Abstract
Anterior cruciate ligament (ACL) deficient patients have an increased rate of patellofemoral joint (PFJ) osteoarthritis (OA) as compared to the general population. Although the cause of post-injury OA is multi-factorial, alterations in joint biomechanics may predispose patients to cartilage degeneration. This study aimed to compare in vivo PFJ morphology and mechanics between ACL deficient and intact knees in subjects with unilateral ACL ruptures. Eight male subjects underwent baseline MRI scans of both knees. They then performed a series of 60 single-legged hops, followed by a post-exercise MRI scan. This process was repeated for the contralateral knee. The MR images were converted into three-dimensional surface models of cartilage and bone in order to assess cartilage thickness distributions and strain following exercise. Prior to exercise, patellar cartilage was significantly thicker in intact knees as compared to ACL deficient knees by 1.8%. In response to exercise, we observed average patellar cartilage strains of 5.4 ± 1.1% and 2.5 ± 1.4% in the ACL deficient and intact knees, respectively. Importantly, the magnitude of patellar cartilage strain in the ACL deficient knees was significantly higher than in the intact knees. However, while trochlear cartilage experienced a mean strain of 2.4 ± 1.6%, there was no difference in trochlear cartilage strain between the ACL deficient and uninjured knees. In summary, we found that ACL deficiency was associated with decreased patellar cartilage thickness and increased exercise-induced patellar cartilage strain when compared to the uninjured contralateral knees.
Keywords: biomechanics, strain, patella, osteoarthritis, MRI
INTRODUCTION
Patellofemoral joint (PFJ) pain and cartilage degeneration are commonly observed sequelae of anterior cruciate ligament (ACL) rupture (Asano et al., 2004; Culvenor et al., 2013; Lohmander et al., 2007; Neuman et al., 2009; Sachs et al., 1989). The radiographic prevalence of PFJ osteoarthritis (OA) is estimated to be 50% 10–15 years following ACL injury, even if the patient had their ligament surgically reconstructed (Culvenor et al., 2013). Moreover, patients who have sustained ACL rupture have a higher risk of patellar cartilage loss 7–11 years post-injury, identified via magnetic resonance imaging (MRI) (Potter et al., 2012). The limitations in function and pain associated with knee OA (Farrokhi et al., 2013) are of grave concern for the young population experiencing ACL injuries.
A contributing factor to post-injury OA development may include alterations in PFJ loading resulting from ACL deficiency. While changes in PFJ kinematics have been identified, the effect of ACL deficiency on in vivo cartilage mechanics has not been well quantified. It has been suggested that alterations in tibial translation and rotation with ACL deficiency may lead to abnormal patellar rotation, tilt, and translation (Hsieh et al., 1998; Van de Velde et al., 2008). These altered patellofemoral kinematics may ultimately lead to altered PFJ cartilage contact (Hsieh et al., 2002). While normal mechanical loading of cartilage is critical for cartilage development and maintenance (Beaupre et al., 2000; Coleman et al., 2013), abnormal cartilage loading in the setting of ligamentous injury may contribute to cartilage degeneration and early-onset OA (Griffin and Guilak, 2005; Van de Velde et al., 2009). Therefore, measuring how ACL deficiency affects the in vivo PFJ cartilage response in patients is crucial for understanding the link between altered loading and OA progression.
Cartilage can be modeled as a biphasic material (Mow et al., 1984; Mow et al., 1980), consisting of the extracellular matrix and interstitial fluid. As a result, following the removal of a mechanical load, fluid that was exuded from the tissue during loading reenters the tissue in a time-dependent manner (Eckstein et al., 2006; Mow et al., 1984). This time-dependent recovery of fluid allows for the measurement of cartilage strain in response to activity using MR imaging, by comparing cartilage thickness distributions prior to and following load-bearing activities (Coleman et al., 2013; Sutter et al., 2015). As there is currently limited in vivo data quantifying the influence of ACL deficiency on PFJ cartilage mechanics, we employed this MRI-based technique to compare site-specific PFJ cartilage thickness changes and strain in ACL deficient and intact knees following exercise. We hypothesized that ACL deficient knees would demonstrate differences in both patellofemoral cartilage thickness and strain as compared to the intact contralateral knees.
METHODS
Subject Demographics
Institutional Review Board approval was obtained for this study, and subjects provided written consent prior to participation. Eight male subjects with unilateral ACL injuries (confirmed clinically and radiographically) were recruited for participation (mean age 31 years, range 21–47 years; mean body mass index (BMI) 25.6 kg/m2, range 21.7–34.7 kg/m2). The chronicity of the injuries ranged from 24 days to 13 years and 11 months prior to data collection. One subject could not recall the date of injury, but estimated the injury to have occurred between 14–25 months prior. At the time of the study, subjects had not undergone ACL reconstruction and had no history of injury or surgery to the intact contralateral knee.
Data Collection
The protocol used in this study has been previously described (Sutter et al., 2015). Subjects were instructed to refrain from strenuous activity the night before and the morning of the study. They arrived at the laboratory at 7am to reduce the effects of diurnal changes in cartilage thickness (Coleman et al., 2013; Widmyer et al., 2013). Upon arrival, the subjects completed a standard International Knee Documentation Committee (IKDC) questionnaire to help gauge each individual’s functionality and pain levels (Collins et al., 2011). The participants were then asked to lie supine near the MR scanner for 45 minutes prior to data collection (Sutter et al., 2015) in order to allow the cartilage to relax to a baseline unloaded state (Eckstein et al., 2006). The subjects then underwent MRI of both knees prior to activity. All MRI scans were acquired with subjects in the supine position and their knees relaxed. Sagittal plane images were obtained using a 3T MR scanner (Trio Tim; Siemens; Malvern, PA) with an eight-channel dedicated knee coil using a double echo steady-state (DESS) pulse sequence (resolution: 0.3x0.3x1 mm, flip angle: 25 degrees, repetition time (TR): 17 ms, echo time (TE): 6 ms) (Taylor et al., 2013; Utturkar et al., 2013). The duration of each DESS acquisition was approximately 9 minutes.
Subjects were then transported via wheelchair about 10 m to the hallway adjacent to the MR scanner, where they performed 60 single-legged hops, each of which was 0.6 m in horizontal distance. During hopping, the contralateral knee remained in a flexed, non-weight bearing position. Immediately after, subjects returned to the wheelchair and were transported back to the MR scanner for a post-activity MRI scan of the exercised knee using the same parameters outlined above. This process was then repeated to perform the hopping activity and subsequent post-activity MRI scan on the opposite knee. Half of the subjects had their ACL deficient knee tested first, while the other half had their intact knee tested first. The mean time from the completion of the hopping activity to the start of the post-exercise MRI scan was 3 minutes and 48 seconds overall (range 3:14 – 4:35; ACL deficient mean 3:48; intact mean 3:47).
Model Creation and Analysis
MR images were imported into a solid modeling software (Rhinoceros; Robert McNeel and Associates; Seattle, Washington), in which a single investigator manually traced the outer bony cortices and articular cartilage surfaces of the patella and femur on each slice. This segmentation technique has been previously shown to be repeatable to within 0.03 mm, corresponding to differences in cartilage thickness of approximately 1.2% (Coleman et al., 2013). The tracings were stacked to create wireframe models, which were then converted into three-dimensional surface mesh models of the cartilage and bone (Geomagic Studio; Morrisville, NC) (Okafor et al., 2014).
The bony surfaces of the pre- and post-exercise models were aligned using an iterative closest-point technique (Geomagic Studio; Morrisville, NC), thereby allowing for the quantification of changes in cartilage thickness pre- versus post-exercise at corresponding locations (Okafor et al., 2014). Patellar and trochlear cartilage thickness, in both the pre- and post-exercise models, was defined as the distance between each vertex on the cartilage surface mesh and the closest vertex on the corresponding bony surface mesh. These calculations were used to create cartilage thickness maps of the patellofemoral joint (Figure 1). Cartilage thickness was then measured at several sampling regions, each 2.5 mm in radius, spaced evenly across the patellar and trochlear cartilage surfaces. Eleven regions were used across the patellar cartilage surface and fifteen regions were used across the femoral trochlea (Figure 2), employing a technique modified from a previous investigation in our laboratory (Coleman et al., 2013). Strain within each of these regions was defined as the normalized change in cartilage thickness following exercise (post-exercise thickness minus pre-exercise thickness, divided by pre-exercise thickness). The average compartmental strain was calculated as the mean strain across all sampling regions on the patellar and trochlear cartilage surfaces, respectively.
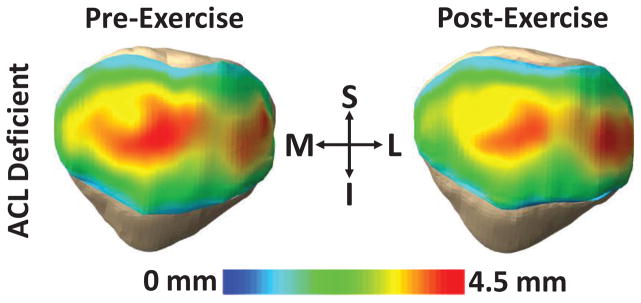
Figure 1.
Exercise decreased cartilage thickness in the patella, as demonstrated in an ACL deficient knee. Red regions represent thicker cartilage, while blue regions represent thinner cartilage. (S = superior; I = inferior; M = medial; L = lateral).
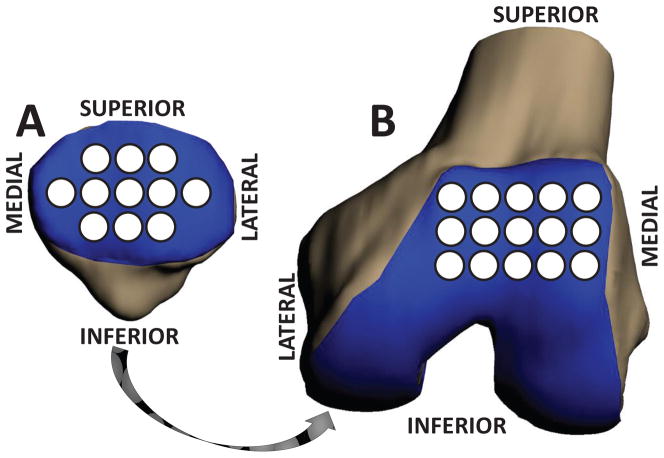
Figure 2.
Patellofemoral cartilage was sampled within 2.5 mm radius regions spanning the cartilage surfaces, enabling site-specific comparisons of cartilage thickness before and after exercise and between ACL deficient and intact knees. (A) Patellar cartilage. (B) Trochlear cartilage.
All statistical analyses were performed using Statistica (StatSoft, Inc.; Tulsa, OK), with significance defined as p<0.05. Two separate repeated measures ANOVAs were performed (for patellar cartilage and for trochlear cartilage) to compare cartilage thicknesses before and after exercise in the ACL deficient versus intact knees. Significant ANOVA results were followed up with Fisher’s LSD post-hoc tests. Single mean t-tests were used to determine if average compartmental strains were significantly different from zero, and average compartmental strains for the ACL deficient and intact contralateral knees were compared using paired t-tests. All results are reported as the mean ± 95% confidence interval.
RESULTS
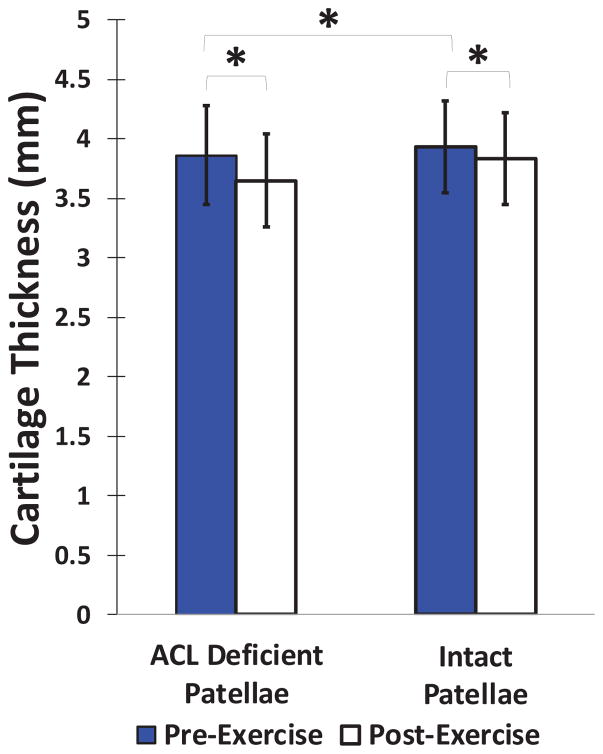
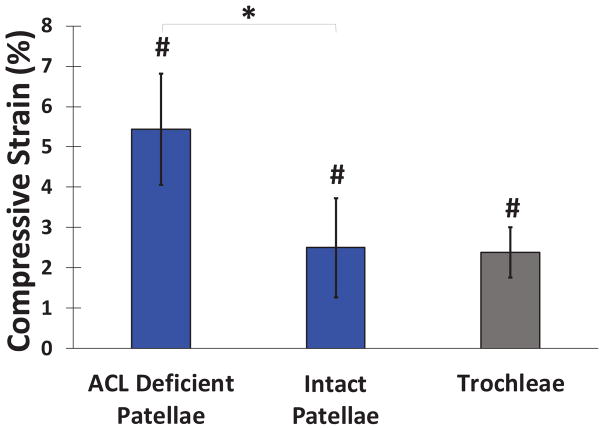
At baseline, the patellar cartilage was, on average, significantly thicker in the intact contralateral knees compared to their ACL deficient counterparts by 1.8% (p=0.028; Figure 3). There was a significant decrease in patellar cartilage thickness following hopping in both the intact and ACL deficient knees (Figure 4). In the intact knees, patellar cartilage demonstrated an average compartmental compressive strain of 2.5±1.4% (p=0.011). In the ACL deficient knees, the average compartmental compressive strain was 5.4±1.1% (p<0.0001). The magnitude of patellar cartilage strain in the ACL deficient knees was significantly greater than that observed in the intact knees (p=0.025). There were no significant differences according to location on the surface of the patellar cartilage.
Figure 3.
Mean (±95% CI) patellar cartilage thickness before and after performing a series of 60 single-legged hops in both ACL deficient and intact knees. Patellar cartilage was significantly thinner post-exercise for both groups, and ACL deficient knees had significantly thinner patellar cartilage at baseline than their intact counterparts (*p<0.05).
Figure 4.
Mean (±95% CI) articular cartilage compressive strain after hopping exercise. Patellar cartilage compressive strain in ACL deficient knees was greater as compared to intact knees (*p<0.05), while trochlear cartilage compressive strain was not statistically different in ACL deficient and intact knees (p=0.97). Average compressive strains were non-zero (#p<0.05).
Additionally, simple linear regression models were used to determine the effect of ACL deficiency duration on patellar cartilage strain and baseline patellar cartilage thickness. One subject was unable to recall the precise date of injury (estimated 14–25 months prior to testing); therefore, this subject was excluded from the linear regression models. The duration of ACL deficiency was not found to have a significant effect on patellar cartilage strain (p=0.61) or baseline patellar cartilage thickness (p=0.55) for the 7 subjects analyzed.
ACL deficiency did not significantly affect trochlear cartilage thickness (p=0.97). The overall average trochlear cartilage experienced an exercise-induced compressive strain of 2.4±1.6%, which was significantly different from zero (p=0.023). Furthermore, ACL deficiency did not affect exercise-induced trochlear cartilage strain (p=0.64).
DISCUSSION
While previous investigations have demonstrated a link between ACL deficiency and increased cartilage deformations in the tibiofemoral joint (Van de Velde et al., 2009), there is little data quantifying the effect of ACL deficiency on in vivo PFJ cartilage deformations. The results of the present study demonstrate that patellar cartilage in ACL deficient knees is significantly thinner than patellar cartilage in intact knees by 1.8%. Furthermore, after single-legged hopping, an exercise that has been previously shown to generate measurable tibiofemoral cartilage strain (Sutter et al., 2015), patellar cartilage strain was evident in both ACL deficient and intact knees. Importantly, we found significantly greater exercise-induced patellar cartilage strain in ACL deficient knees as compared to intact knees. These findings are indicative of altered PFJ loading and potentially degenerative changes in the PFJ cartilage in ACL deficient knees.
In this study, we found that patellar cartilage experienced an average compartmental compressive strain of 2.5±1.4% in intact knees, versus 5.4±1.1% in ACL deficient knees. Prior work has demonstrated comparable magnitudes of exercise-induced tibial cartilage strain with single-legged hopping (5%) (Sutter et al., 2015) and treadmill walking (2–5%) in uninjured subjects (Lad et al., 2016; Liu et al., 2017). Additionally, the degree of patellar cartilage strain in intact knees in this study was slightly higher than that of patellar cartilage diurnal strain resulting from daily activities (2%) (Coleman et al., 2013). These small differences are likely due to differences between the loading patterns experienced during the controlled single-legged hopping activity used here versus the loading patterns experienced during the unrestricted activities of daily living that lead to diurnal changes (Coleman et al., 2013).
In our study, there was a slight delay (average 3 minutes and 48 seconds) between the completion of the hopping activity and the start of the post-exercise MRI scan. Although this delay was minimized by the completion of the activity in the hallway adjacent to the MR scanner, it could not be completely avoided. However, the average time delay was nearly identical for the ACL deficient and intact knees (3:48 and 3:47, respectively), minimizing the potential for between-group differences in cartilage recovery prior to the post-exercise scan. Previous work has shown that patellar cartilage regains approximately 50% of its volume within 45 minutes of performing 100 deep knee bends due to fluid reentry in the articular cartilage upon removal of mechanical load (Eckstein et al., 2006; Mow et al., 1984). This information, coupled with our 3 minute and 48 second average delay between the conclusion of the exercise and the start of MRI acquisition, indicates that our strain findings are likely an under-estimation of the cartilage deformation present immediately after completion of the hopping activity.
The finding of increased patellar cartilage strain in ACL deficient knees relative to contralateral uninjured knees may be due to altered tibiofemoral kinematics resulting from ACL deficiency. Specifically, there have been multiple studies indicating that the absence of the ACL alters the motion of the tibia relative to the femur (Butler et al., 1980; Defrate et al., 2006; Fukubayashi et al., 1982; Grood et al., 1984; Hsieh et al., 1998; Li et al., 2007a; Van de Velde et al., 2009; Van de Velde et al., 2007). These changes in the motion of the tibiofemoral joint may alter patellofemoral mechanics (Ali et al., 2016; Li et al., 2007b). To this point, in a biomechanical study in cadaveric knees, Hsieh et al. noted that sectioning of the ACL resulted in a significant increase in patellar lateral shift and posterior translation with knee flexion (Hsieh et al., 1998). In a follow-up study, the authors showed that ACL deficiency shifted the patellofemoral contact region laterally (Hsieh et al., 2002). In line with these findings, Van de Velde et al. used MRI-based 3D knee models and biplanar fluoroscopy to compare ACL deficient knees with intact contralateral knees in vivo (Van de Velde et al., 2008). They found that patellofemoral cartilage contact was shifted proximally and laterally with ACL deficiency during a lunge (Van de Velde et al., 2008). These kinematic changes may cause abnormal loading of patellofemoral cartilage, which may contribute to the increased patellar cartilage strains in ACL deficient knees reported here.
Alterations in the magnitude and distribution of load across the joint may also contribute to the elevation of PFJ strain in ACL deficient knees. In support of this hypothesis, ACL deficiency has been associated with abnormal elongation of the patellar tendon (Van de Velde et al., 2008). As quadriceps muscle force acts via the patellar tendon to allow for knee extension, an increased patellar tendon length could reduce quadriceps efficiency and thus the ability of the quadriceps to absorb the loading in the PFJ (Draganich et al., 1987). Furthermore, quadriceps weakness has a high incidence following ACL injury (Sachs et al., 1989), which may result in increased PFJ contact forces and thus a larger patellar cartilage strain (Amin et al., 2006; Hurley, 1999). Congruently, Ali et al. demonstrated that ACL deficiency increased PFJ joint reaction forces during simulated deep knee bends using finite element modeling (Ali et al., 2016). Together, these studies suggest that alterations in load transfer through the joint may contribute to the elevated patellofemoral strains in ACL deficient knees observed in this study.
Alterations in PFJ loading due to ACL deficiency have important implications in the development of post-injury OA. Normal chondrocyte metabolism and health is sensitive to alterations in cartilage composition and mechanical compression (Griffin and Guilak, 2005; Guilak, 2011; Guilak et al., 1994). While physiologic cyclic loading of the cartilage is believed to enhance the anabolic metabolism of chondrocytes (Sanchez-Adams et al., 2014; Wong et al., 1999), chondrocyte matrix synthesis may be inhibited in instances of either super-physiologic (Wong et al., 1999) or static cartilage loading (Guilak et al., 1994). It has been proposed that such alterations may predispose chondrocytes to catabolic metabolism of the extracellular matrix, thereby affecting cartilage composition (Griffin and Guilak, 2005; Guilak, 2011; Sanchez-Adams et al., 2014). These compositional changes may include loss of proteoglycan and disruption of the collagen matrix, which would affect the mechanical function of the cartilage (Han et al., 2017; Hatcher et al., 2017; Makela et al., 2014).
The observed increase in cartilage deformation with ACL deficiency may also reflect altered mechanical properties of cartilage associated with early (pre-OA) degenerative changes in cartilage composition (Han et al., 2017; Sanchez-Adams et al., 2014; Setton et al., 1995; Setton et al., 1994). In support of this hypothesis, Han et al. found that ACL transection resulted in alterations of cartilage collagen fibrils and reduction of proteoglycan concentration as early as nine weeks post-injury in a rabbit model (Han et al., 2017). Furthermore, Van Ginckel et al. noted macroscopic morphological changes in knee cartilage using MRI even following surgical reconstruction of the ACL (Van Ginckel et al., 2013). Disruption of proteoglycan and collagen organization alters cartilage mechanical properties (Hatcher et al., 2017; Setton et al., 1994), resulting in a decrease in modulus and thus increased strain during loading. In the future, quantitative MRI techniques (such as T1ρ and T2 mapping) can be utilized alongside the strain measurements described in the present study to evaluate early compositional changes in the cartilage following an ACL injury (Amano et al., 2016; Hatcher et al., 2017; Kumar et al., 2014; Neu, 2014).
In addition to an increased magnitude of patellar cartilage strain, we found that the ACL deficient knees had thinner patellar cartilage compared to the intact contralateral knees at baseline. This is consistent with earlier investigations that have described alterations in cartilage thickness following ACL rupture (Frobell, 2011; Frobell et al., 2009). For instance, Frobell et al. demonstrated decreased cartilage volume in the femoral trochlea at one and two years post-injury (Frobell, 2011; Frobell et al., 2009). Cartilage thinning has also been observed in ACL reconstructed knees with non-anatomic graft placement (DeFrate, 2017; Okafor et al., 2014). Similar to our findings, Potter et al. showed that the risk of patellar cartilage loss triples between years one and two post-ACL injury (Potter et al., 2012). The observed loss of cartilage thickness may reflect degeneration in the ACL deficient knee. A future longitudinal study could measure patellofemoral cartilage thickness changes in a cohort of ACL deficient patients at specific time intervals post-injury to determine a more exact timeline for this apparent cartilage thinning.
In summary, we measured PFJ cartilage thickness before and after hopping in ACL deficient and intact knees. We observed significantly thinner patellar cartilage prior to exercise and a greater patellar cartilage strain in ACL deficient knees when compared to the corresponding intact contralateral knees. These findings suggest that alterations in native PFJ biomechanics after ACL injury may contribute to cartilage changes post-ACL injury. An improved understanding of the effect of ACL deficiency on cartilage thickness and strain can aid the development of prevention strategies for post-injury OA. The techniques implemented in the current study can be used in the future to evaluate the effectiveness of various treatment methods for preserving the articular cartilage of the PFJ.
Acknowledgments
We would like to thank the Duke University Center for Advanced Magnetic Resonance Development (CAMRD) for their assistance with this project. This work was funded by the National Institutes of Health (R01 AR065527).
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ali AA, Shalhoub SS, Cyr AJ, Fitzpatrick CK, Maletsky LP, Rullkoetter PJ, Shelburne KB. Validation of predicted patellofemoral mechanics in a finite element model of the healthy and cruciate-deficient knee. J Biomech. 2016;49:302–309. doi: 10.1016/j.jbiomech.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K, Pedoia V, Su F, Souza RB, Li X, Ma CB. Persistent Biomechanical Alterations After ACL Reconstruction Are Associated With Early Cartilage Matrix Changes Detected by Quantitative MR. Orthopaedic Journal of Sports Medicine. 2016;4:2325967116644421. doi: 10.1177/2325967116644421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, Grigoryan M, Hunter DJ, Felson DT. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2006;60:189–198. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano H, Muneta T, Ikeda H, Yagishita K, Kurihara Y, Sekiya I. Arthroscopic evaluation of the articular cartilage after anterior cruciate ligament reconstruction: a short-term prospective study of 105 patients. Arthroscopy. 2004;20:474–481. doi: 10.1016/j.arthro.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Beaupre GS, Stevens SS, Carter DR. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37:145–151. [PubMed] [Google Scholar]
- Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62:259–270. [PubMed] [Google Scholar]
- Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT, 3rd, Guilak F, DeFrate LE. Diurnal variations in articular cartilage thickness and strain in the human knee. J Biomech. 2013;46:541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS) Arthritis Care & Research. 2011;63:S208–S228. doi: 10.1002/acr.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culvenor AG, Cook JL, Collins NJ, Crossley KM. Is patellofemoral joint osteoarthritis an under-recognised outcome of anterior cruciate ligament reconstruction? A narrative literature review. Br J Sports Med. 2013;47:66–70. doi: 10.1136/bjsports-2012-091490. [DOI] [PubMed] [Google Scholar]
- DeFrate LE. Effects of ACL graft placement on in vivo knee function and cartilage thickness distributions. J Orthop Res. 2017;35:1160–1170. doi: 10.1002/jor.23541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34:1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- Draganich LF, Andriacchi TP, Andersson GB. Interaction between intrinsic knee mechanics and the knee extensor mechanism. J Orthop Res. 1987;5:539–547. doi: 10.1002/jor.1100050409. [DOI] [PubMed] [Google Scholar]
- Eckstein F, Hudelmaier M, Putz R. The effects of exercise on human articular cartilage. Journal of Anatomy. 2006;208:491–512. doi: 10.1111/j.1469-7580.2006.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrokhi S, Piva SR, Gil AB, Oddis CV, Brooks MM, Fitzgerald GK. Association of severity of coexisting patellofemoral disease with increased impairments and functional limitations in patients with knee osteoarthritis. Arthritis Care Res (Hoboken) 2013;65:544–551. doi: 10.1002/acr.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93:1096–1103. doi: 10.2106/JBJS.J.00929. [DOI] [PubMed] [Google Scholar]
- Frobell RB, Le Graverand MP, Buck R, Roos EM, Roos HP, Tamez-Pena J, Totterman S, Lohmander LS. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009;17:161–167. doi: 10.1016/j.joca.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Fukubayashi T, Torzilli PA, Sherman MF, Warren RF. An in vitro biomechanical evaluation of anterior-posterior motion of the knee. Tibial displacement, rotation, and torque. J Bone Joint Surg Am. 1982;64:258–264. [PubMed] [Google Scholar]
- Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Grood ES, Suntay WJ, Noyes FR, Butler DL. Biomechanics of the knee-extension exercise. Effect of cutting the anterior cruciate ligament. J Bone Joint Surg Am. 1984;66:725–734. [PubMed] [Google Scholar]
- Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- Han SK, Ronkainen AP, Saarakkala S, Rieppo L, Herzog W, Korhonen RK. Alterations in structural macromolecules and chondrocyte deformations in lapine retropatellar cartilage 9 weeks after anterior cruciate ligament transection. J Orthop Res. 2017 doi: 10.1002/jor.23650. [DOI] [PubMed] [Google Scholar]
- Hatcher CC, Collins AT, Kim SY, Michel LC, Mostertz WC, 3rd, Ziemian SN, Spritzer CE, Guilak F, DeFrate LE, McNulty AL. Relationship between T1 rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. J Biomech. 2017;55:18–26. doi: 10.1016/j.jbiomech.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YF, Draganich LF, Ho SH, Reider B. The effects of removal and reconstruction of the anterior cruciate ligament on patellofemoral kinematics. Am J Sports Med. 1998;26:201–209. doi: 10.1177/03635465980260020901. [DOI] [PubMed] [Google Scholar]
- Hsieh YF, Draganich LF, Ho SH, Reider B. The effects of removal and reconstruction of the anterior cruciate ligament on the contact characteristics of the patellofemoral joint. Am J Sports Med. 2002;30:121–127. doi: 10.1177/03635465020300010601. [DOI] [PubMed] [Google Scholar]
- Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25:283–298. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- Kumar D, Kothari A, Souza RB, Wu S, Benjamin Ma C, Li X. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: A pilot study. The Knee. 2014;21:881–885. doi: 10.1016/j.knee.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad NK, Liu B, Ganapathy PK, Utturkar GM, Sutter EG, Moorman CT, 3rd, Garrett WE, Spritzer CE, DeFrate LE. Effect of normal gait on in vivo tibiofemoral cartilage strains. J Biomech. 2016;49:2870–2876. doi: 10.1016/j.jbiomech.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. Acta Orthop. 2007a;78:355–360. doi: 10.1080/17453670710013924. [DOI] [PubMed] [Google Scholar]
- Li G, Papannagari R, Nha KW, DeFrate LE, Gill TJ, Rubash HE. The coupled motion of the femur and patella during in vivo weightbearing knee flexion. J Biomech Eng. 2007b;129:937–943. doi: 10.1115/1.2803267. [DOI] [PubMed] [Google Scholar]
- Liu B, Lad NK, Collins AT, Ganapathy PK, Utturkar GM, McNulty AL, Spritzer CE, Moorman CT, 3rd, Sutter EG, Garrett WE, DeFrate LE. In Vivo Tibial Cartilage Strains in Regions of Cartilage-to-Cartilage Contact and Cartilage-to-Meniscus Contact in Response to Walking. Am J Sports Med. 2017 doi: 10.1177/0363546517712506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- Makela JT, Rezaeian ZS, Mikkonen S, Madden R, Han SK, Jurvelin JS, Herzog W, Korhonen RK. Site-dependent changes in structure and function of lapine articular cartilage 4 weeks after anterior cruciate ligament transection. Osteoarthritis Cartilage. 2014;22:869–878. doi: 10.1016/j.joca.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- Neu CP. Functional imaging in OA: role of imaging in the evaluation of tissue biomechanics. Osteoarthritis and Cartilage. 2014;22:1349–1359. doi: 10.1016/j.joca.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman P, Kostogiannis I, Friden T, Roos H, Dahlberg LE, Englund M. Patellofemoral osteoarthritis 15 years after anterior cruciate ligament injury--a prospective cohort study. Osteoarthritis Cartilage. 2009;17:284–290. doi: 10.1016/j.joca.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Okafor EC, Utturkar GM, Widmyer MR, Abebe ES, Collins AT, Taylor DC, Spritzer CE, Moorman CT, 3rd, Garrett WE, DeFrate LE. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J Biomech. 2014;47:96–101. doi: 10.1016/j.jbiomech.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40:276–285. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- Sachs RA, Daniel DM, Stone ML, Garfein RF. Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17:760–765. doi: 10.1177/036354658901700606. [DOI] [PubMed] [Google Scholar]
- Sanchez-Adams J, Leddy HA, McNulty AL, O’Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep. 2014;16:451. doi: 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton LA, Mow VC, Howell DS. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. Journal of Orthopaedic Research. 1995;13:473–482. doi: 10.1002/jor.1100130402. [DOI] [PubMed] [Google Scholar]
- Setton LA, Mow VC, Müller FJ, Pita JC, Howell DS. Mechanical Properties of Canine Articular Cartilage Are Significantly Altered Following Transection of the Anterior Cruciate Ligament. Journal of Orthopaedic Research. 1994;12:451–463. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- Sutter EG, Widmyer MR, Utturkar GM, Spritzer CE, Garrett WE, Jr, DeFrate LE. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. Am J Sports Med. 2015;43:370–376. doi: 10.1177/0363546514559821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Cutcliffe HC, Queen RM, Utturkar GM, Spritzer CE, Garrett WE, DeFrate LE. In vivo measurement of ACL length and relative strain during walking. J Biomech. 2013;46:478–483. doi: 10.1016/j.jbiomech.2012.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utturkar GM, Irribarra LA, Taylor KA, Spritzer CE, Taylor DC, Garrett WE, Defrate LE. The effects of a valgus collapse knee position on in vivo ACL elongation. Ann Biomed Eng. 2013;41:123–130. doi: 10.1007/s10439-012-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde SK, Bingham JT, Hosseini A, Kozanek M, DeFrate LE, Gill TJ, Li G. Increased tibiofemoral cartilage contact deformation in patients with anterior cruciate ligament deficiency. Arthritis Rheum. 2009;60:3693–3702. doi: 10.1002/art.24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Velde SK, DeFrate LE, Gill TJ, Moses JM, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency on the in vivo elongation of the medial and lateral collateral ligaments. Am J Sports Med. 2007;35:294–300. doi: 10.1177/0363546506294079. [DOI] [PubMed] [Google Scholar]
- Van de Velde SK, Gill TJ, DeFrate LE, Papannagari R, Li G. The effect of anterior cruciate ligament deficiency and reconstruction on the patellofemoral joint. Am J Sports Med. 2008;36:1150–1159. doi: 10.1177/0363546508314404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ginckel A, Verdonk P, Witvrouw E. Cartilage adaptation after anterior cruciate ligament injury and reconstruction: implications for clinical management and research? A systematic review of longitudinal MRI studies. Osteoarthritis Cartilage. 2013;21:1009–1024. doi: 10.1016/j.joca.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Widmyer MR, Utturkar GM, Leddy HA, Coleman JL, Spritzer CE, Moorman CT, 3rd, DeFrate LE, Guilak F. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 2013;65:2615–2622. doi: 10.1002/art.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M, Siegrist M, Cao X. Cyclic compression of articular cartilage explants is associated with progressive consolidation and altered expression pattern of extracellular matrix proteins. Matrix Biol. 1999;18:391–399. doi: 10.1016/s0945-053x(99)00029-3. [DOI] [PubMed] [Google Scholar]