall increasing trend (P = 0.0013) was found when including all regions quantified.
Fig. 4.
In vitro autoradiography of [123I]TZ6019 for P301S mice and wild type control. The 20 µm coronal ipsilateral slices from P301S tau transgenic and healthy wild type mice, were incubated with [123I]TZ6019 alone (A) or [123I]TZ6019 plus 100 µM cold GSK1482160 (B) at room temperature for 60 min. Brain region keys: (a) Cortex, (b) Hippocampus, and (c) Thalamus.
Table 2.
Summary of P2X7 receptor expression quantified from the in vitro autoradiography in P301S and wild type mouse brain using [123I]TZ6019
| ROI | P2X7 receptor expression quantified by autoradiography (PSL/mm2, mean± S.D., n = 3) |
P-value (t-test) |
|
|---|---|---|---|
|
| |||
| Wild type | P301S | ||
| Hippocampus | 154.18 ± 16.14 | 255.05 ± 10.90 | 0.00043a |
| Cortex | 135.23 ± 32.55 | 330.53 ± 91.08 | 0.012a |
| Thalamus | 187.41 ± 61.02 | 337.98 ± 93.45 | 0.039a |
| Total brain | 193.05 ± 39.92 | 260.86 ± 48.93 | 0.043a |
P < 0.05
Fig. 5.
Elevation of P2X7 receptor expression in P301S mice was quantified in vitro autoradiography by [123I]TZ6019. Quantitative histogram of higher binding of [123I]TZ6019 in total brain (A), hippocampus (B), cortex (C), and thalamus (D) for P301S than wild type mice (n = 3, *** indicated student test P < 0.05). PSL/mm2, photo-stimulated luminescence signals per square millimeter.
3.7 Expression of P2X7 receptor and astrocytes staining in P301S mice
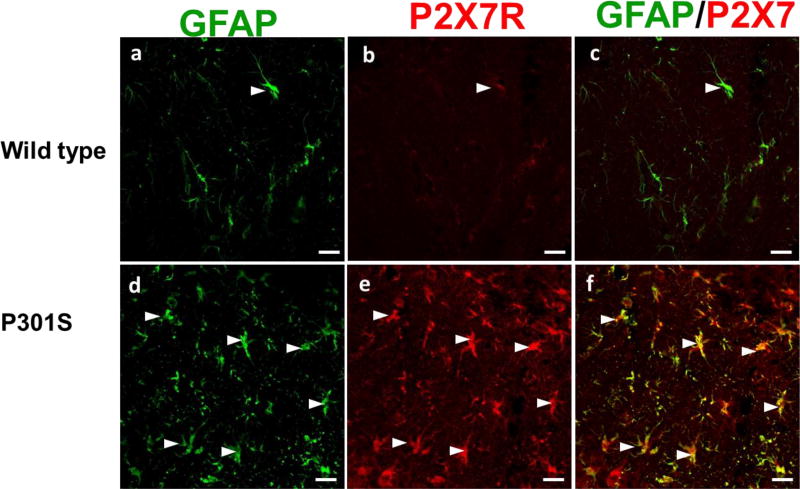
Immunofluorescence (IF) staining was done in the other half of the brain tissues from P301S and wild type mice. The data revealed that astrocytes (GFAP, green) colocalized with P2X7 receptor (red) in the hippocampal region as visualized by the dual labeled yellow cells (Fig. 6). Overall, our IF microscopy results confirmed that expression of P2X7 receptor was up-regulated in brains of P301S mice, and colocalized with astrocytes.
Fig. 6.
Immunofluorescence (IF) staining of elevation of P2X7 receptor and astrocyte activation in P301S mice. Representative IF confocal images demonstrate astrocytes expression (GFAP, green) in hippocampus of P301S mice (d, f), compared to wild type control mice (a, c). Representative IF images demonstrate elevation of P2X7 receptor (red) expression in brain of P301S mice (e), compared to wild type control mice (c). The colocalized astrocytes (green) and P2X7 receptor (red) in hippocampus region are depicted with white arrows. The white scale bar is 20 µm.
4. Discussion
A number of radiolabeled P2X7 ligands have been reported (Donnelly-Roberts et al., 2009; Han et al., 2017; Honore et al., 2006; Janssen et al., 2014; Lord et al., 2015; Ory et al., 2016; Territo et al., 2016) (Fig. 1), but none of them have been successfully translated to human studies. Two tritiated radioligand [3H]A804598 and [3H]JNJ54232334, have been characterized in rat brain membranes (Donnelly-Roberts et al., 2009; Lord et al., 2015). However, nonspecific binding may compromise the accurate analysis of P2X7 receptor binding with these radioligands (Lord et al., 2015). An analogue of GSK1482160 was originally discovered from GlaxoSmithKline with a high throughput screening for P2X7 receptor specific antagonists (Abdi et al., 2010; Chambers et al., 2010). Continuing the work on a C-11 labeled analogue, we synthesized and characterized the iodinated analogue TZ6019 in this study. We observed that the binding potency of TZ6019 was in nanomolar range. We applied both [123I]TZ6019 and [3H]A804598 to screen compounds by the radioligand competitive assay, slightly lower IC50 values were observed using [123I]TZ6019 compared to [3H]A804598 (Table 1). A larger pool of compounds will need to be screened to confirm this relationship. Compounds that show significant differences in binding potency using these two different radioligands may provide valuable insight information for the P2X7 binding properties for different chemical classes, represented by TZ6019 vs A804598. Compared to the relatively higher non-specific binding of [3H]804598 (Lord et al., 2015), [123I]TZ6019 may provide higher specific binding to P2X7 receptor. In addition, the synthesis of [123I]TZ6019 is easily achieved with significantly higher yields compared to [3H]804598. [123I]TZ6019 has advantages for use as a screening radioligand to determine the binding potency of compounds to P2X7 receptor.
Transgenic P301S mice display neuroinflammation with microglial activation and astrogliosis at 9 month old age (Lopez-Gonzalez et al., 2015). The ultrastructure of the neurofibrillay tangle-like lesions in these mice is similar to that found in brain lesions of human AD and tauopathy patients. In addition to the promising in vitro binding studies, we quantified the P2X7 receptor expression in brain of P301S mice by [123I]TZ6019. The in vitro autoradiography revealed that [123I]TZ6019 has significantly higher specific binding in P301S mice (Fig 4, 5). This suggests that [123I]TZ6019 may be used to assess neruroinflammatory response in AD or tauopathy. Athough the expression of P2X7 receptor in brain regions was globally increased, the hippocampus, cortex, and thalamus regions were significantly increased (Tabel 2). The IF results further support that the P2X7 receptor elevation in hippocampus, associating with an increased expression of astrocytes in P301S mice (Fig. 6).
These results agree with previous report for astrocytic activation in P301S mice, but previous studies did not measure the P2X7 receptor expression (Lopez-Gonzalez et al., 2015). In mouse models of AD, astrocytes or microglia exhibit abnormal activation and produce pro-inflammatory mediators (Lopez-Gonzalez et al., 2015; Parvathenani et al., 2003). P2X7 receptor plays a vital role in astrocytes/microglia-mediated IL-1β processing and release through the membrane receptors, which promotes inflammatory damage in CNS (Grygorowicz et al., 2016; Jimenez-Pacheco et al., 2016). The elevated expression of P2X7 receptor in AD mice model of tg2576 mice was reported (Parvathenani et al., 2003), but the expression of P2X7 receptor in a tauopathy model has not been reported. In this study, we characterized in vitro binding of [123I]TZ6019 with brain tissue of P301S mice, a tauopathy mouse model. To the best of our knowledge, this is the first investigation of P2X7 receptor in the P301S mice.
P2X7 radioligands have demonstrated utility for in vivo imaging studies; the P2X7 radioligand [11C]GSK1482160 as a PET radioligand has been reported recently (Han et al., 2017; Ory et al., 2016). Understanding the interaction of P2X7 receptor with its ligands is key to developing therapeutic strategies targeting this receptor. However the weak binding affinity to rodent P2X7 receptor may limit the application of this radiotracer (Gao et al., 2015; Han et al., 2017; Ory et al., 2016). Additional new radioligands are still needed in the future. Compared to compound GSK1482160, the fluoroethane or iodinated modification showed similar binding potency to P2X7 receptor with minor reduction of binding potency based on IC50 values. In vivo binding specificity is a key parameter for a novel radiotracer. Without using P2X7 receptor knockout mice to characterize the in vivo binding specificity of [123I]TZ6019 was a limitation. Our future study of promising P2X7 receptor imaging radiotracers will use P2X7 receptor knockout mice to confirm their in vivo binding specificity. Nevertheless, the properties of [123I]TZ6019 reported in current study, will facilitate the development of new selective P2X7 ligands for potential imaging and therapeutic applications.
5. Conclusion
A potent iodinated labeled radiotracer [123I]TZ6019 for P2X7 receptor was successfully radiosynthesized and evaluated in this study. Our in vitro cell binding assays demonstrated that this radioligand specifically binds to recombinant human P2X7 receptor with nanomolar affinity. Our in vitro autoradiography revealed that [123I]TZ6019 was able to detect the increase of P2X7 receptor expression in brain from P301S mutant transgenic mice. This compound represents a promising radioligand for screening new P2X7 compounds and assessing P2X7 receptor expression in neuroinflammatory related diseases.
Acknowledgments
This work was supported by USA Department of Energy (DOE)-Training in Techniques and Translation: Novel Nuclear Medicine Imaging Agents for Oncology and Neurology, DESC0008432, and Interdisciplinary Training in Translational Radiopharmaceutical Development and Nuclear Medicine Research for Oncologic, Neurologic, and Cardiovascular Imaging, DESC0012737, the Fixel Foundation, and USA National Institute of health/National Institute of Neurological Disorders and Stroke and National Institute on Aging (NIH/NINDS and NIA) NS075527, NIH/NINDS, NS103988 and NS103957, USA National Institute of health/National Institute of mental health (NIH/NIMH) MH092797.
Abbreviations
- ACN
Acetonitrile
- AD
Alzheimer’s disease
- AMF
ammonium formate
- ATP
adenosine-triphosphate
- BzATP
3'-O-(4-Benzoyl)benzoyl ATP, strong agonist for P2X7 receptor
- CNS
central nervous system
- DMEM
Dulbecco's Modified Eagle's Medium
- DMF
dimethylformamide
- EDAC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
- EtOAc
ethyl acetate
- ESI
electrospray ionization
- GFAP
Glial fibrillary acidic protein
- FACS
fluorescence activation cell sorting
- HEK-293
human embryonic kidney cells 293
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HOBt
1-hydrobenzotrizole
- HPLC
high-performance liquid chromatography
- HRMS
high resolution mass spectrum
- hr
hour
- IC50
the concentration of an inhibitor where the response (or binding) is reduced by half
- IF
Immunofluorescence
- Kd
the equilibrium dissociation constant, inverse of association constant (Ka)
- Ki
inhibition dissociation constant
- LC-MS
liquid chromatography–mass spectrometry
- LiHMDS
Lithium bis(trimethylsilyl)amide
- MAPT
human microtubule-associated protein tau
- NHS
normal horse serum
- NMR
nuclear magnetic resonance
- PBS
phosphate buffered saline
- PD
Parkinson’s disease
- PET
positron emission tomography
- Prnp
prion protein promoter
- PSL/mm2
photo-stimulated luminescence signals per square millimeter
- P2X7 receptor
P2X ligand-gated ion channel 7
- ROI
region of interest
- SDS
sodium dodecyl sulfate
- TMS
tetramethylsilane
- THF
tetrahydrofuran
- TLC
Thin layer chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors do not have any conflict of interest.
References
- Abdi MH, et al. Discovery and structure-activity relationships of a series of pyroglutamic acid amide antagonists of the P2X7 receptor. Bioorg Med Chem Lett. 2010;20:5080–5084. doi: 10.1016/j.bmcl.2010.07.033. [DOI] [PubMed] [Google Scholar]
- Able SL, et al. Receptor localization, native tissue binding and ex vivo occupancy for centrally penetrant P2X7 antagonists in the rat. Br J Pharmacol. 2011;162:405–414. doi: 10.1111/j.1476-5381.2010.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett R, et al. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66:638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- Basso AM, et al. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia. 2016;64:1772–1787. doi: 10.1002/glia.23001. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol. 2013;170:624–640. doi: 10.1111/bph.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco F, et al. A role for P2X7 in microglial proliferation. J Neurochem. 2006;99:745–758. doi: 10.1111/j.1471-4159.2006.04101.x. [DOI] [PubMed] [Google Scholar]
- Boumechache M, et al. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J Biol Chem. 2009;284:13446–13454. doi: 10.1074/jbc.M901255200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic cotransmission. Exp Physiol. 2009a;94:20–24. doi: 10.1113/expphysiol.2008.043620. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling: past, present and future. Braz J Med Biol Res. 2009b;42:3–8. doi: 10.1590/s0100-879x2008005000037. [DOI] [PubMed] [Google Scholar]
- Chambers LJ, et al. Synthesis and structure-activity relationships of a series of (1H-pyrazol-4-yl)acetamide antagonists of the P2X7 receptor. Bioorg Med Chem Lett. 2010;20:3161–3164. doi: 10.1016/j.bmcl.2010.03.096. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chessell IP, et al. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br J Pharmacol. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collo G, et al. Tissue distribution of the P2X7 receptor. Neuropharmacology. 1997;36:1277–1283. doi: 10.1016/s0028-3908(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts DL, et al. [3H]A-804598 ([3H]2-cyano-1-[(1S)-1-phenylethyl]-3-quinolin-5-ylguanidine) is a novel, potent, and selective antagonist radioligand for P2X7 receptors. Neuropharmacology. 2009;56:223–229. doi: 10.1016/j.neuropharm.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Ferrari D, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Franke H, Illes P. Pathological potential of astroglial purinergic receptors. Adv Neurobiol. 2014;11:213–256. doi: 10.1007/978-3-319-08894-5_11. [DOI] [PubMed] [Google Scholar]
- Gao M, et al. Synthesis of [(11)C]GSK1482160 as a new PET agent for targeting P2X(7) receptor. Bioorg Med Chem Lett. 2015;25:1965–1970. doi: 10.1016/j.bmcl.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Grygorowicz T, et al. Early P2X7R-related astrogliosis in autoimmune encephalomyelitis. Mol Cell Neurosci. 2016;74:1–9. doi: 10.1016/j.mcn.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Han J, et al. Pharmacologic characterizations of a P2X7 receptor-specific radioligand, [11C]GSK1482160 for neuroinflammatory response. Nucl Med Commun. 2017;38:372–382. doi: 10.1097/MNM.0000000000000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore P, et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther. 2006;319:1376–1385. doi: 10.1124/jpet.106.111559. [DOI] [PubMed] [Google Scholar]
- Janssen B, et al. Synthesis and initial preclinical evaluation of the P2X7 receptor antagonist [(1)(1)C]A-740003 as a novel tracer of neuroinflammation. J Labelled Comp Radiopharm. 2014;57:509–516. doi: 10.1002/jlcr.3206. [DOI] [PubMed] [Google Scholar]
- Jimenez-Pacheco A, et al. Transient P2X7 Receptor Antagonism Produces Lasting Reductions in Spontaneous Seizures and Gliosis in Experimental Temporal Lobe Epilepsy. J Neurosci. 2016;36:5920–5932. doi: 10.1523/JNEUROSCI.4009-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letavic MA, et al. Synthesis and Pharmacological Characterization of Two Novel, Brain Penetrating P2X7 Antagonists. ACS Med Chem Lett. 2013;4:419–422. doi: 10.1021/ml400040v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Gonzalez I, et al. Neuroinflammatory Gene Regulation, Mitochondrial Function, Oxidative Stress, and Brain Lipid Modifications With Disease Progression in Tau P301S Transgenic Mice as a Model of Frontotemporal Lobar Degeneration-Tau. J Neuropathol Exp Neurol. 2015;74:975–999. doi: 10.1097/NEN.0000000000000241. [DOI] [PubMed] [Google Scholar]
- Lord B, et al. A novel radioligand for the ATP-gated ion channel P2X7: [3H] JNJ-54232334. Eur J Pharmacol. 2015;765:551–559. doi: 10.1016/j.ejphar.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Michel AD, et al. Decavanadate, a P2X receptor antagonist, and its use to study ligand interactions with P2X7 receptors. Eur J Pharmacol. 2006;534:19–29. doi: 10.1016/j.ejphar.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Monif M, et al. Microglia: proliferation and activation driven by the P2X7 receptor. Int J Biochem Cell Biol. 2010;42:1753–1756. doi: 10.1016/j.biocel.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Monif M, et al. The P2X7 receptor drives microglial activation and proliferation: a trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory D, et al. Preclinical Evaluation of a P2X7 Receptor-Selective Radiotracer: PET Studies in a Rat Model with Local Overexpression of the Human P2X7 Receptor and in Nonhuman Primates. J Nucl Med. 2016;57:1436–1441. doi: 10.2967/jnumed.115.169995. [DOI] [PubMed] [Google Scholar]
- Park JH, et al. Potent Suppressive Effects of 1-Piperidinylimidazole Based Novel P2X7 Receptor Antagonists on Cancer Cell Migration and Invasion. J Med Chem. 2016;59:7410–7430. doi: 10.1021/acs.jmedchem.5b01690. [DOI] [PubMed] [Google Scholar]
- Parvathenani LK, et al. P2X7 mediates superoxide production in primary microglia and is upregulated in a transgenic mouse model of Alzheimer's disease. J Biol Chem. 2003;278:13309–13317. doi: 10.1074/jbc.M209478200. [DOI] [PubMed] [Google Scholar]
- Territo PR, et al. Characterization of [11C]-GSK1482160 for targeting the P2X7 receptor as a biomarker for neuroinflammation. J Nucl Med. 2016;58:458–465. doi: 10.2967/jnumed.116.181354. [DOI] [PubMed] [Google Scholar]
- Yoshiyama Y, et al. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53:337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]