Abstract
In Parkinson disease (PD), a complex neurodegenerative disorder that affects nearly 10 million people worldwide, motor skills are significantly impaired. However, onset and progression of motor deficits and the neural correlates of these deficits are poorly understood. We used a genetic mouse model of PD (Pink1 −/−), with phenotypic similarities to human PD, to investigate the manifestation of early-onset sensorimotor deficits. We hypothesized this mouse model would show early vocalization and gross motor dysfunction that would be progressive in nature. Pink1 −/− mice, compared to wild type (WT) controls, were evaluated at 2, 3, 4, 5, and 6 months of age. To quantify deficit progression, ultrasonic vocalizations and spontaneous locomotor activity (cylinder test and pole test) were analyzed. Although somewhat variable, in general, Pink1 −/− mice produced significantly more simple calls with reduced intensity as well as a larger percentage of cycle calls compared to WT counterparts. However, there were no significant differences in duration, bandwidth, or peak frequency for any of the ultrasonic call types between genotypes. Pink1 −/− mice showed a significant impairment in limb motor skills with fewer hindlimb steps, forelimb steps, and rears and lands in the cylinder test compared to WT. Additionally, Pink1 −/− mice took significantly longer to turn and traverse during the pole test. Immunohistochemical staining showed no significant difference in the number of tyrosine hydroxylase (TH) positive cells in the substantia nigra or density of TH staining in the striatum between genotypes. These data suggest the Pink1 −/− mouse model may be instrumental in defining early motor biomarkers of PD in the absence of nigrostriatal dopamine loss.
Keywords: Parkinson disease, mouse, ultrasonic vocalization, Pink1
1. Introduction
Parkinson disease (PD), a complex neurodegenerative disorder affecting over 10 million individuals (de Lau et al., 2004), is primarily the result of progressive decline in the dopaminergic activity of the substantia nigra (SN) and the corresponding neural pathways (Lees et al., 2009). Hallmark motor dysfunctions associated with PD include deficits in the limbs, such as bradykinesia, muscular rigidity, and resting tremor, and are associated with the loss of central nervous system dopamine (Bernheimer et al., 1973). The cranial sensorimotor dysfunction that occurs with vocalization, articulation, and swallowing in PD is not well understood and is thought to involve extra-dopaminergic mechanisms outside of the hallmark pathology (Braak et al., 2003).
Communication (including vocalization) deficits affect up to 90% of individuals, appear early in the disease process, and are among the most debilitating features of PD (Holmes et al., 2000; Miller et al., 2006; Volonté et al., 2002). A reduction in loudness, pitch, and duration decreases a patient’s ability to successfully interact with others, consequently negatively influencing their overall life quality (Goberman and Blomgren, 2008; Harel et al., 2004; Ho et al., 1998; Holmes et al., 2000; Marras et al., 2008; Stewart et al., 1995). Voice dysfunction in PD does not respond to standard pharmaceutical (dopamine replacement therapies) and surgical (deep brain stimulation) treatments (Baijens and Speyer, 2009; Ciucci et al., 2013; Schulz and Grant, 2000). Therefore, identification and characterization of early and progressive models to study vocal deficits is critical to understanding disease progression as well as developing effective, sustainable therapies.
While most cases of PD are sporadic in nature, approximately 10% of cases are due to genetic mutations. In human populations, mutations in specific genes have been linked to early-onset hereditary forms of the disease (Farrer, 2006; Rochet et al., 2012). Of these genetic cases, mutations in Pink1 are the second most common cause of autosomal recessive PD and cause progressive peripheral deficits as well as nigrostriatal pathology (Bonifati et al., 2002; Bonifati et al., 2005; Guo et al., 2011). The Pink1 protein is a master regulator of mitochondrial function and mitophagy (Geisler et al., 2010; Kawajiri et al., 2011; Poole et al., 2008; Yang et al., 2008) and multiple studies suggest that mitochondrial dysfunction plays a central role in the pathogenesis of PD. Genetic PD can be indistinguishable from idiopathic PD, therefore, studying gene responsible for inherited PD can yield invaluable information about pathogenesis as a whole (Albanese et al., 2005; Hatano et al., 2009; Kawajiri et al., 2011).
Using ultrasonic vocalization measures, previous work in male rats with complete knock-out of the Pink1 gene demonstrated significant vocal dysfunction including reductions in pitch and loudness (peak frequency and bandwidth) (Grant et al., 2015b), as well as reductions in social conspecific responses to their vocalizations (Pultorak et al., 2015). Additionally, several studies in the rat Pink1 −/− model have shown significant brain pathology including dopaminergic and noradrenergic cell loss in the substantia nigra (Dave et al., 2014) and locus coeruleus (Grant et al., 2015b), and also alpha synuclein aggregations in brainstem motor regions including the periaqueductal gray (Grant et al., 2015b), as well as metabolic and mitochondrial pathogenesis (Villeneuve et al., 2014). Importantly, in the rat model these behavioral deficits correlate to brain pathology. Likewise, mice overexpressing alpha-synuclein (Thy1-aSyn) have significant early-onset vocalization behaviors associated with alpha-synuclein brainstem pathology suggesting the presence of early alpha-synuclein may be a mechanism underlying cranial sensorimotor deficits in this mouse model (Grant et al., 2014).
Currently, a single study suggests that striatal dopamine release and synaptic plasticity is affected in the Pink1 −/− mouse model compared to age-matched wild type (WT) mice (Kitada et al., 2007). However, vocalization and sensorimotor function has not been evaluated. In the current study, we assessed ultrasonic vocalizations, spontaneous motor activity (cylinder test), and gross limb sensorimotor control (pole test). We hypothesized that Pink1 −/− mice would exhibit early and progressive vocalization and sensorimotor impairments compared to WT controls.
2. Results
All inter-rater and intra-rater reliability tests for USV, cylinder, and pole analysis were above 0.90 (Table 1). Body weights were significantly different between genotypes at 5 mo of age. Pink1 −/− mice (average 32.67 g +/− 0.28 were heavier on average compared to WT (29.78 g +/− 0.29) mice (Mann-Whitney Rank Sum Tests U-statistic=63.0, p=0.002). There were no significant relationships between body weight and any of the motor behavioral variables measured at 5 mo of age (p>0.05).
Table 1. Inter- and intra-rater reliability.
10% of the behavioral files were randomly selected and re-analyzed, user data was analyzed using an intra-class correlation coefficient.
| Behavioral Assay | Inter-reliability | Intra-reliability |
|---|---|---|
| USV Call Category | 0.95 | 0.98 |
| USV Bandwidth | 0.91 | 0.93 |
| USV Intensity | 0.94 | 0.97 |
| USV Duration | 0.93 | 0.96 |
| USV Peak Frequency | 0.91 | 0.95 |
| Cylinder Test | 0.90 | 0.97 |
| Adhesive Test | n/a | 0.95 |
| Pole Test | n/a | 0.95 |
2.1 Ultrasonic vocalizations
USV data (means and SEM) are reported in Table 2.
Table 2. Ultrasonic vocalization means (standard error of the mean).
Abbreviations: mo=month; WT=wildtype; sec = seconds; kHz = kilohertz; dB = decibel
| Acoustic Variable |
Testing Timepoint (age of animals) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 mo | 3 mo | 4 mo | 5 mo | 6 mo | ||||||
| WT | Pink1 −/− | WT | Pink1 −/− | WT | Pink1 −/− | WT | Pink1 −/− | WT | Pink1 −/− | |
| Number of calls | 61.72 (25.64) | 58.94 (25.64) | 122.22 (25.64) | 110.33 (25.64) | 118.00 (25.64) | 65.72 (25.64) | 176.10 (34.40) | 144.17 (31.41) | 125.75 (27.20) | 95.43 (29.08) |
| Call rate (#/min) | 1.03 (0.43) | 0.98 (0.43) | 2.04 (0.3) | 1.84 (0.43) | 1.97 (0.43) | 1.10 (0.43) | 1.63 (0.43) | 1.60 (0.43) | 2.10 (0.46) | 1.48 (0.47) |
| % simple calls | 0.36 (0.058) | 0.35 (0.06) | 0.37 (0.04) | 0.31 (0.05) | 0.25 (0.05) | 0.42 (0.05) | 0.27 (0.05) | 0.31 (0.05) | 0.38 (0.04) | 0.26 (0.04) |
| % jump calls | 0.26 (0.04) | 0.27 (0.04) | 0.27 (0.03) | 0.25 (0.04) | 0.23 (0.03) | 0.24 (0.04) | 0.29 (0.04) | 0.22 (0.03) | 0.27 (0.03) | 0.26 (0.03) |
| % cycle calls | 0.093 (0.02) | 0.15 (0.02) | 0.084 (0.02) | 0.088 (0.02) | 0.079 (0.02) | 0.084 (0.02) | 0.082 (0.02) | 0.11 (0.02) | 0.069 (0.01) | 0.10 (0.01) |
| % complex calls | 0.29 (0.062) | 0.23 (0.06) | 0.28 (0.05) | 0.35 (0.05) | 0.43 (0.05) | 0.33 (0.05) | 0.36 (0.05) | 0.36 (0.05) | 0.28 (0.04) | 0.37 (0.04) |
| Average duration (simple) (sec) | 0.018 (0.002) | 0.018 (0.002) | 0.019 (0.002) | 0.019 (0.002) | 0.021 (0.002) | 0.018 (0.002) | 0.022 (0.002) | 0.022 (0.002) | 0.020 (0.001) | 0.024 (0.002) |
| Average duration (jump) (sec) | 0.026 (0.0065) | 0.027 (0.006) | 0.027 (0.005) | 0.027 (0.005) | 0.028 (0.005) | 0.028 (0.006) | 0.032 (0.005) | 0.049 (0.005) | 0.032 (0.004) | 0.029 (0.005) |
| Average duration (cycle) (sec) | 0.026 (0.0083) | 0.032 (0.008) | 0.029 (0.006) | 0.042 (0.007) | 0.039 (0.007) | 0.041 (0.008) | 0.054 (0.007) | 0.060 (0.006) | 0.050 (0.005) | 0.051 (0.006) |
| Average duration (complex) (sec) | 0.037 (0.0069) | 0.047 (0.006) | 0.048 (0.005) | 0.055 (0.006) | 0.054 (0.005) | 0.051 (0.007) | 0.074 (0.006) | 0.076 (0.006) | 0.060 (0.005) | 0.070 (0.005) |
| Average bandwidth (simple) (kHz) | 12638.63 (2265.62) | 12255.63 (2119.30) | 13659.63 (1662.51) | 14317.23 (1895.56) | 15267.47 (1730.40) | 14172.81 (1998.09) | 11909.23 (1895.56) | 14901.55 (1807.34) | 14312.75 (1498.57) | 15633.99 (1602.04) |
| Average bandwidth (jump) (kHz) | 25056.74 (1927.052) | 25022.99 (1802.59) | 25124.42 (1414.07) | 25071.24 (1612.29) | 24817.78 (1471.81) | 23574.62 (1699.50) | 21421.56 (1612.29) | 26631.47 (1471.81) | 24470.67 (1274.63 | 23883.80 (1362.63 |
| Average bandwidth (cycle) (kHz) | 15707.91 (2589.85) | 17346.56 (2422.58) | 13589.83 (1900.43) | 21056.76 (2284.03) | 17494.71 (2065.99) | 18873.003 (2422.58) | 18878.71 (2166.82) | 18669.51 (1978.03) | 20396.092 (1713.02) | 21777.63 (1900.45) |
| Average bandwidth (complex) (kHz) | 34696.15 (2901.30) | 37504.50 (2713.96) | 36842.94 (2128.97) | 38977.72 (2427.40) | 42086.87 (2215.90) | 41188.56 (2713.92) | 42243.82 (2427.40) | 42149.85 (2314.43) | 38926.20 (2051.53) | 39925.72 (2051.53) |
| Average peak frequency (simple) (kHz) | 79632.70 (1870.07 | 78443.97 (1749.30) | 78610.26 (1372.25) | 81458.13 (1564.61) | 80348.12 (1428.29) | 79387.15 (1649.25) | 79324.46 (1564.61) | 81272.85 (1491.80) | 77401.23 (1236.93) | 81457.50 (1322.34) |
| Average peak frequency (jump) (kHz) | 78365.82 (3352.503) | 76600.00 (3135.98) | 78190.91 (2460.07) | 78866.47 (2804.91) | 80293.14 (2560.52) | 77259.55 (2956.63) | 67474.19 (2804.91) | 77845.89 (2560.52) | 77005.71 (2217.47) | 78443.31 (2370.58) |
| Average peak frequency (cycle) (kHz) | 79484.81 (3608.74) | 80711.47 (3375.67) | 79806.59 (2648.09) | 79178.95 (3182.61) | 77298.52 (2878.78) | 76439.30 (3375.67) | 75173.56 (3019.20) | 69836.56 (2756.22) | 76127.49 (2386.96) | 74869.44 (2648.091) |
| Average peak frequency (complex) (kHz) | 77473.48 (1610.67) | 78064.83 (1506.65) | 75723.88 (1181.91) | 76398.82 (1347.59) | 76561.66 (1230.17) | 77083.21 (1506.65) | 74503.70 (1347.59) | 76776.09 (1284.87) | 74991.63 (1138.92) | 76247.81 (1138.92) |
| Average intensity (simple) (dB) | −53.40 (1.05) | −53.75 (0.99) | −54.03 (0.77) | −55.22 (0.88) | −51.71 (0.81) | −55.31 (0.93) | −55.70 (0.88) | −55.65 (0.84) | −55.39 (0.70) | −56.21 (0.75) |
| Average intensity (jump) (dB) | −50.40 (2.56) | −48.95 (2.39) | −50.27 (1.88) | −50.68 (2.14) | −48.41 (1.95) | −51.45 (2.25) | −46.16 (2.14) | −51.85 (1.95) | −52.22 (1.69) | −53.09 (1.81) |
| Average intensity (cycle) (dB) | −51.80 (2.55) | −50.22 (2.38) | −52.82 (1.87) | −49.61 (2.25) | −47.85 (2.03) | −51.96 (2.38) | −52.36 (2.13) | −49.26 (1.95) | −52.71 (1.69) | −51.77 (1.87) |
| Average intensity (complex) (dB) | −49.61 (1.55) | −47.39 (1.45) | −49.17 (1.14) | −48.11 (1.30) | −45.22 (1.18) | −48.27 (1.45) | −48.48 (1.30) | −50.03 (1.24) | −50.52 (1.095) | −50.16 (1.010) |
2.1.1 Number of calls and call rate
There was no significant interaction between genotype (Pink1−/− and WT) and time (2, 3, 4, 5, 6, mo) for number of calls (F(4,159)=0.278, p=0.892) and call rate (F(4,174)=0.366, p=0.833). Additionally, number of calls and call rate demonstrated no main effect of genotype (F(1,159)=2.158, p=0.143), F(1,174)=1.592, p=0.209, respectively). There was a main effect of time for the number of calls produced regardless of genotype (F(4,159)=3.153, p=0.016; Figure 2A). Specifically, post-hoc comparisons revealed differences (increases) between 2 and 3 mo (p=0.031), 2 and 5 mo (p<0.001), and 4 and 5 mo (p=0.022). For call rate, there was no main effect of time (F(4,174)=1.332, p=0.260).
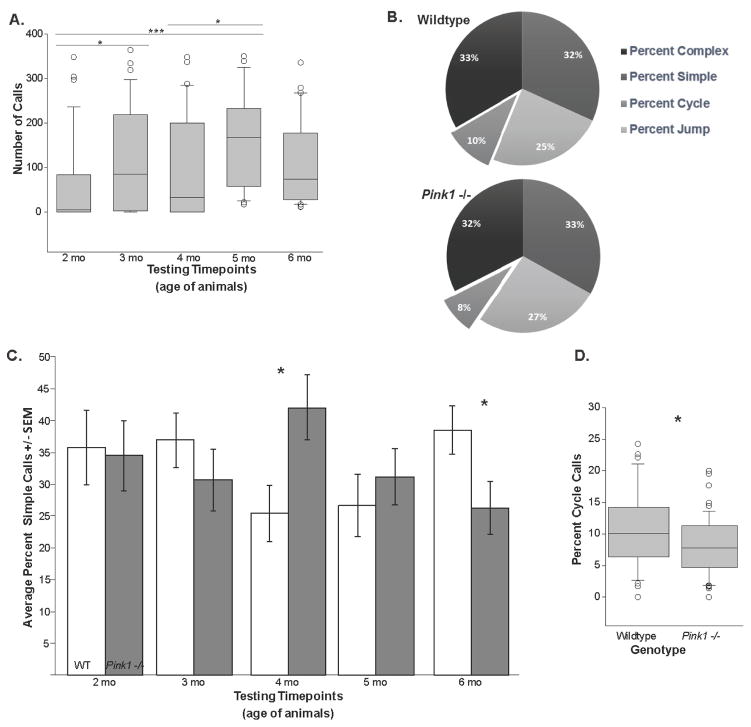
Figure 2. Call rate and composition.
(A) The number of calls produced during the data collection timepoints (corresponding to age of the animals; 2–6 months; main effect of age). (B) The distribution (%) of each call type for wild type (WT) and Pink1 −/− mice. Displaced segment corresponds to significant difference between genotypes. (C) The average (+/− SEM) percent simple calls at each testing timepoint. White bar is WT, gray bar is Pink1 −/−. Asterisks represents statistical significance between genotypes (* p<0.05; ** p<0.01, ***p<0.001). (D) The percentage of the call profile that were cycle calls. Data reflects significant difference between WT and Pink1 −/− mice, collapsed across timepoints (main effect of time). For box plot, the boundary of the box closest to zero indicates the 25th percentile. The line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. Outlying points are indicated by open circles.
2.1.2 Call composition
Mouse USVs were divided into their respective call categories (Figure 1 and 2B). For the percent simple calls, there was a significant interaction between genotype and time (F(4,110)=2.999, p=0.022; Figure 2C). Pink1−/− mice produced more simple calls compared to the WT controls at 4 mo (p=0.016). This was reversed at the 6 mo timepoint, where the WT controls produced a larger percentage of simple calls (p=0.034) (Figure 2C).
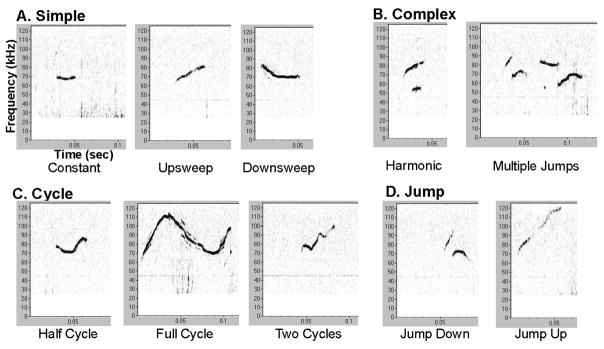
Figure 1. Mouse ultrasonic vocalizations.
Overview of the 4 call categories and 10 within-call types: (A) Simple (constant, upsweep, downsweep); (B) Complex (harmonic, multiple); (C) Cycle (half, full, two); (D) Jump (down, up). Vocalization spectrograms are representative of a 6-month wild type (WT) mouse. Spectrogram X-axis is time (seconds (sec)); Y-axis is frequency (kilohertz (kHz)). A high pass filter was used to eliminate noise below 25kHz. Relative intensity (“loudness”) is measured in decibels (dB) and encoded by darkness of the signal; louder is darker.
For the remaining call categories (jump, cycle, and complex); there were no significant age × genotype interactions (F(4,110)=0.532, p=0.712; F(4,110)=0.856, p=0.493; F(4,111)=1.621, p=0.175). Additionally, for percent jump calls there was no main effect of genotype (F(1,110)=0.558, p=0.457) or main effect of time (F(4,110)=0.292, p=0.882). For the percent cycle calls, there was a main effect of genotype (F(1,110)=6.137, p=0.015); Pink1−/− mice produced a larger percentage of cycle calls relative to the WT controls (Figure 2D). However, there was no main effect of time (F(4,110)=1.791, p=0.136). For the percent complex calls, there was no main effect of time (F(4,111)=1.471, p=0.216) or genotype (F(1, 102)=0.0056, p=0.941).
2.1.3 Duration of calls
There was no significant interaction between genotype and time for simple call duration (F(4,109)=0.644, p=0.632), jump call duration (F(4,110)=1.205, p=0.313), cycle call duration (F(4,106)=0.275, p=0.894), and complex call duration (F(4,106)=0.478, p=0.752). Additionally, there was not a main effect of genotype or main effect of time for simple call duration (F(1,109)=0.051, p=0.822; F(4,109)=1.479, p=0.214) and jump call duration (F(1,110)=0.930, p=0.337; F(4,110)=2.279, p=0.066).
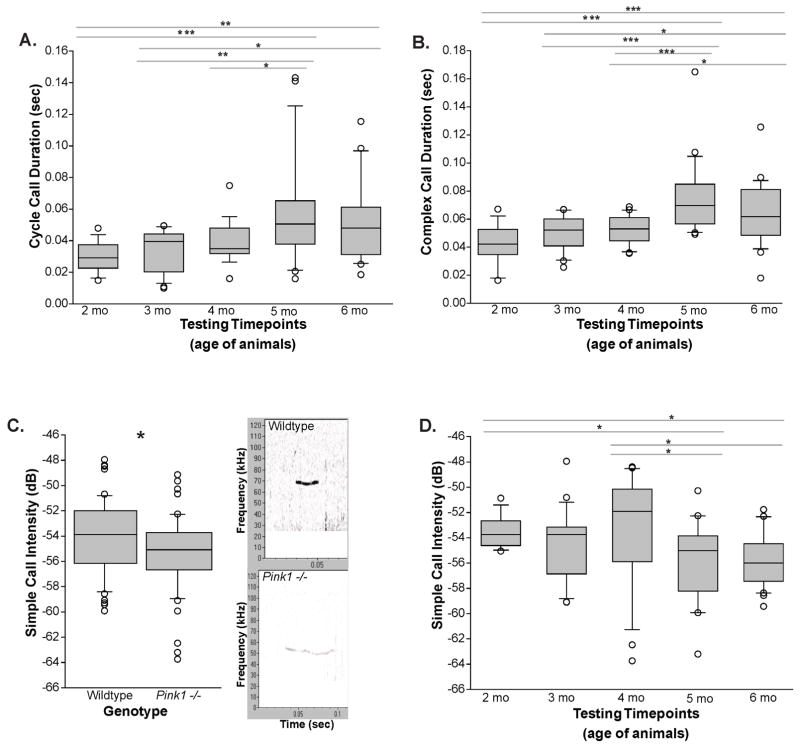
There was not a main effect of genotype for both cycle call duration and complex call duration (F(1,106)=1.598, p=0.209; F(1,106)=1.928, p=0.162), but there were significant differences among timepoints (F(4,106)=9.334, p<0.001, Figure 3A; F(4,106)=9.334, p<0.001, Figure 3B). Specifically, for cycle call duration, post-hoc comparison revealed significant increases between 2 and 5 mo (p<0.001), 2 and 6 mo (p=0.002), 3 and 5 mo (p=0.002), 3 and 6 mo (p=0.016), and 4 and 5 mo (p=0.017). Furthermore, for complex call duration, post-hoc comparisons showed significant differences between 2 and 5 mo (p<0.001), 2 and 6 mo (p<0.001), 3 and 5 mo (p<0.001), 3 and 6 mo (p=0.011), 4 and 5 mo (p<0.001), and 4 and 6 mo (p=0.023).
Figure 3. Duration and intensity.
(A) The cycle call duration (sec) and (B) complex call duration (sec) at each data collection timepoint (corresponding to age of the animals; 2–6 months; main effect of time). (C) The simple call intensity (dB) between genotypes with representative call spectrogram (frequency (kHz) on the Y-axis, time (sec) on the X-axis) for wild type (WT) and Pink1 −/− mice at 6 months of age (main effect of genotype). (D) The simple call intensity (dB) across data collection timepoints for all animals (main effect of time). The boundary of the box closest to zero indicates the 25th percentile. The line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. Outlying points are indicated by open circles. Asterisks represents statistical significance between genotypes (* p<0.05; ** p<0.01, ***p<0.001). Bars indicate significance between timepoints with asterisks showing levels of significance (* p<0.05; ** p<0.01, ***p<0.001).
2.1.4 Bandwidth of calls
There was no significant interaction between genotype and time for simple call bandwidth (F(4,109)=0.355, p=0.840), jump call bandwidth (F(4,110)=1.421, p=0.232), cycle call bandwidth (F(4,106)=1.001, p=0.411), and complex call bandwidth (F(4,106)=0.182, p=0.947). Additionally, there was no main effect of genotype for simple call bandwidth (F(1,109)=0.352, p=0.554), jump call bandwidth (F(1,110)=0.436, p=0.511), cycle call bandwidth (F(1,106)=2.910, p=0.091), and complex call bandwidth (F(1,106)=0.421, p=0.518). There were not main effects of time for simple call bandwidth (F(4,109)=0.569, p=0.686), jump call bandwidth (F(4,110)=0.213, p=0.931), cycle call bandwidth (F(4,106)=1.487, p=0.212), and complex call bandwidth (F(4,106)=1.991, p=0.102).
2.1.5 Peak frequency of calls
There was no significant interaction between genotype and time for simple call peak frequency (F(4,109)=1.192, p=0.319), jump call peak frequency (F(4,110)=1.832, p=0.128), cycle call peak frequency (F(4,106)=0.314, p=0.868), and complex call peak frequency (F(4,106)=0.151, p=0.962). Additionally, there was no main effect of genotype for simple call peak frequency (F(1,109)=1.904, p=0.171), jump call peak frequency (F(1,110)=0.785, p=0.378), cycle call peak frequency (F(1,106)=0.518, p=0.473), and complex call peak frequency (F(1,106)=1.577, p=0.212). There was no main effect of time for simple call peak frequency (F(4,109)=0.195, p=0.941), jump call peak frequency (F(4,110)=1.740, p=0.147), cycle call peak frequency (F(4,106)=2.092, p=0.088), and complex call peak frequency (F(4,106)=0.832, p=0.508).
2.1.6 Intensity of calls
There was no significant interaction between genotype and time for simple call intensity (F(4,109)=1.330, p=0.264), jump call intensity (F(4,110)=0.808, p=0.523), cycle call intensity (F(4,106)=0.948, p=0.440), and complex call intensity (F(4,106)=1.212, p=0.311). Additionally, there was no main effect of genotype on jump call intensity (F(1,110)=1.677, p=0.198), cycle call intensity (F(1,106)=0.494, p=0.484), and complex call intensity (F(1,106)=0.055, p=0.816). There was no main effect of time for jump call intensity (F(4,110)=1.115, p=0.354), cycle call intensity (F(4,106)=0.363, p=0.835), and complex call intensity (F(4,106)=2.286, p=0.066).
However, simple call intensity did demonstrate a main effect of genotype (F(1,109)=4.649, p=0.033). Notably, Pink1−/− mice produced calls with a lower intensity (quieter) compared to the WT controls (Figure 3C). Also, there was a main effect of time regardless of genotype (F(4,109)=3.323, p=0.013; Figure 3D). Specifically, post-hoc comparisons revealed decreases between 2 and 5 mo (p=0.028), 2 and 6 mo (p=0.013), 4 and 5 mo (p=0.014), and 4 and 6 mo (p=0.005).
2.2 Cylinder Test
Means and standard errors of the means are listed in Table 3.
Table 3.
Average motor behavioral variables (standard error of the mean).
| Motor Behavioral Variable | Testing Timepoint (age of animals) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2mo | 3mo | 4mo | 5mo | 6mo | ||||||
| WT | Pink1 −/− | WT | Pink1 −/− | WT | Pink1 −/− | WT | Pink1 −/− | WT | Pink1 −/− | |
| Hindlimb Steps | n/a | n/a | 106.84 (5.15) | 91.06 (5.29) | 85.72 (5.30) | 75.83 (5.29) | 80.88 (5.45) | 55.56 (5.29) | 59.94 (5.29) | 52.39 (5.29) |
| Forelimb Steps | n/a | n/a | 128.11 (5.58) | 102.83 (5.73) | 99.06 (5.73) | 87.67 (5.73) | 88.59 (5.90) | 69.28 (5.73) | 69.44 (5.73) | 60.17 (5.73) |
| Rears + Lands | n/a | n/a | 57.74 (3.10) | 53.94 (3.18) | 40.89 (3.18) | 37.83 (3.18) | 43.53 (3.27) | 34.94 (3.18) | 31.11 (3.18) | 23.44 (3.18) |
| Average Turn Time | 3.30 (0.60) | 3.83 (0.60) | 3.25 (0.47) | 3.10 (0.60) | 2.24 (0.47) | 2.89 (0.48) | 2.74 (0.47) | 2.51 (0.47) | 2.60 (0.47) | 3.31 (0.47) |
| Average Traverse Time | 6.26 (4.23) | 7.82 (4.23) | 7.51 (3.30) | 6.87 (3.40) | 5.66 (3.3) | 7.32 (3.30) | 6.77 (3.30) | 6.31 (3.30) | 25.81 (3.30) | 21.85 (3.3) |
2.2.1 Hindlimb steps
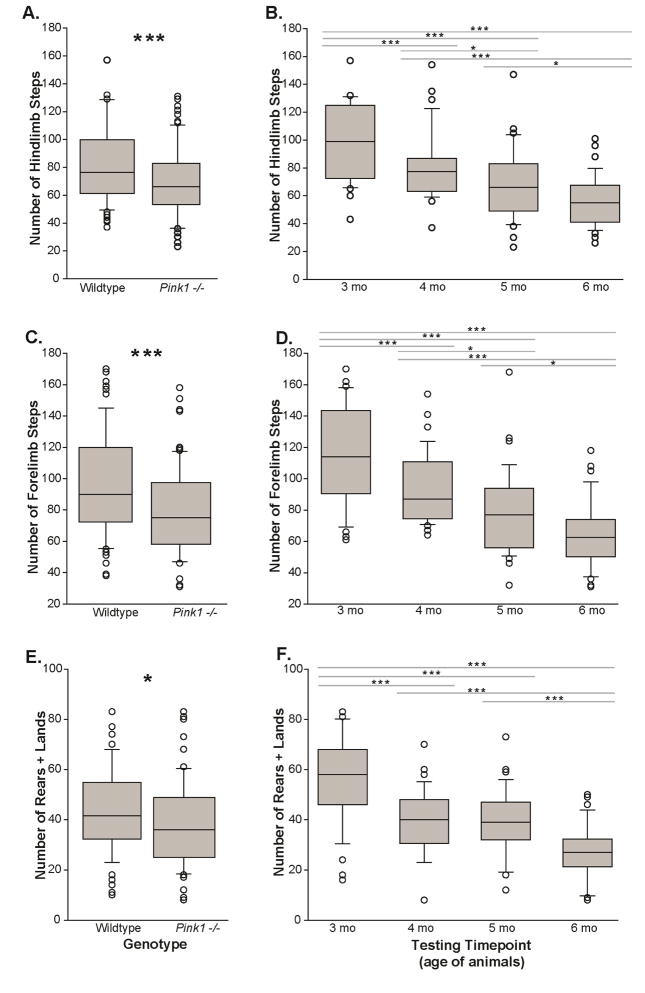
There was no significant interaction between genotype and time (F(3,143)=1.101, p=0.351). There was a main effect of genotype with Pink1−/− mice having fewer steps than WT controls. (F(1,143)=15.29, p<0.001, Figure 4A). Additionally, there was a main effect of time for all mice (F(3,143)=24.17, p<0.001, Figure 4B). Specifically, post-hoc comparisons revealed decreases between 3 and 4 mo (p<0.001), 3 and 5 mo (p<0.001), 3 and 6 mo (p<0.001) as well as 4 and 5 mo (p=0.020), 4 and 6 mo (p<0.001), and 5 and 6 mo (p=0.025). All mice, regardless of genotype, showed a decrease in the number of hindlimb steps with age and habituation; this has been previously reported in mice (Fleming et al., 2013) as well as the rat (Grant et al., 2015a; Kelm-Nelson et al., 2015).
Figure 4. Cylinder locomotor activity test.
The number of hindlimb steps in the cylinder (A) between genotypes and (B) across data collection timepoints (corresponding to age of the animals; 2–6 months; main effect of time). The number of forelimb steps (C) between genotypes and (D) across timepoints. The number of rears + lands; main effect between (E) genotypes and main effect (F) across timepoints. X-axis is testing the genotype or timepoint/animal age (mo); Y-axis is number of steps. The boundary of the box closest to zero indicates the 25th percentile. The line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. Outlying points are indicated by open circles. Asterisks represents statistical significance between genotypes (* p<0.05; ** p<0.01, ***p<0.001). Bars indicate significance between timepoints with asterisks showing levels of significance (* p<0.05; ** p<0.01, ***p<0.001).
2.2.2 Forelimb steps
There was no significant interaction between genotype and time (F(3,143)=0.837, p=0.476). There was a main effect of genotype with Pink1−/− mice having fewer forelimb steps than WT controls (F(1,143)=16.19, p<0.001, Figure 4C). For all mice, there was a main effect of time (F(3,143)=28.85, p<0.001, Figure 4D). Specifically, post-hoc comparisons revealed decreases between 3 and 4 mo (p<0.001), 3 and 5 mo (p<0.001), 3 and 6 mo (p<0.001) as well as 4 and 5 mo (p=0.014), 4 and 6 mo (p<0.001), and 5 and 6 mo (p=0.016). In general, all mice showed a decrease in the number of total forelimb steps over time.
2.2.3 Rears + lands
There was no significant interaction between genotype and time (F(3,143)=0.373, p=0.772). There was a main effect of genotype (F(1,143)=6.584, p=0.011, Figure 4E); Pink1 −/− mice had fewer rears + lands than WT controls. There was a main effect of time (F(3,143)=27.62, p<0.001, Figure 4F). Specifically, post-hoc comparisons revealed decreases between 3 and 4 mo (p<0.001), 3 and 5 mo (p<0.001), 3 and 6 mo (p<0.001), 4 and 6 mo (p<0.001), and 5 and 6 mo (p<0.001). In general, all mice showed a decrease in the number of rears + lands over time.
2.3 Pole Test
The performance of animals in pole test can be influenced by the animal body weight. Thus, we collected body weight at 5 mo of age and found that it was significantly different between genotypes, as stated above. Nevertheless, there were no significant relationships between body weight and pole test behavioral variables measured at this timepoint (p>0.05). Means and standard errors of the means are listed in Table 3.
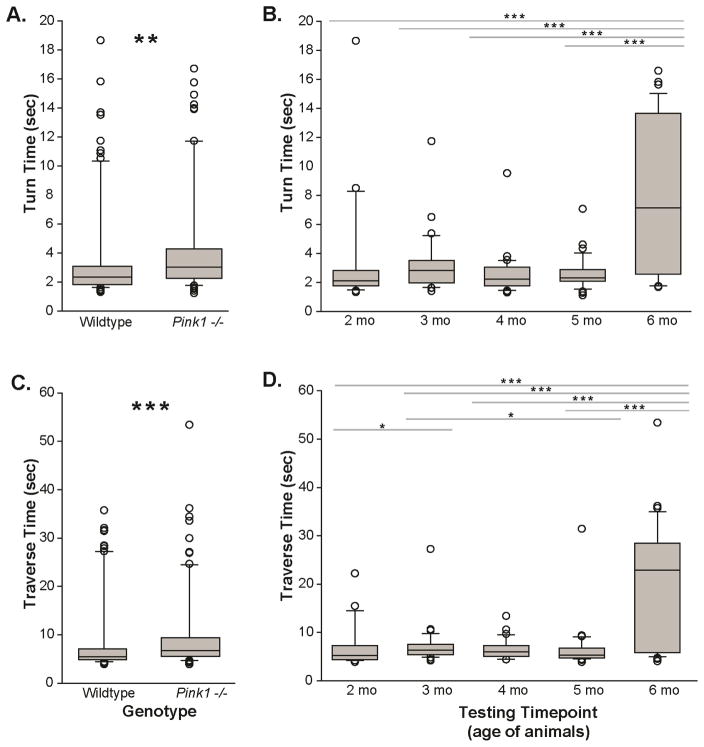
2.3.1 Turn time
There was no significant interaction between genotype and time (F(4,163)=1.443, p=0.223). There was a main effect of genotype (F(1,163)=8.378, p=0.004, Figure 5A); Pink1−/− mice took longer to complete the task than WT controls. There was also a main effect of time (F(4,163)=8.782, p<0.001, Figure 5B). Specifically, post-hoc comparisons revealed differences between 2 and 6 mo (p<0.001), 3 and 6 mo (p=0.001), 4 and 6 mo (p<0.001), and 5 and 6 mo (p<0.001). Mice took longer to complete the task at the 6 months timepoint compared to all other timepoints.
Figure 5. Pole locomotion test.
The time (sec) taken to turn at the top of the pole for (A) genotypes main effect (wild type (WT) vs. Pink1 −/−) and (B) main effect of time (corresponding to age of the animals; 2–6 months). The time (sec) required to traverse the pole into respective mouse homecage for (C) main effect of genotype and (D) main effect of timepoint. X-axis is testing the genotype or timepoint/animal age (mo); Y-axis is time (sec). The boundary of the box closest to zero indicates the 25th percentile. The line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. Outlying points are indicated by open circles. Asterisks represents statistical significance between genotypes (* p<0.05; ** p<0.01, ***p<0.001). Bars indicate significance between timepoints with asterisks showing levels of significance (* p<0.05; ** p<0.01, ***p<0.001).
2.3.2 Traverse time
There was no significant interaction between genotype and time (F(4,164)=1.150, p=0.335). There was a main effect of genotype (F(1,164)=13.72, p<0.001, Figure 5C); Pink1−/− mice took longer to complete the task than WT controls. There was also a main effect of time (F(4,164)=9.928, p<0.001, Figure 5D). Specifically, post-hoc comparisons revealed differences between 2 and 3 mo (p=0.043), 2 and 6 mo (p<0.001), 3 and 5 mo (p=0.030), 3 and 6 mo (p=0.001), 4 and 6 mo (p<0.001), and 5 and 6 mo (p<0.001). Generally, mice took longer to complete the traversal of the pole at 6 months compared to all other timepoints.
2.4 Immunohistochemistry analysis
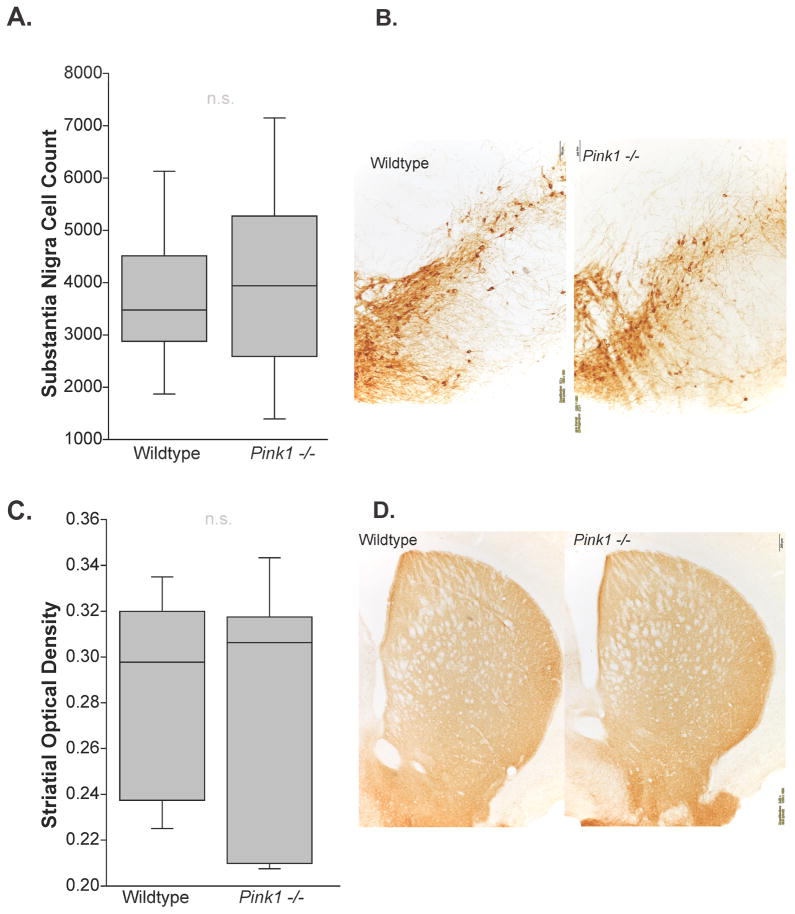
For immunohistochemical analysis at 6 mo, there were no significant differences between genotype (WT mean=3696.27 (SEM=415.072); Pink1 −/− mean=3969.01 (SEM=590.208); Table 4) and the number of TH positive cells in the substantia nigra (t(16)=0.38, p=0.36) (Figure 6A, B). Additionally, there were no differences between genotypes (WT mean=0.286 (SEM =0.0152); Pink1 −/− mean=0.275 (SEM=0.0182)) in TH optical density in the striatum (t(15)=0.44, p=0.33) (Figure 6C, D).
Table 4.
Average TH immunohistochemistry measures (standard error of the mean) at 8 months of age.
| Genotype | Substantia Nigra Cell Count | Striatum Optical Density |
|---|---|---|
| WT | 3696.27 (415.072) | 0.286 (0.015) |
| Pink1−/− | 3969.01 (590.21) | 0.275 (0.018) |
Figure 6. Tyrosine hydroxylase immunohistochemistry.
At 8 months of age, (A) stereological cell counts in the substantia nigra of wild type (WT) vs Pink1 −/− mice. The boundary of the box closest to zero indicates the 25th percentile. The line within the box marks the median and the boundary of the box farthest from zero indicates the 75th percentile. Whiskers (error bars) above and below the box indicate the 90th and 10th percentiles. (B) Representative photomicrographs taken at 10x magnification (833.3 us exposure time with white balance) of a WT and a Pink1 −/− mouse. Substantia nigra quantification where the 3rd cranial nerve is visible; approximate coordinates are Bregma −3.51 millimeter (mm), Interaural 0.28 mm. Scale bar is 100 μm. (C) Optical density in the striatum of WT and Pink1 −/− mice. (D) Representative photomicrographs for each genotype at 4x magnification (555.5 us exposure time with white balance); approximate coordinates are Bregma 0.61 mm, Interaural 4.4 mm. Scale bar is 200 μm. “n.s” is non-significant.
3. Discussion
To gain insight into the early pathogenesis of PD this study evaluated sensorimotor function including ultrasonic vocalizations, motor activity, and limb motor control, in the Pink1 −/− mouse model of PD compared to age-matched controls. We hypothesized that over time Pink1 −/− mice would have progressive declines in vocal sensorimotor function, the measurable acoustic properties of their calls. These data show Pink1 −/− mice produced significantly more simple calls with reduced intensity as well as a larger percentage of cycle calls compared to WT counterparts. However, there were no significant differences in duration, bandwidth, or peak frequency for any of the ultrasonic call types between genotypes, meaning the deficits were modest. We also hypothesized that Pink1 −/− mice would have progressive declines in limb motor performance. Here, we show that Pink1 −/− mice have reduced hindlimb steps and rearing and landing in the cylinder test (overall activity) as well as significant increases in the time to turn and traverse during the pole test (locomotor skill) compared to control animals. Interestingly, these motor deficits occur in the absence of nigra cell loss or reductions in TH immunolabeling in the striatum suggesting that other mechanisms may be responsible for the observable declines in motor performance or that motor dysfunction may precede dopaminergic neurodegeneration. In general, the Pink1 −/− model may be a useful model in studying the onset of sensorimotor deficits, limb deficits, and nervous system pathology.
This study represents the first behavioral characterization of the B6.129S4-Pink1Tm1SHN/J Pink1 −/− mouse model of PD. Ultrasonic vocalizations are analogous to human vocalizations as the source is the larynx, they are produced on an egressive airflow, and they are communicative by nature (Arriaga et al., 2012; Brudzynski, 2013; Costantini and D’Amato F, 2006; Riede, 2011; Riede, 2013). The deficits in the Pink1 −/− mouse model are not as robust as previously reported models, but do show some similar trends. For example, within the Pink1 −/− rat model, the 6-OHDA rat model, and the alpha-synuclein overexpression (Thy1-aSyn) mouse model of PD, vocalization intensity is significantly reduced (Ciucci et al., 2009; Grant et al., 2014; Grant et al., 2015b). Here, we show that, with age, both the Pink1 −/− and the WT mice have a reduction in simple call intensity (“loudness”); however, the Pink1−/− model vocalization is significantly reduced compared to WT. These male vocalizations are generated in response to conspecific female interactions (Whitney and John, 1979). Thus, a decrease in call intensity may be a result of decreased sexual motivation, although other sexual behaviors (mounting, sniffing) did not appear to be decreased in this study (data not shown). Previous reports suggest anosmia in Pink1 −/− mice (Glasl et al., 2012), this important PD biomarker, in addition to characterizing motivation with specific assays, should be evaluated over time in this model.
Changes in the call repertoire (profile) over time could also represent the effect of experience, or a shift from anticipation to consumption of sexual reward/behavior (Berridge, 2006). Overtime, all mice demonstrate increases in the total number of calls produced, the proportion of cycle calls, and complex call duration, but otherwise show no other differences. The call profile in the Pink1 −/− mouse is skewed, with Pink1 −/− mice showing an increase in simple calls at 4 months of age and then declines in simple calls at 6 months of age. While testing parameters remained similar over timepoints, we cannot rule out the effect of the experimenter or environmental conditions (i.e. female stimulus animals, stress). Moreover, in general, Pink1 −/− mice produce an increase in cycle calls compared to WT. The significance of the call repertoire in this strain of mouse is not known, however it is hypothesized that calls are used in specific social situations; future studies should evaluate pathology influence on specific call types.
Similar to the 6-OHDA-rat neurotoxin model and the Thy1-aSyn mouse model, call profile and intensity appear to be most vulnerable to manipulation (Ciucci et al., 2007; Ciucci et al., 2009; Grant et al., 2014). While we do not report nigrostriatal cell loss as a result of comparing stereological cell counts in the nigra and optical density in the striatum, previous studies do report a decrease in dopamine release and synaptic plasticity in the striatum (Kitada et al., 2007). Thus, disruption of dopaminergic synaptic transmission may have consequences on cranial sensorimotor performance without altering the number of dopaminergic neurons. Moreover, Gispert et al. demonstrated aging Pink1−/− mice show increased mitochondrial dysfunction, resulting in impaired neuronal activity, in the absence of overt neuronal death (Gispert et al., 2009). Consistent with these findings, there is a critical role for Pink1 in dopamine release, and this pathology may precede nigrostriatal depletion (Kitada et al., 2007). PD is not solely characterized based on basal ganglia dopamine depletion; multiple other pathologies in the periphery and within the central nervous system exist. In fact, animal models of alpha-synuclein aggregation, such as the Thy1-aSyn mouse, do not display nigral dopamine death, but do show motor deficits including vocal motor (Fernagut et al., 2007; Fleming et al., 2004; Grant et al., 2014) and are still considered useful models for specific research questions.
Pink1 −/− mice show significant decreases in hindlimb, forelimb, and overall motor activity in the cylinder test, suggesting that this is a translational model for the onset of limb deficits in PD. Furthermore, Pink1 −/− mice take significantly longer to turn and traverse the pole. In another knockout model and different background strain, after 1 year of age Pink1 −/− mice showed reductions in body weight and significant reduction of locomotor activity at 16 months of age (the background strain is known for low motor activity) (Gispert et al., 2009). Kitada et al (2007) also reports no significant difference in the number of dopaminergic neurons or levels of striatal dopamine and receptors. However, they do report decreases in striatal dopamine release and propose that altered dopamine physiology may be a precursor to nigral degeneration, which may be responsible for the motor deficits reported here. Consistent with our previous studies, in general, all mice show a reduction in activity over time, usually corresponding with increases in body weight (Grant et al., 2015b; Kelm-Nelson et al., 2015). Specifically, the pole test times to turn and traverse are sensitive to changes in weight which likely contributes to the increased times at 6 mo of age in both WT and Pink1 −/− mice. We did not co-vary for weight in this study because we did not find that the motor behavioral variables correlated to body weight.
In addition to impairing dopamine release, deletion of the Pink1 gene has significant implications on mitochondrial quality and function (Deng et al., 2008; Gómez-Sánchez et al., 2014; Kawajiri et al., 2011; Poole et al., 2008). Experiments suggest that loss of Pink1 accelerates neurodegenerative phenotypes by impairing mitochondrial function and removal of misfolded proteins by autophagy (Gandhi et al., 2012). Manczak et al. reported numerous and complex changes in mitochondrial function and structure with aging (Manczak et al., 2005); thus, it is possible that the variability within the Pink1−/− group is a result of dissimilar phenotypic expressions at the mitochondrial level of the same initial genotype. Additionally, a study that followed mitochondrial activity and structure after knocking out the Pink1 gene in mice found a rapid compensation within five days. It is possible the Pink1−/− mice have effective compensatory mechanisms for the genetic disruption of mitochondrial activity, thereby explaining the mild-moderate (versus severe) deficits and high variability between genotypes and over time. Future studies should then evaluate whether these deficits can be rescued with pharmacological dopamine replacements, such as levodopa, or exercise. Additionally, evaluation of non-dopaminergic pathology including alpha synuclein aggregation and peripheral neuro-muscular pathology should be quantified in this model.
In general, characterization of rodent models of PD are key to elucidating disease mechanisms as well as developing effective interventions for parkinsonian deficits that will ultimately lead to optimized patient treatments. Animal models of PD more fully represent the widespread and progressive neurochemical and behavioral abnormalities seen in human idiopathic PD. Overall, our findings demonstrate the Pink1−/− mouse to be a promising model for aspects of sensorimotor behavior related to non-dopaminergic PD pathology. Future research will need to address behavioral variability among time points for more subtle deficits such as vocalization.
4. Experimental procedure
4.1 Animals and habituation
Animals (Pink1 −/− n=18, Strain: B6.129S4-Pink1Tm1SHN/J and WT n=18, Background Strain: C57BL/6J) (Kitada et al., 2007) were obtained from Jackson® Laboratories (Pink1 −/−, stock #017946) at 6 weeks of age and housed as single-sex social groups (n=2 or 3 per group) in standard polycarbonate cages (17 centimeter [cm] × 28 cm × 12 cm) on a 12:12 hour (hr) reverse light cycle. Testing occurred during the dark period in red light. All mice were handled and habituated to testing procedures (room, experimenters, and testing apparatus). General habituation occurred daily for 2 weeks prior to the first test timepoint. Additional habituation to testing procedures occurred daily for 3 consecutive days prior to each test timepoint. Female mice were used to elicit vocalization behavior from the males but were not included in any of the analyses. Body weights were measured at 5 months of age. All mice were euthanized at 6 months (mo) of age (see below). Procedures were approved by the University of Wisconsin-Madison Animal Care and Use Committee (IACUC-Protocol M02646) and were conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MA) (2011).
4.2 Behavioral tests
4.2.1 Ultrasonic vocalization
Ultrasonic vocalizations were recorded for all mice at 2, 3, 4, 5, and 6 mo of age. To elicit ultrasonic vocalizations, each male mouse was individually placed in his home cage and introduced to a sexually receptive conspecific WT female. The female was removed as soon as the male showed typical socio-sexual behaviors (e.g. sniffing, chasing). An ultrasonic microphone (CM16, Avisoft, Germany) with a 16-bit depth and a sampling rate of 250-kilohertz (kHz), flat frequency response of up to 150-kHz, and a working frequency response range of 10–180-kHz was attached to a panel 20 cm above the male’s home cage. The male-only vocalization recording started immediately after the female was removed from the testing cage and continued for 60 seconds (sec).
Offline acoustic analysis was performed with a customized automated program SASLab Pro (Avisoft, Germany). The WAV files were analyzed with Avisoft-generated spectrograms using a Fast Fourier Transform (FFT) of 512 points, frame size of 100%, flat top window, and temporal resolution set to display 75% overlap. A high pass filter was used to eliminate noise below 25 kHz. Calls were slowed down in order for an experienced rater, masked to experimental conditions, to categorize each call.
There are 12 call types, which fall into 4 general call categories (Figure 1A–D). These categories were defined in terms of complexity: simple, jump, cycle, and complex, similar to (Grant et al., 2014; Portfors, 2007). The following variables were measured within each call type and averaged for each animal: bandwidth (hertz, Hz), peak frequency (kHz), intensity (decibels, dB), and duration (milliseconds, ms).
4.2.2 Cylinder test
All mice underwent this test at 3, 4, 5, and 6 mo of age. Spontaneous motor activity was measured for 120 sec in a transparent cylinder similar to Fleming et al. (2004) (31 cm height × 19 cm diameter). Recordings (HDR-CS210; Sony, New York, NY, 100 frames/sec) were viewed in slow motion by a rater, masked to genotype, using Windows Media Player (Microsoft Windows). The following dependent variables were measured: total number of forelimb steps, total number of hindlimb steps, total number of rears, and total number of lands.
4.2.3 Pole test
All mice were tested at 2, 3, 4, 5, and 6 mo of age and recorded at 100 frames/sec (HDR-CX210; Sony, New York, NY). Each mouse was placed at the top of a vertical pole (10 millimeter [mm] diameter, 480 mm length), head positioned upwards, and then allowed to turn and climb down the pole to enter the homecage (adapted from (Matsuura et al., 1997; Ogawa et al., 1985)). Each mouse performed 5 trials and the following variables were averaged across all trials: time to orient towards the bottom of the pole (sec) and time to traverse the pole from the top to the homecage (sec). Video recordings were analyzed with Adobe Photoshop CC (Adobe Systems Inc., San Jose, CA) by a rater masked to genotype.
4.3 Tissue extraction and immunohistochemistry
Twenty-four hours after testing at 6 mo of age, all mice were euthanized. Briefly, mice were deeply anesthetized with 5% isoflurane, transcardially perfused with 200 milliliters (ml) of cold saline followed by 500 ml of cold 4% paraformaldehyde in 1% phosphate buffered saline (PBS). Fixed brains were excised, post-fixed for 24 hr in 4% paraformaldehyde at 4 °C, cryoprotected in 0.02% sodium azide in 0.1M PBS solution, and flash frozen in isopentane. Brains were mounted on a freezing microtome and 40 micron (μm) coronal sections were harvested throughout the cortex and brainstem. Free-floating sections were stored in cryoprotectant at −20 °C until they were stained for immunoreactivity over every 5th section. Anatomically equivalent sections were used from each animal.
Tissue sections were stained for tyrosine hydroxylase (TH) immunoreactivity in a single batch to minimize differences in batch variability. Antibody specificity for TH was verified by omitting the primary step. Briefly, tissue sections were rinsed in 0.01M phosphate buffered saline (PBS, pH=7.4) with 0.3% Triton X-100 (PBS-T; Tx-100 #21568-2500, Acros®, NJ) for 34 minutes (min), incubated in 0.5% H202 for 10 min, rinsed for 34 min, blocked in 20% normal goat serum (NGS, Equitech-Bio Inc, Kerrville, TX) made in PBS-T with 2% Bovine Serum Albumin (BSA, Fisher Scientific #BP1600-100) solution for 1 hour (hr), and then agitated and incubated overnight (24 hr) in 1% NGS (made in PBS-T with 2% BSA) primary solution at 4° C (polyclonal rabbit anti-TH at 1:2000 (AB152, Millipore, Billerica, MA)). Sections were then rinsed in PBS-T for 34 min and incubated in conjugated biotinylated secondary solution for 3 hrs at room temperature (goat anti-rabbit IgG at 1:500; Millipore LV1602347). Sections were then rinsed in PBS-T for 34 min, incubated in avidin-biotin solution (Vectastain Elite ABC, Vector Laboratories) for 1 hr, rinsed in 0.1M PBS for 30 min, and the avidin–biotin complex was labeled using filtered 3,3-diaminobenzidine (DAB Easy Tablets, 10mg/tablet; 0.04%, Acros®, New Jersey) with 0.01% hydrogen peroxide solution. Tissue was rinsed for 25 min in 0.01M PBS, float mounted onto gelatin-coated slides, dried overnight, dehydrated in a graded series of alcohols and xylenes, and cover slipped (Fisherbrand® Microscope Cover Glass, 12-545-M, 24X60-1) using Cytoseal® 60 (Thermo Scientific Richard-Allan Scientific, 23-244256) mounting medium.
4.4 Stereology and quantification
Cell bodies positively stained for TH were counted using Stereo Investigator® (MBF Bioscience, Williston, VT) and the Optical Fractionator Probe, while working on an Olympus BX53 microscope fitted with a QImaging Retiga 1300c monochrome camera, a Prior XYZ Proscan III motorized stage kit, and a plasma screen monitor. This methodological combination allowed for unbiased nigrostriatal cell count estimation in every 5th tissue section throughout the substantia nigra pars compacta (SNpc) of each mouse brain (Bregma −2.69 to −4.03 mm). Modified from stereological methods described previously (West et al., 1991), cell body counts for each of the 3–5 brain slices per animal (Pink1 −/− n=9, WT n=9) consisted of: using a 4x magnification lens to outline the SNpc based on a stereotaxic atlas of the mouse brain (Paxinos and Franklin, 2001), randomly sampling the outlined regions of the 19–22 μm-thick slice using a counting grid of optical dissectors (each being 100 μm × 100 μm) at 40x magnification, counting cell bodies if the top of the leading edge came into focus within the inclusion lines of the dissector (nucleoli weren’t visible at this level of magnification), exempting the 2 μm guard zones above and below the tissue from estimation, and finally, sampling 100% of the total outlined area per tissue section due to the low number of sections available per mouse. The cell counts were then averaged for individual data and then combined to create an average for each genotype.
Optical density measurements of TH-positive neurons in the striatum were taken every 5th tissue section (Bregma 1.33 to −0.11 mm) using Olympus cellSens Dimension software (version 1.15) with a Dual CCD DP80 color/monochrome camera (Olympus), and the freely available open-source imaging and analysis software, ImageJ (US National Institutes of Health, Bethesda, MD). Between 3 and 5 slices per animal (Pink1 −/− n=9, WT n=8) were imaged at 10x magnification in cellSens Dimension to ensure similar focus between hemispheres, if the focus was markedly different they were imaged separately. Following this, ImageJ was used to trace around the striatum in individual hemispheres to omit irrelevant data. The pixels in the images were calibrated on a provided gray scale (ImageJ), the images were converted from RGB color to 8-bit, and optical density was measured as a level of mean gray value. The optical density measures were then averaged for individual data and then combined to create an average for each genotype.
4.5 Statistics
All statistical analyses were performed in SigmaPlot ® 12.5 System (Systat Software, Inc., San Jose, CA). Variables were rank transformed if data failed to conform to assumptions for Analysis of Variance (ANOVA) as determined by statistical models for normality (Shapiro-Wilk test) and equal variance (Levene’s test). For behavior data, a mixed-model repeated measures 2×5 ANOVA for the independent variables (genotype [2 levels: WT and Pink1−/−] and time points [5 levels: 2, 3, 4, 5, and 6 mo]) was used with Fisher’s Least Significance Difference test for post-hoc analyses. Due to the exploratory nature of the experiments and the associated risks of statistical type II error, no corrections were made for multiple comparisons. For tissue analyses, independent Students’ t-tests were used to compare optical density and stereological cell counts between genotypes. The critical level for significance was set at 0.05 for all testing.
For reliability purposes, 10% of the behavioral files were randomly sampled and re-analyzed. Inter- and intra-rater reliability was calculated using an intra-class correlation coefficient (ICC); a correlation coefficient of 0.9 or greater was considered reliable.
Research Highlights.
Pink1 −/− mice exhibit variations in simple call production and intensity
Pink1 −/− mice demonstrate reduced gross motor activity
Pink1 −/− mice show a slower locomotor activity time
Pink1 −/− mice do not have nigrostriatal dopamine loss at 6 months of age
Acknowledgments
Funding: F32 D014399 (Kelm-Nelson), R01 DC014358 (Ciucci), UW Department of Surgery Pilot Funds (Kelm-Nelson), UW Department of Surgery (Ciucci); Hilldale Undergraduate Research Award (Brauer); JPB Foundation Support (Marongiu and Kaplitt).
We gratefully acknowledge John C. Szot, Martin Martinez, Thomas Houghland, Isabel Hart, Brooke Rogers, Kia Smurawa, Mroj Alassaf for their assistance with data aquisition and analysis.
Footnotes
Financial Disclosure/Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Guide for the Care and Use of Laboratory Animals. National Academy of Sciences; Washington DC: 2011. [Google Scholar]
- Albanese A, Valente EM, Romito LM, Bellacchio E, Elia AE, Dallapiccola B. The PINK1 phenotype can be indistinguishable from idiopathic Parkinson disease. Neurology. 2005;64:1958–1960. doi: 10.1212/01.WNL.0000163999.72864.FD. [DOI] [PubMed] [Google Scholar]
- Arriaga G, Zhou EP, Jarvis ED. Of Mice, Birds, and Men: The Mouse Ultrasonic Song System Has Some Features Similar to Humans and Song-Learning Birds. PLoS ONE. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baijens LW, Speyer R. Effects of therapy for dysphagia in Parkinson’s disease: systematic review. Dysphagia. 2009;24:91–102. doi: 10.1007/s00455-008-9180-1. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacol. 2006:191. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Dekker MCJ, Vanacore N, Fabbrini G, Squitieri F, Marconi R, Antonini A, Brustenghi P, Dalla Libera A, De Mari M, Stocchi F, Montagna P, Gallai V, Rizzu P, van Swieten JC, Oostra B, van Duijn CM, Meco G, Heutink P. Autosomal recessive early onset parkinsonism is linked to three loci: PARK2, PARK6, and PARK7. Neurological Sciences. 2002;23:s59–s60. doi: 10.1007/s100720200069. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rohé CF, Breedveld GJ, Fabrizio E, De Mari M, Tassorelli C, Tavella A, Marconi R, Nicholl DJ, Chien HF, Fincati E, Abbruzzese G, Marini P, De Gaetano A, Horstink MW, Maat-Kievit JA, Sampaio C, Antonini A, Stocchi F, Montagna P, Toni V, Guidi M, Libera AD, Tinazzi M, De Pandis F, Fabbrini G, Goldwurm S, de Klein A, Barbosa E, Lopiano L, Martignoni E, Lamberti P, Vanacore N, Meco G, Oostra BA Network, T.I.P.G. Early-onset parkinsonism associated with PINK1 mutations: Frequency, genotypes, and phenotypes. Neurology. 2005;65:87–95. doi: 10.1212/01.wnl.0000167546.39375.82. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Ethotransmission: communication of emotional states through ultrasonic vocalization in rats. Curr Opin Neurobiol. 2013;23:310–7. doi: 10.1016/j.conb.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: A preliminary study. Behavioural Brain Research. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: Degradation of 50-khz ultrasonic vocalization in rats. Behavioral Neuroscience. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Grant LM, Rajamanickam ESP, Hilby BL, Blue KV, Jones CA, Kelm-Nelson CA. Early Identification and Treatment of Communication and Swallowing Deficits in Parkinson Disease. Seminars in Speech and Language. 2013;34:185–202. doi: 10.1055/s-0033-1358367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F, D’Amato FR. Ultrasonic vocalizations in mice and rats: social contexts and functions. Acta zoologica Sinica. 2006;52:619–633. [Google Scholar]
- Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer RC, Iii, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, McCoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MAS, Fiske BK, Sherer TB, Frasier MA. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiology of Disease. 2014;70:190–203. doi: 10.1016/j.nbd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- de Lau LML, Giesbergen PCLM, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MMB. Incidence of parkinsonism and Parkinson disease in a general population: The Rotterdam Study. Neurology. 2004;63:1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–8. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and Histopathological Consequences of Paraquat Intoxication in Mice: Effects of α-Synuclein Over-Expression. Synapse (New York, NY) 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and Progressive Sensorimotor Anomalies in Mice Overexpressing Wild-Type Human α-Synuclein. J Neurosci. 2004;24:9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Ekhator OR, Ghisays V. Assessment of Sensorimotor Function in Mouse Models of Parkinson’s Disease. JOVE. 2013:e50303. doi: 10.3791/50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Vaarmann A, Yao Z, Duchen MR, Wood NW, Abramov AY. Dopamine Induced Neurodegeneration in a PINK1 Model of Parkinson’s Disease. PLOS ONE. 2012;7:e37564. doi: 10.1371/journal.pone.0037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken H-H, Becker D, Voos W, Leuner K, Müller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, García-Arencíbia M, Fernández-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert AS, Rüb U, Chen A, Nussbaum RL, Auburger G. Parkinson Phenotype in Aged PINK1-Deficient Mice Is Accompanied by Progressive Mitochondrial Dysfunction in Absence of Neurodegeneration. PLOS ONE. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasl L, Kloos K, Giesert F, Roethig A, Di Benedetto B, Kuhn R, Zhang J, Hafen U, Zerle J, Hofmann A, de Angelis MH, Winklhofer KF, Holter SM, Vogt Weisenhorn DM, Wurst W. Pink1-deficiency in mice impairs gait, olfaction and serotonergic innervation of the olfactory bulb. Exp Neurol. 2012;235:214–27. doi: 10.1016/j.expneurol.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Goberman AM, Blomgren M. Fundamental frequency change during offset and onset of voicing in individuals with Parkinson disease. Journal of Voice. 2008;22:178–91. doi: 10.1016/j.jvoice.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Gómez-Sánchez R, Gegg ME, Bravo-San Pedro JM, Niso-Santano M, Alvarez-Erviti L, Pizarro-Estrella E, Gutiérrez-Martín Y, Alvarez-Barrientos A, Fuentes JM, González-Polo RA, Schapira AHV. Mitochondrial impairment increases FL-PINK1 levels by calcium-dependent gene expression. Neurobiology of Disease. 2014;62:426–440. doi: 10.1016/j.nbd.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant LM, Richter FR, Miller JE, White SA, Fox CM, Zhu C, Chesselet M-F, Ciucci MR. Vocalization deficits in mice over-expressing alpha-synuclein, a model of pre-manifest Parkinson’s disease. Behavioral Neuroscience. 2014;128:110–121. doi: 10.1037/a0035965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant LM, Kelm-Nelson CA, Hilby BL, Blue KV, Paul Rajamanickam ES, Pultorak JD, Fleming SM, Ciucci MR. Evidence for early and progressive ultrasonic vocalization and oromotor deficits in a PINK1 gene knockout rat model of Parkinson’s disease. J Neurosci Res. 2015a doi: 10.1002/jnr.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant LM, Kelm-Nelson CK, Hilby BL, Blue KV, Rajamanickam ESP, Pultorak J, Fleming SM, Ciucci MR. Evidence for early and progressive ultrasonic vocalization and oromotor deficits in a PINK1 knockout rat model of Parkinson disease. Journal of Neuroscience Research. 2015b;93:1713–27. doi: 10.1002/jnr.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-f, Wang L, He D, Yang Q-hO, Duan Z-x, Zhang X-w, Nie L-l, Yan X-x, Tang B-s. Clinical features and [11C]-CFT PET analysis of PARK2, PARK6, PARK7-linked autosomal recessive early onset Parkinsonism. Neurological Sciences. 2011;32:35–40. doi: 10.1007/s10072-010-0360-z. [DOI] [PubMed] [Google Scholar]
- Harel BT, Cannizzaro MS, Cohen H, Reilly N, Snyder PJ. Acoustic characteristics of Parkinsonian speech: a potential biomarker of early disease progression and treatment. Journal of Neurolinguistics. 2004;17:439–453. [Google Scholar]
- Hatano T, Kubo S, Sato S, Hattori N. Pathogenesis of familial Parkinson’s disease: new insights based on monogenic forms of Parkinson’s disease. J Neurochem. 2009;111:1075–93. doi: 10.1111/j.1471-4159.2009.06403.x. [DOI] [PubMed] [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson’s disease. Behavioural neurology. 1998;11:131. [PubMed] [Google Scholar]
- Holmes RJ, Oates JM, Phyland DJ, Hughes AJ. Voice characteristics in the progression of Parkinson’s disease. International Journal of Language & Communication Disorders/Royal College of Speech & Language Therapists. 2000;35:407–18. doi: 10.1080/136828200410654. [DOI] [PubMed] [Google Scholar]
- Kawajiri S, Saiki S, Sato S, Hattori N. Genetic mutations and functions of PINK1. Trends Pharmacol Sci. 2011;32:573–80. doi: 10.1016/j.tips.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Kelm-Nelson CA, Yang KM, Ciucci MR. Exercise Effects on Early Vocal Ultrasonic Communication Dysfunction in a PINK1 Knockout Model of Parkinson’s Disease. Journal of Parkinson’s Disease. 2015;5:749–63. doi: 10.3233/JPD-150688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, Yamaguchi H, Tscherter A, Martella G, Bonsi P, Zhang C, Pothos EN, Shen J. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. Parkinson’s disease. The Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- Manczak M, Jung Y, Park BS, Partovi D, Reddy PH. Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. Journal of Neurochemistry. 2005;92:494–504. doi: 10.1111/j.1471-4159.2004.02884.x. [DOI] [PubMed] [Google Scholar]
- Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE Parkinson Study Group, D.I. Predictors of deterioration in health-related quality of life in Parkinson’s disease: results from the DATATOP trial. Movement disorders: official journal of the Movement Disorder Society. 2008;23:653. doi: 10.1002/mds.21853. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. Journal of Neuroscience Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- Miller N, Noble E, Jones D, Burn D. Life with communication changes in Parkinson’s disease. Age and Ageing. 2006;35:235–239. doi: 10.1093/ageing/afj053. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y. A simple quantitative bradykinesia test in MPTP-treated mice. Research communications in chemical pathology and pharmacology. 1985;50:435–441. [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic; San Diego, CA: 2001. [Google Scholar]
- Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV. Types and Functions of Ultrasonic Vocalizations in Laboratory Rats and Mice. American Association for Laboratory Animal Science. 2007;46:28–34. [PubMed] [Google Scholar]
- Pultorak J, Kelm-Nelson CK, Holt LR, Blue KV, Ciucci MR, Johnson AM. Decreased approach behavior and nucleus accumbens immediate early gene expression in response to Parkinsonian ultrasonic vocalizations in rats. Social Neuroscience. 2015;11:365–79. doi: 10.1080/17470919.2015.1086434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol. 2011;106:2580–2592. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T. Stereotypic laryngeal and respiratory motor patterns generate different call types in rat ultrasound vocalization. Journal of Experimental Zoology. Part A, Ecological Genetics and Physiology. 2013;319:213–24. doi: 10.1002/jez.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochet JC, Hay BA, Guo M. Molecular insights into Parkinson’s disease. Prog Mol Biol Transl Sci. 2012;107:125–88. doi: 10.1016/B978-0-12-385883-2.00011-4. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: a review of the literature. J Commun Disord. 2000;33:59–88. doi: 10.1016/s0021-9924(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Stewart C, Winfield L, Hunt A, Bressman SB, Fahn S, Blitzer A, Brin MF. Speech dysfunction in early Parkinson’s disease. Movement Disorders. 1995;10:562–5. doi: 10.1002/mds.870100506. [DOI] [PubMed] [Google Scholar]
- Villeneuve L, Purnell P, Boska M, Fox H. Early Expression of Parkinson’s Disease-Related Mitochondrial Abnormalities in PINK1 Knockout Rats. Molecular Neurobiology. 2014:1–16. doi: 10.1007/s12035-014-8927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonté MA, Porta M, Comi G. Clinical assessment of dysphagia in early phases of Parkinson’s disease. Neurological Sciences. 2002;23:s121–s122. [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whitney G, John N. Cues That Elicit Ultrasounds from Adult Male Mice. American Zoologist. 1979;19:457–463. [Google Scholar]
- Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, Lu B. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–5. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]