Abstract
Myasthenia gravis (MG) is an archetypal autoimmune disease. The pathology is characterized by autoantibodies to the acetylcholine receptor (AChR) in most patients, or to muscle-specific tyrosine kinase (MuSK) in others, and to a growing number of other postsynaptic proteins in smaller subsets. A decrease in the number of functional acetylcholine receptors (AChR), or functional interruption of the AChR, within the muscle end plate of the neuromuscular junction is caused by pathogenic autoantibodies. Although the molecular immunology underpinning the pathology is well understood, much remains to be learned about the cellular immunology contributing to the production of autoantibodies. This review will document research concerning the immunopathology of MG, bringing together evidence principally from human studies with an emphasis on the role of adaptive immunity and B cells in particular. Proposed mechanisms for autoimmunity, which take into account that different types of MG may incorporate divergent immunopathology, are offered.
Keywords: Myasthenia Gravis, B cells, B lymphocytes, Autoimmunity, Immunopathology, Autoantibodies, AChR, MuSK
Introduction
Patients with myasthenia gravis (MG) experience skeletal muscle weakness, worsened by activity 1,2. MG is a multifactorial disease that includes immune dysregulation, predisposing genetics, and environmental factors. The disease is rare; the estimated annual incidence is 1–2 per 100,000, and the prevalence ranges from approximately 7–20 per 100,000 based on regional studies performed since 1990 3–5. Recent epidemiological investigations indicate that, like other autoimmune diseases, the incidence of MG is rising considerably 6. Such increases can be partly attributed to improved diagnostic precision and rising longevity of the populace, but a genuine rise in incidence may point toward the important role of environmental contributions.
MG is an archetype for B cell-mediated autoimmune disorders. The molecular immunopathology (Figure 1) is attributed to the presence of autoantibodies specifically targeting components of the acetylcholine receptor (AChR). The specific disease mechanism is defined by these autoantibodies and their recognition of a number of molecular elements of the AChR, which impairs neuromuscular transmission in the postsynaptic membrane. The specific end-plate abnormalities mediated by the autoantibodies include disruption of receptor signaling and complement-directed tissue damage. Unlike many autoimmune diseases, MG autoantibodies are demonstrably pathogenic 7–12. This has been substantiated through numerous in-vitro approaches and perhaps demonstrated most convincingly through passive transfer of patient-derived serum or immunoglobulin, which reproduces features of the disease in experimental animals 13. Further evidence is provided by documented examples of maternal-fetal autoantibody transmission 14,15 and neonatal transfer 16,17, both of which can generate disease symptoms.
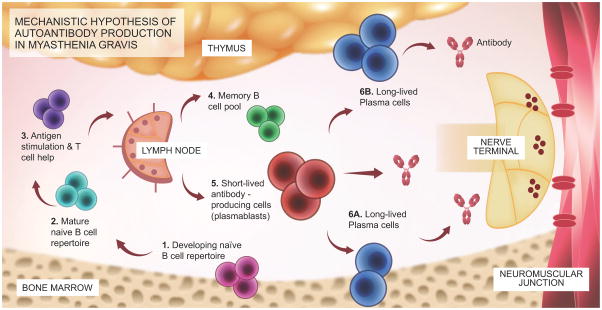
Figure 1.
Schematic diagram outlining the mechanistic hypothesis for the production of AChR or MuSK MG autoantibodies.
The proposed mechanistic path to autoantibody production in MG begins with naïve B cells (Steps 1 and 2), which likely encounter antigen(s) and receive T cell help in the lymph node (3). They then differentiate into memory B cells (4), antibody-secreting plasmablasts (5), and antibody-secreting long-lived plasma cells, which reside in the bone marrow (6A) and may also be present in the thymus (6B) of some patients with AChR MG. Plasmablasts and plasma cells may contribute to MG autoantibody production. B cell depletion therapy eliminates CD20+ memory and naïve B cells but does not directly eliminate plasmablasts or plasma cells, which are CD20-negative. After CD20-targeted depletion, MG serum autoantibody titers markedly diminish (especially in MuSK MG), suggesting that plasma cells are unlikely candidates for autoantibody production. Rather, short-lived plasmablasts are more viable candidates. As only a small fraction of these cells express CD20, the effectiveness of B cell depletion therapy may depend upon depletion of a pool of plasmablast-progenitor CD20+ memory B cells. Conversely, autoantibody titers that remain elevated following CD20-targeted depletion may be the product of long-lived plasma cells.
Genetic factors partly contribute to MG susceptibility 18. Although families in whom more than one member has MG are rare, limited MG twin-pair studies suggest rough approximations on MG concordance to be near 35% in monozygotic twins, and near 5% in dizygotic twins 19. These values, which are similar to a number of other autoimmune diseases, re-emphasize that varying degrees of both genetic and environmental factors contribute to the development of the disease 20. Nearly all of the MG-associated genes identified to date are involved in the immune response; a pattern common to nearly all autoimmune diseases 21. The human leukocyte antigen (HLA) locus remains the most strongly associated risk factor for the disease, especially HLA-DQA1 22. Examples of other genes encoding molecules that are involved in immune modulation include CTLA4, PTPN22, TNFRSF11A (RANK), 22 and TNFAIP3 interacting protein 1 (TNIP1) 23, all of which participate in cell-signaling pathways.
The incidence of MG with AChR autoantibodies has been observed to distribute in a bimodal pattern. Cases of early-onset MG, defined as patients in whom symptoms occur before approximately age 40, are predominantly women. Conversely, the incidence of late-onset disease is higher in men than in women. MG with muscle-specific tyrosine kinase (MuSK) autoantibodies is predominantly found in women and has a peak incidence of less than 40 years of age 24. Clinical classifications of MG include a number of subgroups 25,26. Ocular MG, which is restricted to isolated ptosis, diplopia, or both, with no signs or symptoms of weakness elsewhere, is often the first manifestation of the disease. In 40–50% of ocular MG cases, autoantibodies are not detected 27. This, however, does not exclude the possibility that they are present and causal. During this early stage of the disease they may be below the level of detection of commonly used assays and/or may be enriched at the neuromuscular junction (NMJ), the site of disease pathology, and thus not measurable in the serum.
Generalized MG usually includes symptoms associated with ocular disease, as well as weakness in extremity, bulbar, and/or respiratory muscles. Autoantibody status is used to classify the disease and has treatment implications in some cases. AChR, MuSK, and low-density lipoprotein (LDL) receptor-related protein 4 (LRP4) autoantibody positive and autoantibody seronegative represent additional major subsets. Within the AChR positive population, further subdivision categorizes early and late onset, early onset MG (EOMG) and late onset MG (LOMG) respectively. EOMG is often characterized by thymic hyperplasia, while cases of LOMG have thymic abnormalities less frequently. Concomitant autoimmune diseases in AChR MG patients are not uncommon, being reported in 13–22% 28. The most frequently associated autoimmune disease is thyroid disease, followed by systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).
The production of autoantibodies clearly implicates a principal role for B cells in the disease pathogenesis. Dysregulation in immune cell types extending beyond B lineages have been documented, indicating that a combination of factors contributes to disease manifestation; this theme is largely consistent throughout human autoimmunity. The contributions of B cells to the production of MG autoantibodies have been the focus of investigative interests, especially with the successful introduction of biological therapeutics that target these cells. In this review, we focus on the contributions that B cells make to the immunopathology of AChR, MuSK, and other subtypes of MG, although studies outside of AChR MG remain limited in number. We highlight T cell subsets that affect B cell responses, tissue compartments harboring B cells, B cell and antibody secreting subsets, and therapeutic intervention. Thymoma-associated MG and animal models are not included.
B cell-mediated MG immunopathology influenced by T cells
The autoantibodies in AChR MG are class switched, somatically mutated, and primarily of the IgG1 subclass. The initiation of the process that produces this affinity-driven maturation is dependent on T cell help. Accordingly, CD4 T cells are the main drivers in the immunopathogenesis of MG disease 29–33. They play a multi-faceted role in immunity, from promoting inflammation to inducing immune tolerance and supporting B cell function. In MG, each of these roles has been demonstrated to be dysregulated, resulting in the production of autoantibodies and secretion of pro-inflammatory cytokines. Specifically, AChR-specific CD4 T cells produce IFN-γ and IL-17 in response to AChR peptide stimulation, supporting the role of Th1 and Th17 cells in the pathogenesis of MG 34,35. Further evidence demonstrating Th1 and Th17 cells act as mediators of MG pathogenesis comes from experimental autoimmune MG (EAMG) studies used to evaluate the susceptibility of MG in the absence of Th1 and Th17 responses. Mice deficient for the IL-17 or T-bet gene, a Th1 transcription factor, were less susceptible to EAMG as demonstrated by decreases in clinical score, cytokine production, and AChR antibody production 36,37. The only two studies profiling T cells in MuSK MG mirror the observations in AChR MG, in which CD4 T cells from MuSK MG patients exhibited enhanced frequencies of Th1 and Th17 cytokines 38,39. Highlighting the infancy of study in this field is the fact that the identification of antigen-specific T cells in MuSK remains to be accomplished.
Regulatory T cells
The detection of AChR-specific Th1 and Th17 cells also coincides with the generation of defective regulatory T cell (Treg) responses in MG patients. Qualitatively, thymic, and peripheral Tregs from MG patients are impaired in their ability to suppress T cell responses 40,41. This impairment of Tregs is further complicated by data demonstrating that the T cells in MG become resistant to suppression when cultured with functional Treg 42. Quantitatively, reports on the frequency of Tregs in MG patients have been mixed, with some groups showing a decrease in the frequency of Tregs in MG 43–46, while others show no such alteration 40,47,48,41. The differences could be attributed to the markers that were used to identify Tregs. Before FOXP3 was identified as the transcription factor for Tregs, Tregs were identified only by the expression of CD25. Collectively, the functional assays for Tregs provide the best support for their role in the disease, because they show that Tregs in MG patients are defective in regulating immune responses compared to those in healthy subjects.
T-follicular helper (Tfh) & T-follicular regulatory (Tfr) cells
AChR MG is a CD4 dependent B cell mediated disease; therefore, the interaction of Tfh, Tfr, and B cells are critical in dictating the development of MG disease. In 1988, before Tfh cells were recognized as a distinct CD4 T cell subset, it was demonstrated that CD4 T cells were critical in the production of anti-AChR autoantibodies by B cells 30–33,49. Now it is well understood that CD4 T cells differentiate into Tfh cells upon expression of the master transcription factor Bcl6 and surface marker CXCR5, which allows migration into the germinal center where they support differentiation of B cells into memory B cells and antibody-secreting subsets (plasmablasts and plasma cells) 50,51. In the thymus of MG patients, the frequency of Tfh cells and B cells increased along with the expression of the Tfh associated markers Bcl6, IL-21, PD-1, and ICOS on thymocytes 52,53. CXCR5+ CD4 T cells are also found in the periphery, and it is suspected that they are memory Tfh cells that have migrated out of the germinal center 54. Circulating Tfh cells, IL-21, and CXCL13 were found at a higher frequency in MG patients and correlated with disease severity 55,56. To demonstrate the pathogenic role of Tfh cells in EAMG, knockdown of the Tfh transcription factor Bcl6 decreased the frequency of Tfh cells and IL-21 production, and importantly, diminished disease severity 57.
A counterpart to Tfh cells, which suppress Tfh and B cell interactions in the germinal center, are follicular regulatory (Tfr) T cells 58–60. Similar to Tfh cells, Tfr cells express CXCR5, PD-1, ICOS, and Bcl6. But unlike Tfh cells, Tfr cells are derived from natural Treg precursors, and express FOXP3 and BLIMP-1 61. Tfr can suppress the production of IL-21 and IL-4 by Tfh cells and inhibit class switching and antibody production by B cells. In MG, the increase in Tfh cells is likely due to a dysregulation in Tfr cells as MG patients exhibit lower ratios of Tfr/Tfh cells and frequency of Tfr cells 62,63. Moving forward, it is of interest to better understand the dynamics between Tfh, Tfr, and B cells in immune tolerance and disease pathogenesis.
B cell products: Immunoglobulins that drive MG pathology
Studies conducted many decades ago aimed to identify the circulating agent(s) that caused blocking of neuromuscular transmission in MG 64,65. Through continued efforts that built upon these, and many other early investigations, it is now well understood that the molecular immunopathology of MG is due to the presence of circulating autoantibodies specifically targeting the AChR, MuSK, or LRP4. Patient-derived AChR, MuSK, and LRP4 autoantibodies in MG are demonstrably pathogenic 7–12,66. Patients most often harbor only one of these autoantibody specificities. In approximately 75–80% of MG patients, AChR autoantibodies are detectable, MuSK autoantibodies are found in another 5–10% and LRP4 autoantibodies account for up to 20–30% of those without AChR or MuSK autoantibodies 67,68. This leaves about 5–10% of MG patients without detectable serum autoantibodies to either AChR, MuSK, or LRP4; those individuals are termed seronegative 67,68. However, the presumption that autoantibodies are absent in such cases is likely incorrect and requires alternative (autoantibody-independent) models to describe the pathology. The introduction of cell-based assays that express MuSK or clustered AChR was instrumental in demonstrating that a considerable portion of MG patients previously characterized as seronegative, did indeed have autoantibodies present in their serum 69,70. Their autoantibodies were simply not detectable by the commercially available and commonly used radioimmunoassay 71. Accordingly, it is anticipated that seronegative MG will continue to yield to the influence of higher sensitivity assays and to the discovery of new autoantigen targets 72–74.
Acetylcholine receptor autoantibodies
In the most common form of MG, autoantibodies directed against AChR are predominantly IgG1 and IgG3. These two subclasses effectively activate complement, leading to the formation of membrane attack complex 75–77. Autoantibody and complement-mediated damage to the NMJ impairs neuromuscular transmission through damage to the postsynaptic neuromuscular junction in the form of simplification of postsynaptic junctional folds, removal of AChR from the membrane, and widening of the synapse 78. Thus, AChR function is impaired by a number of autoantibody-mediated functions: complement-mediated tissue injury and removal of AChR from the muscle membrane (probably a major factor), cross-linkage of divalent AChR autoantibodies that results in internalization of AChR (by modulating autoantibodies), and rarely, a direct blockade of AChR by the autoantibodies 79–84.
Muscle specific kinase autoantibodies
MuSK is thought to play an important role in clustering AChR at the NMJ to promote efficient neuromuscular transmission. Passive transfer and active immunization studies in animals have shown that MuSK autoantibodies are pathogenic 85,86. In contrast to AChR MG, MuSK autoantibodies are predominantly IgG4 and do not effectively activate complement 87. Thus, the pathogenesis of MuSK MG is likely not complement mediated, and disruption of the interaction between MuSK and the postsynaptic protein LRP4 and collagen Q may be the predominant pathologic mechanism of MuSK autoantibodies 88–90. An interesting feature of secreted IgG4 antibodies is their ability to engage in “Fab-arm exchange” where a monospecific IgG4 protein may swap a heavy and light chain pair with another IgG4 to become bispecific 91. It has recently been shown that Fab-arm exchanged antibodies are present in MuSK MG and are pathogenic 92.
Other autoantibodies in MG
Recent studies of patients with AChR or MuSK autoantibodies undetectable through using standard assays have revealed additional autoantibodies specific to other NMJ proteins, including LRP4; agrin; collagen Q; cortactin; and the voltage-gated potassium channel, Kv1.4 93–100. LRP4 autoantibodies are predominantly IgG1, and studies suggest they are pathogenic 66. Pathogenicity of the other autoantibodies remains unclear at the moment. Titin autoantibodies, which are known to be present in AChR autoantibody positive patients with thymoma and in LOMG, have recently been observed in patients without identifiable autoantibodies to AChR, MuSK, or LRP4 using a high-sensitivity radioimmunoprecipitation (RIA) assay 101. These findings suggest that titin autoantibodies may have a role as a diagnostic marker, but independent replication using a large collection of MG patients including those with and without thymoma is needed.
The contributions of B cell subsets to MG immunopathology
B cell tissue compartments in MG
The compartmentalized enrichment of disease-associated B cells can occur in tissue specific autoimmunity 102. For example, at the synovium, the site of tissue injury in patients with rheumatoid arthritis, B cells producing autoantibodies that are directed toward citrullinated protein/peptide antigens can be observed 103. Although the site of tissue injury in AChR MG is the muscle end plate, the residence of B cells that express AChR autoantibodies is widely diverse (Figure 1). Over four decades ago, AChR-specific IgG was found in the MG thymus 104. Shortly afterward, the first identification of AChR autoantibody-producing B cells that occupied the thymus was reported 105. Thymic abnormality, defined by an AChR specific immune infiltrate, is now recognized as a fundamental characteristic of many (but not all) AChR MG patients.
The thymus in a subset of MG patients includes 106 AChR expression by thymic epithelial cells and myoid cells, the presence of proinflammatory cytokines, and defective regulatory T cells 42. B cell supporting CD4+ helper T cells are also present 107. B cells populating the hyperplastic thymus express markers of activation and display functional signs of activation 108. B cells often organize in the hyperplastic thymus within tertiary lymphoid organs, frequently exemplifying many characteristics of germinal centers. The presence and frequency of these structures positively associate with circulating AChR autoantibodies, reflecting a contributory role in their production. While these characteristics of AChR MG thymus tissue are seminal, they are not applicable to the entire MG population given that the thymus of approximately 30% of AChR MG patients is not hyperplastic and therefore may contain only few, if any, disease-associated B cells 109.
The B cell subset in the thymus tissue that is responsible for the detectable AChR autoantibody has not been precisely defined, but spontaneous production of AChR autoantibodies was demonstrated as most likely due to resident plasma cells 110,111 with possible contributions from plasmablasts. Additional studies demonstrated that AChR autoantibody production by thymic lymphocytes can occur spontaneously or with mitogen stimulation, suggesting that heterogeneous B cell populations make such contributions 112,113. AChR-specific CD27+ memory B cells are also likely to be present in the hyperplastic MG thymus, although specific identification of such has not been formally demonstrated. B cell repertoire sequencing and B cell immortalization studies have shown that the B cells resident in the MG thymus are broadly, clonally heterogeneous as they lack a dominant clone(s) among the infiltrate 114,115. They harbor the characteristics of antigen experience, including somatic hypermutation 116–119,114 and biased usage of variable region gene segments, which include over-representation of the VH3 family at the expense of VH4 genes 114. Of course, among these sequenced B cells are those producing autoantibodies directed toward AChR. However, they represent a minor fraction of the total B cell infiltrate as they are not highly enriched 120,111.
Given the major role of the thymus in MG AChR autoantibody production, thymectomy has been a long-standing treatment strategy. While thymus resection does not extinguish the disease in most cases, a recent, large, placebo-controlled clinical trial has confirmed the long-held belief that the procedure is beneficial 121. The AChR antibody titer in the majority of MG patients who have had a thymectomy does fall, but invariably does not reach undetectable levels 122,123. Provided that all of the thymus tissue harboring immune infiltrate is surgically removed, the persistence of both disease and autoantibody is a strong indication that additional locations host autoantibody-producing B cells.
AChR autoantibody-producing B cells can also be found in the circulation 124,30 and lymph nodes 125. Measurement of AChR-specific B cells present in the circulation are often positively associated with thymic hyperplasia and high serum autoantibody titers 124. AChR autoantibody-producing B cells have also been identified in the bone marrow 126. The bone marrow is a well-recognized niche for long-lived plasma cells that directly contribute to the majority of serum immunoglobulin and are accordingly responsible for serological memory. Plasma cells in the bone marrow that produce AChR autoantibodies may contribute to persistently elevated titers after thymectomy 127 and other treatments 128 (discussed below).
Do lymphocytes, including autoantibody-producing B cells, gather at the site of tissue injury, the neuromuscular junction? Lymphocytes that localize to the end-plate region have been observed in the muscle tissue of AChR MG patients 129–131. Localization in regions that include evidence of tissue damage has also been reported 132 although the specific lineage of such cells was not always elucidated. A subsequent investigation reported macrophages and T cells in MG muscle tissue but overall these infiltrates include few cells, are infrequently found at end-plates and are present in only a subset of patients 76. Overall, the presence of lymphocytic infiltrates does not appear to associate with the loss of AChR from the end-plates 76. MG autoantibody-producing B cells, at the end-plate, have not been unambiguously identified to date. Fittingly, it would also be of interest to associate such an infiltrate directly with autoimmune mechanisms to confirm that it is not non-specific inflammation that originates subsequent to the autoimmune-mediated tissue damage. Such investigations would require MG muscle biopsies, which are rarely obtained and represent a recognized constraint in the field.
While clonal B cell enrichment in the hyperplastic thymus tissue is conspicuous, perturbations in periphery are harder to observe. The circulating B cell repertoire was recently characterized through analysis of over 500,000 unique sequences, however only minor, albeit important, deviations from normal controls were evident 133. This indicates that pathogenic B cells make small changes to the global repertoire that are not readily perceived without very large data sets. Finally, the thymus of patients with MuSK MG presents a picture that is quite different than that of AChR MG patients. Immune cell infiltrates and ectopic germinal centers that are frequently found in the AChR MG thymus tissue are rarely present in MuSK MG 134. This infers that MuSK autoantibodies may develop and reside in a compartment other than the thymus.
Circulating Memory B cells and Antibody-secreting B cells
Studies directed toward understanding peripheral B cell functional abnormalities in MG are few. The few thorough immunophenotyping studies that have been conducted uncovered some abnormalities in the circulating B cell lineages. Similar to the findings of the repertoire sequencing studies, these perturbations are small and are observed only in B cell subsets. The difference in the frequency of B cell populations between AChR MG and healthy controls is unremarkable and there is no evidence of a general defect in B cell differentiation in MG patients. Double negative (CD27−IgD−) B cells, the frequency of which is elevated in some autoimmune diseases 135 and associated with disease-specific autoantibody titer 136, do not appear to be altered in AChR MG 137. A subpopulation of B cells express CD5+. These cells may be associated with autoantibody production and regulation, although their role in immunobiology is not unambiguously defined. Several autoimmune diseases, such as SLE and Sjogren’s syndrome, are associated with enhanced frequencies of CD5(+) B cells. Similarly, there appears to be an increase in CD5+ B cells in a subset of AChR MG patients 138. Plasmablast frequency, including recently activated subsets (HLA-DRhi) in ocular MG, are elevated in a some AChR MG patients 137. While such phenotyping studies can identify subset abnormalities that are likely related to autoimmunity, their limitation lies in that identification of autoantibody producers is not possible.
Regulatory B cells
Regulatory B cells (Bregs) are a rare B cell subset with a profound effect in promoting immune tolerance. They inhibit T cell expansion through IL-10, TGF-β, and IL-35 and support the differentiation of T cells into regulatory T cells (Tregs) 139–141. Unlike Tregs, a specific transcription factor or a surface phenotype that identifies Bregs remains undefined, although several B cell populations including CD24hiCD27+ 142, CD24hiCD38hi 143,144, and CD25hiCD71hi 145 B cells have been described to contain an enriched population of Bregs. Recent studies suggest that Bregs are antigen-specific and expand in the presence of an inflammatory event 140,146. However, the mechanisms driving their generation and maintenance remain undefined.
One of the better-described subsets of Bregs are B10 cells, which are B cells identified by the production of IL-10, an immunosuppressive cytokine. In humans, B10 cells represent 0.3–0.8% of total B cells and decrease in number with age 142. Due to their low frequency in circulation, and in attempt to better profile B10 cells, B10 cells are expanded into B10 progenitor cells by a multi-day TLR stimulation with recombinant CD40L, followed up by a re-stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin to detect IL-10 by flow cytometry 142. In 2014, the first MG study examining B10 cells revealed a decrease in the frequency of B10 cells and a subset of CD24+CD38+ B cells in AChR and MuSK MG compared to healthy controls 147. This decrease in the frequency of B10 cells correlated with disease severity based on MGFA clinical classification assignments. Disease severity also correlated with the functional ability of B10 cells to suppress CD4 T cell proliferation 148. Additionally, examination of patients undergoing rituximab treatment revealed that the repopulation of B10 cells was associated with the responsiveness to rituximab treatment. Although several EAMG and patient studies have supported the decrease in the frequency of B10 cells in MG, determining the mechanisms that limit B10 differentiation and their function remain an unmet need 149–151. Moving forward, further research is needed to validate B10 cell frequencies as a biomarker of MG disease severity, and methods to increase B10 cell frequencies may hold promise as a treatment for MG.
Naïve B cell repertoire formation and immunological tolerance
Gene segment recombination that occurs in developing B cells is among the defining features of the adaptive immune system. The process involves stochastically recombined gene segments to generate functional antibodies (B cell receptors) that are expressed on the cell surface. The arbitrary joining is the basis for the vast diversity of the B cell repertoire needed for complete immunity, but this comes at a price; the developing B cell repertoire invariably includes autoreactive antibodies. However, control mechanisms are in place. The majority of autoreactive B cells are eliminated at two separate steps152,153 during B cell development. First, a central checkpoint in the bone marrow between early immature and immature B cell stages removes the vast majority of developing B cells that express autoreactive antibodies. A second B cell tolerance checkpoint operates in the periphery, and selects against these autoreactive new emigrant B cells before they enter the mature naïve B cell compartment. Patients with autoimmune diseases often exhibit defective B cell tolerance checkpoints; this has been clearly demonstrated in RA, SLE, and type-1 diabetes (T1D) 152,154,155.
B cell tolerance also functions improperly in both AChR and MuSK MG patients 156. Accordingly, the naïve B cell repertoire in MG is shaped in the context of abnormal counter-selection during B cell development (that is, self-reactive cells are not eliminated). It follows that the manifestation of this abnormality is a naïve repertoire that is shaped differently from those in which B cell tolerance functions normally (Figure 1). Next generation deep sequencing allows for the comprehensive evaluation of the B cell receptor (BCR) repertoire properties in health and disease and provides the depth necessary to adequately depict the circulating peripheral repertoire, which includes up to 1011 B cells in humans 157. Application of this approach to the naïve B cell compartments in AChR and MuSK MG patients revealed repertoire features that were not observed where B cell tolerance functioned properly 133. This emphasizes the impact of tolerance defects on peripheral autoimmune repertoires. It remains to be determined whether the autoreactive naïve B cell pool is the reservoir from which disease-associated autoantibodies are derived.
Therapeutics
Rituximab and new biologicals that target B cells
Rituximab is a clinically approved B cell directed biologic. It is a chimeric monoclonal antibody that targets the CD20 antigen found on subsets of the B cell lineage. CD20 is a 33-kDa protein expressed by all mature B cells, but not on pre-B or differentiated plasmablasts and plasma cells. Rituximab has been used as part of the standard therapy for non-Hodgkin lymphoma (NHL) and has emerged as a highly effective tool in the management of certain autoimmune diseases 158,159. Interest in its use for MG began after a patient with both lymphoma and MG responded favorably to rituximab160. Several recent studies 128,161–164 have demonstrated the benefits of rituximab treatment in MG. In addition to significant clinical improvement, rituximab also allowed for tapering and subsequent discontinuation of other immunotherapies in both AChR and MuSK MG patients 163. The beneficial effect, although durable, is not permanent as post-rituximab relapses have been observed 165. Interestingly, the B cell repertoire that reemerges following CD20-mediated B cell depletion therapy includes evidence of tolerance dysfunction in autoimmune conditions shown to have the checkpoint abnormalities prior to treatment 166. This indicates that CD20-mediated depletion does not restore checkpoint function and suggests that disease manifestation may reemerge if the abnormal repertoire contributes to pathology.
Given that MG is a B cell mediated disease, the mechanism by which rituximab takes action may seem apparent. That is, B cells are depleted, which results in diminished pathogenic autoantibody production. However, a careful look uncovers that it may be more complicated (Figure 1). Long-lived plasma cells secrete the majority of circulating immunoglobulin. Given the absence of CD20 on their surface, they are not directly affected by rituximab. This is supported by unchanged immunization-generated and plasma cell-dependent antibody titers, such as those for tetanus, following anti-CD20 B cell depletion 128. As described above, it is apparent that plasma cells and/or plasmablasts produce AChR autoantibodies. In AChR MG the autoantibody titer can remain positive after treatment with rituximab, even during clinical improvement 128. Thus, it is not exactly clear how the treatment affects improvement. Similarly, NMO autoantibodies directed toward the AQP4 water channel do not fall correspondingly even though remarkable clinical improvement follows rituximab treatment 167. While these collective data may reflect compartmentalization (in the muscle or CNS) of the respective autoantibodies and/or discordance of serum titer with disease activity, they may also suggest that additional mechanisms, besides those associated with autoantibody, may promote disease activity, at least in the case of NMO. In AChR MG, given the clear dependence of immunopathology on autoantibodies, it is likely that rituximab eliminates autoantibody-producing cells, but the compartment and specific cells remain to be identified. The persistence of a positive autoantibody titer in responding patients may reflect the inability of the assays to discriminate between detection of these autoantibodies and their pathogenic properties, and/or that the serum does not reflect autoantibody status in the central nervous system (CNS) tissue (in NMO) or neuromuscular junction (in MG).
The situation in MuSK MG may be quite different than that in AChR MG (Figure 1). The markedly diminished MuSK autoantibody titer, as early as three months after rituximab-mediated CD20+ B cell depletion 128, suggests that long-lived plasma cells are not likely to be major contributors to MuSK autoantibody production. Rather, short-lived antibody-secreting cells such as plasmablasts are viable candidates as the primary driver of MuSK autoantibody production. As only a small fraction of these cells expresses CD20 168, the effectiveness of rituximab in MuSK MG may depend upon depletion of a pool of plasmablast-progenitor CD20+ memory B cells 168,169. Our group has experimentally tested this model, the results of which suggest that plasmablasts, do indeed, contribute to the production of MuSK-specific autoantibodies 170.
Provided that plasmablasts or plasma cells, the majority of which lack CD20 168, are a major source of pathogenic AChR autoantibodies, there are existing biologics that can effectively target these cells through their expression of CD19. Inebilizumab (formerly MEDI-551) is an anti-CD19 antibody with enhanced antibody-dependent cell-mediated cytotoxicity against B cell lineages. The drug is currently being evaluated in NMO given the known role of plasmablasts in AQP4 autoantibody production 171,172. A similar evaluation in AChR MG has not been initiated, but given the similarities in these diseases, testing in rituximab-resistant patients could be considered.
MG induction; a side effect of cancer immunotherapy
The immune system is equipped with checkpoints that enforce immune homeostasis. Our immune system utilizes these checkpoints as a balance to prevent an immune response against self-antigens and thus the onset of autoimmune diseases like MG. Moreover, these checkpoints fine tune the immune response against pathogens to efficiently activate immune cells, and subsequently shut down their effector functions once the pathogen is removed to prevent immunopathogenesis. With an improved understanding of the mechanisms and the key proteins surrounding immune checkpoints, recent developments in cancer immunotherapy have focused on therapies that intervene in the biology of immune checkpoints to promote T cell activation 173.
In the tumor microenvironment, one mechanism of immune evasion by tumor cells is the binding of inhibitory ligands with its corresponding receptor. Notably, T cells in the tumor microenvironment highly express inhibitory receptors and this interaction shuts down the effector function of T cells. Thus, the goal of checkpoint blockade therapy is to tilt this balance towards immune activation by blocking the interaction of inhibitory receptors with its ligand, promoting the generation of efficient tumor-eliminating T cells. Two of the well-studied immune checkpoint targets that have changed the landscape of cancer therapy are CTLA-4 and PD-1 174,175. CTLA-4 is upregulated after T cell activation and competes with CD28 for binding to B7-1 and B7-2. This competition favors CTLA-4 because CTLA-4 has a higher affinity to B7-1 and B7-2 than CD28 and subsequently results in immune suppression. PD-1 is an inhibitory receptor that is upregulated following T cell activation and inhibits T cell function.
Based on the mechanisms behind immunotherapy, it is no surprise that the main side effect of unleashing the control of the immune response has been immune-related disease manifesting as dermatologic, endocrine, gastrointestinal, and hepatic events. Although cases of MG after such immunotherapy are rare, the exacerbation of MG is a vital health concern that must be carefully monitored 176. The first record of ipilimumab-induced (anti-CTLA4) MG was described in two patients with melanoma 177. The functional burden caused by the development of MG contributed to the death of one patient, while the other patient improved after plasmapheresis. In lung cancer, MG symptoms were reported after combination therapy with either PD-1 or PD-L1 and CTLA-4 inhibitors 178,179. In these particular cases, the patients developed complications associated with MG exacerbation and did not survive. Interestingly, there are also cases of checkpoint blockade therapy in patients with known history of MG 180,181. In each of these cases, the patients were given anti-PD-1 therapy to treat melanoma, but developed an exacerbation of their MG disease. Fortunately, their MG symptoms resolved, and although their anti-PD-1 therapy was discontinued, the patients had stable disease or saw a shrinkage of their tumors.
The mechanism by which anti-CTLA-4, anti-PD-1, or anti-PD-L1 therapy induces MG disease is undefined. Available case reports describe a rapid progression to myasthenic crisis; therefore, any neurologic event must be recognized early and immunotherapy must be discontinued immediately, and then likely followed by initiation of high-dose steroids along with IVIg or plasmapheresis. Immunologically, these studies suggest that the MG-specific B and T cells are present in people with no prior clinical evidence of MG pathology. While these autoreactive cells are normally suppressed through immune-tolerance mechanisms, the introduction of checkpoint blockade therapies decreases the ability of T cells to discriminate self and non-self. It is not clear whether the checkpoint-inhibitor induced disease emulates the typical form in terms of immunobiology. Autoantibody specificity and participating cell types first need to be identified so similarities can be defined.
Collectively, with immunotherapy becoming front-line treatment for many cancer types, the number of reports describing exacerbated or emerging MG following the use of checkpoint-inhibitor immunotherapy continues to grow rapidly 182,183,184. Therefore, prior to treatment, patients must be carefully scrutinized for any autoimmune diseases, family history of such, and current physical condition. Knowing a patient’s likelihood of an adverse event following immunotherapy assists clinicians in maximizing the power of immunotherapy while limiting side effects.
Future directions and conclusions
Our understanding of MG immunopathology remains incomplete. It appears that the mechanism used by B cells for autoantibody production in AChR and MuSK MG differ, but details of both are needed to understand the immunopathology that will guide the development of more effective therapies. Clinicians need superior biomarkers that accurately associate with disease activity and severity to help guide management. Directly measuring autoantibody-producing B cells may fulfill this requirement. The B cell response in MG most certainly requires T cell help. While it is understood that pro-inflammatory antigen-specific T cells are at work in AChR MG, very little is known about the role of T cells in MuSK or LRP4 MG.
Translational B cell studies that will have the most impact on the care and treatment of patients are likely to include further investigation of B cell depletion in MG disease subsets, including MuSK. Evaluation of second-generation B cell directed biologics such as ocrelizumab, which appear to have improved efficacy and are better tolerated over the first generation, should be considered 185. However, highly specific treatment is desired over broad approaches that target an entire immune-cell lineage. Autoantigen-based chimeric immunoreceptors that can direct T cells to kill autoreactive B cells through the specificity of the B cell receptor (BCR) have been developed for the B cell-mediated autoimmune disease, pemphigus 186. Such an approach may provide an effective strategy for specific targeting of autoreactive B cells in MG, and not only result in clinical improvement but also in an improved safety profile over other less specifically-targeted agents.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health through a Grant to KCO, under award number R01AI114780 and supported, in part, by a pilot research award to KCO from Conquer Myasthenia Gravis. RJN is supported, in part, by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under award number U01NS084495. JSY is supported by an award from Conquer Myasthenia Gravis. JTG is supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number K23NS085049. The authors thank Karen Boss for copy editing and proofreading the manuscript and Charlotte and Eliza Gurley for administrative assistance in its preparation.
List of acronyms and/or abbreviations
- MG
Myasthenia gravis
- AChR
Acetylcholine receptor
- MuSK
Muscle specific kinase
- NMO
Neuromyelitis optica
- NMJ
Neuromuscular junction
- NINDS
Neurological Disorders and Stroke
- HLA
Human leukocyte antigen
- RANK
TNFRSF11A
- TNIP1
TNFAIP3 interacting protein 1
- LDL
Low-density lipoprotein
- LRP4
Low-density lipoprotein receptor-related protein 4
- EAMG
Experimental autoimmune MG
- Treg
Regulatory T cell
- Tfr
Follicular regulatory T cell
- RIA
Radio-immunoprecipitation assay
- BCR
B cell receptor
- NHL
Non-Hodgkin’s lymphoma
- T1D
Type 1 diabetes
- BAFF
B cell activating factor
- PMA
Phorbol 12 myristate 13 acetate
- Tregs
Regulatory T cells
- CNS
Central nervous system
Footnotes
Conflict of interests/financial disclosures: KCO has received personal compensation in the past year from Genentech for educational activities and from Proclara Biosciences and Editas Medicine for consulting services. JTG has received personal compensation in the past year from Jacobus Pharmaceuticals, argenx, and UCB Pharma for consulting services and from Grifols for educational activities. RJN reports support through an investigator-initiated trial agreement from Genentech for placebo/drug for the currently underway clinical trial (www.clincial trials.gov, NCT02110706).
References
- 1.Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2(10):797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- 2.Gilhus NE. Myasthenia Gravis. N Engl J Med. 2016;375(26):2570–2581. doi: 10.1056/NEJMra1602678. [DOI] [PubMed] [Google Scholar]
- 3.Phillips LH., 2nd The epidemiology of myasthenia gravis. Ann N Y Acad Sci. 2003;998:407–412. doi: 10.1196/annals.1254.053. [DOI] [PubMed] [Google Scholar]
- 4.Santos E, Coutinho E, Moreira I, Silva AM, Lopes D, Costa H, Silveira F, Nadais G, Morais H, Martins J, Branco MC, Veiga A, Silva RS, Ferreira A, Sousa F, Freijo M, Matos I, Andre R, Negrao L, Fraga C, Santos M, Sampaio M, Lopes C, Leite MI, Goncalves G. Epidemiology of myasthenia gravis in Northern Portugal: Frequency estimates and clinical epidemiological distribution of cases. Muscle Nerve. 2016;54(3):413–421. doi: 10.1002/mus.25068. [DOI] [PubMed] [Google Scholar]
- 5.Lefter S, Hardiman O, Ryan AM. A population-based epidemiologic study of adult neuromuscular disease in the Republic of Ireland. Neurology. 2016 doi: 10.1212/WNL.0000000000003504. [DOI] [PubMed] [Google Scholar]
- 6.Cetin H, Fulop G, Zach H, Auff E, Zimprich F. Epidemiology of myasthenia gravis in Austria: rising prevalence in an ageing society. Wien Klin Wochenschr. 2012;124(21–22):763–768. doi: 10.1007/s00508-012-0258-2. [DOI] [PubMed] [Google Scholar]
- 7.Vincent A, Beeson D, Lang B. Molecular targets for autoimmune and genetic disorders of neuromuscular transmission. Eur J Biochem. 2000;267(23):6717–6728. doi: 10.1046/j.1432-1033.2000.01785.x. [DOI] [PubMed] [Google Scholar]
- 8.Koneczny I, Cossins J, Vincent A. The role of muscle-specific tyrosine kinase (MuSK) and mystery of MuSK myasthenia gravis. J Anat. 2013 doi: 10.1111/joa.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viegas S, Jacobson L, Waters P, Cossins J, Jacob S, Leite MI, Webster R, Vincent A. Passive and active immunization models of MuSK-Ab positive myasthenia: electrophysiological evidence for pre and postsynaptic defects. Exp Neurol. 2012;234(2):506–512. doi: 10.1016/j.expneurol.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Lindstrom JM, Engel AG, Seybold ME, Lennon VA, Lambert EH. Pathological mechanisms in experimental autoimmune myasthenia gravis. II. Passive transfer of experimental autoimmune myasthenia gravis in rats with anti-acetylcholine recepotr antibodies. J Exp Med. 1976;144(3):739–753. doi: 10.1084/jem.144.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda K, Korenaga S, Ito Y. Myasthenia gravis: passive transfer to mice of antibody to human and mouse acetylcholine receptor. Neurology. 1981;31(3):282–287. doi: 10.1212/wnl.31.3.282. [DOI] [PubMed] [Google Scholar]
- 12.Sterz R, Hohlfeld R, Rajki K, Kaul M, Heininger K, Peper K, Toyka KV. Effector mechanisms in myasthenia gravis: end-plate function after passive transfer of IgG, Fab, and F(ab′)2 hybrid molecules. Muscle Nerve. 1986;9(4):306–312. doi: 10.1002/mus.880090404. [DOI] [PubMed] [Google Scholar]
- 13.Toyka KV, Brachman DB, Pestronk A, Kao I. Myasthenia gravis: passive transfer from man to mouse. Science. 1975;190(4212):397–399. doi: 10.1126/science.1179220. [DOI] [PubMed] [Google Scholar]
- 14.Melber D. Maternal-fetal transmission of myasthenia gravis with acetylcholine-receptor antibody. N Engl J Med. 1988;318(15):996. doi: 10.1056/nejm198804143181518. [DOI] [PubMed] [Google Scholar]
- 15.Vernet-der Garabedian B, Lacokova M, Eymard B, Morel E, Faltin M, Zajac J, Sadovsky O, Dommergues M, Tripon P, Bach JF. Association of neonatal myasthenia gravis with antibodies against the fetal acetylcholine receptor. J Clin Invest. 1994;94(2):555–559. doi: 10.1172/JCI117369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson JO, Penn AS, Lisak RP, Abramsky O, Brenner T, Schotland DL. Antiacetylcholine receptor antibody in neonatal myasthenia gravis. Am J Dis Child. 1981;135(3):222–226. doi: 10.1001/archpedi.1981.02130270014006. [DOI] [PubMed] [Google Scholar]
- 17.Keesey J, Lindstrom J, Cokely H. Anti-acetylcholine receptor antibody in neonatal myasthenia gravis. N Engl J Med. 1977;296(1):55. doi: 10.1056/NEJM197701062960125. [DOI] [PubMed] [Google Scholar]
- 18.Avidan N, Le Panse R, Berrih-Aknin S, Miller A. Genetic basis of myasthenia gravis - a comprehensive review. J Autoimmun. 2014;52:146–153. doi: 10.1016/j.jaut.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Ramanujam R, Pirskanen R, Ramanujam S, Hammarstrom L. Utilizing twins concordance rates to infer the predisposition to myasthenia gravis. Twin Res Hum Genet. 2011;14(2):129–136. doi: 10.1375/twin.14.2.129. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanos DP, Smyk DS, Rigopoulou EI, Mytilinaiou MG, Heneghan MA, Selmi C, Gershwin ME. Twin studies in autoimmune disease: genetics, gender and environment. J Autoimmun. 2012;38(2–3):J156–169. doi: 10.1016/j.jaut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Uddin M, Sturge M, Rahman P, Woods MO. Autosome-wide copy number variation association analysis for rheumatoid arthritis using the WTCCC high-density SNP genotype data. J Rheumatol. 2011;38(5):797–801. doi: 10.3899/jrheum.100758. [DOI] [PubMed] [Google Scholar]
- 22.Renton AE, Pliner HA, Provenzano C, Evoli A, Ricciardi R, Nalls MA, Marangi G, Abramzon Y, Arepalli S, Chong S, Hernandez DG, Johnson JO, Bartoccioni E, Scuderi F, Maestri M, Gibbs JR, Errichiello E, Chio A, Restagno G, Sabatelli M, Macek M, Scholz SW, Corse A, Chaudhry V, Benatar M, Barohn RJ, McVey A, Pasnoor M, Dimachkie MM, Rowin J, Kissel J, Freimer M, Kaminski HJ, Sanders DB, Lipscomb B, Massey JM, Chopra M, Howard JF, Jr, Koopman WJ, Nicolle MW, Pascuzzi RM, Pestronk A, Wulf C, Florence J, Blackmore D, Soloway A, Siddiqi Z, Muppidi S, Wolfe G, Richman D, Mezei MM, Jiwa T, Oger J, Drachman DB, Traynor BJ. A genome-wide association study of myasthenia gravis. JAMA Neurol. 2015;72(4):396–404. doi: 10.1001/jamaneurol.2014.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregersen PK, Kosoy R, Lee AT, Lamb J, Sussman J, McKee D, Simpfendorfer KR, Pirskanen-Matell R, Piehl F, Pan-Hammarstrom Q, Verschuuren JJ, Titulaer MJ, Niks EH, Marx A, Strobel P, Tackenberg B, Putz M, Maniaol A, Elsais A, Tallaksen C, Harbo HF, Lie BA, Raychaudhuri S, de Bakker PI, Melms A, Garchon HJ, Willcox N, Hammarstrom L, Seldin MF. Risk for myasthenia gravis maps to a (151) Pro-->Ala change in TNIP1 and to human leukocyte antigen-B*08. Ann Neurol. 2012;72(6):927–935. doi: 10.1002/ana.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guptill JT, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical findings and response to treatment in two large cohorts. Muscle Nerve. 2011;44(1):36–40. doi: 10.1002/mus.22006. [DOI] [PubMed] [Google Scholar]
- 25.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023–1036. doi: 10.1016/S1474-4422(15)00145-3. [DOI] [PubMed] [Google Scholar]
- 26.Berrih-Aknin S, Frenkian-Cuvelier M, Eymard B. Diagnostic and clinical classification of autoimmune myasthenia gravis. J Autoimmun. 2014;48–49:143–148. doi: 10.1016/j.jaut.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Jacob S, Viegas S, Leite MI, Webster R, Cossins J, Kennett R, Hilton-Jones D, Morgan BP, Vincent A. Presence and pathogenic relevance of antibodies to clustered acetylcholine receptor in ocular and generalized myasthenia gravis. Arch Neurol. 2012;69(8):994–1001. doi: 10.1001/archneurol.2012.437. [DOI] [PubMed] [Google Scholar]
- 28.Nacu A, Andersen JB, Lisnic V, Owe JF, Gilhus NE. Complicating autoimmune diseases in myasthenia gravis: a review. Autoimmunity. 2015;48(6):362–368. doi: 10.3109/08916934.2015.1030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuchroo VK, Greer JM, Kaul D, Ishioka G, Franco A, Sette A, Sobel RA, Lees MB. A single TCR antagonist peptide inhibits experimental allergic encephalomyelitis mediated by a diverse T cell repertoire. Journal of Immunology. 1994;153(7):3326–3336. [PubMed] [Google Scholar]
- 30.Lisak RP, Laramore C, Zweiman B, Moskovitz A. In vitro synthesis of antibodies to acetylcholine receptor by peripheral blood mononuclear cells of patients with myasthenia gravis. Neurology. 1983;33(5):604–608. doi: 10.1212/wnl.33.5.604. [DOI] [PubMed] [Google Scholar]
- 31.Lisak RP, Laramore C, Levinson AI, Zweiman B, Moskovitz AR, Witte A. In vitro synthesis of antibodies to acetylcholine receptor by peripheral blood cells: role of suppressor T cells in normal subjects. Neurology. 1984;34(6):802–805. doi: 10.1212/wnl.34.6.802. [DOI] [PubMed] [Google Scholar]
- 32.Authier FJ, De Grissac N, Degos JD, Gherardi RK. Transient myasthenia gravis during HIV infection. Muscle Nerve. 1995;18(8):914–916. doi: 10.1002/mus.880180819. [DOI] [PubMed] [Google Scholar]
- 33.Nath A, Kerman RH, Novak IS, Wolinsky JS. Immune studies in human immunodeficiency virus infection with myasthenia gravis: a case report. Neurology. 1990;40(4):581–583. doi: 10.1212/wnl.40.4.581. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Amezquita RA, Kleinstein SH, Stathopoulos P, Nowak RJ, O’Connor KC. Autoreactive T Cells from Patients with Myasthenia Gravis Are Characterized by Elevated IL-17, IFN-gamma, and GM-CSF and Diminished IL-10 Production. Journal of immunology. 2016;196(5):2075–2084. doi: 10.4049/jimmunol.1501339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang ZY, Okita DK, Howard J, Jr, Conti-Fine BM. T-cell recognition of muscle acetylcholine receptor subunits in generalized and ocular myasthenia gravis. Neurology. 1998;50(4):1045–1054. doi: 10.1212/wnl.50.4.1045. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Hao J, Dayao CS, Shi FD, Campagnolo DI. T-bet deficiency decreases susceptibility to experimental myasthenia gravis. Exp Neurol. 2009;220(2):366–373. doi: 10.1016/j.expneurol.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Schaffert H, Pelz A, Saxena A, Losen M, Meisel A, Thiel A, Kohler S. IL-17-producing CD4(+) T cells contribute to the loss of B-cell tolerance in experimental autoimmune myasthenia gravis. European journal of immunology. 2015;45(5):1339–1347. doi: 10.1002/eji.201445064. [DOI] [PubMed] [Google Scholar]
- 38.Yi JS, Guidon A, Sparks S, Osborne R, Juel VC, Massey JM, Sanders DB, Weinhold KJ, Guptill JT. Characterization of CD4 and CD8 T cell responses in MuSK myasthenia gravis. J Autoimmun. 2014;52:130–138. doi: 10.1016/j.jaut.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yilmaz V, Oflazer P, Aysal F, Durmus H, Poulas K, Yentur SP, Gulsen-Parman Y, Tzartos S, Marx A, Tuzun E, Deymeer F, Saruhan-Direskeneli G. Differential Cytokine Changes in Patients with Myasthenia Gravis with Antibodies against AChR and MuSK. PLoS One. 2015;10(4):e0123546. doi: 10.1371/journal.pone.0123546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balandina A, Lecart S, Dartevelle P, Saoudi A, Berrih-Aknin S. Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105(2):735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thiruppathi M, Rowin J, Ganesh B, Sheng JR, Prabhakar BS, Meriggioli MN. Impaired regulatory function in circulating CD4(+)CD25(high)CD127(low/-) T cells in patients with myasthenia gravis. Clin Immunol. 2012;145(3):209–223. doi: 10.1016/j.clim.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gradolatto A, Nazzal D, Truffault F, Bismuth J, Fadel E, Foti M, Berrih-Aknin S. Both Treg cells and Tconv cells are defective in the Myasthenia gravis thymus: roles of IL-17 and TNF-alpha. J Autoimmun. 2014;52:53–63. doi: 10.1016/j.jaut.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 43.Fattorossi A, Battaglia A, Buzzonetti A, Ciaraffa F, Scambia G, Evoli A. Circulating and thymic CD4 CD25 T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology. 2005;116(1):134–141. doi: 10.1111/j.1365-2567.2005.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Xiao BG, Xi JY, Lu CZ, Lu JH. Decrease of CD4(+)CD25(high)Foxp3(+) regulatory T cells and elevation of CD19(+)BAFF-R(+) B cells and soluble ICAM-1 in myasthenia gravis. Clin Immunol. 2008;126(2):180–188. doi: 10.1016/j.clim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Masuda M, Matsumoto M, Tanaka S, Nakajima K, Yamada N, Ido N, Ohtsuka T, Nishida M, Hirano T, Utsumi H. Clinical implication of peripheral CD4+CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. Journal of neuroimmunology. 2010;225(1–2):123–131. doi: 10.1016/j.jneuroim.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Xu WH, Zhang AM, Ren MS, Zhang XD, Wang F, Xu XC, Li Q, Wang J, Din BS, Wu YB, Chen GH. Changes of Treg-associated molecules on CD4+CD25 +Treg cells in myasthenia gravis and effects of immunosuppressants. J Clin Immunol. 2012;32(5):975–983. doi: 10.1007/s10875-012-9685-0. [DOI] [PubMed] [Google Scholar]
- 47.Battaglia A, Di Schino C, Fattorossi A, Scambia G, Evoli A. Circulating CD4+CD25+ T regulatory and natural killer T cells in patients with myasthenia gravis: a flow cytometry study. J Biol Regul Homeost Agents. 2005;19(1–2):54–62. [PubMed] [Google Scholar]
- 48.Matsui N, Nakane S, Saito F, Ohigashi I, Nakagawa Y, Kurobe H, Takizawa H, Mitsui T, Kondo K, Kitagawa T, Takahama Y, Kaji R. Undiminished regulatory T cells in the thymus of patients with myasthenia gravis. Neurology. 2010;74(10):816–820. doi: 10.1212/WNL.0b013e3181d31e47. [DOI] [PubMed] [Google Scholar]
- 49.Fujii Y, Lindstrom J. Regulation of antibody production by helper T cell clones in experimental autoimmune myasthenia gravis. J Immunol. 1988;141(10):3361–3369. [PubMed] [Google Scholar]
- 50.Zhu Y, Zou L, Liu YC. T follicular helper cells, T follicular regulatory cells and autoimmunity. International immunology. 2015 doi: 10.1093/intimm/dxv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunology and cell biology. 2014;92(1):64–71. doi: 10.1038/icb.2013.55. [DOI] [PubMed] [Google Scholar]
- 52.Song Y, Zhou L, Miao F, Chen G, Zhu Y, Gao X, Wang Y, Pang L, Zhao C, Sun X, Chen Z. Increased frequency of thymic T follicular helper cells in myasthenia gravis patients with thymoma. J Thorac Dis. 2016;8(3):314–322. doi: 10.21037/jtd.2016.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang X, Liu S, Chang T, Xu J, Zhang C, Tian F, Sun Y, Song C, Yi W, Lin H, Li Z, Yang K. Intrathymic Tfh/B Cells Interaction Leads to Ectopic GCs Formation and Anti-AChR Antibody Production: Central Role in Triggering MG Occurrence. Mol Neurobiol. 2016;53(1):120–131. doi: 10.1007/s12035-014-8985-1. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends in immunology. 2014;35(9):436–442. doi: 10.1016/j.it.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo C, Li Y, Liu W, Feng H, Wang H, Huang X, Qiu L, Ouyang J. Expansion of circulating counterparts of follicular helper T cells in patients with myasthenia gravis. Journal of neuroimmunology. 2013;256(1–2):55–61. doi: 10.1016/j.jneuroim.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Saito R, Onodera H, Tago H, Suzuki Y, Shimizu M, Matsumura Y, Kondo T, Itoyama Y. Altered expression of chemokine receptor CXCR5 on T cells of myasthenia gravis patients. Journal of neuroimmunology. 2005;170(1–2):172–178. doi: 10.1016/j.jneuroim.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Xin N, Fu L, Shao Z, Guo M, Zhang X, Zhang Y, Dou C, Zheng S, Shen X, Yao Y, Wang J, Wang J, Cui G, Liu Y, Geng D, Xiao C, Zhang Z, Dong R. RNA interference targeting Bcl-6 ameliorates experimental autoimmune myasthenia gravis in mice. Mol Cell Neurosci. 2014;58:85–94. doi: 10.1016/j.mcn.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 58.Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. Journal of immunology. 2011;187(9):4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 59.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nature medicine. 2011;17(8):975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, Fan HM, Liu ZM, Neelapu SS, Dong C. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature medicine. 2011;17(8):983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sage PT, Sharpe AH. T follicular regulatory cells. Immunological reviews. 2016;271(1):246–259. doi: 10.1111/imr.12411. [DOI] [PubMed] [Google Scholar]
- 62.Zhang CJ, Gong Y, Zhu W, Qi Y, Yang CS, Fu Y, Chang G, Li Y, Shi S, Wood K, Ladha S, Shi FD, Liu Q, Yan Y. Augmentation of Circulating Follicular Helper T Cells and Their Impact on Autoreactive B Cells in Myasthenia Gravis. Journal of immunology. 2016;197(7):2610–2617. doi: 10.4049/jimmunol.1500725. [DOI] [PubMed] [Google Scholar]
- 63.Wen Y, Yang B, Lu J, Zhang J, Yang H, Li J. Imbalance of circulating CD4(+)CXCR5(+)FOXP3(+) Tfr-like cells and CD4(+)CXCR5(+)FOXP3(-) Tfh-like cells in myasthenia gravis. Neuroscience letters. 2016;630:176–182. doi: 10.1016/j.neulet.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 64.Nastuk WL, Strauss AJ, Osserman KE. Search for a neuromuscular blocking agent in the blood of patients with myasthenia gravis. Am J Med. 1959;26(3):394–409. doi: 10.1016/0002-9343(59)90248-7. [DOI] [PubMed] [Google Scholar]
- 65.Bergh NP. Biologic assays in myasthenia gravis for any agents causing a neuromuscular block. Scand J Clin Lab Invest. 1953;5(Suppl 5):5–47. [PubMed] [Google Scholar]
- 66.Shen C, Lu Y, Zhang B, Figueiredo D, Bean J, Jung J, Wu H, Barik A, Yin DM, Xiong WC, Mei L. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. J Clin Invest. 2013;123(12):5190–5202. doi: 10.1172/JCI66039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Binks S, Vincent A, Palace J. Myasthenia gravis: a clinical-immunological update. J Neurol. 2016;263(4):826–834. doi: 10.1007/s00415-015-7963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis - autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259–268. doi: 10.1038/nrneurol.2016.44. [DOI] [PubMed] [Google Scholar]
- 69.Huda S, Waters P, Woodhall M, Leite MI, Jacobson L, De Rosa A, Maestri M, Ricciardi R, Heckmann JM, Maniaol A, Evoli A, Cossins J, Hilton-Jones D, Vincent A. IgG-specific cell-based assay detects potentially pathogenic MuSK-Abs in seronegative MG. Neurol Neuroimmunol Neuroinflamm. 2017;4(4):e357. doi: 10.1212/NXI.0000000000000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leite MI, Jacob S, Viegas S, Cossins J, Clover L, Morgan BP, Beeson D, Willcox N, Vincent A. IgG1 antibodies to acetylcholine receptors in ‘seronegative’ myasthenia gravis. Brain. 2008;131(Pt 7):1940–1952. doi: 10.1093/brain/awn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devic P, Petiot P, Simonet T, Stojkovic T, Delmont E, Franques J, Magot A, Vial C, Lagrange E, Nicot AS, Risson V, Eymard B, Schaeffer L. Antibodies to clustered acetylcholine receptor: expanding the phenotype. Eur J Neurol. 2014;21(1):130–134. doi: 10.1111/ene.12270. [DOI] [PubMed] [Google Scholar]
- 72.Cortes-Vicente E, Gallardo E, Martinez MA, Diaz-Manera J, Querol L, Rojas-Garcia R, Illa I. Clinical Characteristics of Patients With Double-Seronegative Myasthenia Gravis and Antibodies to Cortactin. JAMA Neurol. 2016;73(9):1099–1104. doi: 10.1001/jamaneurol.2016.2032. [DOI] [PubMed] [Google Scholar]
- 73.Kaminski HJ. Seronegative Myasthenia Gravis-A Vanishing Disorder? JAMA Neurol. 2016;73(9):1055–1056. doi: 10.1001/jamaneurol.2016.2277. [DOI] [PubMed] [Google Scholar]
- 74.Hong Y, Zisimopoulou P, Trakas N, Karagiorgou K, Stergiou C, Skeie GO, Hao HJ, Gao X, Owe JF, Zhang X, Yue YX, Romi F, Wang Q, Li HF, Gilhus NE, Tzartos SJ. Multiple antibody detection in ‘seronegative’ myasthenia gravis patients. Eur J Neurol. 2017;24(6):844–850. doi: 10.1111/ene.13300. [DOI] [PubMed] [Google Scholar]
- 75.Rodgaard A, Nielsen FC, Djurup R, Somnier F, Gammeltoft S. Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin Exp Immunol. 1987;67(1):82–88. [PMC free article] [PubMed] [Google Scholar]
- 76.Nakano S, Engel AG. Myasthenia gravis: quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. 1993;43(6):1167–1172. doi: 10.1212/wnl.43.6.1167. [DOI] [PubMed] [Google Scholar]
- 77.Lefvert AK, Cuenoud S, Fulpius BW. Binding properties and subclass distribution of anti-acetylcholine receptor antibodies in myasthenia gravis. J Neuroimmunol. 1981;1(1):125–135. doi: 10.1016/0165-5728(81)90015-1. [DOI] [PubMed] [Google Scholar]
- 78.Engel AG, Sakakibara H, Sahashi K, Lindstrom JM, Lambert EH, Lennon VA. Passively transferred experimental autoimmune myasthenia gravis. Sequential and quantitative study of the motor end-plate fine structure and ultrastructural localization of immune complexes (IgG and C3), and of the acetylcholine receptor. Neurology. 1979;29(2):179–188. doi: 10.1212/wnl.29.2.179. [DOI] [PubMed] [Google Scholar]
- 79.Engel AG, Lambert EH, Howard FM. Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc. 1977;52(5):267–280. [PubMed] [Google Scholar]
- 80.Hara H, Hayashi K, Ohta K, Itoh N, Nishitani H, Ohta M. Detection and characterization of blocking-type anti-acetylcholine receptor antibodies in sera from patients with myasthenia gravis. Clin Chem. 1993;39(10):2053–2057. [PubMed] [Google Scholar]
- 81.Whiting PJ, Vincent A, Newsom-Davis J. Acetylcholine receptor antibody characteristics in myasthenia gravis. Fractionation of alpha-bungarotoxin binding site antibodies and their relationship to IgG subclass. J Neuroimmunol. 1983;5(1):1–9. doi: 10.1016/0165-5728(83)90022-x. [DOI] [PubMed] [Google Scholar]
- 82.Almon RR, Andrew CG, Appel SH. Serum globulin in myasthenia gravis: inhibition of alpha-bungarotoxin binding to acetylcholine receptors. Science. 1974;186(4158):55–57. doi: 10.1126/science.186.4158.55. [DOI] [PubMed] [Google Scholar]
- 83.Drachman DB, Angus CW, Adams RN, Michelson JD, Hoffman GJ. Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. The New England journal of medicine. 1978;298(20):1116–1122. doi: 10.1056/NEJM197805182982004. [DOI] [PubMed] [Google Scholar]
- 84.Loutrari H, Kokla A, Tzartos SJ. Passive transfer of experimental myasthenia gravis via antigenic modulation of acetylcholine receptor. European journal of immunology. 1992;22(9):2449–2452. doi: 10.1002/eji.1830220939. [DOI] [PubMed] [Google Scholar]
- 85.Punga AR, Lin S, Oliveri F, Meinen S, Ruegg MA. Muscle-selective synaptic disassembly and reorganization in MuSK antibody positive MG mice. Experimental neurology. 2011;230(2):207–217. doi: 10.1016/j.expneurol.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 86.Klooster R, Plomp JJ, Huijbers MG, Niks EH, Straasheijm KR, Detmers FJ, Hermans PW, Sleijpen K, Verrips A, Losen M, Martinez-Martinez P, De Baets MH, van der Maarel SM, Verschuuren JJ. Muscle-specific kinase myasthenia gravis IgG4 autoantibodies cause severe neuromuscular junction dysfunction in mice. Brain. 2012;135(Pt 4):1081–1101. doi: 10.1093/brain/aws025. [DOI] [PubMed] [Google Scholar]
- 87.Niks EH, van Leeuwen Y, Leite MI, Dekker FW, Wintzen AR, Wirtz PW, Vincent A, van Tol MJ, Jol-van der Zijde CM, Verschuuren JJ. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol. 2008;195(1–2):151–156. doi: 10.1016/j.jneuroim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 88.Koneczny I, Cossins J, Waters P, Beeson D, Vincent A. MuSK myasthenia gravis IgG4 disrupts the interaction of LRP4 with MuSK but both IgG4 and IgG1–3 can disperse preformed agrin-independent AChR clusters. PLoS One. 2013;8(11):e80695. doi: 10.1371/journal.pone.0080695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohno K, Ito M, Kawakami Y, Krejci E, Engel AG. Specific binding of collagen Q to the neuromuscular junction is exploited to cure congenital myasthenia and to explore bases of myasthenia gravis. Chem Biol Interact. 2013;203(1):335–340. doi: 10.1016/j.cbi.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ohno K, Otsuka K, Ito M. Roles of collagen Q in MuSK antibody-positive myasthenia gravis. Chem Biol Interact. 2016;259(Pt B):266–270. doi: 10.1016/j.cbi.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 91.van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martinez-Martinez P, Vermeulen E, den Bleker TH, Wiegman L, Vink T, Aarden LA, De Baets MH, van de Winkel JG, Aalberse RC, Parren PW. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007;317(5844):1554–1557. doi: 10.1126/science.1144603. [DOI] [PubMed] [Google Scholar]
- 92.Koneczny I, Stevens JA, De Rosa A, Huda S, Huijbers MG, Saxena A, Maestri M, Lazaridis K, Zisimopoulou P, Tzartos S, Verschuuren J, van der Maarel SM, van Damme P, De Baets MH, Molenaar PC, Vincent A, Ricciardi R, Martinez-Martinez P, Losen M. IgG4 autoantibodies against muscle-specific kinase undergo Fab-arm exchange in myasthenia gravis patients. Journal of autoimmunity. 2016 doi: 10.1016/j.jaut.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Romi F, Suzuki S, Suzuki N, Petzold A, Plant GT, Gilhus NE. Anti-voltage-gated potassium channel Kv1. 4 antibodies in myasthenia gravis. J Neurol. 2012;259(7):1312–1316. doi: 10.1007/s00415-011-6344-y. [DOI] [PubMed] [Google Scholar]
- 94.Higuchi O, Hamuro J, Motomura M, Yamanashi Y. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol. 2011;69(2):418–422. doi: 10.1002/ana.22312. [DOI] [PubMed] [Google Scholar]
- 95.Pevzner A, Schoser B, Peters K, Cosma NC, Karakatsani A, Schalke B, Melms A, Kroger S. Anti-LRP4 autoantibodies in AChR- and MuSK-antibody-negative myasthenia gravis. J Neurol. 2012;259(3):427–435. doi: 10.1007/s00415-011-6194-7. [DOI] [PubMed] [Google Scholar]
- 96.Zhang B, Tzartos JS, Belimezi M, Ragheb S, Bealmear B, Lewis RA, Xiong WC, Lisak RP, Tzartos SJ, Mei L. Autoantibodies to lipoprotein-related protein 4 in patients with double-seronegative myasthenia gravis. Arch Neurol. 2012;69(4):445–451. doi: 10.1001/archneurol.2011.2393. [DOI] [PubMed] [Google Scholar]
- 97.Gasperi C, Melms A, Schoser B, Zhang Y, Meltoranta J, Risson V, Schaeffer L, Schalke B, Kroger S. Anti-agrin autoantibodies in myasthenia gravis. Neurology. 2014;82(22):1976–1983. doi: 10.1212/WNL.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 98.Gallardo E, Martinez-Hernandez E, Titulaer MJ, Huijbers MG, Martinez MA, Ramos A, Querol L, Diaz-Manera J, Rojas-Garcia R, Hayworth CR, Verschuuren JJ, Balice-Gordon R, Dalmau J, Illa I. Cortactin autoantibodies in myasthenia gravis. Autoimmun Rev. 2014;13(10):1003–1007. doi: 10.1016/j.autrev.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 99.Zoltowska Katarzyna M, Belaya K, Leite M, Patrick W, Vincent A, Beeson D. Collagen Q--a potential target for autoantibodies in myasthenia gravis. J Neurol Sci. 2015;348(1–2):241–244. doi: 10.1016/j.jns.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang B, Shen C, Bealmear B, Ragheb S, Xiong WC, Lewis RA, Lisak RP, Mei L. Autoantibodies to agrin in myasthenia gravis patients. PLoS One. 2014;9(3):e91816. doi: 10.1371/journal.pone.0091816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stergiou C, Lazaridis K, Zouvelou V, Tzartos J, Mantegazza R, Antozzi C, Andreetta F, Evoli A, Deymeer F, Saruhan-Direskeneli G, Durmus H, Brenner T, Vaknin A, Berrih-Aknin S, Behin A, Sharshar T, De Baets M, Losen M, Martinez-Martinez P, Kleopa KA, Zamba-Papanicolaou E, Kyriakides T, Kostera-Pruszczyk A, Szczudlik P, Szyluk B, Lavrnic D, Basta I, Peric S, Tallaksen C, Maniaol A, Gilhus NE, Casasnovas Pons C, Pitha J, Jakubikova M, Hanisch F, Bogomolovas J, Labeit D, Labeit S, Tzartos SJ. Titin antibodies in “seronegative” myasthenia gravis--A new role for an old antigen. J Neuroimmunol. 2016;292:108–115. doi: 10.1016/j.jneuroim.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 102.Corsiero E, Nerviani A, Bombardieri M, Pitzalis C. Ectopic Lymphoid Structures: Powerhouse of Autoimmunity. Front Immunol. 2016;7:430. doi: 10.3389/fimmu.2016.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, Pitzalis C. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6(1):e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mittag T, Kornfeld P, Tormay A, Woo C. Detection of anti-acetylcholine receptor factors in serum and thymus from patients with myasthenia gravis. N Engl J Med. 1976;294(13):691–694. doi: 10.1056/NEJM197603252941303. [DOI] [PubMed] [Google Scholar]
- 105.Vincent A, Scadding GK, Thomas HC, Newsom-Davis J. In-vitro synthesis of anti-acetylcholine-receptor antibody by thymic lymphocytes in myasthenia gravis. Lancet. 1978;1(8059):305–307. doi: 10.1016/s0140-6736(78)90073-9. [DOI] [PubMed] [Google Scholar]
- 106.Marx A, Pfister F, Schalke B, Saruhan-Direskeneli G, Melms A, Strobel P. The different roles of the thymus in the pathogenesis of the various myasthenia gravis subtypes. Autoimmun Rev. 2013;12(9):875–884. doi: 10.1016/j.autrev.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 107.Newsom-Davis J, Willcox N, Calder L. Thymus cells in myasthenia gravis selectively enhance production of anti-acetylcholine-receptor antibody by autologous blood lymphocytes. N Engl J Med. 1981;305(22):1313–1318. doi: 10.1056/NEJM198111263052203. [DOI] [PubMed] [Google Scholar]
- 108.Leprince C, Cohen-Kaminsky S, Berrih-Aknin S, Vernet-Der Garabedian B, Treton D, Galanaud P, Richard Y. Thymic B cells from myasthenia gravis patients are activated B cells. Phenotypic and functional analysis J Immunol. 1990;145(7):2115–2122. [PubMed] [Google Scholar]
- 109.Berrih-Aknin S, Le Panse R. Myasthenia gravis: a comprehensive review of immune dysregulation and etiological mechanisms. J Autoimmun. 2014;52:90–100. doi: 10.1016/j.jaut.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 110.Willcox HN, Newsom-Davis J, Calder LR. Cell types required for anti-acetylcholine receptor antibody synthesis by cultured thymocytes and blood lymphocytes in myasthenia gravis. Clin Exp Immunol. 1984;58(1):97–106. [PMC free article] [PubMed] [Google Scholar]
- 111.Scadding GK, Vincent A, Newsom-Davis J, Henry K. Acetylcholine receptor antibody synthesis by thymic lymphocytes: correlation with thymic histology. Neurology. 1981;31(8):935–943. doi: 10.1212/wnl.31.8.935. [DOI] [PubMed] [Google Scholar]
- 112.Lisak RP, Levinson AI, Zweiman B, Kornstein MJ. Antibodies to acetylcholine receptor and tetanus toxoid: in vitro synthesis by thymic lymphocytes. J Immunol. 1986;137(4):1221–1225. [PubMed] [Google Scholar]
- 113.Levinson AI, Zweiman B, Lisak RP, Dziarski A, Moskovitz AR. Thymic B-cell activation in myasthenia gravis. Neurology. 1984;34(4):462–468. doi: 10.1212/wnl.34.4.462. [DOI] [PubMed] [Google Scholar]
- 114.Sims GP, Shiono H, Willcox N, Stott DI. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J Immunol. 2001;167(4):1935–1944. doi: 10.4049/jimmunol.167.4.1935. [DOI] [PubMed] [Google Scholar]
- 115.Vrolix K, Fraussen J, Losen M, Stevens J, Lazaridis K, Molenaar PC, Somers V, Bracho MA, Le Panse R, Stinissen P, Berrih-Aknin S, Maessen JG, Van Garsse L, Buurman WA, Tzartos SJ, De Baets MH, Martinez-Martinez P. Clonal heterogeneity of thymic B cells from early-onset myasthenia gravis patients with antibodies against the acetylcholine receptor. J Autoimmun. 2014;52:101–112. doi: 10.1016/j.jaut.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 116.Graus YF, de Baets MH, Parren PW, Berrih-Aknin S, Wokke J, van Breda Vriesman PJ, Burton DR. Human anti-nicotinic acetylcholine receptor recombinant Fab fragments isolated from thymus-derived phage display libraries from myasthenia gravis patients reflect predominant specificities in serum and block the action of pathogenic serum antibodies. J Immunol. 1997;158(4):1919–1929. [PubMed] [Google Scholar]
- 117.Cardona A, Pritsch O, Dumas G, Bach JF, Dighiero G. Evidence for an antigen-driven selection process in human autoantibodies against acetylcholine receptor. Mol Immunol. 1995;32(16):1215–1223. doi: 10.1016/0161-5890(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 118.Farrar J, Portolano S, Willcox N, Vincent A, Jacobson L, Newsom-Davis J, Rapoport B, McLachlan SM. Diverse Fab specific for acetylcholine receptor epitopes from a myasthenia gravis thymus combinatorial library. Int Immunol. 1997;9(9):1311–1318. doi: 10.1093/intimm/9.9.1311. [DOI] [PubMed] [Google Scholar]
- 119.Zuckerman NS, Howard WA, Bismuth J, Gibson K, Edelman H, Berrih-Aknin S, Dunn-Walters D, Mehr R. Ectopic GC in the thymus of myasthenia gravis patients show characteristics of normal GC. Eur J Immunol. 2010;40(4):1150–1161. doi: 10.1002/eji.200939914. [DOI] [PubMed] [Google Scholar]
- 120.Hill ME, Shiono H, Newsom-Davis J, Willcox N. The myasthenia gravis thymus: a rare source of human autoantibody-secreting plasma cells for testing potential therapeutics. J Neuroimmunol. 2008;201–202:50–56. doi: 10.1016/j.jneuroim.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 121.Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, Strobel P, Mazia C, Oger J, Cea JG, Heckmann JM, Evoli A, Nix W, Ciafaloni E, Antonini G, Witoonpanich R, King JO, Beydoun SR, Chalk CH, Barboi AC, Amato AA, Shaibani AI, Katirji B, Lecky BR, Buckley C, Vincent A, Dias-Tosta E, Yoshikawa H, Waddington-Cruz M, Pulley MT, Rivner MH, Kostera-Pruszczyk A, Pascuzzi RM, Jackson CE, Garcia Ramos GS, Verschuuren JJ, Massey JM, Kissel JT, Werneck LC, Benatar M, Barohn RJ, Tandan R, Mozaffar T, Conwit R, Odenkirchen J, Sonett JR, Jaretzki A, 3rd, Newsom-Davis J, Cutter GR, Group MS. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med. 2016;375(6):511–522. doi: 10.1056/NEJMoa1602489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vincent A, Newsom-Davis J, Newton P, Beck N. Acetylcholine receptor antibody and clinical response to thymectomy in myasthenia gravis. Neurology. 1983;33(10):1276–1282. doi: 10.1212/wnl.33.10.1276. [DOI] [PubMed] [Google Scholar]
- 123.Okumura M, Ohta M, Takeuchi Y, Shiono H, Inoue M, Fukuhara K, Kadota Y, Miyoshi S, Fujii Y, Matsuda H. The immunologic role of thymectomy in the treatment of myasthenia gravis: implication of thymus-associated B-lymphocyte subset in reduction of the anti-acetylcholine receptor antibody titer. J Thorac Cardiovasc Surg. 2003;126(6):1922–1928. doi: 10.1016/s0022-5223(03)00938-3. [DOI] [PubMed] [Google Scholar]
- 124.Newsom-Davis J, Willcox N, Scadding G, Calder L, Vincent A. Anti-acetylcholine receptor antibody synthesis by cultured lymphocytes in myasthenia gravis: thymic and peripheral blood cell interactions. Ann N Y Acad Sci. 1981;377:393–402. doi: 10.1111/j.1749-6632.1981.tb33747.x. [DOI] [PubMed] [Google Scholar]
- 125.Fujii Y, Monden Y, Hashimoto J, Nakahara K, Kawashima Y. Acetylcholine receptor antibody-producing cells in thymus and lymph nodes in myasthenia gravis. Clin Immunol Immunopathol. 1985;34(1):141–146. doi: 10.1016/0090-1229(85)90018-2. [DOI] [PubMed] [Google Scholar]
- 126.Fujii Y, Monden Y, Hashimoto J, Nakahara K, Kawashima Y. Acetylcholine receptor antibody production by bone marrow cells in a patient with myasthenia gravis. Neurology. 1985;35(4):577–579. doi: 10.1212/wnl.35.4.577. [DOI] [PubMed] [Google Scholar]
- 127.Fujii Y, Hashimoto J, Monden Y, Ito T, Nakahara K, Kawashima Y. Specific activation of lymphocytes against acetylcholine receptor in the thymus in myasthenia gravis. J Immunol. 1986;136(3):887–891. [PubMed] [Google Scholar]
- 128.Diaz-Manera J, Martinez-Hernandez E, Querol L, Klooster R, Rojas-Garcia R, Suarez-Calvet X, Munoz-Blanco JL, Mazia C, Straasheijm KR, Gallardo E, Juarez C, Verschuuren JJ, Illa I. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78(3):189–193. doi: 10.1212/WNL.0b013e3182407982. [DOI] [PubMed] [Google Scholar]
- 129.Rowland LP, Hoefer PF, Aranow H, Jr, Merritt HH. Fatalities in myasthenia gravis; a review of 39 cases with 26 autopsies. Neurology. 1956;6(5):307–326. doi: 10.1212/wnl.6.5.307. [DOI] [PubMed] [Google Scholar]
- 130.Russell DS. Histological changes in the striped muscles in myasthenia gravis. J Pathol Bacteriol. 1953;65(2):279–289. doi: 10.1002/path.1700650202. [DOI] [PubMed] [Google Scholar]
- 131.Pascuzzi RM, Campa JF. Lymphorrhage localized to the muscle end-plate in myasthenia gravis. Arch Pathol Lab Med. 1988;112(9):934–937. [PubMed] [Google Scholar]
- 132.Maselli RA, Richman DP, Wollmann RL. Inflammation at the neuromuscular junction in myasthenia gravis. Neurology. 1991;41(9):1497–1504. doi: 10.1212/wnl.41.9.1497. [DOI] [PubMed] [Google Scholar]
- 133.Vander Heiden JA, Stathopoulos P, Zhou JQ, Chen L, Gilbert TJ, Bolen CR, Barohn RJ, Dimachkie MM, Ciafaloni E, Broering TJ, Vigneault F, Nowak RJ, Kleinstein SH, O’Connor KC. Dysregulation of B Cell Repertoire Formation in Myasthenia Gravis Patients Revealed through Deep Sequencing. J Immunol. 2017;198(4):1460–1473. doi: 10.4049/jimmunol.1601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leite MI, Strobel P, Jones M, Micklem K, Moritz R, Gold R, Niks EH, Berrih-Aknin S, Scaravilli F, Canelhas A, Marx A, Newsom-Davis J, Willcox N, Vincent A. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57(3):444–448. doi: 10.1002/ana.20386. [DOI] [PubMed] [Google Scholar]
- 135.Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, Hupperts R, Somers V. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J Immunol. 2016;197(12):4576–4583. doi: 10.4049/jimmunol.1502448. [DOI] [PubMed] [Google Scholar]
- 136.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178(10):6624–6633. doi: 10.4049/jimmunol.178.10.6624. [DOI] [PubMed] [Google Scholar]
- 137.Kohler S, Keil TO, Swierzy M, Hoffmann S, Schaffert H, Ismail M, Ruckert JC, Alexander T, Hiepe F, Gross C, Thiel A, Meisel A. Disturbed B cell subpopulations and increased plasma cells in myasthenia gravis patients. J Neuroimmunol. 2013 doi: 10.1016/j.jneuroim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 138.Ragheb S, Lisak RP. The frequency of CD5+ B lymphocytes in the peripheral blood of patients with myasthenia gravis. Neurology. 1990;40(7):1120–1124. doi: 10.1212/wnl.40.7.1120. [DOI] [PubMed] [Google Scholar]
- 139.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. Journal of immunology. 2015;194(4):1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 140.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. doi: 10.1016/j.immuni.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 141.Lykken JM, Candando KM, Tedder TF. Regulatory B10 cell development and function. International immunology. 2015;27(10):471–477. doi: 10.1093/intimm/dxv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Das A, Ellis G, Pallant C, Lopes AR, Khanna P, Peppa D, Chen A, Blair P, Dusheiko G, Gill U, Kennedy PT, Brunetto M, Lampertico P, Mauri C, Maini MK. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection. Journal of immunology. 2012;189(8):3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Science translational medicine. 2013;5(173):173ra123. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 145.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, Ruckert B, Akdis CA, Akdis M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131(4):1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 146.Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity. 2011;34(5):703–714. doi: 10.1016/j.immuni.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 147.Sun F, Ladha SS, Yang L, Liu Q, Shi SX, Su N, Bomprezzi R, Shi FD. Interleukin-10 producing-B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve. 2014;49(4):487–494. doi: 10.1002/mus.23951. [DOI] [PubMed] [Google Scholar]
- 148.Yi JS, Russo MA, Massey JM, Juel V, Hobson-Webb LD, Gable K, Raja SM, Balderson K, Weinhold KJ, Guptill JT. B10 Cell Frequencies and Suppressive Capacity in Myasthenia Gravis Are Associated with Disease Severity. Front Neurol. 2017;8:34. doi: 10.3389/fneur.2017.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Guptill JT, Yi JS, Sanders DB, Guidon AC, Juel VC, Massey JM, Howard JF, Jr, Scuderi F, Bartoccioni E, Evoli A, Weinhold KJ. Characterization of B cells in muscle-specific kinase antibody myasthenia gravis. Neurol Neuroimmunol Neuroinflamm. 2015;2(2):e77. doi: 10.1212/NXI.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sheng JR, Quan S, Soliven B. IL-10 derived from CD1dhiCD5(+) B cells regulates experimental autoimmune myasthenia gravis. Journal of neuroimmunology. 2015;289:130–138. doi: 10.1016/j.jneuroim.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 151.Sheng JR, Rezania K, Soliven B. Impaired regulatory B cells in myasthenia gravis. Journal of neuroimmunology. 2016;297:38–45. doi: 10.1016/j.jneuroim.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 152.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 154.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201(10):1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee J-Y, Stathopoulos P, Gupta S, Bannock JM, Barohn RJ, Cotzomi E, Dimachkie MM, Jacobson L, Lee CS, Morbach H, Querol L, Shan J-L, Vander Heiden JA, Waters P, Vincent A, Nowak RJ, O’Connor KC. Compromised fidelity of B-cell tolerance checkpoints in AChR and MuSK myasthenia gravis. Annals of Clinical and Translational Neurology. 2016;3(6):443–454. doi: 10.1002/acn3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Glanville J, Zhai W, Berka J, Telman D, Huerta G, Mehta GR, Ni I, Mei L, Sundar PD, Day GMR, Cox D, Rajpal A, Pons J. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20216–20221. doi: 10.1073/pnas.0909775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Ann Oncol. 2003;14(4):520–535. doi: 10.1093/annonc/mdg175. [DOI] [PubMed] [Google Scholar]
- 159.Dorner T, Isenberg D, Jayne D, Wiendl H, Zillikens D, Burmester G International Roundtable on BcaTTfI. Current status on B-cell depletion therapy in autoimmune diseases other than rheumatoid arthritis. Autoimmun Rev. 2009;9(2):82–89. doi: 10.1016/j.autrev.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 160.Gajra A, Vajpayee N, Grethlein SJ. Response of myasthenia gravis to rituximab in a patient with non-Hodgkin lymphoma. Am J Hematol. 2004;77(2):196–197. doi: 10.1002/ajh.20169. [DOI] [PubMed] [Google Scholar]
- 161.Lebrun C, Bourg V, Tieulie N, Thomas P. Successful treatment of refractory generalized myasthenia gravis with rituximab. Eur J Neurol. 2009;16(2):246–250. doi: 10.1111/j.1468-1331.2008.02399.x. [DOI] [PubMed] [Google Scholar]
- 162.Illa I, Diaz-Manera J, Rojas-Garcia R, Pradas J, Rey A, Blesa R, Juarez C, Gallardo E. Sustained response to Rituximab in anti-AChR and anti-MuSK positive Myasthenia Gravis patients. J Neuroimmunol. 2008;201–202:90–94. doi: 10.1016/j.jneuroim.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 163.Nowak RJ, Dicapua DB, Zebardast N, Goldstein JM. Response of patients with refractory myasthenia gravis to rituximab: a retrospective study. Ther Adv Neurol Disord. 2011;4(5):259–266. doi: 10.1177/1756285611411503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zebardast N, Patwa HS, Novella SP, Goldstein JM. Rituximab in the management of refractory myasthenia gravis. Muscle Nerve. 2010;41(3):375–378. doi: 10.1002/mus.21521. [DOI] [PubMed] [Google Scholar]
- 165.Robeson KR, Kumar A, Keung B, DiCapua DB, Grodinsky E, Patwa HS, Stathopoulos PA, Goldstein JM, O’Connor KC, Nowak RJ. Durability of the Rituximab Response in Acetylcholine Receptor Autoantibody-Positive Myasthenia Gravis. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.4190. [DOI] [PubMed] [Google Scholar]
- 166.Chamberlain N, Massad C, Oe T, Cantaert T, Herold KC, Meffre E. Rituximab does not reset defective early B cell tolerance checkpoints. J Clin Invest. 2016;126(1):282–287. doi: 10.1172/JCI83840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pellkofer HL, Krumbholz M, Berthele A, Hemmer B, Gerdes LA, Havla J, Bittner R, Canis M, Meinl E, Hohlfeld R, Kuempfel T. Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology. 2011;76(15):1310–1315. doi: 10.1212/WNL.0b013e3182152881. [DOI] [PubMed] [Google Scholar]
- 168.Quach TD, Rodriguez-Zhurbenko N, Hopkins TJ, Guo X, Hernandez AM, Li W, Rothstein TL. Distinctions among Circulating Antibody-Secreting Cell Populations, Including B-1 Cells, in Human Adult Peripheral Blood. J Immunol. 2016;196(3):1060–1069. doi: 10.4049/jimmunol.1501843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lanzillotta M, Della-Torre E, Stone JH. Roles of Plasmablasts and B Cells in IgG4-Related Disease: Implications for Therapy and Early Treatment Outcomes. Curr Top Microbiol Immunol. 2017 doi: 10.1007/82_2016_58. [DOI] [PubMed] [Google Scholar]
- 170.Stathopoulos P, Kumar A, Nowak RJ, O’Connor KC. Post B cell depletion autoantibody-producing plasmablasts identified in muscle-specific kinase myasthenia gravis. J Clin Invest Insight. 2017 doi: 10.1172/jci.insight.94263. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chihara N, Aranami T, Oki S, Matsuoka T, Nakamura M, Kishida H, Yokoyama K, Kuroiwa Y, Hattori N, Okamoto T, Murata M, Toda T, Miyake S, Yamamura T. Plasmablasts as migratory IgG-producing cells in the pathogenesis of neuromyelitis optica. PLoS One. 2013;8(12):e83036. doi: 10.1371/journal.pone.0083036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chihara N, Aranami T, Sato W, Miyazaki Y, Miyake S, Okamoto T, Ogawa M, Toda T, Yamamura T. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci U S A. 2011;108(9):3701–3706. doi: 10.1073/pnas.1017385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol. 2016;29(6):806–812. doi: 10.1097/WCO.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 174.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 175.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England journal of medicine. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Johnson DB, Saranga-Perry V, Lavin PJ, Burnette WB, Clark SW, Uskavitch DR, Wallace DE, Dickson MA, Kudchadkar RR, Sosman JA. Myasthenia Gravis Induced by Ipilimumab in Patients With Metastatic Melanoma. J Clin Oncol. 2015;33(33):e122–124. doi: 10.1200/JCO.2013.51.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Antonia S, Goldberg SB, Balmanoukian A, Chaft JE, Sanborn RE, Gupta A, Narwal R, Steele K, Gu Y, Karakunnel JJ, Rizvi NA. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17(3):299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Loochtan AI, Nickolich MS, Hobson-Webb LD. Myasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancer. Muscle & nerve. 2015;52(2):307–308. doi: 10.1002/mus.24648. [DOI] [PubMed] [Google Scholar]
- 180.Lau KH, Kumar A, Yang IH, Nowak RJ. Exacerbation of myasthenia gravis in a patient with melanoma treated with pembrolizumab. Muscle & nerve. 2016;54(1):157–161. doi: 10.1002/mus.25141. [DOI] [PubMed] [Google Scholar]
- 181.Maeda O, Yokota K, Atsuta N, Katsuno M, Akiyama M, Ando Y. Nivolumab for the treatment of malignant melanoma in a patient with pre-existing myasthenia gravis. Nagoya J Med Sci. 2016;78(1):119–122. [PMC free article] [PubMed] [Google Scholar]
- 182.Gonzalez NL, Puwanant A, Lu A, Marks SM, Zivkovic SA. Myasthenia triggered by immune checkpoint inhibitors: New case and literature review. Neuromuscul Disord. 2017;27(3):266–268. doi: 10.1016/j.nmd.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 183.Cooper DS, Meriggioli MN, Bonomi PD, Malik R. Severe Exacerbation of Myasthenia Gravis Associated with Checkpoint Inhibitor Immunotherapy. J Neuromuscul Dis. 2017;4(2):169–173. doi: 10.3233/JND-170219. [DOI] [PubMed] [Google Scholar]
- 184.Makarious D, Horwood K, Coward JIG. Myasthenia gravis: An emerging toxicity of immune checkpoint inhibitors. Eur J Cancer. 2017;82:128–136. doi: 10.1016/j.ejca.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 185.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L, Opera I Investigators OIC. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 186.Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, Di Zenzo G, Lanzavecchia A, Seykora JT, Cotsarelis G, Milone MC, Payne AS. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]