Abstract
There is a growing body of research on matching- and non-matching-to-sample (MTS, NMTS) relations with rats using olfactory stimuli; however, the specific characteristics of this relational control are unclear. In the current study we examine MTS and NMTS in rats with an automated olfactometer using a successive (go, no-go) procedure. Ten rats were trained to either match- or non-match-to-sample with common scents (apple, cinnamon, etc.) as olfactory stimuli. After matching or non-matching training with four odorants, rats were tested for transfer twice with four new odorants on each test. Most rats trained on MTS showed immediate transfer to new stimuli, and most rats trained on NMTS showed full transfer by the second set of new odors. After meeting criterion on the second transfer test, the contingencies were reversed with four new odor stimuli such that subjects trained on matching were shifted to non-matching and vice versa. Following these reversed contingencies, the effects of the original training persisted for many trials with new odorants. These data extend previous studies on same-different concept formation in rats, showing strong generalization requiring few exemplars. The critical role of olfactory stimuli is discussed.
Keywords: olfaction, matching-to-sample, identity, same-different, rats, abstract concepts
1. Introduction
As identity and oddity are two of the most elemental concepts of learning, they have been the focus of most recent research on concept learning in nonhumans. Identity and oddity can be operationalized by same/different or match-/non-match-to-sample (MTS/NMTS) procedures, such that successful transfer to novel stimuli defines the emergence of concept learning. Using such procedures, identity/oddity has been demonstrated in a number of species, including primates (D'Amato, Salmon, & Colombo, 1985; Katz, Wright, & Bachevalier, 2002; Vonk, 2003), dolphins (Herman, Hovancik, Gory, & Bradshaw, 1989), sea lions (Kastak & Schusterman, 1994), harbor seals (Scholtyssek, Kelber, Hanke, Dehnhardt, 2013); echidna (Russell & Burke, 2016), pigeons and other birds (Bodily, Katz, & Wright, 2008; Magnotti, Katz, Wright, & Kelly, 2015; Wright, Cook, Rivera, Sands, & Delius, 1988), and honeybees (Giurfa, Zhang, Jenett, Menzel, & Srinivasan, 2001). Initial studies with rodents using visual stimuli (e.g., Iversen, 1993; 1997) failed to show identity/oddity but with the use of olfactory stimuli there has been more success (e.g., April, Bruce, & Galizio, 2011; Lu, Slotnick & Silberberg, 1993; Otto & Eichenbaum, 1992; Peña, Pitts, & Galizio, 2006; Prichard, Panoz-Brown, Bruce, & Galizio, 2015).
For example, using an olfactory discrimination procedure, Peña, et al. (2006) trained rats to dig in sand scented with common household spices to obtain sucrose pellets (cf. Dusek & Eichenbaum, 1997) and found evidence for generalized matching-to-sample. Rats were initially trained on a single conditional discrimination (two olfactory stimuli) and novel olfactory stimuli were added as criterion level performances were reached. At the end of the study, rats were matching at high levels of accuracy with 20 or more different stimuli and responses to novel stimuli were well above chance levels in three of the four rats tested. However, as novel stimuli were only introduced one or two at a time, it was not possible to identify precisely at what point generalized matching developed.
April, et al. (2011) used a similar olfactory discrimination procedure to train six rats on either MTS or NMTS. In this study, a reversal procedure based on the Zentall and Hogan (1974) study with pigeons was used such that after initial MTS or NMTS training, contingencies were switched and transfer assessed. Zentall and Hogan inferred concept learning from response persistence to the originally trained contingency. April et al. trained rats on either MTS or NMTS with five scent stimuli (set A); once rats responded with 90% accuracy, they were switched to five new stimuli (set B) and showed evidence of savings in these transfer tests. After 15 sessions with these stimuli (set B), a new stimulus set of five odors (set C) was presented with the previous contingencies reversed. Initial levels of accuracy were quite low as all animals continued to respond in line with the original MTS or NMTS contingency from sets A and B. Even after extended training, most animals failed to exceed chance levels of responding on the new contingency with set C. This study showed that rats developed the identity or oddity relation with as few as 10 exemplars.
The studies noted above used scented sand to present the odorants and a manual procedure with simultaneous conditional discrimination training. In the Pena, et al. (2006) study, rats were tested in an operant chamber modified to allow the experimenter to manually insert a tray with the sample and the two comparisons. In the April et al. (2011) study, rats were presented with sample stimuli in a holding cage, then moved to a circular arena in which the comparison stimuli were presented. An alternative to this approach is to use automated presentation of the odorants in an operant chamber and record nose-poking behavior at the port where the scent is introduced. Instead of a two-alternative choice procedure for presentation of comparison stimuli, a go, no-go procedure is trained. After the sample odor is presented, one comparison odor is presented, and the rat learns to “go” (nose-poke) to earn reinforcement when the comparison matches the sample (in a MTS paradigm) and to withhold responding (no-go) when the comparison does not match the sample. The automated procedure increases experimental control by minimizing effects of handling and other distractions for the subject, as well as providing a more precise dependent measure. Slotnick and colleagues (see Slotnick, 2001 for a review) developed this procedure for rats and mice, demonstrating both discrimination and MTS with odor stimuli. In particular, Lu, et al. (1993) used an automated procedure to test odor matching in rats, using successive conditional discrimination training (go, no-go). They found that rats learned an olfactory MTS task even with delays with a masking odor up to 10 seconds between stimuli and showed rapid transfer of learning to new sets of odor stimuli. However, because performance on initial transfer tests (before reinforcement) was not presented, it was not possible to determine whether transfer involved generalized control by the identity relation or by rapid learning of new stimulus sets.
Using a similar automated procedure, Prichard, et al. (2015) trained six rats on a go, no-go MTS procedure with four odor stimuli. Stimuli were presented in pairs and nose-poke responses to matching odor pairs, but not non-matching pairs, were reinforced. Once rats met criterion responding, non-reinforced probe trials with novel odors were intermittently introduced. Most rats showed high levels of transfer, suggesting that four exemplars (i.e., four different odors and eight trial-type combinations) may be sufficient for emergence of the identity relation. This outcome was surprising as most studies have found that many more trained exemplars are necessary to produce reliable transfer in other species (Katz & Wright, 2006; Wright, Magnotti, Katz, Leonard, & Kelly, 2016).
In the current study, we were interested in extending the research of Prichard et al. (2015) to include an analysis of the non-identity as well as the identity relation. Further, we wanted to examine transfer across stimuli and persistence of the original contingencies to infer concept learning. Thus, we used the same automated olfactometer set-up to present odor stimuli with a successive discrimination procedure to replicate and extend Prichard, et al. (2015), and employed a reversal design, similar to Zentall and Hogan (1974) and April, et al. (2011).
2. Method
2.1. Subjects
The subjects of this experiment were 15 male Sprague-Dawley albino rats approximately 90–150 days old at the beginning of training. Some of the animals were trained to lever-press prior to beginning the present study, but all were naïve to training with odor stimuli and the olfactometer procedures. All rats were individually housed on a reversed 12-hour light-dark cycle. The rats were maintained at 85 percent of their free feeding weight and received ad libitum access to water in their home cages. All experiments were performed during the dark phase, between 7:00 a.m. and 7:00 p.m. Rats were fed Lab Diet Rat Chow daily approximately 1 hr following their individual experimental session. Animals were maintained and data were collected in accordance with the NIH Guide for the Care and Use of Laboratory Animals; all researchers completed IACUC training and the study was approved by the UNCW Animal Care and Use Committee.
2.2. Apparatus
Sessions were conducted in Med Associates operant chambers with three response ports located across the front panel; however, only the center port (2.5 cm diameter) was activated during the experiment. Inside the center port was a stimulus light, infrared photo beam response detector, and openings for scents to be pumped in and drawn out. The chamber measured 30.5 cm long by 24 cm wide by 21 cm high with a pellet dispenser located on the opposite side of the chamber from the response ports. Chambers were housed in sound attenuating cubicles. Each chamber was interfaced to a computer equipped with MED-PC software. Three five-channel Med Associates olfactometer systems (ENV-275-5) were added to each chamber. An input pump (Linear AC0102, 2.84 pound per square inch with an airflow of .177 cubic feet per min) delivered air through glass jars containing an odorant solution to solenoids that, when operated, forced scented air through Teflon tubing and a manifold into the center nose port of the chamber. A vacuum pump (Linear VP0125, −9.84 Hg vacuum and air displacement of .247 cubic feet/min) removed air from a tube located at the bottom of the center port. Thus, the system was capable of delivering 15 separate odors through the center response port [see Prichard, et al. (2015) for an illustration].
2.3. Odorants
Liquid odorants purchased from The Great American Spice Company, Nature’s Garden, and local stores were used to create four sets of stimuli: Set A (cinnamon, apricot, bubblegum, root beer), Set B (brandy, vanilla butternut, almond, licorice), Set C (apple, grass, coconut, sandalwood), Set D (clove, honey, blueberry, geraniol). A fifth set (E: lemon, maple, lavender, peppermint) was used with one rat but the peppermint oil appeared to contaminate the apparatus and disrupt performance, so these scents were discontinued. Odorants were diluted to a solution of 6.7 ml oil per 100 ml distilled water. Glassware was cleaned at the end of each testing day and solutions were refilled every morning.
2.4. Procedure
2.4.1. Shaping phase
An initial session of magazine training was followed by response training in which both the center port light and house light were illuminated. During this phase, a single nose-poke turned off both lights, and delivered a sugar pellet accompanied by a light above the food hopper. After a 5-s period, the hopper light went out and the house and center-port lights came on and the procedure continued to provide reinforcement on a FR1 schedule. Once regular responding was established, the reinforcement schedule was progressively increased to FI-5s over several sessions. To acclimate animals to scent delivery through the center port, four odorants were introduced for each rat (see Table 1). Each trial began with the onset of the house and center port lights and delivery of one of the four odorants; completion of the FI-5s schedule terminated the lights and odorant delivery and produced reinforcement and the onset of the hopper light for 5 s.
Table 1.
Odor sequence for each rat
| Initial Training | Transfer 1 | Transfer 2 | Reversal | |
|---|---|---|---|---|
| Rat | MTS1 | MTS2 | MTS3 | NMTS |
|
| ||||
| K2 | A | E/A* | C | D |
| K3 | A | B | C | D |
| M19 | B | A | D | C |
| M18 | A | D | B | C |
| K35 | B | A | C | D |
| Rat | NMTS1 | NMTS2 | NMTS3 | MTS |
|
| ||||
| K31 | A | B | C | D |
| M15 | A | B | D | C |
| M16 | A | B | C | D |
| M12 | B | A | D | C |
| K19 | A | D | C | B |
Initially Set E (lemon, maple, lavender, and peppermint) was used but we found that peppermint appeared to contaminate the apparatus. Thus K2 had 8 days of transfer phase 1 with odor Set E before he was switched back to Set A; he met criterion again before the transfer phase 2 with Set C. No other rats were tested with Set E.
2.4.2. Initial conditional discrimination training phase
Once rats were consistently responding to all four scents throughout the session, conditional discrimination training began. Rats were randomly assigned to either MTS or NMTS training and began training with the initial stimulus set used in shaping (see Table 1). All trials consisted of stimulus pairs presented through the center port, and only center port responses were effective throughout the experiment. Trials began with the onset of the house light and center port light. Following an initial observing nose-poke response, a sample odor was presented, with the first nose poke after 5 s (FI-5s schedule) resulting in a 1 s termination of the house and center port lights, followed by the onset of the comparison odor and both lights. On positive trials (matching for the MTS group and non-matching for the NMTS group), responding was reinforced on an FI-5s schedule. The first response after 5 s resulted in termination of the comparison odor, the house light, and the center port light and a 5 s onset of the hopper light along with delivery of a sugar pellet. On negative trials, the comparison was presented for 5 s and then terminated, along with the house and center port lights. A 30 s inter-trial interval separated the termination of the comparison stimuli and the initiation of the following trial. While the number of nose-pokes was recorded for each element of the trial [observing response, sample presentation, comparison (S+ or S−) presentation], only those nose-pokes occurring during the 5 s when the S+ or S− was presented were used to calculate discrimination ratios or response rates (see below).
Sessions were conducted five days/week. Each session was composed of 48 trials and included eight different trial types (four sets of exemplars), four positive and four negative. For example, for Set A scents, MTS positive trial types were cinnamon-cinnamon, apricot-apricot, bubblegum-bubblegum, and root beer-root beer; negative trial types were cinnamon-bubblegum, apricot-root beer, bubblegum-cinnamon, and root beer-apricot. For the subjects initially trained on NMTS, Set A positive trial types were cinnamon-bubblegum, apricot-root beer, bubblegum-cinnamon, and root beer-apricot; negative trial types were cinnamon-cinnamon, apricot-apricot, bubblegum-bubblegum, and root beer-root beer. Trial types were randomly determined with the constraint that no more than four consecutive positive (reinforced) or negative (non-reinforced) trial types were permitted. Further, odor pairings were constructed so that each odorant occurred equally often as a sample and comparison. Initially, training continued until a mastery criterion was met such that an average discrimination ratio (DR: responses to S+ divided by responses to both S+ and S−) of .85 with a minimum DR of .80 on each set of trial types was met on two consecutive sessions. Although some rats met this criterion, many developed discriminative performances but failed to meet criterion even after extensive training, and the criterion was reduced to a mean DR of .80 on each set of trial types for two consecutive sessions, which was further weakened to .80 for a single session. Despite these changes, five rats failed to meet training criterion after more than 75 sessions of training and were dropped from the study (two in MTS and three in NMTS—four never met criterion on the initial set, and one failed to meet criterion on Set 3).
2.4.3. Transfer to novel odors phase
When the prevailing criterion was met, rats were advanced to transfer phase 1 in which a new set of four odorants was presented (see Table 1) under the same contingencies used in the initial training phase (MTS or NMTS). Training continued on the second set of odors until rats met the mastery criterion as described above. When the criterion was met, rats were advanced to another new set of four odorants with the same contingencies (transfer phase 2).
2.4.4. Reversal phase
Once mastery was met on this third set of odors, rats were moved to the reversal phase in which four new odorants were presented, but the contingencies were reversed (i.e., MTS animals were reversed to NMTS and vice versa), and these reversal contingencies were maintained for 10 sessions.
2.5. Data analysis
The primary measure of interest was transfer of matching or non-matching on the initial exposure to novel stimuli in the transfer and reversal phases. This was assessed by comparing response rates on positive and negative trial types during the first session with each novel stimulus set to those obtained during the final (criterion) session of the previous set. In addition, response rates on the initial exposure to each novel trial type during the first session with a new stimulus set were analyzed separately. Finally, DRs obtained during the initial sessions of training to the three training stimulus sets and the final reversal set were compared to assess the effects of the changed contingencies. Other measures of savings (e.g., sessions to criterion) were not evaluated because the changes in mastery criteria that were imposed through the experiment as well as apparatus problems that affected some rats’ performance during training made such analyses difficult to interpret.
3. Results
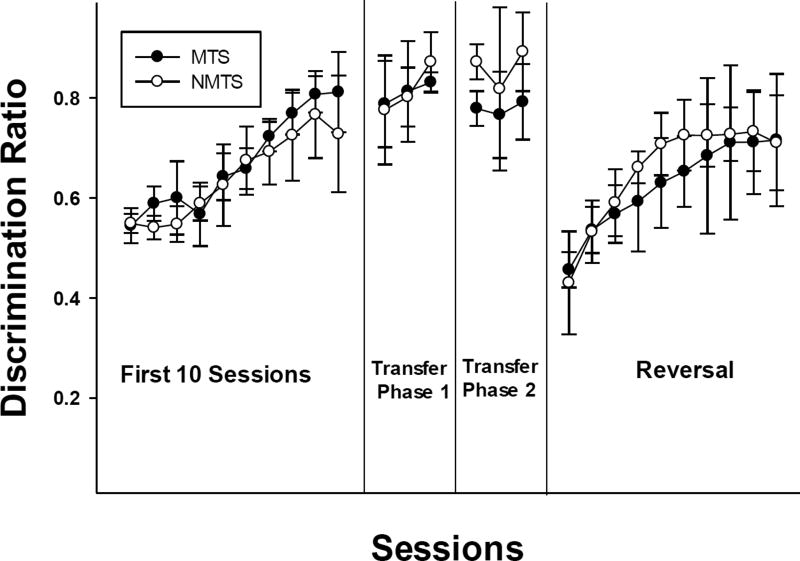
Figure 1 presents the average DR for the 10 rats that completed all phases of the study. Shown are the mean DRs for the first 10 sessions of initial training, the first three sessions for transfer phases 1 and 2 (only three sessions are shown because some rats met criterion that rapidly in transfer task 1 and 2) and the first 10 sessions for the reversal phase. In general, acquisition on matching and non-matching was quite rapid with mean DRs above .7 in the NMTS group and above .8 in the MTS group by the end of 10 sessions of initial training. There was considerable variability between subjects and the differences between groups were not significant. A 2-way mixed design ANOVA confirmed the improvement in DR across sessions during acquisition [F(9,72)=23.0, p<.001], with no main effect of training group (MTS or NMTS) and no interaction of training group and sessions. After meeting criterion with the initial stimulus set, accuracy with new stimuli was high on the first sessions of the transfer phases (second and third panels of Figure 1). For both transfer phases 1 and 2, there were no main effects of sessions or training group and no interaction between sessions and training group (all ps>.05). Finally, note that when contingencies were reversed, DRs declined to below .50 with a gradual adjustment to the changed contingencies during the following 10 sessions. In the reversal phase, ANOVA confirmed the significant improvement in DR across sessions [F(9,72)=19.7, p<.001], with no main effect of training group or interaction of training group and sessions.
Figure 1.
Mean discrimination ratios for the MTS and NMTS groups during the first 10 sessions of initial training, the first three sessions of transfer phases 1 and 2, and the first 10 sessions of the reversal phase for all 10 rats. Error bars indicate standard deviations.
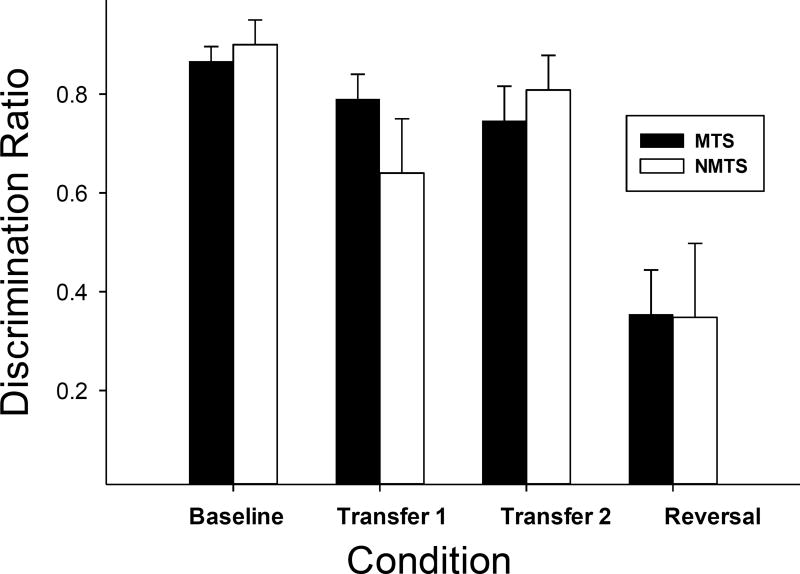
Although the highly accurate performances on the transfer phases with novel stimuli suggest that rats may have developed generalized MTS/NMTS, it is possible that the high DRs are based on rapid learning during the initial session. Figure 2 presents an analysis of DRs based only on the initial exposure to each novel trial type during the first session of the transfer phases. DRs were above .5 in both groups on both transfer tasks, providing evidence that the same/different relation transferred to novel stimuli. Rats trained on MTS showed strong evidence of generalized matching on both transfer phases; indeed, transfer DRs were similar to those obtained during baseline. However, rats trained on NMTS showed less complete transfer to new stimuli in the first transfer phase, although they showed stronger transfer on the second. The reversal resulted in a dramatic drop in accuracy on the first day of reversed contingencies in both groups.
Figure 2.
Mean discrimination is presented for the final session of the initial baseline training and the first exposure to novel stimuli during the transfer and reversal phases.
Using a 2-way mixed design ANOVA to compare the DR of initial training at criterion to the DR on the first exposure to new scents in transfer phase 1, the first exposure to new scents on transfer phase 2, and the first exposure to new scents with reversed contingencies, we found a main effect of phase [F(3,24)=62.7, p<.001), such that the baseline DR with the initial training set was significantly higher than performance on the first presentation of new scents in transfer 1 (p<.01, Tukey’s HSD), but equal to performance on the first presentation of new scents in the second transfer. There was no significant difference in DRs across the two transfer tasks. As expected, reversal DRs were significantly lower than DRs during baselines and both transfer tasks. While there was no main effect of training group [MTS or NMTS; F(1,8) < 1], the interaction between phase and training group approached significance, [F(3,24)=2.61, p=.074], and warranted inspection of the individual subject data to untangle the effect. There was high variability in DRs, especially for the NMTS subjects at transfer phase 1 and this contributed to the statistical trend for an interaction. Individual subject data in Figures 3 and 4 highlight the strong transfer for the MTS group and the weaker transfer for the NMTS group. Response rates on positive and negative trials (the basis of the DR measure) are compared directly in these figures.
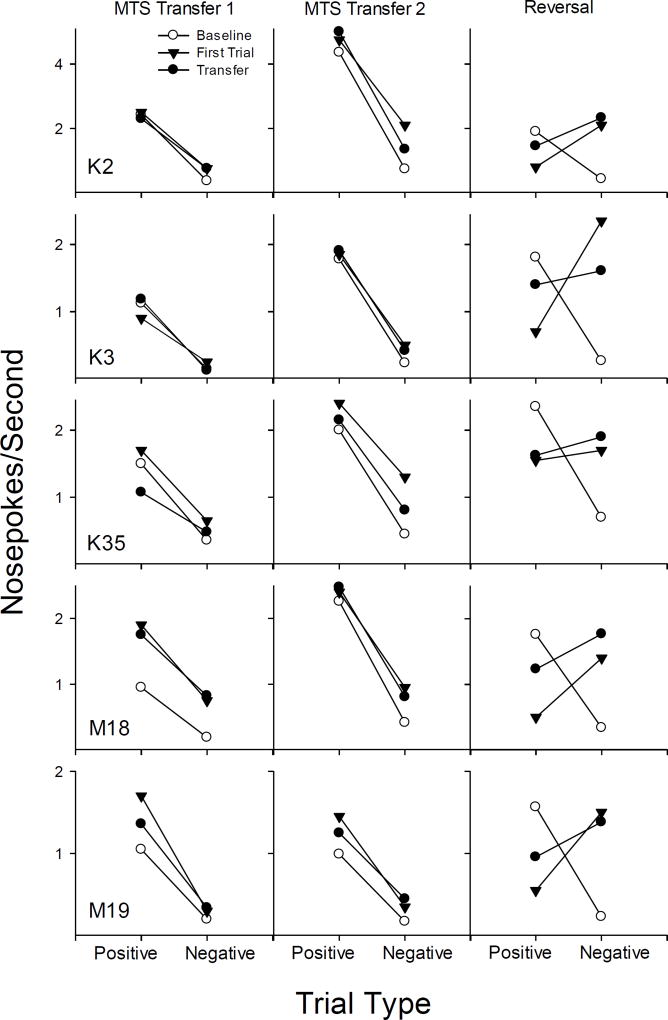
Figure 3.
Response rates on positive and negative trial types for subjects trained initially on MTS. The left panel shows baseline performance on the set of odors initially trained (open circles) compared to performance on the first day of transfer phase 1 (filled circles) with performance on the first exposure to the novel trial types depicted (filled triangles) separately. The center panel shows performance on the second set of odors after criterion was met (open circles) compared to performance on the first day of transfer day 2 (filled circles), with performance on the first exposure to the novel trial types depicted (filled triangles) separately. The right panel shows the effects of reversal with the last day of performance on the MTS trained contingencies (open circles) compared to performance on the first day of the reversed contingencies with a set of four novel scents (filled circles), with performance on the first exposure to the novel trial types depicted (filled triangles) separately.
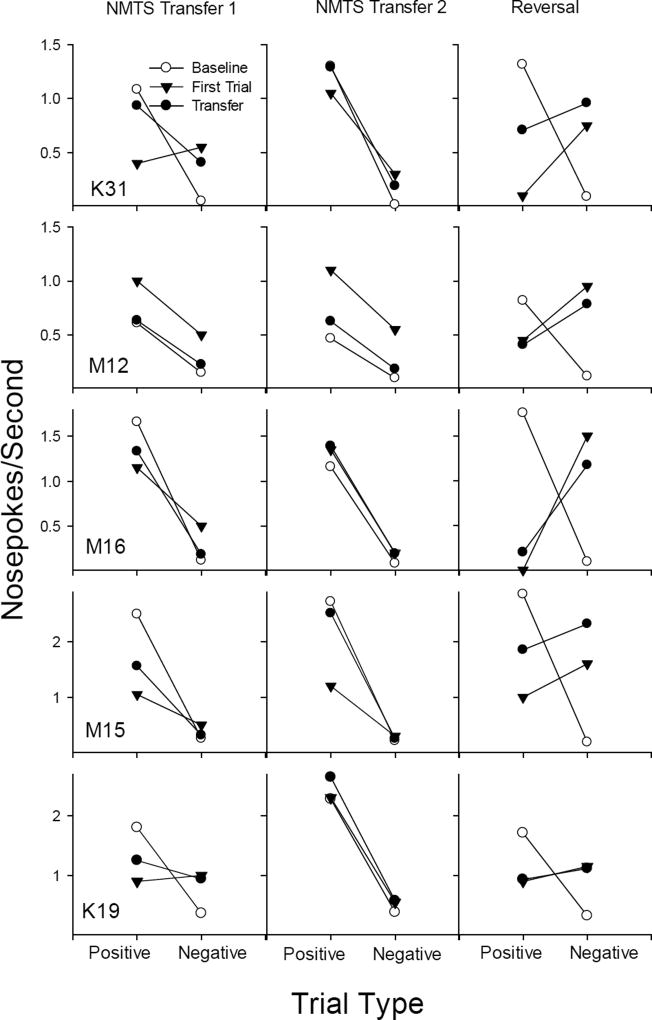
Figure 4.
Response rates on positive and negative trial types for subjects trained initially on NMTS. The left panel shows baseline performance on the set of odors initially trained (open circles) compared to performance on the first day of transfer phase 1 (filled circles) with performance on the first exposure to the novel trial types depicted (filled triangles) separately. The center panel shows performance on the second set of odors after criterion was met (open circles) compared to performance on the first day of transfer day 2 (filled circles), with performance on the first exposure to the novel trial types depicted (filled triangles) separately. The right panel shows the effects of reversal with the last day of performance on the NMTS contingencies (open circles) compared to performance on the first day of the reversed contingencies with a set of four novel scents (filled circles), with performance on the first exposure to the novel trial types depicted (filled triangles) separately.
Figure 3 shows mean responses per second for baseline and transfer tasks for the five rats trained initially on MTS. For each rat, three vertical panels depict performance on the first transfer phase (left), the second transfer phase (center) and the reversal phase (right). Each panel shows baseline performance on the set of odors initially trained (open circles; response rates are based on the last day of training when rats had met criterion) compared to performance on the first day of exposure to a set of four novel scents (filled circles). In addition, performance on the first exposure to the novel trial types is depicted (filled triangles) separately.
All five rats showed relatively high response rates on positive trials and much lower rates on negative trials as expected based on their training histories. The expected outcome of emergent identity would result in performance in transfer phases (filled circles) that would look similar to baselines with high rates of responding on novel positive identity trials and low rates on negative trials. As seen clearly in the left panel of Figure 3, all rats showed this pattern on the first exposure to the novel scents (filled triangles) indicating emergent identity. The same pattern was clear in the second transfer phase (middle panel for each rat), again indicating emergent identity from the first trial of the exposure to a set of another four novel scents.
Further evidence of generalized identity is shown in the right panel during the reversal phase when contingencies were shifted to NMTS as a set of four new scents was presented. As the right panel of Figure 3 shows for each rat, baseline performances on the last day of MTS training on a set of odors (open circles) showed high rates of responding to positive trial types and low rates of responding to negative trial types. When a set of four novel scents was first presented with reversed contingencies (filled triangles), four of the five rats maintained responding according to the identity relation; that is, they responded at high rates to the negative trial types (consistent with the identity relation) and at low rates to the positive trial types (consistent with the oddity relation). As the session continued, four of the rats maintained responding fairly consistent with the originally trained MTS contingencies, though the slope of the lines depicting responding to negative and positive trial types becomes less steep, indicating that the rats (especially K3) were adjusting to the changed contingency (filled circles). There was one exception to the finding of strong transfer in the reversal phase. Despite showing transfer on the first two tests, K35 appeared to respond equally to the new scents in the reversal, with similar rates of responding to positive and negative trial types. Thus, across all phases, rats trained on MTS showed clear transfer of the matching relation on 14 out of 15 exposures to novel stimuli.
Individual data for the five rats initially trained with NMTS are presented in Figure 4, which shows response rates during baseline (open circles) and transfer phase 1 (filled circles) in the left panel. Four of five rats (K31, M15, M16, and M12) showed clear evidence of emergent non-matching in the first transfer phase, though performance on the first presentation of the new scents (filled triangles) was not as consistent as overall performance on the first session (filled circles). Indeed, only one rat (M12) showed response rates on the first presentation that were as well differentiated as during baseline. In contrast, two rats (K19 and K31) showed no evidence of transfer during the initial exposure to the new odors, although K31 showed a rapid adjustment during the course of the session. Finally, rats M15 and M16 showed evidence of transfer on the first test, but responses rates were not as well differentiated. However, by transfer phase 2 (middle panel of Figure 4), all five rats showed discrimination of positive and negative trial types of the four new scents (filled circles) at the same level as their baseline performance (open circles). The slopes of the lines are remarkably consistent, and generally showed emergent non-matching on the first exposures of the new scents (filled triangles) with the exception of Rat M15.
For K31, M16 and M12, performance on the reversal (right panel of Figure 4) indicates that rats responded to the new scents as if the original non-matching contingencies were in effect; that is, they responded at high rates to the negative trial types (non-matching) and at low rates to the positive trial types (matching). This was evident from the first presentation of the new scents under the reversed contingencies (filled triangles), with some discrimination apparent as the rats were in contact with the reversed contingencies during the rest of the session (filled circles); that is, the difference between responding to positive and negative trial types began to level off. Rats K19 and M15 all showed discriminative baseline performance (open circles), but their responding to the four new scents in the reversal was similar for positive and negative trial types (filled circles and filled triangles). Thus, three of five rats showed evidence of emergent non-matching in the reversal phase, but two did not. Overall, transfer of non-matching was observed on 12 of 15 tests.
4. Discussion
Most of the rats showed rapid and strong transfer as early as the first transfer phase and those animals that did not show transfer in phase 1 showed it in phase 2. Further, during the reversal phase, most subjects maintained responding on the original contingencies with novel stimuli, showing interference with the acquisition of reversed contingencies. Thus, the present data replicate and extend the work of April et al. (2011), Lu et al., (1993), Peña et al (2006), and Prichard et al. (2015) and support the hypothesis that rats tested with olfactory stimuli can develop same-different relations with few exemplars. It is noteworthy that the only previous demonstration of generalized oddity in rats used a simultaneous NMTS arrangement in which the sample and one of the comparison stimuli were identical—thus selection of the “odd” stimulus was reinforced (April et al., 2011). In the present study, the use of a successive discrimination procedure meant that only two stimuli were used in each trial so non-identity is a more appropriate term than oddity to describe our results.
Transfer to novel stimuli is the critical criterion for assessment of concept learning (Katz, et al., 2007; Lazareva & Wasserman, 2008). In the current study, rats trained initially with MTS displayed evidence of immediate transfer to novel stimuli as early as the first transfer test. Further, as shown in Figure 3, left and center panels, there was equivalent performance for baseline and novel stimuli, with discriminative responding comparable to baseline even at the first presentation of novel scents. Katz, et al. (2007), Lazereva and Wasserman (2008), and Wright (1997) argued that demonstration of equivalent performances on baseline and novel stimuli is necessary to make the claim of full concept learning. The MTS subjects clearly demonstrated full concept learning for identity after training with only four odor.
However, the subjects trained initially on NMTS showed less robust effects. Only M16 and M12 (Figure 4, left and center panels) show full transfer in both transfer phases, with response rates for the initial exposure to new scents equivalent to baseline performance. K31 and K19 show immediate and equivalent transfer by the second test. M15 shows partial transfer on both transfer phases, with full discrimination developing during the first session with novel scents, but not at the first presentation of novel scents.
The differential effects of training with MTS and NMTS were a puzzling feature of the present study. Comparisons between MTS and NMTS have generally shown more rapid acquisition of the baseline conditional discriminations in the NMTS conditions (e.g., Wright & Delius, 2005). This was not observed in the present study, and although acquisition rates appeared to be about equal, rats trained on MTS showed stronger transfer than those trained on NMTS. As noted above, all five MTS rats showed full transfer on the initial test as compared to only two in the NMTS group. Of course, by the second transfer test the NMTS rats were also showing strong transfer; clearly with exposure to a greater number of exemplars the NMTS subjects were able to catch up. It may be worth noting that most of the previous research showing more rapid acquisition and transfer with NMTS than MTS used simultaneous discrimination procedures. Perhaps the “oddity preference effect” is less evident in a go, no-go procedure in which there is only one stimulus available to the animal and response rate, rather than choice, is the metric.
In both the MTS and NMTS groups it is noteworthy that transfer occurred after training with a fairly limited number of odorants. Six of the 10 rats showed virtually full transfer after initial training with only four odorants and eight showed full transfer in phase 2 (after training with eight odorants). By comparison, monkeys and pigeons generally do not show full transfer of same/different concept learning unless training includes 60 or more stimuli (Bodily, et al., 2008; Katz, et al., 2002; Katz & Wright, 2006; Wright, Rivera, Katz, & Bachevalier, 2003), although fewer stimuli have proven necessary in studies with chimpanzees (Oden, Thompson, & Premack, 1988), Clark’s nutcrackers (Wright, et al., 2016) and several species using the combined-stimulus method (Castro, Kennedy & Wasserman, 2010; Russell & Burke, 2016; Smirnova, Zorina, Obozova, & Wasserman, 2015; Wasserman, Castro, & Freeman, 2012).
The basis of the strong transfer with relatively few exemplars in the present study is unknown, and warrants further analysis. A possibility that must be considered is that with a short (1 sec) interstimulus interval, some of the sample odor may have lingered in the chamber and stimulus control based on scent duration or stimulus change, rather than by same-different relations, could have been a factor controlling behavior. However, Lu, et al. (1993), using a similar procedure, noted that matching was not disrupted by the introduction of a masking scent during the ISI, thereby creating a stimulus change on both positive and negative trials. Also, April et al., (2011) found generalized MTS and NMTS in rats with odor stimuli with relatively few exemplars in a simultaneous procedure in which control by stimulus change is not applicable. Still, further research is needed to clarify the sources of stimulus control in the present go, no-go procedure.
It does seem likely that the use of olfactory stimuli is critical to the development of emergent stimulus relations in rats. Other successful demonstrations of generalized MTS and NMTS in rats have been achieved with olfactory stimuli (April, et al. 2011; Peña, et al. 2006; Prichard, et al., 2015) and, by comparison, studies with rats using visual stimuli have had little success (Iverson; 1993; 1997; but note also Wasserman, et al., 2012). Through the use of olfactory stimuli, we expect that the research on concept formation could be extended to other rodents and other species, such as dogs and elephants, which also have exceptional olfactory perception. Further, although the present study shows generalized MTS and NMTS across a range of odors, some have argued that generalization across sensory modalities is necessary to truly demonstrate same/different concept learning (see Mackintosh, 2000; Scholtyssek, et al., 2013). Thus, it would be of considerable interest to explore the possibility of MTS and NMTS using different stimulus modalities in future studies.
Highlights.
The present study showed that rats can learn both identity and non-identity conditional discriminations using a successive, go, no-go procedure with olfactory stimuli.
Evidence for emergent same-different concept learning was demonstrated using a reversal procedure.
Same-different concept learning emerged after training with only four to eight exemplars.
Acknowledgments
Funding
This research was supported in part by grant DA029252 to Mark Galizio and by a UNCW Center for the Support of Undergraduate Research and Fellowships Supplies Award to Katherine Dyer.
The authors would like to thank Samantha Hess, Erin Lackey, Danielle Panoz-Brown, and Alyssa Cawley for assistance with data collection and Angela Goolsby for editorial comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- April LB, Bruce K, Galizio M. Matching- and nonmatching-to-sample concept learning in rats using olfactory stimuli. Journal of the Experimental Analysis of Behavior. 2011;96(2):139–54. doi: 10.1901/jeab.2011.96-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodily KD, Katz JS, Wright AA. Matching-to-sample abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34(1):178–184. doi: 10.1037/0097-7403.34.1.178. [DOI] [PubMed] [Google Scholar]
- Castro L, Kennedy PL, Wasserman EA. Conditional same-different discrimination by pigeons: Acquisition and generalization to novel and few-item displays. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36(1):23–38. doi: 10.1037/a0016326. [DOI] [PubMed] [Google Scholar]
- D'Amato MR, Salmon DP, Colombo M. Extent and limits of the matching concept in monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11(1):35–51. doi: 10.1037//0097-7403.11.1.35. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank AJ, Wasserman EA. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84(2):147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giurfa M, Zhang S, Jenett A, Menzel R, Srinivasan MV. The concepts of ‘sameness’ and ‘difference’ in an insect. Nature. 2001;410(6831):930–933. doi: 10.1038/35073582. [DOI] [PubMed] [Google Scholar]
- Herman LM, Hovancik JR, Gory JD, Bradshaw GL. Generalization of visual matching by a bottlenosed dolphin (Tursiops truncatus): Evidence for invariance of cognitive performance with visual and auditory materials. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15(2):124–136. [Google Scholar]
- Iversen IH. Acquisition of matching-to-sample performance in rats using visual stimuli on nose keys. Journal of the Experimental Analysis of Behavior. 1993;59(3):471–482. doi: 10.1901/jeab.1993.59-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH. Matching-to-sample performance in rats: A case of mistaken identity? Journal of the Experimental Analysis of Behavior. 1997;68(1):27–45. doi: 10.1901/jeab.1997.68-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastak D, Schusterman RJ. Animal transfer of visual identity matching-to-sample in two California sea lions (Zalophus californianus) Animal Learning & Behavior. 1994;22(4):427–435. [Google Scholar]
- Katz JS, Wright AA. Same/different abstract-concept learning by pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:80–86. doi: 10.1037/0097-7403.32.1.80. [DOI] [PubMed] [Google Scholar]
- Katz JS, Wright AA, Bachevalier J. Mechanisms of same/different abstract-concept learning by rhesus monkeys (Macaca mulatta) Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:358–368. [PubMed] [Google Scholar]
- Katz JS, Wright AA, Bodily KD. Issues in the comparative cognition of abstract-concept learning. Comparative Cognition & Behavior Reviews. 2007;2:79–92. doi: 10.3819/ccbr.2008.20005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazareva OF, Wasserman EA. Categories and concepts in animals. In: Menzel R, et al., editors. Learning and memory- A comprehensive reference. Vol. II. Behavioral approaches. Elsevier; 2008. pp. 197–226. [Google Scholar]
- Lu XM, Slotnick BM, Silberberg AM. Odor matching and odor memory in the rat. Physiology & Behavior. 1993;53:795–804. doi: 10.1016/0031-9384(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. Abstraction and discrimination. In: Heyes C, Huber L, editors. The evolution of cognition. Cambridge, MA: MIT Press; 2000. pp. 123–141. [Google Scholar]
- Magnotti JF, Katz JS, Wright AA, Kelly DM. Superior abstract-concept learning by Clark's nutcrackers (Nucifraga columbiana) Biology Letters. 2015;11(5):148. doi: 10.1098/rsbl.2015.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigrosh BJ, Slotnick BM, Nevin JA. Olfactory discrimination, reversal learning, and stimulus control in rats. Journal of Comparative and Physiological Psychology. 1975;89(4):285. doi: 10.1037/h0076821. [DOI] [PubMed] [Google Scholar]
- Oden DL, Thompson KR, Premack D. Spontaneous transfer of matching by infant chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14(2):140–145. [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed non-matching-to-sample task. Behavioral Neuroscience. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Peña T, Pitts RC, Galizio M. Identity matching-to-sample with olfactory stimuli in rats. Journal of the Experimental Analysis of Behavior. 2006;85(2):203–221. doi: 10.1901/jeab.2006.111-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard A, Panoz-Brown D, Bruce K, Galizio M. Emergent identity but not symmetry following successive olfactory discrimination training in rats. Journal of the Experimental Analysis of Behavior. 2015;104(2):133–145. doi: 10.1002/jeab.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F, Burke D. Conditional same/different concept learning in the short-beaked echidna (Tachyglossus aculeatus) Journal of the Experimental Analysis of Behavior. 2016;105(1):133–154. doi: 10.1002/jeab.185. [DOI] [PubMed] [Google Scholar]
- Scholtyssek C, Kelber A, Hanke FD, Dehnhardt G. A harbor seal can transfer the same/different concept to new stimulus dimensions. Animal Cognition. 2013;16:915–925. doi: 10.1007/s10071-013-0624-0. [DOI] [PubMed] [Google Scholar]
- Slotnick B. Animal cognition and the rat olfactory system. Trends in Cognitive Sciences. 2001;5(5):216–222. doi: 10.1016/s1364-6613(00)01625-9. [DOI] [PubMed] [Google Scholar]
- Smirnova A, Zorina Z, Obozova T, Wasserman E. Crows spontaneously exhibit analogical reasoning. Current Biology. 2015;25(2):256–260. doi: 10.1016/j.cub.2014.11.063. [DOI] [PubMed] [Google Scholar]
- Vonk J. Gorilla (Gorilla gorilla gorilla) and orangutan (Pongo abelii) understanding of first- and second-order relations. Animal Cognition. 2003;6(2):77–86. doi: 10.1007/s10071-003-0159-x. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Castro L, Freeman JH. Same–different categorization in rats. Learning & Memory. 2012;19(4):142–145. doi: 10.1101/lm.025437.111. [DOI] [PubMed] [Google Scholar]
- Wright AA. Concept learning and learning strategies. Psychological Science. 1997;8(2):119–123. [Google Scholar]
- Wright AA, Cook RG, Rivera JJ, Sands SF, Delius JD. Concept learning by pigeons: Matching to sample with trial-unique video picture stimuli. Animal Learning & Behavior. 1988;16:436–444. [Google Scholar]
- Wright AA, Delius JD. Learning processes in matching and oddity: The oddity preference effect and sample reinforcement. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31(4):425–432. doi: 10.1037/0097-7403.31.4.425. [DOI] [PubMed] [Google Scholar]
- Wright AA, Magnotti JF, Katz JS, Leonard K, Kelly DM. Concept learning set-size functions for Clark’s nutcrackers. Journal of the Experimental Analysis of Behavior. 2016;105(1):76–84. doi: 10.1002/jeab.174. [DOI] [PubMed] [Google Scholar]
- Wright AA, Rivera JJ, Katz JS, Bachevalier J. Abstract-concept learning and list-memory processing by capuchin and rhesus monkeys. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29(3):184–198. doi: 10.1037/0097-7403.29.3.184. [DOI] [PubMed] [Google Scholar]
- Zentall T, Hogan D. Abstract concept learning in the pigeon. Journal of Experimental Psychology. 1974;102(3):393–398. [Google Scholar]