Abstract
Objective
We sought to determine whether abnormalities in emotion processing underlie functional (psychogenic) dystonia, one of the most common functional movement disorders.
Methods
Motor and emotion circuits were examined in 12 subjects with functional dystonia, 12 with primary organic dystonia and 25 healthy controls using functional magnetic resonance imaging at 4T and a finger-tapping task (motor task), a basic emotion-recognition task (emotional faces task), and an intense-emotion stimuli task.
Results
There were no differences in motor task activation between groups. In the Faces task, compared to the other groups, functional dystonia subjects showed areas of decreased activation in the right middle temporal gyrus and bilateral precuneus; and increased activation in the right inferior frontal gyrus, bilateral occipital cortex and fusiform gyrus, and bilateral cerebellum. In the intense-emotion task, compared to the other groups, functional dystonia subjects showed decreased activation in the left insular and left motor cortices (compared to organic dystonia, they showed an additional decrease in activation in the right opercular cortex and right motor cortex) and increased activation in the left fusiform gyrus.
Conclusions
Functional dystonia subjects exhibited stimulus-dependent altered activation in networks involved in motor preparation and execution, spatial cognition, and attentional control. These results support the presence of network dysfunction in functional dystonia.
Key terms: Functional dystonia, psychogenic dystonia, functional movement disorders, conversion disorder, fMRI, emotion processing
INTRODUCTION
Functional (psychogenic) dystonia (FD) is one of the most common functional movement disorders but its pathophysiological underpinnings remain poorly understood and, currently, no specific treatments are available. The term FD applies to the excessive posturing or twisting in a focal, segmental, or generalized distribution, presumed to result from psychological conflicts, and not following the “rules” of dystonia.1 Unlike patients with organic dystonia (OD), who predominantly exhibit action-induced, position-dependent, task-sensitive progressive posturing or twisting of a limb, patients with FD manifest sudden-onset fixed posturing of a distal limb at rest, a unique phenotype that yields a clinically definite diagnostic certainty.2
Neuroimaging studies have documented that the basal ganglia and limbic systems are integral parts of the neural pathways for emotion processing and are involved in emotional conflict resolution.3 In patients with FD, functional neuroimaging studies have demonstrated aberrant regional cerebral blood flow during simple motor tasks4 and activation of the right amygdala during response to simple emotional stimuli (e.g., fear or happiness).5 Thus, in this mechanistic functional magnetic resonance imaging (fMRI) study, we sought to examine the extent to which neuronal circuits underlying movement in adult patients with FD are different from those of primary OD and to determine whether disturbances in the somatosensory and emotion circuitry associated with FD can be used to distinguish it from its organic counterpart. We used facial emotion processing fMRI tasks because of their ability to examine cortical underpinnings of emotions and to provide insights regarding the emotional state of the investigated individual. Also, the brain regions responsible for facial emotion recognition and processing, which include visual (spatial cognition) and executive (attentional control) networks6, may be involved directly or indirectly in the generation or maintenance of FD. Thus, we hypothesized that patients with FD may have a differential fMRI activation pattern in response to emotional stimuli when compared to patients with OD and healthy controls (HCs). Furthermore, we hypothesized that there are differences in activation with simple (e.g., happy) versus intense emotional stimuli (e.g., disgust). Our hypotheses were generated based on findings from studies that evaluated emotion processing in other neurological disorders7 and from studies that have focused on the effect of stress on neuroimaging findings in another frequently encountered functional disorder, psychogenic non-epileptic seizures.8
SUBJECTS AND METHODS
Study Population
We prospectively recruited 12 consecutively consenting patients with clinically definite functional (psychogenic) unilateral or asymmetric limb dystonia (FD group) diagnosed based on established clinical criteria1 who were willing to undergo the fMRI procedure. Patients were referred to the study over a 3-year period from within the University of Cincinnati Movement Disorders Center by movement disorders neurologists. Their clinical presentation, among other features, included incongruity or inconsistency of dystonic movements, false weakness, non-anatomic sensory changes, onset of dystonia at rest, pain, excessive slowness of movements, multiple somatizations, bizarre nature of the movements, and tenderness to light touch. In all cases, final diagnosis was confirmed at a consensus conference in the recruiting center. We also prospectively recruited 12 consecutively consenting patients with idiopathic limb dystonia (OD group) and, 25 HCs with no history of neurological or general medical conditions. We chose this number of subjects based on prior sample size calculations suggesting that for a liberal significance threshold of 0.05, about 12 subjects are required to achieve 80% power at the single voxel level for typical activations, accounting for intra- and inter-subject variability.9 We note that this sample size was selected for examination of group differences, and did not account for covariates controlling for all comorbid psychological and psychiatric conditions often present in patients with functional neurological disorders. This study was approved by the local IRB and all subjects signed an informed consent.
Clinical measurements
To examine participants for the presence of psychopathology, all underwent a 15-minute structured diagnostic interview (Mini International Neuropsychiatric Interview; MINI) developed to screen for axis I DSM-IV and ICD-10 psychiatric disorders.10 The MINI is the structured psychiatric interview of choice for psychiatric evaluation and outcome tracking in clinical trials and epidemiological studies.11 It allows the ascertainment of psychiatric disorders, including depression, anxiety, bipolar disorder, and post-traumatic stress disorder (PTSD). In addition to the MINI, for depression and anxiety specifically, we administered two clinician-rated instruments, the 17-item Hamilton Depression Rating Scale (HAM-D),12 which evaluates depressed mood and vegetative and cognitive symptoms of depression; and the 14-item Hamilton Anxiety Rating Scale (HAM-A),13 which evaluates psychic and somatic anxiety. These scales were administered as part of a structured interview.14
Functional MRI procedure
Anatomical and functional brain images were obtained using a 4T MRI/MRS system (Varian Inc.). The behavioral experiment was programmed in E-Prime, version 1 (www.pstnet.com). All participants wore MR-compatible VGA goggles and headphones (Resonance Technologies, Inc.). For each imaging session, once the participant was positioned in the scanner, a three-plane scout scan was performed to confirm isocenter positioning prior to the functional tasks. An echo-planar imaging (EPI) was performed while subjects carried out the behavioral paradigms using a T2*-weighted gradient-echo EPI pulse sequence: TR/TE 3000/29msec, FOV 256×256mm, matrix 64×64, slice thickness 4mm, flip angle 75°. A multi-echo reference scan was performed to correct for geometric distortion and Nyquist ghost artifacts.11 After completion of all functional MRI (fMRI) tasks a T1-weighted three-dimensional anatomical high-resolution scan using modified equilibrium Fourier transform (MDEFT) sequence (TR/TE 13/6msec, T(MD) 1.1sec, FOV 192×256×256mm, matrix 192×256×256mm, slice thickness 1mm, flip angle 20°) was acquired.15 The MRI system triggered the behavioral paradigms to ensure precise timing of the task with respect to image acquisition. All subjects performed the tasks in the same order with a break in-between for the reference scan.
Imaging paradigms
Finger-tapping motor task was designed to assess and monitor the motor system while in the scanner. The task consisted of a 30-second paced block of right-only finger tapping followed by a 30-second paced block of left-only tapping followed by a 30-second block of rest prior to repeating the cycle for 4 times. Subjects were instructed to adhere to the provided rate with the visual prompt presented regularly at a rate of 1 Hz while tapping on a lever using their right or left index finger in response to the “R” or “L” flashing on the screen. The total task duration was 6 minutes. Task adherence was monitored visually. The task was modeled such that the blocks of rest were treated as “baseline” in the analyses.
The “emotional faces” task (EFT) was designed to assess response to emotional stimuli. Over the span of 14 minutes, subjects were presented with 120 different faces, corresponding to unique (non-repeating) facial identities each depicting a particular emotion (sadness, happiness, or fear) or a neutral expression.7 Rather than focusing subjects’ attention on explicit judgements of emotion, which may alter their processing, we used a method consistent with previous studies that documented the presence of implicit processing of emotions during an unrelated face judgement task (identifying correct gender).7 This allowed us to monitor subjects’ attention to the stimuli without asking them to attend explicitly to emotional content. Thus, subjects were instructed to decide the gender of each face by pressing one of two buttons with the right thumb. Subjects were exposed to 30 prototypically happy, 30 sad, 30 fearful and 30 neutral expressions presented in random order selected from the NimStim set of facial expressions.16 Each stimulus was presented for 2 seconds with variable inter-stimulus interval of 3.9±2.4 seconds; during the delay subjects viewed a fixation cross. Subjects were asked to press button “1” for males and button “2” for females while viewing each image.
The intense-emotion stimuli task (CPT-END) consisted of a series of offensive or disgusting images probing intense emotional circuitry.17 This task utilized a visual oddball paradigm where 70% of the cues were squares, 10% were circles (targets), 10% were emotionally unpleasant pictures, and 10% were emotionally neutral pictures. Subjects held the same response box as for the emotional faces task and were asked to press with the right thumb a “2” for circles and “1” for all other images. There were two runs of the task in the imaging session. There were 158 total cues with 3 seconds per cue and a constant display time of 2.75 seconds with a 0.25-second interval with fixation cross. Emotional and neutral pictures originated from the International Affective Picture System (University of Florida, Gainesville, Florida) and were selected based on criteria utilized by us and others previously.18
Image Processing and data analysis
Reconstruction of the raw data was performed with 3D Hamming filter using in-house software developed in IDL (www.ittvis.com).19 First-level fMRI data processing was carried out using FSL (FMRIB Software Library)20, 21 and AFNI (Analysis of Functional Neuroimages).22
Anatomical Data
Data were first reoriented using FSL’s fslreorient2std. Next, the T1 data were bias corrected and brain extracted using FSL’s FAST23 and BET respectively.24 The brain extracted image was then normalized and resampled to the 2mm isotropic MNI ICBM 152 non-linear 6th generation template25 using FSL’s FLIRT.26, 27 Subcortical segmentation was performed using FSL’s FIRST28 on the bias corrected image in native space.
Functional Data
Typical pre-processing steps, such as reorientation, slice timing correction and brain extraction, were carried out using FSL’s fslreorient2std, “slicetimer” and BET24, respectively. Outlying functional volumes were detected using FSL’s “fsl_motion_outliers”. Motion correction of the BOLD time-series was carried out using MCFLIRT.26 The functional file was interpolated to 2×2×2 mm voxel size and aligned to the Montreal Neurological Institute (MNI) template25 by first co-registering it with the participant’s T1 using FSL’s FLIRT.26, 27 The motion related artifacts were then regressed from the data by setting up a general linear model design using 24 motion parameters (6 motion parameters, the 6 motion parameters squared, a first order autoregressive model of the 6 motion parameters and a first order autoregressive model of the 6 motion parameters squared) plus an additional parameter for each detected outlier.29 The residuals from the GLM were high-pass filtered in accordance with the task timing (0.008 Hz, 0.04 Hz and 0.04 Hz for the finger-tapping, emotional faces and CPT-END tasks respectively) and smoothed with a 6mm FWHM filter using AFNI’s 3dBandpass.
Group Analyses
For the finger-tapping motor task, differences in left-hand tapping versus rest and right-hand tapping versus rest were examined. For the EFT, differences at the group level were examined for emotional faces (happy, sad, fearful) versus neutral faces, fearful faces versus neutral faces, and all faces (happy, sad, fearful, neutral) versus fixation cross. With the CPT-END task, group differences were examined for emotional images versus squares and for emotional images versus neutral images. For each task, pairwise comparisons between the FD and OD groups were performed without correction for multiple comparisons due to the exploratory nature of the study. Each dystonia group was then compared to healthy controls (HC) in order to establish differences in emotion processing between each dystonia group and healthy subjects. To control for the presence of anxiety and depression, HAM-A and HAM-D scores were regressed out in the group comparisons. We also examined whether there was any correlation of task activation with HAM-A/HAM-D scores. All task-based group results were corrected for multiple comparisons using FSL’s threshold free cluster enhancement (TFCE), a nonparametric permutation test, with 5000 iterations. Significant results were only reported for group comparisons that survived an initial Z threshold greater than 2.3 and a corrected threshold of p<0.05.
Region-of-interest (ROI)-to-ROI connectivity analysis
Connectivity analysis was performed using regions of interest based on the cortical regions of group difference in task activation in any of the three contrasts (FD vs. HC, OD vs. HC, FD vs. OD) for the respective tasks. A set of subcortical anatomical regions defined from the Harvard-Oxford subcortical atlas30–33 and the sub-thalamic nucleus atlas34 was also included in the connectivity analyses due to their known roles in motor and emotional function (Figure 1). These anatomical regions were the anterior cingulate gyrus, posterior cingulate gyrus and left and right regions of the amygdala, thalamus, caudate, putamen, and subthalamic nuclei. The average time course over all voxels in each ROI was extracted and correlated with the average time course from each of the other ROIs. The correlation coefficients were then converted to Fisher Z scores and used in a GLM analysis to examine differences in functional connectivity between the three groups. Seed-seed correlations were corrected for multiple comparisons using False Discovery Rate (FDR) correction35. This correlation analysis was implemented using in-house software implemented in Python.
Figure 1. Atlas-based subcortical regions of interest selected for connectivity analysis.
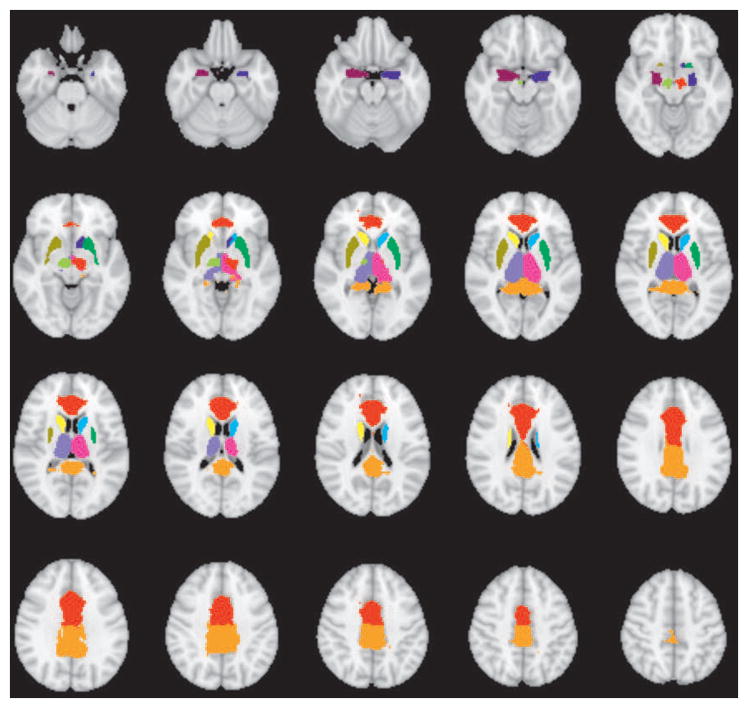
The anatomical regions of interest included the anterior cingulate gyrus, posterior cingulate gyrus and left and right regions of the uncus/amygdala, thalamus, caudate, and putamen from the Harvard-Oxford Subcortical Atlas and left and right subthalamic nuclei from the Subthalamic Nucleus atlas.
RESULTS
Population characteristics
The study population includes 12 patients with FD (age: 34.2 ± 10.6 years, 9 females), 12 patients with OD (57.4 ± 9.4 years, 5 females) and 25 HCs (43.6 ± 14.6 years, 21 females). Given pattern of referral, disease duration was shorter in FD compared to OD subjects (3.5 ± 3.4 [range, 0.2 to 11 years] vs. 11.8 ± 12.9 years [range, 2 to 33 years], respectively). Because of disease-specific demographic differences between FD and OD, as expected, age and gender were different between the groups (p <0.01).
Psychiatric features
Depression (HAM-D, 16.6 ± 9.9 vs. 2.5 ± 4.0 [HAM-D score 14–18 = moderate depression]) and anxiety scores (HAM-A, 13.8 ± 11.1 vs. 1.5 ± 2.1 [HAM-A score 14–17 = mild anxiety) were higher in the FD group compared to OD (all p <0.05). Major depression was ascertained in 5/12 patients with FD and PTSD in 4/12 per MINI screen (Table 1) which is typical for patient with psychogenic disorders.36, 37
Table 1.
Clinical features of study subjects
| Subject Number | Age | Duration (years) | Body Involvement | HAM-D | HAM-A | Positive MINI Results/Diagnosis in OD | |
|---|---|---|---|---|---|---|---|
| Functional Dystonia | 1 | 41 | 5 | Bilateral hand curling | 15 | 6 | Substance abuse and dependence (ongoing) |
| 2 | 38 | 4 | Left arm flexion | 23 | 20 | MDD, OCD, GAD (all ongoing), past manic episode | |
| 3 | 40 | 3 | Left hand flexion, jaw pulling, torticollis, and right eye closure | 18 | 13 | PTSD (ongoing) | |
| 4 | 21 | 0.4 | Right hand flexion, neck tremor | 9 | 5 | none | |
| 5 | 21 | 2 | Right leg posturing | 2 | 3 | none | |
| 6 | 44 | 11 | All limbs and trunk | 34 | 35 | none | |
| 7 | 30 | 1 | Head, full body dystonia | 13 | 23 | MDD (ongoing) | |
| 8 | 21 | 0.33 | right jaw deviation, torticollis, right arm weakness | 0 | 0 | none | |
| 9 | 37 | 7 | Bilateral foot flexion and inversion | 22 | 23 | MDD, PTSD, GAD (all ongoing) | |
| 10 | 53 | 5 | face, hands, and feet dystonia | 28 | 25 | MDD, PTSD, GAD (all ongoing) | |
| 11 | 24 | 0.2 | Bilateral foot flexion and inversion | 16 | 6 | MDD (ongoing), PD (lifetime), PTSD (ongoing) | |
| 12 | 40 | 20 | Left hand flexion, then right hand | 19 | 6 | None | |
| Organic Dystonia | 1 | 52 | 2 | Jaw and fingers | 9 | 3 | None/Segmental dystonia |
| 2 | 64 | 2 | Left thumb | 0 | 1 | None/Focal dystonia, dystonic tremor | |
| 3 | 63 | 2.5 | Right hand | 12 | 6 | None/Hand dystonia | |
| 4 | 53 | 11 | Right hand | 3 | 2 | None/Writer’s cramp | |
| 5 | 71 | 3 | Right hand, neck | 0 | 0 | None/Segmental dystonia, dystonic tremor | |
| 6 | 48 | 12 | Right hand | 0 | 0 | None/Writer’s cramp | |
| 7 | 71 | 32 | Arm and neck | 2 | 1 | None/Segmental dystonia, dystonic tremor | |
| 8 | 63 | 1.5 | Left hand | 0 | 0 | None/Focal dystonia, dystonic tremor | |
| 9 | 55 | 32 | Right hand | 0 | 0 | None/Focal dystonia, dystonic tremor | |
| 10 | 39 | 2 | Left hand | 0 | 0 | None/Focal dystonia, dystonic tremor | |
| 11 | 54 | 33 | Bilateral hands/arms | 4 | 5 | GSP (lifetime)/Dystonic tremor, spasmodic dysphonia | |
| 12 | 56 | 9 | Right hand | 0 | 0 | None/Focal dystonia and tremor |
HAM-D: 17-item Hamilton Depression Rating Scale; HAM-A: 14-item Hamilton Anxiety Rating Scale; MINI: Mini International Neuropsychiatric Interview to screen for axis I DSM-IV and ICD-10 psychiatric disorders; OCD: obsessive-compulsive disorder; GAD: generalized anxiety disorder; GSP: Generalized social phobia; MDD: major depression; PD: panic disorder; PTSD: post-traumatic stress disorder.
Motor processing: finger-tapping task
There were no significant differences in activation patterns between the two dystonia groups, nor between either of the dystonia groups and healthy controls.
Emotional faces task
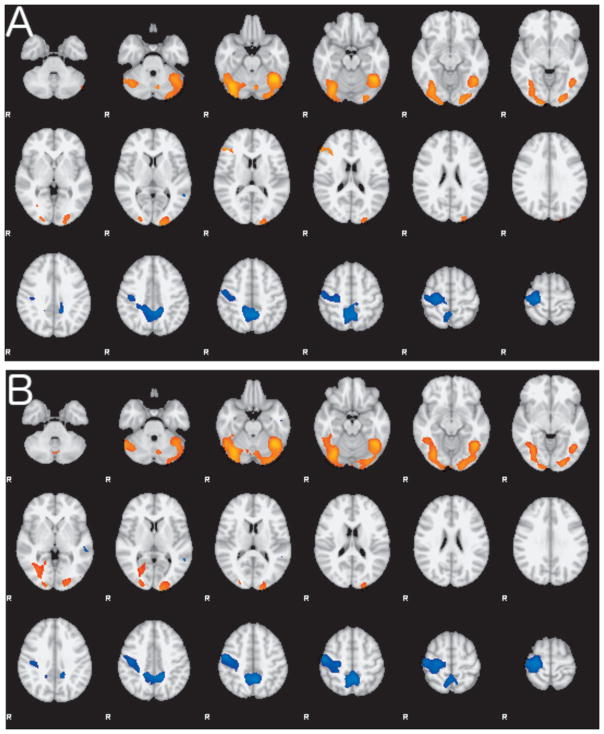
Compared with HCs, FD subjects showed areas of decreased activation in the right middle temporal gyri and bilateral precuneus; and increased activation in the right inferior frontal gyrus, bilateral occipital cortex and fusiform gyrus, and bilateral cerebellar hemispheres (Figure 2A). There was a similar pattern observed when comparing subjects with FD to those with OD (Figure 2B). These differences in activation were observed comparing the recognition of faces vs. fixation cross. There were a few small regions of difference between FD and HCs when comparing differences in emotional vs. neutral faces in the white matter near the right lateral ventricle and in right temporal fusiform cortex.
Figure 2. Group differences in the Emotional Faces task-related activation.
A. Composite map shows the differences in activation between FD and HC during the Faces task contrasting faces versus a fixation cross as control. Regions of greater activity in FD are shown in red-yellow hues; regions of lower activity in FD in blue hues. B. Composite map showing the differences in activation between FD and OD during the Faces task contrasting faces versus a fixation cross as control. Regions of greater activity in FD are shown in red-yellow hues; regions of lower activity in FD in blue hues. Both group contrasts are thresholded at Z > 2.3 and corrected p<0.05.
CPT-END task
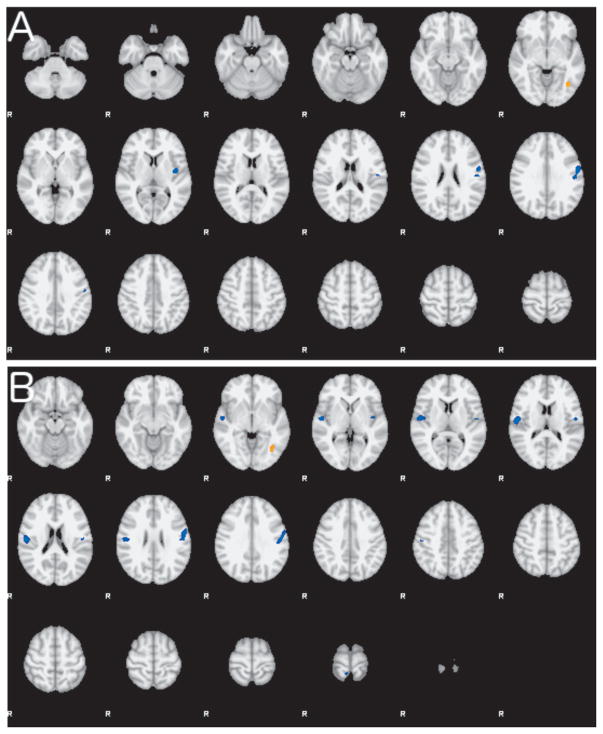
Compared with HCs, FD subjects showed decreased activation in the left insular and left motor cortices and increased activation in the left occipital fusiform gyrus (Figure 3A). Compared with OD, FD subjects showed decreased activation in the same regions but with additional decrease in activation in the right opercular cortex and right motor cortex (Figure 3B). These differences were similar when contrasting emotional images versus squares and emotional images versus neutral images. The findings for these two tasks have been summarized in Table 2 (Centroids available as online supplementary material).
Figure 3. Group differences in the CPT-END task-related activation.
A. Composite map shows the differences in activation between FD and HC during the CPT-END task contrasting intense emotional images versus squares as control. Regions of lower activity in FD are shown in blue hues and a small region of greater activity in FD is shown in orange. B. Composite map showing differences in activation between FD and OD during the CPT-END task contrasting intense emotional images versus squares. Regions of lower activity in FD are shown in blue hues. Both group contrasts are thresholded at Z > 2.3 and corrected p<0.05.
Table 2.
Summary of fMRI comparative activity between study groups
| Emotional Faces task | CPT-END | |
|---|---|---|
|
| ||
| FD v HC | FD<HC | FD<HC |
| ↓ R middle temporal gyri | ↓ L insular cortex | |
| ↓ B precuneus | ↓ L motor cortex | |
| FD>HC | FD>HC | |
| ↑ R inferior frontal gyrus | ↑ L occipital fusiform gyrus | |
| ↑ B occipital cortex | ||
| ↑ B fusiform gyrus | ||
| ↑ Multiple cerebellar regions | ||
|
| ||
| FD v OD | Similar to above | Similar to above plus |
| ↓ R central opercular cortex | ||
| ↓ R motor cortex | ||
|
| ||
| OD v HC | No significant differences | No significant differences |
For centroids, see Supplementary Table
Connectivity Analysis
Differences in ROI-ROI connectivity between the three groups were assessed in each of the three fMRI tasks. For the finger-tapping motor task and emotional faces task, there were no significant differences in connectivity between any of the groups. In the CPT-END task a significant increase in connectivity for OD vs. HC appeared between the posterior cingulate gyrus and left postcentral gyrus (t = 5.74; FDR p value = 0.0001) and between the posterior cingulate gyrus and right central opercular cortex (t =3.93; FDR p value =0.02).
Effects of psychiatric features on activation pattern
There was no significant correlation between HAM-A and HAM-D scores and activation levels for any task.
DISCUSSION
This first evaluation of the emotional circuitry in patients with FD shows that compared to OD, patients with FD have (1) no differences in activation in response to a motor task (indicating lack of involvement of the primary motor system during motor-only tasks); (2) decreased activation in response to basic emotional stimuli in selected motor and sensory areas, mostly right middle temporal gyri and bilateral precuneus; and (3) decreased activation in response to intense emotional stimuli in the left insular and left motor cortices. These findings provide insights into the neurobiology of FD and suggest that there are distinct stimulus-dependent abnormalities in emotion processing in both FD and OD, which may represent endophenotypic traits or be associated with the maintenance of the motor behaviors in these disorders.
A PET study compared regional cerebral blood flow, a measure of local metabolic rate, in six patients with right-leg FD at rest, during fixed posturing of the right leg, and during paced ankle movements to six patients with OD.4 Blood flow was higher in the cerebellum and basal ganglia and lower in the primary motor cortex in patients with FD, whereas it was high in primary motor, premotor and parietal cortices, but low in the cerebellum in patients with OD.4 Our emotion processing paradigms confirmed changes in similar brain regions and also documented several striking differences between these groups and HCs. Another study examined the brain activation patterns using fMRI and a simple facial emotion paradigm in 16 patients with a variety of functional disorders (including 2 with FD) and found no group differences on primary analysis compared to HCs even after controlling for concurrent depression and anxiety.5 However, this study identified greater right amygdala activity during happy stimuli on post-hoc analysis in the functional group.5 While important, that study did not assess differences between simple and intense emotion processing paradigms. Altogether, the combined data suggest that there may be stimulus-dependent differential abnormalities in activation in the sensorimotor (motor planning), visual (spatial cognition), and executive (attentional control) networks between FD and OD. Further, these are complementary to the findings in patients with organic cervical dystonia of reduced connectivity within the sensorimotor and primary visual networks and increased connectivity within the executive control network as measured by task-free (resting) fMRI38 and consistent with neurophysiological evidence suggesting defects in neural inhibitory processes, sensorimotor integration, and maladaptive plasticity across different brain regions in patients with OD.39 These changes appear to be dependent on emotional stimuli and may be influenced by associated psychiatric comorbidities, although our study was underpowered to properly assess the latter.
It is also possible that some of the aberrant activation patterns may represent an epiphenomenon secondary to “dystonic postures”, which has not been adequately excluded in prior electrophysiological studies.40 While some of these differences have been attributed to changes in cortical plasticity,41 there have been recent concerns regarding the reliability over time of these observations in OD, based on the variability of response to the paired associative stimulation paradigm.42 Similarly, the fMRI paradigms used in the current study have not been studied for intra-individual consistency over time, and changes in cortical activation may be altered without implying true biological changes. Nevertheless, it has been postulated that patients with FD exhibit abnormalities in subcortical processing, as suggested by studies in other functional neurological disorders.5, 43, 44 These findings, when compared to HCs, suggest that abnormal processing of emotional information is associated with limbic activation via changes in connectivity between basal ganglia and thalamocortical circuits to produce a deficit in sensory or motor processing.45 Alternatively, simple emotional stimuli may instead lead to “functional deafferentation” due to active inhibition of somatosensory processing by limbic areas concerned with emotion and attention,46 thus resulting in the overall decreased fMRI responses to the emotion processing paradigm. It is plausible that such deactivation is the neurobiological correlate of alexithymia (the inability to identify and describe emotions), which has long been used to explain conversion disorder as the result of emotions that cannot be experienced consciously as feeling states or put into words.47
Our study has several limitations. Although it was powered to detect major differences in activation by emotional processing tasks, the sample size of 12 precludes an adequate assessment of the effect of all psychological and psychiatric co-morbidities (such as depression, PTSD, and anxiety) on the activation patterns in the motor and emotional tasks. It is possible that at least some of the differences in activation patterns identified in subjects with FD may relate to their psychiatric co-morbidities and be a sign of maladaptive cognitive interpretations of emotions,48 but the presence or absence of these co-morbidities was not monitored in our relatively small sample, powered for major differences across disorders rather than psychiatric comorbidities. Nevertheless, we deliberately excluded patients with severe depression to avoid this potential confound. The effectiveness of this exclusion criterion is supported by the lack of a main effect of depression on the fMRI results. Similarly, due to the limited sample size, we were unable to determine the effects, if any, of sidedness, clinical variability (including age or gender, for which the groups were different based on anticipated demographic differences between FD and OD), and topographical involvement in the dystonia phenotype (e.g., left vs. right, arm vs. leg). As many forms of dystonia do not share the same set of clinical features, it may be possible to argue that within FD there may be findings that are overlap with those of OD at the neural level, forming an “organic spectrum” that includes functional manifestations. Nevertheless, the variance in phenomenological involvement, which is typical of a specialized movement disorders center, would have expected to dilute the statistical differences between groups rather than create false positive findings. Finally, we did not measure alexithymia in our patients, which has been recently shown to contribute to the development of functional disorders49 and to impaired facial emotion recognition in these patients,50 and may have mediated at least some of the cortical activation in response to emotion stimuli.
Prospective studies will be needed to determine whether the observed changes are relevant to the pathogenesis of these disorders or may represent changes that are either compensatory or secondary to primary motor or psychological states. Finally, it will be important to examine the extent to which promising interventions, such as cognitive behavioral therapy,51 may be capable of normalizing the abnormalities in emotion processing in FD identified in this study.
Supplementary Material
Acknowledgments
This study was supported by a Career Development Award through a Dystonia Coalition grant NS065701 from the NIH Office of Rare Diseases Research in the National Center for Advancing Translational Sciences. AJE is currently supported by NIH grant 1K23MH092735. While this research was conducted, JPS was supported by NIH K23 NS052468, and JCE was supported by K01 DA020485.
Footnotes
Author Contributions
Dr. Espay - Study concept and design, acquisition of data, analysis and interpretation, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision.
Mr. Maloney – analysis and interpretation, critical revision of the manuscript for important intellectual content.
Dr. Vannest - analysis and interpretation, critical revision of the manuscript for important intellectual content.
Mr. Norris - analysis and interpretation, critical revision of the manuscript for important intellectual content.
Dr. Eliassen – task design, analysis and interpretation, critical revision of the manuscript for important intellectual content.
Mrs. Neefus - acquisition of data, study supervision.
Dr. Allendorfer - acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content.
Dr. Chen - analysis and interpretation, critical revision of the manuscript for important intellectual content.
Dr. Szaflarski - Study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
Conflicts of Interest/Financial Disclosures over last 24 months:
Dr. Espay has received grant support from NIH (NIMH, 1K23MH092735), Great Lakes Neurotechnologies and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, TEVA, Impax, Merz, Acadia, Cynapsus, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from Abbvie, UCB, USWorldMeds, Lundbeck, Acadia, the American Academy of Neurology, and the Movement Disorders Society.
Dr. Vannest receives funding from the NIH.
Mr. Maloney Norris, Dr. Eliassen, and Ms. Neefus have nothing to disclose.
Dr. Allendorfer has received funding from the Shor Foundation for Epilepsy Research. She serves as an associate editor of the journal Restorative Neurology and Neuroscience.
Dr. Chen received honoraria from Allergan, and Merz, Ipsen and UCB. He is the Editor-in- Chief of the Canadian Journal of Neurological Sciences. He received research grants from the Canadian Institutes of Health Research, Michael J. Fox Foundation for Parkinson Research, Medtronic Inc, the Weston Foundation and Merz Pharma.
Dr. Szaflarski has received research funding from NIH, Shor Foundation for Epilepsy Research, Eisai, Epilepsy Foundation of America, Food and Drug Administration, Compumedics Neuroscan, Inc., Department of Defense, State of Alabama, and the University of Alabama at Birmingham (UAB). While this research was conducted, he was supported by NIH K23 NS052468. He serves as an associate editor of the journals Restorative Neurology and Neuroscience and Journal of Epileptology and on editorial boards of the journals Epilepsy & Behavior, Folia Medica Copernicana and Journal of Medical Science.
References
- 1.Fahn S, Williams DT. Psychogenic dystonia. Adv Neurol. 1988;50:431–455. [PubMed] [Google Scholar]
- 2.Espay AJ, Lang AE. Phenotype-specific diagnosis of functional (psychogenic) movement disorders. Curr Neurol Neurosci Rep. 2015;15:556. doi: 10.1007/s11910-015-0556-y. [DOI] [PubMed] [Google Scholar]
- 3.Nowak DA, Fink GR. Psychogenic movement disorders: aetiology, phenomenology, neuroanatomical correlates and therapeutic approaches. Neuroimage. 2009;47:1015–1025. doi: 10.1016/j.neuroimage.2009.04.082. [DOI] [PubMed] [Google Scholar]
- 4.Schrag AE, Mehta AR, Bhatia KP, et al. The functional neuroimaging correlates of psychogenic versus organic dystonia. Brain. 2013;136:770–781. doi: 10.1093/brain/awt008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voon V, Brezing C, Gallea C, et al. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calarge C, Andreasen NC, O’Leary DS. Visualizing how one brain understands another: a PET study of theory of mind. Am J Psychiatry. 2003;160:1954–1964. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- 7.Szaflarski JP, Allendorfer JB, Heyse H, Mendoza L, Szaflarski BA, Cohen N. Functional MRI of facial emotion processing in left temporal lobe epilepsy. Epilepsy Behav. 2014;32:92–99. doi: 10.1016/j.yebeh.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Allendorfer JB, Szaflarski JP. Contributions of fMRI towards our understanding of the response to psychosocial stress in epilepsy and psychogenic nonepileptic seizures. Epilepsy Behav. 2014;35C:19–25. doi: 10.1016/j.yebeh.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. Journal of neuroscience methods. 2002;118:115–128. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 11.Pinninti NR, Madison H, Musser E, Rissmiller D. MINI International Neuropsychiatric Schedule: clinical utility and patient acceptance. Eur Psychiatry. 2003;18:361–364. doi: 10.1016/j.eurpsy.2003.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 13.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14:61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- 14.Williams JB, Kobak KA, Bech P, et al. The GRID-HAMD: standardization of the Hamilton Depression Rating Scale. Int Clin Psychopharmacol. 2008;23:120–129. doi: 10.1097/YIC.0b013e3282f948f5. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Garwood M, Menon R, et al. High contrast and fast three-dimensional magnetic resonance imaging at high fields. Magn Reson Med. 1995;34:308–312. doi: 10.1002/mrm.1910340305. [DOI] [PubMed] [Google Scholar]
- 16.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki H, LaBar KS, McCarthy G. Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci U S A. 2002;99:11447–11451. doi: 10.1073/pnas.182176499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strakowski SM, Eliassen JC, Lamy M, et al. Functional magnetic resonance imaging brain activation in bipolar mania: evidence for disruption of the ventrolateral prefrontal-amygdala emotional pathway. Biol Psychiatry. 2011;69:381–388. doi: 10.1016/j.biopsych.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 22.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 24.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9:58–66. doi: 10.1007/11866763_8. [DOI] [PubMed] [Google Scholar]
- 26.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 27.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical image analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 28.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 30.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Frazier JA, Chiu S, Breeze JL, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein JM, Seidman LJ, Makris N, et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry. 2007;61:935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Makris N, Goldstein JM, Kennedy D, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 34.Forstmann BU, Keuken MC, Jahfari S, et al. Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage. 2012;60:370–375. doi: 10.1016/j.neuroimage.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 36.Morgante F, Edwards MJ, Espay AJ. Psychogenic movement disorders. Continuum (Minneap Minn) 2013;19:1383–1396. doi: 10.1212/01.CON.0000436160.41071.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szaflarski JP, Szaflarski M. Seizure disorders, depression, and health-related quality of life. Epilepsy Behav. 2004;5:50–57. doi: 10.1016/j.yebeh.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Delnooz CC, Pasman JW, Beckmann CF, van de Warrenburg BP. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One. 2013;8:e62877. doi: 10.1371/journal.pone.0062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espay AJ, Morgante F, Purzner J, Gunraj CA, Lang AE, Chen R. Cortical and spinal abnormalities in psychogenic dystonia. Ann Neurol. 2006;59:825–834. doi: 10.1002/ana.20837. [DOI] [PubMed] [Google Scholar]
- 41.Quartarone A, Rizzo V, Terranova C, et al. Abnormal sensorimotor plasticity in organic but not in psychogenic dystonia. Brain. 2009;132:2871–2877. doi: 10.1093/brain/awp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadnicka A, Hamada M, Bhatia KP, Rothwell JC, Edwards MJ. A reflection on plasticity research in writing dystonia. Mov Disord. 2014;29:980–987. doi: 10.1002/mds.25908. [DOI] [PubMed] [Google Scholar]
- 43.Vuilleumier P, Chicherio C, Assal F, Schwartz S, Slosman D, Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–1090. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- 44.Voon V, Gallea C, Hattori N, Bruno M, Ekanayake V, Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvey SB, Stanton BR, David AS. Conversion disorder: towards a neurobiological understanding. Neuropsychiatr Dis Treat. 2006;2:13–20. [PMC free article] [PubMed] [Google Scholar]
- 46.Black DN, Seritan AL, Taber KH, Hurley RA. Conversion hysteria: lessons from functional imaging. J Neuropsychiatry Clin Neurosci. 2004;16:245–251. doi: 10.1176/jnp.16.3.245. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan MJ, Dwivedi AK, Privitera MD, Isaacs K, Hughes C, Bowman M. Comparisons of childhood trauma, alexithymia, and defensive styles in patients with psychogenic non-epileptic seizures vs. epilepsy: Implications for the etiology of conversion disorder. J Psychosom Res. 2013;75:142–146. doi: 10.1016/j.jpsychores.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Harrison NA, Critchley HD. Neuroimaging and Emotion. 2. UK: Elsevier; 2007. [Google Scholar]
- 49.Demartini B, Petrochilos P, Ricciardi L, Price G, Edwards MJ, Joyce E. The role of alexithymia in the development of functional motor symptoms (conversion disorder) J Neurol Neurosurg Psychiatry. 2014;85:1132–1137. doi: 10.1136/jnnp-2013-307203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedrosa Gil F, Ridout N, Kessler H, et al. Facial emotion recognition and alexithymia in adults with somatoform disorders. Depress Anxiety. 2009;26:E26–33. doi: 10.1002/da.20456. [DOI] [PubMed] [Google Scholar]
- 51.LaFrance WC, Jr, Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA Psychiatry. 2014;71:997–1005. doi: 10.1001/jamapsychiatry.2014.817. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.