Abstract
Leptospirosis has emerged as an important urban health problem as slum settlements have expanded worldwide. Yet the dynamics of the environmentally transmitted Leptospira pathogen has not been well characterized in these settings. We used a stratified dense sampling scheme to study the dynamics of Leptospira abundance in surface waters from a Brazilian urban slum community. We collected surface water samples during the dry, intermediate and rainy seasons within a seven-month period and quantified pathogenic Leptospira by quantitative PCR (qPCR). We used logistic and linear mixed models to identify factors that explained variation for the presence and concentration of Leptospira DNA. Among 335 sewage and 250 standing water samples, Leptospira DNA were detected in 36% and 34%, respectively. Among the 236 samples with positive results geometric mean Leptospira concentrations were 152 GEq/mL. The probability of finding Leptospira DNA was higher in sewage samples collected during the rainy season when increased leptospirosis incidence occurred, than during the dry season (47.2% vs 12.5%, respectively, p=0.0002). There was a marked spatial and temporal heterogeneity in Leptospira DNA distribution, for which type of water, elevation, and time of day that samples were collected, in addition to season, were significant predictors. Together, these findings indicate that Leptospira are ubiquitous in the slum environment and that the water-related risk to which inhabitants are exposed is low. Seasonal increases in Leptospira presence may explain the timing of leptospirosis outbreaks. Effective prevention will need to consider the spatial and temporal dynamics of pathogenic Leptospira in surface waters to reduce the burden of the disease.
Keywords: Leptospira, leptospirosis, surface water, public health, sewage, urban slum
Graphical Abstract

1. INTRODUCTION
Leptospirosis is a widespread zoonotic disease that causes more than 1 million cases and 50,000 deaths each year (Costa et al., 2015; Torgerson et al., 2015). The disease ranges from mild flu-like symptoms to severe complications, such as Weil’s disease and pulmonary hemorrhagic syndrome, for which case fatality is 5 to >40% (Haake and Levett, 2015; Ko et al., 2009). Pathogenic Leptospira colonize the kidneys of a broad range of mammalian species and are shed in the urine into the environment where they survive for periods that range from a few hours to several months depending on the species, serovar and the characteristics of the environmental matrix (Hellstrom and Marshall, 1978; Khairani-Bejo et al., 2004; Okazaki and Ringen, 1957; Thibeaux et al., 2017; Trueba et al., 2004). Leptospirosis is an environmentally-transmitted disease: human infection occurs primarily through contact of abraded skin or mucous membranes with contaminated environment, most notably water (Ko et al., 2009). However, there is a lack of knowledge regarding the abundance and distribution of pathogenic Leptospira in surface waters that serve as a transmission source in endemic areas. Moreover, the environmental factors that influence their abundance and distribution, and therefore the risk of infection, are poorly understood.
Leptospirosis has recently emerged as a major public health problem among impoverished urban settlements in tropical and subtropical developing countries (Karande et al., 2002; Ko et al., 1999; Kyobutungi et al., 2008; Riley et al., 2007). Inadequate sanitation in these settings, specifically precarious sewer systems and trash accumulation, promotes the thriving of rodents, which are major reservoirs of pathogenic Leptospira (Costa et al., 2014; Panti-May et al., 2016; Riley et al., 2007; Unger et al., 2016). 865 million people resided in urban slums in 2012 and this number is expected to double by 2025 (UN-HABITAT, 2013). Consequently, the burden of the disease will continue to increase in the coming years.
Exposure to contaminated water is a well-recognized risk factor for leptospirosis in urban slums. Climatic conditions leading to an increased human exposure to water appear to be important drivers for disease transmission. Leptospirosis outbreaks frequently occur during periods of seasonal rainfall and flooding in the urban slum setting (Ko et al., 1999; Tassinari et al., 2004), as well as in other epidemiological situations where transmission is endemic (Desvars et al., 2011; Ko et al., 2009; Lau et al., 2016; Smith et al., 2013; Tangkanakul et al., 2005; Weinberger et al., 2014), or following extreme weather events (Agampodi et al., 2014; Amilasan et al., 2012; Karande et al., 2002; Trevejo et al., 1998). In addition, the proximity of households to open drainage systems and direct contact with sewage, flooding water and runoff have been associated with increased risk of infection in prospective, cross-sectional and case control studies (Barcellos and Sabroza, 2001; Felzemburgh et al., 2014; Navegantes de Araújo et al., 2013; Oliveira et al., 2009; Reis et al., 2008; Sarkar et al., 2002). Furthermore, pathogenic Leptospira have been detected in sewers, streams and puddles from endemic areas (Ganoza et al., 2006; Kurilung et al., 2017; Muñoz-Zanzi et al., 2014; Saito et al., 2013; Sumanta et al., 2015). Altogether, this highlights the key role of surface waters in the transmission of leptospirosis in urban slums.
Yet the abundance and distribution of pathogenic Leptospira in the surface waters of endemic areas have not been well characterized. To date, only one study performed in Peru has succeeded in quantifying pathogenic Leptospira in the waters of an urban slum, reporting mean concentrations around 1,000 leptospires/ml (count range 2–1,286) (Ganoza et al., 2006). In this study, we aimed to provide high-resolution information on the presence and concentration of pathogenic Leptospira in an urban slum at high-risk for leptospirosis, and to evaluate whether the spatiotemporal dynamics of the pathogen explained the variation in risk of infection. To this end, we performed a dense sampling of the surface waters from a Brazilian urban slum with high infection rates (37.8 per 1,000 individuals per year) (Hagan et al., 2015) where leptospirosis outbreaks occur each year in the rainy season (Ko et al., 1999; Sarkar et al., 2002). We collected 585 samples of sewage and standing water from different elevations within this urban slum across a seven-month period that spanned the dry, intermediate and rainy seasons. Presence/absence of pathogenic Leptospira, and concentrations in positive samples, were estimated by quantitative-PCR (qPCR) and subsequently modeled using logistic and linear mixed models, respectively, to identify the factors that explained their spatial and temporal variation.
2. METHODS
2.1 Study site
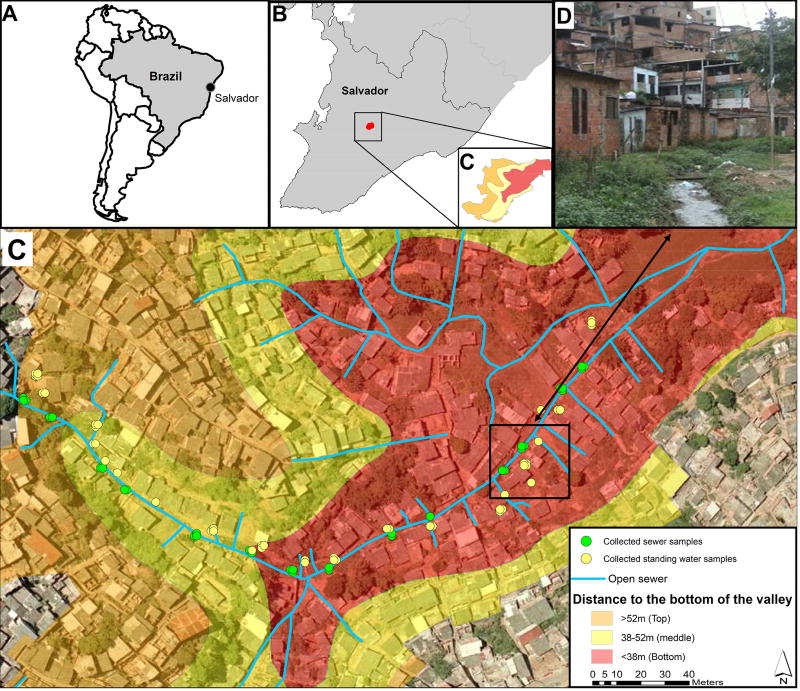
The study was conducted in Pau da Lima, an urban slum community located in the city of Salvador, Brazil (Fig. 1A). The study site has been previously described in detail (Reis et al., 2008; Unger et al., 2016). Briefly, the community consists of four valleys with an area of 0.46 km2 (Fig. 1B) and has a population of 12,651 inhabitants (Felzemburgh et al., 2014). The slum has a precarious sanitary infrastructure with open sewers and rainwater drainage that overflow during heavy rainfall events, leading to frequent flooding in valley bottoms during the rainy season. Salvador has a typical tropical rainforest climate (Köppen classification: Af) with relatively stable temperatures throughout the year daily mean, and average high and low values; 25.3 °C, 28.2 °C, and 22.7 °C, and high relative humidity (average, 80.9%). The average annual precipitation is 2,144 mm, with a monthly average rainfall of over 60 mm, indicating that there is no authentic dry season. However, the period from April to July has an average rainfall of over 200 mm/month (Brazilian National Institute of Meteorology, 2015) and it is locally considered as the rainy season.
Figure 1. Pau da Lima community in the city of Salvador, Brazil.
(A) Location of Salvador in South America. (B) Location of the study site (red) within the city. (C) Sampling sites along the open sewer in the studied valley. In orange, yellow and red, areas of the valley above 52 m (valley top), between 52 and 38 m (valley middle), and below 38 m (valley bottom), respectively, as measured from the lowest point of the valley. (D) Photograph of a representative section of the open sewer at the bottom of the valley.
2.2 Sampling design and collection
One of the valleys in the Pau da Lima community with similar environmental features and risk factors for leptospirosis than the other valleys (Felzemburgh et al., 2014; Hagan, 2016) was selected for the longitudinal survey of surface waters. The valley selected had a slightly smaller surface and a lower incidence of violence, which allowed for a denser sampling and facilitated the access to the sampling sites. The stratified sampling scheme was designed to collect 672 water samples from three strata of sampling sites based on elevation (valley top, middle and bottom) and three collection periods (rainy, intermediate and dry) during the seven-month period from July 2011 to January 2012 inclusive. The valley was divided into three sections of approximately 30,000 m2, which corresponded to above 52 m (valley top), between 38 and 52 m (valley middle), and below 38 m (valley bottom), measured from the lowest point of the valley. We stratified sites according to elevation since previous studies found that leptospirosis infection risk was inversely associated with household elevation (Hagan et al., 2015). Fourteen paired sampling sites were selected along a continuous section of the major open sewer that flows from the top to the bottom of the valley. Among the 14 paired sampling sites, four, eight and sixteen sites were distributed at the valley top, middle and bottom sections, respectively. Within each valley section, paired sampling sites were approximately 30 m apart from each other. For each paired site, sampling was performed at two locations that were 5 m apart between sewer confluences (Fig. 1C). At each of the 28 sampling points, samples were collected from the open sewer and from standing water located in an area contiguous to the sewer. Standing water was defined as any accumulation of water without connection to a sewer or other water flow. If standing water was not available in the area adjacent to the sewer, the sample was collected within a radius of 15 m from the established site, or otherwise not collected.
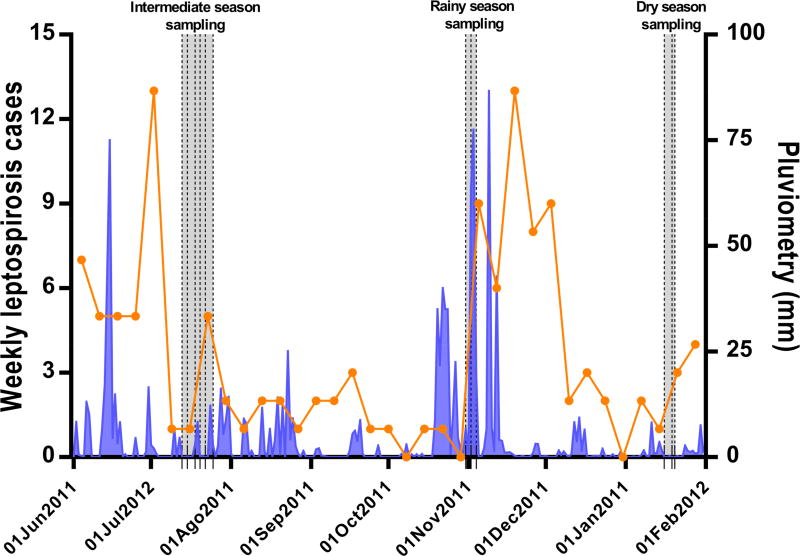
Samples were collected during three sampling campaigns: July 2011, November 2011 and January 2012 (Fig. 2). The sampling months were selected based on the historical average monthly rainfall (1996–2009): those months with an average precipitation higher than 200 mm were considered as the rainy season, those with less than 100 mm were the dry season, and those with a precipitation between 100 and 200 mm were the intermediate season (Fig. S1). Measures of daily rainfall were obtained from a municipal weather station located 0.9 Km away from the study site. Within each sampling period, samples were collected at each of the 28 sampling points on three days each week, both in the morning (from 8 am to 10 am) and in the afternoon (from 4 pm to 6 pm). Because of the correlation between leptospirosis incidence and seasonal rainfall (Ko et al., 1999), samples were collected for two consecutive weeks in July 2011, but only one week in November 2011 and January 2012. Sample collection points were georeferenced and entered in a Geographic Information System (GIS) database (Reis et al., 2008) during the first sampling campaign to facilitate the return to the same sites in the subsequent campaigns. Aliquots of 50 mL of sewage or standing water were collected in sterile polyethylene containers using aseptic techniques at the selected sites and times, and refrigerated at 4 °C up to 18 h before processing.
Figure 2.
Weekly severe leptospirosis cases identified at the state infectious diseases hospital (orange) and precipitation (blue) during the study period. The shaded areas denote the three sample collection campaigns during the intermediate, rainy and dry seasons. The vertical dashed lines indicate the collection days in each sampling campaign.
2.3 Quantification of Leptospira DNA in surface water
DNA was extracted following a procedure described previously (Riediger et al., 2016). Briefly, samples were homogenized by inversion and a 40-mL aliquot was centrifuged at 15,000 × g for 20 min at 4°C. The supernatant was discarded and the pellet was recovered and frozen at −80 °C. Pellets were then thawed in batches of 23 samples and DNA was extracted using the PowerSoil® DNA Isolation kit (MoBio) following the manufacturer’s instructions. An extraction blank consisting of ultrapure water was added to each extraction batch to monitor for cross-contamination.
Pathogenic Leptospira were quantified using a TaqMan® assay targeting a fragment of lipL32 gene (Stoddard et al., 2009) with minor modifications on a 7500 Fast Real-Time PCR thermocycler (Applied Biosystems). Calibration curves based on genomic DNA from L. interrogans serovar Copenhageni strain Fiocruz L1-130 (Nascimento et al., 2004) were run on each plate and used to transform quantification cycles (Cq) to concentrations (genome equivalents (GEq)/reaction). Non-template controls were randomly included in all rows of each plate to discard the presence of contaminating DNA. Samples, controls and calibrators were run in duplicate. All negative controls (extraction blanks and non-template controls) were negative in all cases. qPCR inhibition was monitored using a specifically designed Internal Amplification Control (IAC) plasmid tested in singleplex reactions. See Supplementary Material for further details on the qPCR assay, calibrators, genome equivalent calculations, inhibition assay and estimation of the correction factor. DNA extractions and qPCR analyses were performed according to the minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines (Bustin et al., 2009).
To confirm the specificity of the qPCR in detecting pathogenic Leptospira, 15% of the samples with a positive result in each sampling season were randomly selected for DNA sequencing. The qPCR products were loaded in a 2% agarose gel, submitted to electrophoresis and then purified using the QIAquick Gel Extraction Kit (QIAgen) following the manufacturer’s instructions. Purified products were Sanger sequenced using primer LipL32-45F, edited using BioEdit 7.2.5 (Ibis Biosciences) and compared to the sequences available in GenBank using BLAST.
2.4 Data treatment
Samples were considered positive when both qPCR replicates showed amplification before a Cq of 40. Samples with a single positive reaction were submitted to an additional qPCR in duplicate. If in this second qPCR the sample amplified in either of the replicates, it was considered positive. The GEq per reaction in all positive qPCR replicates were averaged, normalized by the volume of water analyzed, and log10-transformed to obtain concentrations in GEq/mL. To account for the DNA loss during sample processing and DNA extraction, Leptospira GEq concentrations were corrected using a calibration curve generated in sewage spiked with known concentrations of L. interrogans (Riediger et al., 2016) (Supplementary Material).
Positive qPCR samples with concentrations below the 95% hit-rate lower limit of detection of the qPCR (18 GEq/mL) (Riediger et al., 2016) were included in the positivity analysis but were excluded in the concentration analysis. In addition, standing water samples that could not be collected due to the absence of water were treated as negatives for modeling purposes since this absence implied no risk for leptospirosis infection.
2.5 Statistical analysis
Logistic and linear mixed models were used to analyze the occurrence of a positive Leptospira sample and the log10 Leptospira concentration in positive samples, respectively. In both models, we accounted for the repeated-measure structure of the data by including random effect terms for the sampling location, week and day within week. Surface water type, season, period of the day and elevation were included as fixed effects. Elevation was treated as a three-level factor (top, middle and bottom). We first selected only variables that were statistically significant in their respective univariate random effect models (logistic or linear). After including these significant variables in a general model, all possible interactions were tested. As a last step of the modeling strategy, fixed and interactions terms that did not remain significant were eliminated. In all steps, likelihood ratio tests were used for the inclusion or elimination of variables (p <0.05). Random terms were kept in the models even if their respective variances were relatively small given the intrinsic expected correlation in the space and time (location, week and day within the week). In the resulting logistic and linear mixed models, we calculated the predicted probability of finding a surface water sample with Leptospira DNA and the predicted Leptospira DNA concentration according to specific interactions by centering the remaining variables on their observed mean values (Fox, 2003). When a factor with more than two levels was included in the model according to the likelihood ratio criterion described above, we assessed the significance of differences between factor levels using post-hoc pairwise tests (Lenth, 2016). Analyses were conducted using the statistical software R v3.1 (R Core Team, 2013), with lme4 (Bates et al., 2015) , Lsmeans (Lenth, 2016), lmerTest (Kuznetsova et al., 2013) and Effects (Fox and Hong, 2009) packages. Cohen’s kappa was used to estimate the strength of agreement between sewage and standing water samples collected in the same site. Comparisons were made using Welch’s t-test in GraphPad Prism v7.01.
2.6 Leptospirosis incidence
Severe leptospirosis cases in the metropolitan region of Salvador within the study period were identified from an active surveillance program at the state infectious diseases hospital (Couto Maia Hospital). The study team prospectively evaluated admissions to identify suspected cases who met the clinical definition for severe leptospirosis (Ko et al., 1999) and enrolled patients per written informed consent protocols approved by the ethics committees of the Oswaldo Cruz Foundation and Yale University.
3. RESULTS
3.1 Rainfall pattern and leptospirosis incidence
During the study period, the rainfall pattern differed from the historical pattern described for the city of Salvador, Brazil (Brazilian National Institute of Meteorology, 2015). We observed a higher mean cumulative monthly rainfall in November 2011 compared to July 2011 and January 2012 (329.1 mm vs 81.9 and 36.4 mm, respectively, Fig. S1). Therefore, the sampling period in July 2011 was defined as the intermediate season, November 2011 as the rainy season, and January 2012 as the dry season.
A total of 101 severe leptospirosis cases were reported citywide during the 7-months study period, with an incidence of 3.8 cases per 100,000 inhabitants. The number of cases peaked in the rainy season (November 2011) with 6 to 13 cases per week following intense rainfall events (Fig. 2). In the dry and intermediate seasons, 0 to 5 cases were reported each week.
3.2 Specificity of Leptospira qPCR assay
To verify whether the qPCR reaction was specifically detecting pathogenic Leptospira in surface water samples, we partially sequenced the lipL32 amplicon from 36 samples (15.3%) out of 236 qPCR-positive samples. These samples were randomly selected and came from all seasons, types of water, collection times and locations and comprised samples with all the range of estimated concentrations. All 36 sequenced samples showed their highest similarity to other Leptospira lipL32 gene sequences deposited in GenBank (Leptospira sp. (24), L. interrogans (11) and L. borgpetersenii (1)), irrespective of the Leptospira DNA concentration of the sample (see Table S1 for sequence accession numbers and highest hits). This result confirmed that the lipL32 qPCR method is highly specific for the detection of pathogenic Leptospira in complex environmental surface water matrices.
3.3 Distribution and quantification of Leptospira DNA in surface waters
A total of 585 samples (335 sewage and 250 standing water) were collected in Pau da Lima and tested by qPCR for the presence of pathogenic Leptospira. 86 standing water samples could not be collected because no accumulation of water was found in the designated sampling area and one sewage sample was lost during processing (Table 1)
Table 1.
Collection success and occurrence of pathogenic Leptospira in sewage and standing water samples from the urban slum community. The samples are stratified by season, elevation, and period of collection. The overall positivity used for modeling purposes was calculated by considering non-collected standing water samples as negative.
| Sewage samples | Standing water samples | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Targeted | Collected* | Positive | Overall positivity |
Targeted | Collected | Positive | Overall positivity |
|
|
| ||||||||
| TOTAL | 336 | 335 | 121 (36%) | 36% | 336 | 250 (74%) | 115 (46%) | 34% |
| Seasons | ||||||||
| Intermediate | 168 | 168 | 67 (40%) | 40% | 168 | 141 (84%) | 67 (48%) | 40% |
| Rainy | 84 | 84 | 42 (50%) | 50% | 84 | 61 (73%) | 26 (42%) | 31% |
| Dry | 84 | 83 | 12 (15%) | 15% | 84 | 48 (57%) | 22 (46%) | 26% |
| Elevation | ||||||||
| Top | 48 | 48 | 14 (29%) | 29% | 48 | 34 (71%) | 17 (50%) | 35% |
| Middle | 96 | 96 | 28 (29%) | 29% | 96 | 44 (46%) | 21 (48%) | 22% |
| Bottom | 192 | 191 | 79 (41%) | 41% | 192 | 172 (90%) | 77 (45%) | 40% |
| Period | ||||||||
| Morning | 168 | 168 | 65 (39%) | 39% | 168 | 132 (79%) | 64 (49%) | 38% |
| Afternoon | 168 | 167 | 56 (34%) | 34% | 168 | 118 (70%) | 51 (43%) | 30% |
The percentages of collected sewage samples are omitted because only one sample could not be collected and tested for Leptospira presence.
Among 585 samples collected, 236 (40%) were positive for Leptospira DNA (36% of 335 sewage samples, and 46% of 250 standing water samples, respectively). Sewage showed the highest positive proportion in the rainy season, with up to 50% of 84 samples positive, and the lowest in the dry season with only 15% of 83 samples positive. In addition, more sewage samples were positive for pathogenic Leptospira DNA at the bottom of the valley (41% of 191 samples) than in the middle and top areas of the valley. In contrast, the proportion of positive samples in standing water was more stable across seasons and elevations (Table 1). When accounting for non-collected samples the overall positivity decreased to 34% for standing water. Standing water was less frequently found in the middle of the valley and during the dry season with only 46% and 57% of samples collected, respectively. As a result, the overall standing water positivity in the middle of the valley and the dry season was particularly affected, with a reduction of approximately a 50% (Table 1). Furthermore, sewage and standing water samples collected in the morning and afternoon had similar positivity ratios. Finally, the strength of agreement between the results obtained for paired sewage and standing water samples collected in the same site was only ‘fair’ (62% observed agreements; κ= 0.21±0.06) (Table S2).
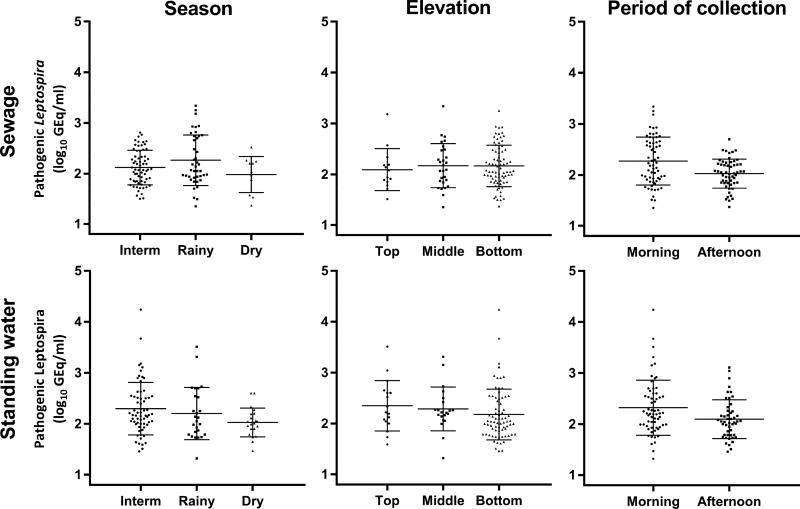
Among 231 qPCR positive samples with concentrations above the lower limit of detection, the geometric mean concentration and count range of Leptospira DNA was 152 [21-17,378] GEq/mL (143 [22 – 2,187] and 166 [20 – 17,378] GEq/mL in sewage and standing water, respectively). Overall, mean geometric Leptospira DNA concentrations in surface water from the urban slum surveys were generally low and did not vary substantially with respect to type of water, season of collection, elevation in the valley and period of collection (Fig. 3).
Figure 3.
Concentration of pathogenic Leptospira spp. in sewage and standing water samples from Pau da Lima stratified by season, elevation and time of collection. The geometric mean and standard deviation are shown for each group of samples.
3.4 Spatial and temporal predictors of Leptospira DNA presence and concentration
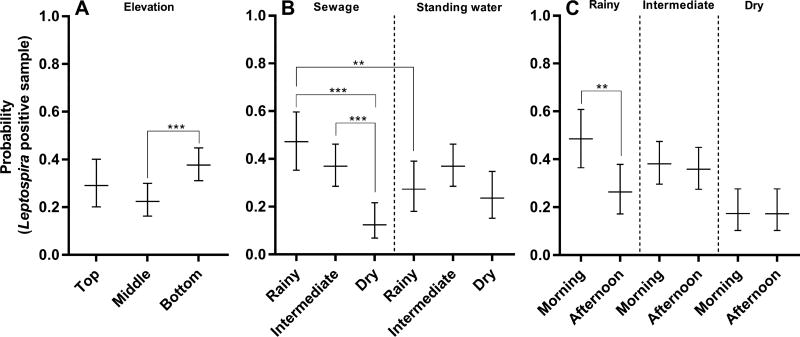
The final logistic mixed model for the probability of finding a positive sample for Leptospira DNA included fixed terms (elevation), fixed terms with interactions (surface water type, season, and period of collection) and random effects (location, week, and day within week) (Table 2). Elevation was included in the model as a fixed term indicating that the localization of the sample in the valley modified their probability of being positive for Leptospira independently of the other variables. The modeled probability of finding a positive sample, with all variables other than elevation set at their observed mean values, was higher in the bottom of the valley (38%) than in the middle (22%) or the top (29%), although the differences with respect to the top section were not statistically significant (p=0.0007 and p=0.269, respectively) (Fig. 4A).
Table 2.
Estimated regression parameters and standard errors in the final logistic and linear mixed models on the probability of finding a positive sample and log10 concentration for Leptospira DNA, respectively.
|
|
||
|---|---|---|
| Coefficient estimate (SE) | ||
|
| ||
| Logistic model for probability |
Linear model for concentration |
|
|
| ||
| Intercept | −1.57 (0.39) *** | 1.92 (0.09) |
| Intermediate season | 1.36 (0.42) ** | 0.16 (0.08) |
| Rainy season | 1.35 (0.47) ** | 0.18 (0.09) * |
| Standing water | 0.77 (0.40) | - |
| Morning period | 0.00 (0.39) | 0.09 (0.15) *** |
| Top elevation | −0.39 (0.20) | - |
| Middle elevation | −0.74 (0.25) *** | - |
| Interaction terms | ||
| Intermediate season X Standing water | −0.77 (0.46) | - |
| Rainy season X Standing water | −1.64 (0.52) ** | - |
| Intermediate season X Morning period | 0.10 (0.45) | - |
| Rainy season X Morning period | 0.97 (0.52) | - |
p≤0.05
p≤0.01;
p≤0.001
Figure 4.
Predicted probability of finding a Leptospira DNA positive sample in the final logistic mixed model according to specific interactions. (A) Elevation (B) Interaction of season and type of water (C) Interaction of season and period. Probabilities were calculated by centering the remaining variables on their observed mean values and are expressed as decimals with 95% confidence intervals. (**) p≤0.01; (***) p≤0.001.
In addition, the model included two significant interaction terms: season and type of water, and season and period of collection. The analysis of the interaction between season and type of water showed that sewage samples in the rainy season and the intermediate season had higher probabilities to be positive (47% and 37%, respectively) than those in the dry season (13%; p=0.0002 and p<0.0001, respectively). In contrast, in standing water samples the probability of being positive did not vary significantly between seasons (Fig 4B). Furthermore, in the rainy season sewage samples showed significantly higher probabilities to be positive than standing water ones (47% and 27%, respectively; p=0.0096). On the contrary, in the dry season, standing water samples were more likely to show positive results, although the difference was not statistically significant (24% and 12%, respectively; p=0.0553) (Fig. 4B). However, when considering all seasons together no difference was found between the overall positivity of sewage and standing water (36% and 34%, respectively, p=0.6028). Regarding the interaction between the season and the period of collection, the model showed that in the rainy season samples had higher probabilities to be positive in the morning than in the afternoon (49% and 26%, respectively; (p=0.0038), whereas no differences were found between the intermediate and dry seasons (Fig. 4C). To sum up, the logistic mixed model revealed that elevation, season, type of water, and period of collection were spatial and temporal predictors of the probabilities of finding Leptospira positive samples in the surface waters of the urban slum
The final linear mixed model for the concentration of Leptospira DNA in positive samples included only season and period of collection as fixed effects and random effects for location, week and day within week. The other variables (type of water and elevation) were not statistically significant in the final model (Table 2). In the rainy season, positive samples had significantly higher concentrations of Leptospira DNA when compared to the dry season (162 and 107 GEq/mL, respectively; p=0.0429). Moreover, samples collected in the morning showed higher concentrations than those collected in the afternoon (180 and 108 GEq/mL, respectively; p<0.0001). However, despite being statistically significant, these differences were small (less than 0.25 log10 units in all cases), which implied that the geometric means in positive samples were virtually the similar regardless of type of water, elevation, season or period of collection.
4. DISCUSSION
In this study, we aimed to determine the abundance of pathogenic Leptospira in the surface waters of an urban slum with high risk for leptospirosis infection, and to evaluate how their presence and concentration varied across space and time. We found that pathogenic Leptospira are ubiquitous in sewage and standing water (>33% positivity) albeit in concentrations that are generally low (around 150 GEq/mL). Our results indicate that pathogenic Leptospira have a heterogeneous spatial and seasonal distribution in our study site, being more prevalent towards the lower areas of the valley and in the rainiest months. Nevertheless, despite the spatial and seasonal variation, there is a widespread and persistent but low environmental burden across the study site.
The probability of finding positive Leptospira samples in the sewage of the urban slum presented a seasonal pattern. The number of positive samples increased during the rainy season, reaching its minimum during the dry season (Table 1 and Fig. 4). This increased positivity may be due to a combination of factors such as a mobilization of pathogenic Leptospira from soil reservoirs because of rainfall, a dissolution of environmental biofilms (Barragan et al., 2011), or an enhanced survival due to higher levels of oxygen or the dilution of sewage toxic compounds (Chang et al., 1948), among others. The specific dynamics of mobilization, dispersion and survival of pathogenic Leptospira in water and soil deserve further studies. The seasonal pattern observed in our study site is consistent with the increased number of severe leptospirosis cases reported in the metropolitan area of Salvador, Brazil 1–4 weeks after intense rainfall events (Fig. 2). This seasonal distribution has been reported in other settings around the world where large epidemics occur in the rainy season preceded by episodes of heavy rainfall such as tropical storms, typhoons or monsoons (Amilasan et al., 2012; Karande et al., 2002; Tangkanakul et al., 2005). The increased contact with potentially contaminated water and soil due to flooding and runoff has been hypothesized as the main driver of leptospirosis outbreaks (Amilasan et al., 2012; Bourhy et al., 2012; Hagan et al., 2015; Karande et al., 2005, 2002). Together with this exposure factor, our results provide the first empirical data showing that in the rainy season surface waters, and sewage in particular, are more likely to contain pathogenic Leptospira and thus, there is a higher environmental risk circulating in the urban slum.
Both sewage and standing water samples were potential reservoirs of pathogenic Leptospira in the environment. Up to 50% of sewage samples were positive in the rainy season, which suggests that in the rainy periods, sewers and its overflow are drivers of infection. In contrast, in the dry season standing water samples showed substantially -although not significantly- higher positivity ratios than sewage and, in general, they presented a diminished temporal variability (Table 1 and Fig. 4B). The differences between sewage and standing water were further accentuated by the weak positivity concordance observed in paired samples (Table S2). Taken together, these results lead us to hypothesize that sewage and standing water are two distinct ecological reservoirs of the pathogen. Consequently, the mechanisms that influence the presence of pathogenic Leptospira in sewage and standing water (input from the animal reservoir, effect of rainfall and run-off, survival kinetics, etc.) may have different spatiotemporal dynamics in each reservoir. Other studies in the Peruvian Amazon, Southern Chile, and Indonesia have also reported high positivity ratios in puddles (Ganoza et al., 2006; Muñoz-Zanzi et al., 2014; Sumanta et al., 2015). Puddles are abundant and ubiquitous in our study site, being found in areas such as the middle of the informal net of unpaved paths that connect the urban slum, and in the yards of houses. These areas are heavily used by community dwellers and may be a more accessible source of pathogenic Leptospira than the open sewers that, although precarious, have some degree of canalization. Since leptospirosis is endemic in the study site with cases occurring year-round (Fig. 2), we believe that standing water may play a role in leptospirosis transmission, particularly in between rainfall events when the accidental contact with sewage and runoff is diminished. Therefore, public health authorities need to consider standing water as a source of pathogenic Leptospira along with sewage when designing interventions aimed at reducing the transmission of the disease.
We identified a spatial distribution of positive samples with a higher environmental risk in the bottom of the valley, despite the small dimensions of our study site. Previous studies in this urban slum showed that lower household elevation was a risk factor for leptospirosis infection presumably because lower elevations are a proxy for higher flooding risk during rainfall events (Hagan et al., 2015) and contact with mud after flooding is associated with higher risks of infection (Felzemburgh et al., 2014; Reis et al., 2008). Since open sewers, rainwater drainages, and non-canalized runoff converge towards the bottom of the valley, surface water in these areas and particularly sewage, may be receiving the influence from all the water basin increasing the probability of finding Leptospira positive samples. Overall, this spatial heterogeneity highlights that small-scale changes in the environmental features may substantially contribute to differences in the risk of infection.
The concentration of pathogenic Leptospira in positive surface water samples was predominantly low. The clear majority of samples had concentrations ranging from 20 to 1,000 GEq/mL, with an average around 150 GEq/mL. To date, there is only one other study that has succeeded in quantifying pathogenic Leptospira in surface waters of urban areas, where they found mean concentrations around 1,000 cells/mL (Ganoza et al., 2006). This discrepancy may be explained by the fact that the 16S rRNA gene-based qPCR used in that study (Smythe et al., 2002) was not completely specific for pathogenic Leptospira (Viau and Boehm, 2011), which resulted in the detection of Leptospira of unknown pathogenicity (Ganoza et al., 2006). On the contrary, the lipL32 qPCR used in our study was highly specific for pathogenic Leptospira (Stoddard et al., 2009), which validates our results.
However, the low surface water loads detected in our study contrasted with the high infection rates reported in the community (35.4 to 37.8 per 1,000 individuals per year) (Felzemburgh et al., 2014; Hagan et al., 2015). The inoculum doses required for human infection are still unknown, but our findings indicate that the concentration circulating in the water is rarely higher than 1,000 GEq/mL. This concentration is several orders of magnitude lower than the doses required to cause infection through natural routes in animal models of infection. The conjunctival route shows LD50 values of 2 × 105 in Guinea Pigs (Lourdault et al., 2009) and doses as high as 108 leptospires to cause 100% death in Golden Syrian hamsters (Wunder et al., 2016a, 2016b). Notably, cuts and abrasions in the skin are an effective route of infection in grivet monkeys and Guinea Pigs (Palmer et al., 1987; Zhang et al., 2012) and have been associated with increased risks for human infection in multiple epidemiological studies (Chusri et al., 2012; Hochedez et al., 2011; Leal-Castellanos et al., 2003). While we cannot rule out the presence of additional infection sources with higher concentrations, previous epidemiological studies performed in this site have consistently pointed out to open sewers as main drivers of infection (Felzemburgh et al., 2014; Hagan et al., 2015; Reis et al., 2008). Thus, we speculate that a mechanism by which the infectious dose substantially decreases, possibly the disruption of skin barriers, enables the transmission of Leptospira in waters with low concentrations. Further epidemiological and experimental studies are required to confirm this hypothesis and to determine whether this route of transmission is the main source of the disease in the study site.
As a limitation of our study, the lipL32 qPCR assay used in our experiments had a detection limit of 18 cells/mL (Riediger et al., 2016). Based on our results, it is possible that concentrations under this limit may be occurring in the surface waters of our study site. If that is the case, the positive proportions reported here might be underestimated. Nevertheless, qPCR does not provide information regarding the viability of bacteria because DNA from metabolically inactive or dead cells can persist for a variable time in the environment (Nocker and Camper, 2009). Since only viable cells have the potential to cause infection, quantitative qPCR-based results may be overestimating the environmental risks. Furthermore, although we identified a higher prevalence of Leptospira positive samples in the rainy season, this study was not designed to explore the specific effect of rainfall events in the dynamics of the pathogen. Thus, we only captured big seasonal differences and not the short-term variability in positivity and concentration that is likely occurring due to mobilization and runoff after rainfall. Such study is needed to understand the immediate impact of rain intensity and frequency in the environmental load and the risks of infection. Finally, this study focused on the surface water reservoirs. Soil and mud are other environmental reservoirs of pathogenic Leptospira that have received little attention in the literature and, may be essential to understanding the global dynamics of pathogenic Leptospira in the environment.
5. CONCLUSIONS
The presence of pathogenic Leptospira exhibited a clear seasonal pattern in the surface waters of the urban slum, particularly in sewage, an epidemiologically proven source of infection. This is the first empirical evidence that the water-related risk to which inhabitants of an endemic area are exposed increases in the rainy season. Thus, the seasonal peaks of severe leptospirosis may be not only due to an increased exposure to contaminated sources, but also to a higher environmental risk, which modifies the current view on leptospirosis transmission after rainfall events.
The water-related risk for leptospirosis was spatially heterogeneous, being more prevalent in sewage samples towards the bottom of the valley. This finding is remarkable when considered the small size of the study site. Furthermore, it indicates that preventive measures need to account for the spatial variation for the risk of the disease.
In addition to sewage, standing water is a reservoir of pathogenic Leptospira in the urban slum environment. Their relatively stable positivity across seasons and elevations, suggests that standing water may be relevant for the transmission of the disease, especially in between rainfall events. Consequently, the closing of open sewers alone, a common public health measure, may not be sufficient to eliminate the water-related transmission of the disease.
The concentration of pathogenic Leptospira in surface waters was generally low (mean concentration 152 GEq/mL) which contrasts with previous environmental studies and the high infection rates reported in this urban slum. Further epidemiological and experimental research is necessary to understand the natural history of leptospirosis infection and its correlation with low infectious doses.
Pau da Lima, our study site in Salvador, Brazil, has similar characteristics to other marginalized communities around the world. Hence, our results may help to understand the drivers of the temporal and spatial variability in urban leptospirosis epidemics. This knowledge is essential to implement timely and efficient measures to reduce the burden of leptospirosis worldwide.
Supplementary Material
-
-
Sewage and standing water are a source of pathogenic Leptospira in urban slums
-
-
Leptospira were ubiquitous in this setting, detected in 33% of sampled surface water
-
-
Pathogen concentrations were low (~150 GEq/mL) in positive surface water samples
-
-
Seasonal leptospirosis risk is associated with increased pathogen detection in water
-
-
Prevention needs to account for the spatiotemporal dynamics of pathogenic Leptospira
Acknowledgments
The authors thank the joint collaborative effort of the resident associations, community leaders and residents, which constitute the Urban Health Council of Pau da Lima. This research was supported by the National Institutes of Health research grants R01 AI052473, U01 AI088752, R25 TW009338, R01 TW009504 and R01 AI121207. INR was supported by Ph.D. scholarship (BEX 066509-6) from the Brazilian Ministry of Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agampodi SB, Dahanayaka NJ, Bandaranayaka AK, Perera M, Priyankara S, Weerawansa P, Matthias MA, Vinetz JM. Regional Differences of Leptospirosis in Sri Lanka: Observations from a Flood-Associated Outbreak in 2011. PLoS Negl. Trop. Dis. 2014;8:e2626. doi: 10.1371/journal.pntd.0002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amilasan AST, Ujiie M, Suzuki M, Salva E, Belo MCP, Koizumi N, Yoshimatsu K, Schmidt WP, Marte S, Dimaano EM, Villarama JB, Ariyoshi K. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg. Infect. Dis. 2012;18:91–94. doi: 10.3201/eid1801.101892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos C, Sabroza PC. The place behind the case: leptospirosis risks and associated environmental conditions in a flood-related outbreak in Rio de Janeiro. Cad. Saude Publica. 2001;17(Suppl):59–67. doi: 10.1590/s0102-311x2001000700014. [DOI] [PubMed] [Google Scholar]
- Barragan VA, Mejia ME, Trávez A, Zapata S, Hartskeerl RA, Haake DA, Trueba GA. Interactions of Leptospira with environmental bacteria from surface water. Curr. Microbiol. 2011;62:1802–1806. doi: 10.1007/s00284-011-9931-3. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015;67:1–48. [Google Scholar]
- Bourhy P, Collet L, Lernout T, Zinini F, Hartskeerl Ra, van der Linden H, Thiberge JM, Diancourt L, Brisse S, Giry C, Pettinelli F, Picardeau M. Human Leptospira isolates circulating in Mayotte (Indian Ocean) have unique serological and molecular features. J. Clin. Microbiol. 2012;50:307–11. doi: 10.1128/JCM.05931-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazilian National Institute of Meteorology (INMET) 2015 Available online: http://www.inmet.gov.br/
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chang SL, Buckingham M, Taylor MP. Studies on Leptospira Iceterohaemorrhagiae: : IV. Survival in Water and Sewage: Destruction in Water by Halogen Compounds, Synthetic Detergents, and Heat. J. Infect. Dis. 1948;82:256–266. doi: 10.1093/infdis/82.3.256. [DOI] [PubMed] [Google Scholar]
- Chusri S, Sritrairatchai S, Hortiwahul T, Charoenmak B, Silpapojakul K. Leptospirosis among river water rafters in Satoon, Southern Thailand. J. Med. Assoc. Thail. 2012;95:874–877. [PubMed] [Google Scholar]
- Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, Stein C, Abela-Ridder B, Ko AI. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015;9:e0003898. doi: 10.1371/journal.pntd.0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Porter FH, Rodrigues G, Farias H, de Faria MT, Wunder EA, Osikowicz LM, Kosoy MY, Reis MG, Ko AI, Childs JE. Infections by Leptospira interrogans, Seoul virus, and Bartonella spp. among Norway rats (Rattus norvegicus) from the urban slum environment in Brazil. Vector Borne Zoonotic Dis. 2014;14:33–40. doi: 10.1089/vbz.2013.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvars A, Jégo S, Chiroleu F, Bourhy P, Cardinale E, Michault A. Seasonality of human leptospirosis in Reunion Island (Indian Ocean) and its association with meteorological data. PLoS One. 2011;6:e20377. doi: 10.1371/journal.pone.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felzemburgh RDM, Ribeiro GS, Costa F, Reis RB, Hagan JE, Melendez AXTO, Fraga D, Santana FS, Mohr S, Dos Santos BL, Silva AQ, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. Prospective study of leptospirosis transmission in an urban slum community: role of poor environment in repeated exposures to the Leptospira agent. PLoS Negl. Trop. Dis. 2014;8:e2927. doi: 10.1371/journal.pntd.0002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. Effect Displays in R for Generalised Linear Models. J. Stat. Softw. 2003;8:1–27. [Google Scholar]
- Fox J, Hong J. Effect Displays in R for Multinomial and Proportional-Odds Logit Models: Extensions to the effects Package. J. Stat. Softw. 2009;32:1–24. [Google Scholar]
- Ganoza CA, Matthias MA, Collins-Richards D, Brouwer K, Cunningham CB, Segura ER, Gilman RH, Gotuzzo E, Vinetz JM. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Med. 2006;3:1329–1340. doi: 10.1371/journal.pmed.0030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA, Levett PN. Leptospirosis in humans. Curr. Top. Microbiol. Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JE, Moraga P, Costa F, Capian N, Ribeiro GS, Wunder EA, Felzemburgh RDM, Reis RB, Nery N, Santana FS, Fraga D, dos Santos BL, Santos AC, Queiroz A, Tassinari W, Carvalho MS, Reis MG, Diggle PJ, Ko AI. Spatiotemporal determinants of urban leptospirosis transmission: Four-year prospective cohort study of slum residents in Brazil. PLoS Negl Trop Dis. 2015;10:e0004275. doi: 10.1371/journal.pntd.0004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom JS, Marshall RB. Survival of Leptospira interrogans serovar pomona in an acidic soil under simulated New Zealand field conditions. Res. Vet. Sci. 1978;25:29–33. [PubMed] [Google Scholar]
- Hochedez P, Rosine J, Théodose R, Abel S, Bourhy P, Picardeau M, Quénel P, Cabié A. Outbreak of leptospirosis after a race in the tropical forest of Martinique. Am. J. Trop. Med. Hyg. 2011;84:621–6. doi: 10.4269/ajtmh.2011.10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karande S, Gandhi D, Kulkarni M, Bharadwaj R, Pol S, Thakare J, De A. Concurrent outbreak of leptospirosis and dengue in Mumbai, India, 2002. J. Trop. Pediatr. 2005;51:174–181. doi: 10.1093/tropej/fmh100. [DOI] [PubMed] [Google Scholar]
- Karande S, Kulkarni H, Kulkarni M, De A, Varaiya A. Leptospirosis in children in Mumbai slums. Indian J. Pediatr. 2002;69:855–858. doi: 10.1007/BF02723705. [DOI] [PubMed] [Google Scholar]
- Khairani-Bejo S, Bahaman AR, Zamri-Saad M, Mutalib AR. The survival of Leptospira interrogans in the Malasyan environment. J. Anim. Vet. Adv. 2004;3:123–129. [Google Scholar]
- Ko AI, Galvão Reis M, Ribeiro Dourado CM, Johnson WD, Riley LW. Urban epidemic of severe leptospirosis in Brazil. Lancet. 1999;354:820–5. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat. Rev. Microbiol. 2009;7:736–47. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurilung A, Chanchaithong P, Lugsomya K, Niyomtham W, Wuthiekanun V, Prapasarakul N. Molecular detection and isolation of pathogenic Leptospira from asymptomatic humans, domestic animals and water sources in Nan province, a rural area of Thailand. Res. Vet. Sci. 2017;115:146–154. doi: 10.1016/j.rvsc.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package) R Package, version. 2013;2 [Google Scholar]
- Kyobutungi C, Ziraba AK, Ezeh A, Yé Y. The burden of disease profile of residents of Nairobi’s slums: results from a demographic surveillance system. Popul. Health Metr. 2008;6:1. doi: 10.1186/1478-7954-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CL, Watson CH, Lowry JH, David MC, Craig SB, Wynwood SJ, Kama M, Nilles EJ. Human Leptospirosis Infection in Fiji: An Eco-epidemiological Approach to Identifying Risk Factors and Environmental Drivers for Transmission. PLoS Negl. Trop. Dis. 2016;10:e0004405. doi: 10.1371/journal.pntd.0004405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal-Castellanos CB, Garcia-Suarez R, Gonzalez-Figueroa E, Fuentes-Allen JL, Escobedo-De la Peña J. Risk factors and the prevalence of leptospirosis infection in a rural community of Chiapas, Mexico. Epidemiol. Infect. 2003;131:1149–1156. doi: 10.1017/s0950268803001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth RV. Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016;69:1–33. [Google Scholar]
- Lourdault K, Aviat F, Picardeau M. Use of quantitative real-time PCR for studying the dissemination of Leptospira interrogans in the guinea pig infection model of leptospirosis. J. Med. Microbiol. 2009;58:648–55. doi: 10.1099/jmm.0.008169-0. [DOI] [PubMed] [Google Scholar]
- Muñoz-Zanzi C, Mason MR, Encina C, Astroza A, Romero A. Leptospira contamination in household and environmental water in rural communities in southern Chile. Int. J. Environ. Res. Public Health. 2014;11:6666–6680. doi: 10.3390/ijerph110706666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento AL, Ko AI, Martins EA, Monteiro-Vitorello CB, Ho PL, Haake DA, Verjovski-Almeida S, Hartskeerl RA, Marques MV, Oliveira MC, Menck CF, Leite LC, Carrer H, Coutinho LL, Degrave WM, Dellagostin OA, El-Dorry H, Ferro ES, Ferro MI, Furlan LR, Gamberini M, Giglioti EA, Goes-Neto A, Goldman GH, Goldman MH, Harakava R, Jeronimo SM, Junqueira-de Azevedo IL, Kimura ET, Kuramae EE, Lemos EG, Lemos MV, Marino CL, Nunes LR, de Oliveira RC, Pereira GG, Reis MS, Schriefer A, Siqueira WJ, Sommer P, Tsai SM, Simpson AJ, Ferro JA, Camargo LE, Kitajima JP, Setubal JC, Van Sluys MA. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J Bacteriol. 2004;186:2164–2172. doi: 10.1128/JB.186.7.2164-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navegantes de Araújo W, Finkmoore B, Ribeiro GS, Reis RB, Felzemburgh RDM, Hagan JE, Reis MG, Ko AI, Costa F. Knowledge, attitudes, and practices related to Leptospirosis among urban slum residents in Brazil. Am. J. Trop. Med. Hyg. 2013;88:359–63. doi: 10.4269/ajtmh.2012.12-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocker A, Camper AK. Novel approaches toward preferential detection of viable cells using nucleic acid amplification techniques. FEMS Microbiol. Lett. 2009;291:137–42. doi: 10.1111/j.1574-6968.2008.01429.x. [DOI] [PubMed] [Google Scholar]
- Okazaki W, Ringen LM. Some effects of various environmental conditions on the survival of Leptospira pomona. Am. J. Vet. Res. 1957;18:219–223. [PubMed] [Google Scholar]
- Oliveira DSC, Guimarães MJB, Portugal JL, Medeiros Z. The socio-demographic, environmental and reservoir factors associated with leptospirosis in an urban area of north-eastern Brazil. Ann. Trop. Med. Parasitol. 2009;103:149–57. doi: 10.1179/136485909X398221. [DOI] [PubMed] [Google Scholar]
- Palmer MF, Waitkins SA, Fitzgeorge RB, Baskerville A. Experimental Infection of Monkeys with Leptospira interrogans Serovar hardjo. Epidemiol. Infect. 1987;98:191–197. doi: 10.1017/s0950268800061902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panti-May JA, Carvalho-Pereira TSA, Serrano S, Pedra GG, Taylor J, Pertile AC, Minter A, Airam V, Carvalho M, Júnior NN, Rodrigues G, Reis MG, Ko AI, Childs JE, Begon M, Costa F. A Two-Year Ecological Study of Norway Rats (Rattus norvegicus) in a Brazilian Urban Slum. PLoS One. 2016;11:e0152511. doi: 10.1371/journal.pone.0152511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. A language and environment for statistical computing 2013 [Google Scholar]
- Reis RB, Ribeiro GS, Felzemburgh RDM, Santana FS, Mohr S, Melendez AXTO, Queiroz A, Santos AC, Ravines RR, Tassinari WS, Carvalho MS, Reis MG, Ko AI. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl. Trop. Dis. 2008;2:e228. doi: 10.1371/journal.pntd.0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediger IN, Hoffmaster AR, Casanovas-Massana A, Biondo AW, Ko AI, Stoddard RA. An Optimized Method for Quantification of Pathogenic Leptospira in Environmental Water Samples. PLoS One. 2016;11:e0160523. doi: 10.1371/journal.pone.0160523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley LW, Ko AI, Unger A, Reis MG. Slum health: diseases of neglected populations. BMC Int. Health Hum. Rights. 2007;7(2) doi: 10.1186/1472-698X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Villanueva SYAM, Chakraborty A, Miyahara S, Segawa T, Asoh T, Ozuru R, Gloriani NG, Yanagihara Y, Yoshida SI. Comparative analysis of Leptospira strains isolated from environmental soil and water in the Philippines and Japan. Appl. Environ. Microbiol. 2013;79:601–609. doi: 10.1128/AEM.02728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar U, Nascimento SF, Barbosa R, Martins R, Nuevo H, Kalofonos I, Kalafanos I, Grunstein I, Flannery B, Dias J, Riley LW, Reis MG, Ko AI. Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am. J. Trop. Med. Hyg. 2002;66:605–10. doi: 10.4269/ajtmh.2002.66.605. [DOI] [PubMed] [Google Scholar]
- Smith JKG, Young MM, Wilson KL, Craig SB. Leptospirosis following a major flood in Central Queensland, Australia. Epidemiol. Infect. 2013;141:585–590. doi: 10.1017/S0950268812001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythe L, Smith I, Smith G, Dohnt M, Symonds M, Barnett L, McKay D. A quantitative PCR (TaqMan) assay for pathogenic Leptospira spp. BMC Infect. Dis. 2002;2:13. doi: 10.1186/1471-2334-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard RA, Gee JE, Wilkins PP, McCaustland K, Hoffmaster AR. Detection of pathogenic Leptospira spp. through TaqMan polymerase chain reaction targeting the LipL32 gene. Diagn. Microbiol. Infect. Dis. 2009;64:247–55. doi: 10.1016/j.diagmicrobio.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Sumanta H, Wibawa T, Hadisusanto S, Nuryati A, Kusnanto H. Spatial Analysis of Leptospira in Rats, Water and Soil in Bantul District Yogyakarta Indonesia. Open J. Epidemiol. 2015;5:22–31. [Google Scholar]
- Tangkanakul W, Smits HL, Jatanasen S, Ashford DA. Leptospirosis: an emerging health problem in Thailand. Southeast Asian J. Trop. Med. Public Health. 2005;36:281–8. [PubMed] [Google Scholar]
- Tassinari WDS, Pellegrini DDCP, Sabroza PC, Carvalho MS. Spatial distribution of leptospirosis in the city of Rio de Janeiro, Brazil, 1996–1999. Cad. saude publica / Minist. da Saude, Fund. Oswaldo Cruz, Esc. Nac. Saude Publica. 2004;20:1721–1729. doi: 10.1590/s0102-311x2004000600031. [DOI] [PubMed] [Google Scholar]
- Thibeaux R, Geroult S, Benezech C, Chabaud S, Soupé-Gilbert M-E, Girault D, Bierque E, Goarant C. Seeking the environmental source of Leptospirosis reveals durable bacterial viability in river soils. PLoS Negl. Trop. Dis. 2017;11:e0005414. doi: 10.1371/journal.pntd.0005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson PR, Hagan JE, Costa F, Calcagno J, Kane M, Martinez-Silveira MS, Goris MGA, Stein C, Ko AI, Abela-Ridder B. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Negl. Trop. Dis. 2015;9:e0004122. doi: 10.1371/journal.pntd.0004122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevejo RT, Rigau-Perez JG, Ashford DA, McClure EM, Jarquin-Gonzalez C, Amador JJ, los Reyes JO, Gonzalez A, Zaki SR, Shieh W-J, McLean RG, Nasci RS, Weyant RS, Bolin CA, Bragg SL, Perkins BA, Spiegel RA. Epidemic leptospirosis associated with pulmonary hemorrhage - Nicaragua, 1995. J. Infect. Dis. 1998;178:1457–1463. doi: 10.1086/314424. [DOI] [PubMed] [Google Scholar]
- Trueba G, Zapata S, Madrid K, Cullen P, Haake D. Cell aggregation: a mechanism of pathogenic Leptospira to survive in fresh water. Int. Microbiol. 2004;7:35–40. [PubMed] [Google Scholar]
- UN-HABITAT. State of the World’s Cities 2012/2013 - Prosperity of Cities 2013 [Google Scholar]
- Unger A, Ko A, Douglass-jaime G. Favela Health in Pau da Lima, Salvador, Brazil. In: Corburn J, Riley L, editors. Slum Health. University of California Press; Berkeley, CA: 2016. pp. 105–117. [Google Scholar]
- Viau EJ, Boehm AB. Quantitative PCR-based detection of pathogenic Leptospira in Hawai’ian coastal streams. J. Water Health. 2011;9:637–646. doi: 10.2166/wh.2011.064. [DOI] [PubMed] [Google Scholar]
- Weinberger D, Baroux N, Grangeon J-P, Ko AI, Goarant C. El Niño Southern Oscillation and leptospirosis outbreaks in New Caledonia. PLoS Negl. Trop. Dis. 2014;8:e2798. doi: 10.1371/journal.pntd.0002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder EA, Figueira CP, Benaroudj N, Hu B, Tong BA, Trajtenberg F, Liu J, Reis MG, Charon NW, Buschiazzo A, Picardeau M, Ko AI. A novel flagellar sheath protein, FcpA, determines filament coiling, translational motility and virulence for the Leptospira spirochete. Mol. Microbiol. 2016a;101(3):457–470. doi: 10.1111/mmi.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunder EA, Figueira CP, Santos GR, Lourdault K, Matthias MA, Vinetz JM, Ramos E, Haake DA, Picardeau M, Dos Reis MG, Ko AI. Real-time PCR reveals rapid dissemination of Leptospira interrogans after intraperitoneal and conjunctival inoculation of hamsters. Infect. Immun. 2016b;84:2105–2015. doi: 10.1128/IAI.00094-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lou X-L, Yang H-L, Guo X-K, Zhang X-Y, He P, Jiang X-C. Establishment of a leptospirosis model in guinea pigs using an epicutaneous inoculations route. BMC Infect. Dis. 2012;12:20. doi: 10.1186/1471-2334-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.