Abstract
BACKGROUND
The United Nations 2015 Millennium Development Goals targeted a 75% reduction in maternal mortality. However, in spite of this goal, the number of maternal deaths per 100,000 live births remains unacceptably high across Sub-Saharan Africa. Because many of these deaths could likely be averted with access to safe surgery, including cesarean delivery, we set out to assess the capacity to provide safe anesthetic care for mothers in the main referral hospitals in East Africa.
METHODS
A cross-sectional survey was conducted at 5 main referral hospitals in East Africa: Uganda, Kenya, Tanzania, Rwanda, and Burundi. Using a questionnaire based on the World Federation of the Societies of Anesthesiologists (WFSA) international guidelines for safe anesthesia, we interviewed anesthetists in these hospitals, key informants from the Ministry of Health and National Anesthesia Society of each country (Supplemental Digital Content, http://links.lww.com/AA/B561).
RESULTS
Using the WFSA checklist as a guide, none of respondents had all the necessary requirements available to provide safe obstetric anesthesia, and only 7% reported adequate anesthesia staffing. Availability of monitors was limited, and those that were available were often nonfunctional. The paucity of local protocols, and lack of intensive care unit services, also contributed significantly to poor maternal outcomes. For a population of 142.9 million in the East African community, there were only 237 anesthesiologists, with a workforce density of 0.08 in Uganda, 0.39 in Kenya, 0.05 in Tanzania, 0.13 in Rwanda, and 0.02 anesthesiologists in Burundi per 100,000 population in each country.
CONCLUSIONS
We identified significant shortages of both the personnel and equipment needed to provide safe anesthetic care for obstetric surgical cases across East Africa. There is a need to increase the number of physician anesthetists, to improve the training of non-physician anesthesia providers, and to develop management protocols for obstetric patients requiring anesthesia. This will strengthen health systems and improve surgical outcomes in developing countries. More funding is required for training physician anesthetists if developing countries are to reach the targeted specialist workforce density of the Lancet Commission on Global Surgery of 20 surgical, anesthetic, and obstetric physicians per 100,000 population by 2030.
The World Health Organizationa estimates that 300,000 women die every year from pregnancy-related causes, predominantly in the developing countries. Sub-Saharan Africab accounts for 50% of the global maternal death burden with a regional average of 500 deaths per 100,000 live births in 2010, followed by Asia with 45%.1 The lifetime risk of dying during pregnancy in the poorest parts of the world is 1 in 62 because of the high incidence of severe pre-eclampsia, cardiac disease, and infectious diseases such as tuberculosis, malaria, and HIV/AIDS.
Anesthetists are an integral part of the obstetric team and participate in the management of more than 50% of parturients in a typical obstetric unit in the United Kingdom.3 They are instrumental in managing critically ill mothers. Safe anesthesia is fundamental for good outcomes in all surgical procedures in the perinatal period. Providing a safe environment that meets the appropriate minimal standards needed for these specific procedures is a basic requirement to ensure good anesthetic and surgical outcomes.
Minimal requirements for safe practice are adequate skills, anesthesia monitors, disposables and drugs, and relevant management protocols for each level of care.4 The World Federation of Societies of Anesthesiologists (WFSA) recommends the international standards for safe practice of anesthesia for anesthesia professionals throughout the world. They are intended to provide guidance and assistance to anesthesia professionals, their professional societies, hospital and facility administrators, and governments for improving and maintaining the quality and safety of anesthesia care.5 In resource-limited settings, the minimal mandatory standards are often not met.
Maternal deaths arise from risks attributable to pregnancy and childbirth as well as from poor-quality care from health services.6 They result from a wide range of indirect and direct causes.c The major direct causes in Africa are hemorrhage (34%), infection (10%), hypertensive disorders (9%), and obstructed labor (4%). This is different from developed countries where hemorrhage only accounts for 13%, and the primary cause of maternal mortality is hypertensive disorders such as eclampsia.c Indirect causes represent 20% of the total deaths; they are attributable to preexisting or concurrent diseases that are not complications of pregnancy itself but are aggravated by it (eg, cardiac disease or HIV/AIDS).d Maternal deaths that happen during childbirth itself ranges from 11% to 17%, and those occurring in the postpartum period range between 50% and 71%. Because a high level of risk is concentrated during childbirth, it is critical to give attention to mothers during delivery and in the immediate postpartum period. Therefore, we did an audit of the main referral hospitals in East Africa to assess the capacity to provide safe anesthesia for obstetric patients according to WFSA guidelines.
This study will assist in understanding the challenges to providing safe anesthesia in the East African community and in identifying the gaps that need to be addressed in this post–Millennium Development Goals era. Future improvements in safety of anesthesia care will be determined by how these countries incorporate this research into their health policies.
METHODS
A cross-sectional survey was conducted from February 2013 to March 2014 at the following 5 East African Community National Referral Hospitals: Mulago Hospital, Uganda; Kenyatta Hospital, Kenya; Muhimbili Hospital, Tanzania; Centre Hospitalier Universite (CHU), Kigali Rwanda; and Centre Hospitalo Universitaire de Kamenge, Burundi.
Ethical approval was obtained from Makerere University School of Medicine Research and Ethics Committee (SOMREC), the Uganda National Council for Science & Technology Ethics Committee, and hospital ethics committees for participating hospitals, including Muhimbili University of Health and Allied Services Ethics Committee, Kenyatta National Hospital/University of Nairobi Ethics and Research Committee, the University of Rwanda Faculty of Medicine Research and Ethics Committee, and Department of Anesthesia Centre Hospitalo Universitaire de Kamenge Burundi. Informed consent was obtained from all individuals participating in the study.
Eighty-six physician and nonphysician anesthetists delivering obstetric anesthesia in the participating hospitals were interviewed by the principal investigator (I.E.). The sample size was calculated using the formula for dichotomous variables with a 95% confidence interval, and anesthetists interviewed in each country were selected proportional to size sampling. We stratified according to the number of physician and nonphysician anesthetists available in each hospital, and the individual anesthetists interviewed were selected by simple random sampling. We obtained a list of the physician and nonphysician anesthetists providing obstetric anesthesia in each hospital and contacted all of them to explain the purpose of the study and to ask them to participate. Contact was continued until the final target number of participants was reached. After we had obtained consent from each individual, we interviewed him or her. We collected quantitative and qualitative data using a structured questionnaire based on WFSA guidelines, which included demographic, administrative, preanesthetic, intraoperative, and postanesthetic variables, and complications. Theaters were checked for the availability of these minimum requirements. The Head of the National Society of Anesthesia and a representative from Ministry of Health of participating countries were interviewed to determine the distribution of anesthetists in the country, challenges faced in delivery of anesthesia, and the possible solutions. A significance level of ≤ 0.05 was used.
WFSA emphasizes the presence of an appropriately trained anesthesia provider with access to recommended monitors and minimum facilities for safe perioperative care and availability of appropriate postanesthesia care services.
RESULTS
The obstetric theaters of the 5 national referral hospitals in the East African Community were assessed (ie, Mulago Hospital, Uganda; Kenyatta Hospital, Kenya; Muhimbili Hospital, Tanzania; Centre Hospitalier Universite de Kigali, Rwanda; and Centre Hospitalo-Universitaire de Kamenge, Burundi. There were a total of 12 operating rooms for obstetric patients in the 5 hospitals. Tables 1 through 3 show the drugs and operating equipment available. As shown in Figure 1, in the study profile, a total of 86 anesthetists were interviewed, and 85 responses were analyzed (99% response rate). One participant in Kigali consented but was unavailable to complete the interview. Table 4 shows the distribution of baseline characteristics of the study participants. Using the WFSA guidelines, none of the facilities had all the requirements available to provide safe anesthesia. When 10 variables were considered, only 4% (3 of 85) of the anesthetists interviewed had access to the facilities with up to 8 of the variables. These included the presence of continuous electrocardiogram (ECG), continuous pulse oximetry, continuous blood pressure monitoring, capnograph, thermometer, stethoscope, difficult airway cart and suction machine intraoperatively, a recovery room for postoperative monitoring, and intensive care unit for critically ill mothers.
Table 1.
Availability of Drugs
| Availability of Drugs
|
|||||
|---|---|---|---|---|---|
| Hospital
|
|||||
| Variable | Mulago, Uganda | CHU Kamenge Burundi | Kenyatta, Kenya | Muhmbili, Tanzania | CHU Kigali, Rwanda |
| Acid-reducing agentsa | Sometimes | Sometimes | Sometimes | Sometimes | Never |
| Sedativesb | Always | Always | Always | Always | Always |
| Antihypertensivesc | Always | Always | Sometimes | Sometimes | Sometimes |
| Vasopressorsd | Always | Always | Always | Always | Always |
| Antibioticse | Always | Sometimes | Always | Always | Sometimes |
| Spinal drugsf | Always | Always | Always | Always | Sometimes |
| General anesthetics IVg | Always | Always | Always | Always | Always |
| Volatile anestheticsh | Always | Always | Always | Always | Always |
| Muscle relaxantsi | Always | Always | Always | Always | Always |
| Opioid analgesicsj | Always | Always | Always | Always | Always |
| Nonopioid analgesicsk | Always | Always | Always | Always | Always |
| Oxytocicsl | Always | Always | Always | Always | Always |
Abbreviation: CHU, Centre Hospitalier Universite.
Drugs available include:
Ranitidine;
Diazepam and Midazolam;
Hydralazine;
Ephedrine, Phenylephrine, and Epinephrine;
Ceftriaxone, Ampicillin, and Gentamicin;
Lignocaine and Bupivacaine;
Thiopentone and Ketamine;
Halothane, Sevoflurane, and Isoflurane;
Suxamethonium and Cisatracurium;
Pethidine and Morphine;
Diclofenac; and
Oxytocin.
Table 3.
Presence and Functionality of Anesthetic Machine
| State of Anesthetic Machine
|
|||||
|---|---|---|---|---|---|
| Hospital
|
|||||
| Variable | Mulago, Uganda | CHU Kamenge, Burundi | Kenyatta, Kenya | Muhimbili, Tanzania | CHU Kigali, Rwanda |
| Oxygen source | Piped and cylinder | Piped | Cylinder | Piped and cylinder | Piped |
| Reserve oxygen cylinder | Absent | Absent | Absent | Absent | Present |
| Inspired oxygen recording | Present | Absent | Absent | Absent | Present |
| Scavenging system | Absent | Absent | Absent | Absent | Absent |
| Breathing system | Present | Present | Present | Present | Present |
| Oxygen supply failure alarm | Not functional | Functional | Not functional | Not functional | Functional |
| Low oxygen pressure alarm | Not functional | Functional | Not functional | Not functional | Functional |
| Ventilator | Not functional | Functional | Functional | Functional | Functional |
| Mechanical ventilation disconnection alarm | Not functional | Functional | Not functional | Functional | Functional |
Abbreviation: CHU, Centre Hospitalier Universite.
Figure 1.
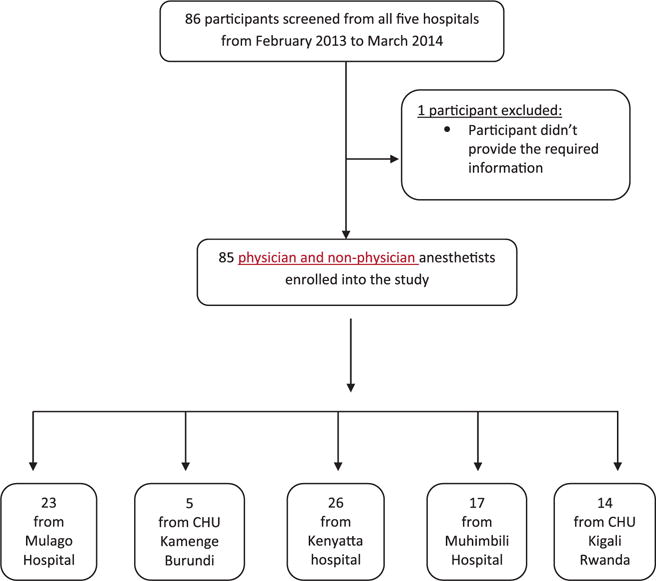
Study profile.
Table 4.
Distribution of Baseline Characteristics of Study Participants by Country
| Country
|
||||||
|---|---|---|---|---|---|---|
| Variable | Mulago, Uganda N = 23 |
CHU Kamenge, Burundi N = 5 |
Kenyatta, Kenya N = 26 |
Muhimbili, Tanzania N = 17 |
CHU Kigali, Rwanda N = 14 |
Overall N = 85 |
| Qualification (% respondents) | ||||||
| Physician anesthetist | 1 (4.35) | 1 (20.00) | 14 (58.33) | 3 (17.65) | 2 (14.29) | 21 (25.30) |
| Nurse anesthetist | 13 (56.52) | 4 (80.00) | 2 (8.33) | 8 (47.06) | 9 (64.29) | 36 (43.37) |
| Clinical officer anesthetist | 8 (34.78) | 0 | 5 (20.83) | 2 (11.76) | 0 | 15 (18.07) |
| Other (anesthesia assistant/anesthesia medical officer) | 1 (4.35) | 0 | 3 (12.50) | 4 (23.53) | 3 (21.43) | 11 (13.25) |
| Mean age in years (SD) | 43.55 (8.03) | 49.00 (11.25) | 41.20 (9.29) | 46.40 (8.11) | 35.15 (6.73) | 42.40 (9.13) |
| Mean years of experience (SD) | 11.22 (6.67) | 19.40 (9.79) | 11.04 (7.64) | 13.29 (8.67) | 11.57 (14.37) | 12.16 (9.21) |
| Gender (%) | ||||||
| Female | 14 (60.87) | 2 (40.00) | 4 (16.67) | 4 (23.53) | 7 (53.85) | 31 (37.80) |
| Male | 9 (39.13) | 3 (60.00) | 20 (83.33) | 13 (76.47) | 6 (46.15) | 51 (62.20) |
| Another place of work (%) | ||||||
| Private | 16 (69.57) | 3 (60) | 20 (79.92) | 13 (76.47) | 8 (57.14) | 60 (70.59) |
| None | 7 (30.43) | 2 (40) | 6 (16.67) | 4 (23.08) | 6 (42.86) | 25 (29.41) |
| Presence of 24-hour recovery room (%) | ||||||
| No | 23 (100) | 5 (100) | 4 (15.38) | 2 (11.76) | 10 (71.43) | 44 (51.76) |
| Yes | 0 | 0 | 22 (84.62) | 15 (88.24) | 4 (28.57) | 41 (48.24) |
Abbreviation: CHU, Centre Hospitalier Universite.
Table 5 reflects the anesthetists’ responses to questions on professional practice and the areas in which improvements could be made. Seventy-four of 85 anesthetists (87%) checked the preoperative informed consent; 58 of 85 anesthetists (68%) performed a preoperative assessment, although this was generally in the operating room (85%) rather than the ward; 19 of 85 anesthetists (22%) had an assistant to perform cricoid pressure. Only 37 of 85 anesthetists (44%) always had access to the postoperative ICU when needed, and only 47 of 85 anesthetists (55%) monitored all patients for 30 minutes after surgery.
Table 5.
Responses to Questions on Professional Practice
| Hospital
|
||||||
|---|---|---|---|---|---|---|
| Variable | Mulago, Uganda N = 23 |
CHU Kamenge, Burundi N = 5 |
Kenyatta, Kenya N = 26 |
Muhimbili, Tanzania N = 17 |
CHU Kigali, Rwanda N = 14 |
Chi P value |
| Preanesthetic surgical check list use | .001 | |||||
| Always | 0 | 0 | 5 (19.23) | 11 (64.71) | 5 (35.71) | |
| Never | 23 (100) | 5 (100) | 21 (80.77) | 6 (35.29) | 9 (64.29) | |
| Perform preanesthetic evaluation | .001 | |||||
| Always | 20 (86.96) | 5 (100) | 21 (80.77) | 8 (47.06) | 4 (30.77) | |
| Never | 3 (13.04) | 0 | 5 (19.23) | 9 (52.94) | 9 (69.23) | |
| Check preoperative informed consent | .001 | |||||
| Always | 21 (91.30) | 0 | 23 (88.46) | 17 (100) | 13 (92.86) | |
| Never | 2 (8.70) | 5 (100) | 3 (11.54) | 0 | 1 (7.14) | |
| Presence of an assistant to give cricoid pressure | .001 | |||||
| Always | 1 (4.35) | 5 (100) | 11 (42.31) | 11 (64.71) | 2 (14.29) | |
| Never | 22 (95.65) | 0 | 15 (57.69) | 6 (35.29) | 12 (85.71) | |
| Continuous education (CPD) in obstetric anesthesia | .001 | |||||
| Always | 0 | 0 | 11 (42.31) | 2 (11.76) | 0 | |
| Never | 23 (100) | 5 (100) | 15 (57.69) | 15 (88.24) | 13 (100) | |
| Adequate supervision | .001 | |||||
| Always | 4 (17.39) | 4 (80.00) | 20 (76.92) | 7 (41.18) | 4 (28.57) | |
| Never | 19 (82.61) | 1 (20.00) | 6 (23.08) | 10 (58.82) | 10 (71.43) | |
| Intraoperative cardiac arrest occurrence in last 1 year (obstetric cases) | .001 | |||||
| Yes | 12 (52.17) | 5 (100) | 7 (26.92) | 11 (64.71) | 12 (85.71) | |
| No | 11 (47.83) | 0 | 19 (73.08) | 6 (35.29) | 2 (14.29) | |
| Supervision for complicated conditionsa | .002 | |||||
| Always | 4 (17.39) | 0 | 16 (61.54) | 7 (41.18) | 2 (14.29) | |
| Never | 19 (82.61) | 5 (100) | 10 (38.46) | 10 (58.82) | 12 (85.71) | |
| Access to new anesthetic technologyb | .001 | |||||
| Always | 3 (13.04) | 1 (20.00) | 21 (80.77) | 7 (43.75) | 4 (28.57) | |
| Never | 20 (86.96) | 4 (80.00) | 5 (19.23) | 9 (56.25) | 10 (71.43) | |
| >50% of patients that are evaluated in the operating roomc | .013 | |||||
| Always | 23 (100) | 2 (40.00) | 21 (80.77) | 15 (88.24) | 11 (78.57) | |
| Never | 0 | 5 (19.23) | 2 (19.23) | 2 (11.76) | 3 (21.43) | |
| Anesthetic practice complies with clinical guidelinesd | .095 | |||||
| Yes | 15 (65.22) | 4 (100) | 9 (36.00) | 9 (52.94) | 8 (57.14) | |
| No | 8 (34.78) | 0 | 16 (64.00) | 8 (47.06) | 6 (42.86) | |
| Patients taken care of after surgery for >0 minutes | .001 | |||||
| Always | 0 | 0 | 20 (76.92) | 14 (82.35) | 13 (92.86) | |
| Never | 23 (100) | 5 (100) | 6 (23.08) | 3 (17.65) | 1 (7.14) | |
| Availability of ICU services for obstetric patients | .001 | |||||
| Always | 2 (8.70) | 0 | 20 (76.92) | 5 (29.41) | 10 (71.43) | |
| Never | 21 (91.30) | 5 (100) | 6 (23.08) | 12 (70.59) | 4 (28.57) | |
Abbreviations: CHU, Centre Hospitalier Universite; CPD, Continuous Professional Development; ICU, intensive care unit.
Support supervision from a senior consultant for anesthesiologists or even from an anesthesiologist – nonphysician.
Access to advances in obstetric anesthesia such as epidurals for cesarean delivery or labor analgesia.
>50% of patients first evaluated preoperatively in the operating room.
Clinical guidelines for spinal anesthesia: aseptic technique.
Only 58% (P = .016) of the anesthesia providers had heard about the WFSA international guidelines for safe anesthesia. Inadequate supervision of emergency conditions was reported by 54% (P = .001). The hospitals were equipped with basic monitors, as shown in Figure 2. However, this equipment was sometimes not functional, as shown in Figure 3. Equipment was not regularly serviced and was usually only repaired when broken down. Sometimes, the ECG was not used because of an intermittent supply of electrodes. There was a particular deficiency in ventilation monitoring, with precordial stethoscope, the standard of care, and capnography not always used in the national referral hospitals (Figure 4).
Figure 2.
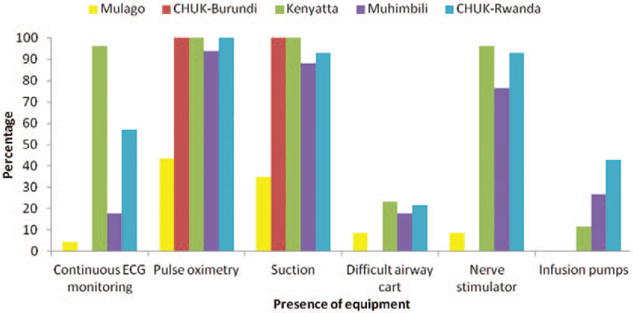
Availability of equipment during surgery at the National Referral Hospital in East Africa.
Figure 3.
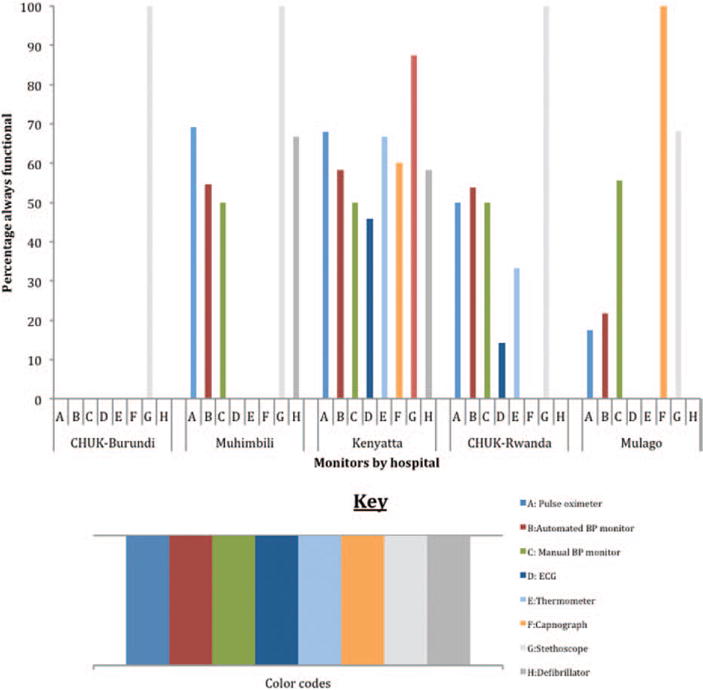
Operational state of monitors at the National Referral Hospitals in East Africa.
Figure 4.
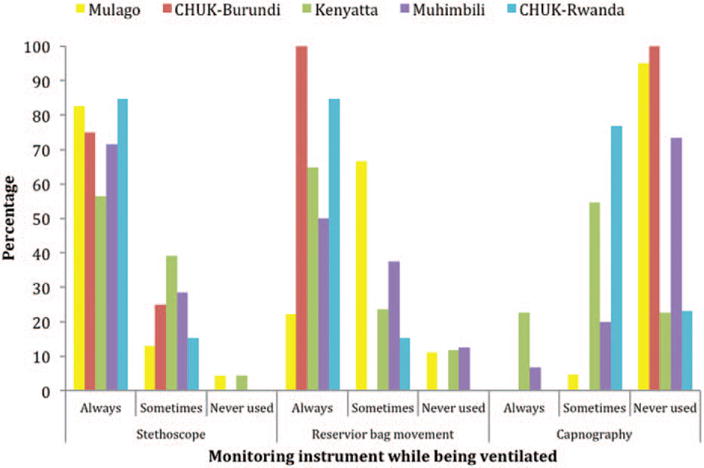
Monitoring during general anesthesia at the National Referral Hospitals in East Africa.
The principal investigator also interviewed the Head of the National Society of Anesthesia and a representative from the Ministry of Health of each country. They all reported similar challenges including few anesthesia personnel and poor supervision of nonphysician anesthetists. Most physician anesthetists are based in the major cities in each country. The map in Figure 5 shows the geographical location of each country and the distribution of anesthesia providers in each country, with anesthesiologists concentrated in urban areas. By March 2014, there were only 30 anesthesiologists in Uganda, 168 in Kenya, 22 in Tanzania, 15 in Rwanda, and 2 in Burundi, for a population of 36.3, 43.2, 43.6, 11.5, and 8.3 million, respectively (Table 6). There is a low ratio of anesthesiologists to the total population in each country, and unsupervised nonphysician anesthetists provide most of the anesthesia in the region. These include nurses or clinical officers with 2 years of training in anesthesia, assistant medical officers with 6 months to 1 year of anesthesia training, and anesthesia assistants who mainly received on-job training. Many of the nonphysician anesthetists in Rwanda and Burundi have found opportunities to upgrade their education in other disciplines and have stopped practicing anesthesia. These are not included in the survey.
Figure 5.
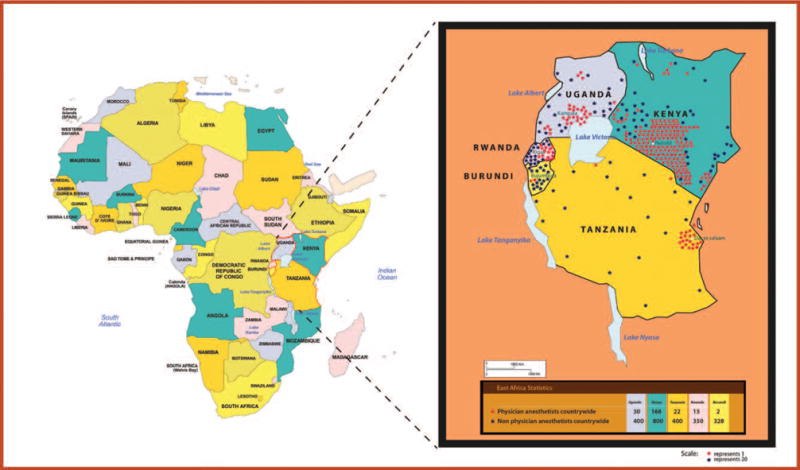
Distribution of physician and non-physician anesthetists in the East African community.
Table 6.
Demographic Data of Each Country
| Variable | Uganda | Kenya | Tanzania | Rwanda | Burundi |
|---|---|---|---|---|---|
| Populationa,b,c7–9 | 36.3 million (2012) | 43.2 million (2012) | 43.6 million (2012) | 11.5 million (2012) | 8.3 million (2010) |
| Number of physician anesthetists countrywide | 30 (7%) | 168 (17%) | 22 (5%) | 15 (4%) | 2 (0.6%) |
| Number of nonphysician anesthetists countrywide | 400 (93%) | 800 (83%) | 400 (95%) | 350 (96%) | 328 (99.4%) |
| % of cesarean deliveries done by physician and nonphysician anesthetists | Largest percentage provided by nonphysician anesthetists: 1 physician anesthetist in the Eastern region (Mbale); 3 in the Western region (Mbarara); and the rest in the Central region. | Physician anesthetists, 20%; nonphysician anesthetists, 80%. | Nonphysician anesthetists provide largest percentage. | Nonphysician anesthetists provide largest percentage. Physician anesthetists are only at the teaching hospitals CHUK, Centre Hospitalier Universitaire Butare (CHUB), and some private hospitals in Kigali. | Two physician anesthetists in the whole country: 1 at CHUK, the second at the Military Hospital. Nonphysician anesthetists provide largest percentage of anesthesia countrywide. |
Abbreviation: CHU, Centre Hospitalier Universite.
UNICEF Uganda Statistics. 2012 [cited 2014 24/April/2014]; Available from: http://www.unicef.org/infobycountry/uganda_statistics.html - 118
UNICEF Kenya Statistics. 2012 [cited 2014 24/April/2014]; Available from: http://www.unicef.org/infobycountry/kenya_statistics.html - 118
UNICEF Rwanda Statistics. 2012 [cited 2014 24/April/2014]; Available from: http://www.unicef.org/infobycountry/rwanda_statistics.html - 118.
Other challenges highlighted from the key informant interviews with representatives of ministry of health and national anesthesia societies included inadequate funding, which has led to lack of basic equipment and poor servicing of the available equipment, making monitoring and delivery of safe anesthesia very difficult. A high patient load is frequently noted, combined with short supplies of drugs, theater material, and blood products necessary for cesarean deliveries. The paucity of local protocols, ineffective referral systems, and lack of intensive care and/or high dependency unit services were also reported to contribute significantly to the poor outcomes.
DISCUSSION
Using the WFSA checklist as a guide, none of the respondents had all the available requirements to provide safe obstetric anesthesia, and only 7% reported adequate anesthesia staffing. Availability of monitors was limited, and those that were available were often nonfunctional. For a population of 142.9 million in the East African community, there were only 237 anesthesiologists, with 30 in Uganda; 168 in Kenya; 22 in Tanzania; 15 in Rwanda; and 2 in Burundi, for a workforce density of 0.08, 0.39, 0.05, 0.13, and 0.02 anesthesiologists per 100,000 population in each country, respectively (Table 6).
The limitations of this study include selection bias because the sample size was stratified according to the number of anesthesia providers in each national referral hospital. Interviewed anesthetists were selected by simple random sampling according to those present in the hospital at the time of the study. The exact number of practicing nonphysician anesthetists could be lower than that reported by the heads of the National Society of Anesthesia because some have changed professions or traveled out of the region.
High-quality obstetric anesthesia is the key component to reducing maternal and fetal deaths during operative interventions for childbirth. Many mothers need more than basic obstetric care.10 In the developing world, many women present to secondary institutions with life-threatening complications; additional lives could be saved if the anesthetic provider was skilled at recognizing the need for, and was able to carry out, prompt and effective resuscitation. The anesthetic provider should have a leading role in the provision of basic intensive care.11
We assessed the main referral hospitals in East Africa, which are also teaching hospitals. No hospital had all the requirements to meet the international standards for safe anesthesia. When the criteria were reduced to 8 variables, only 4% (3 of 85) of the anesthetists had access to good-quality facilities. We found that the anesthetists also lacked access to continuous professional development and medical education on managing obstetric emergencies. ICU services for care of critically ill obstetric patients were not readily available in Mulago, CHU de Kamenge Burundi, or Muhimbili Hospitals.
One of the primary challenges to improving outcomes from anesthesia and surgery is providing access to timely surgical care delivered by adequately trained and resourced health care providers.12 Timing of appropriate and safe surgical intervention has been clearly shown to be critical in preventing maternal death and disability because most perinatal deaths occur during labor and delivery or within the first 48 hours thereafter.e The three-delays model13 identifies some of the readily addressable factors, for which the first 2 delays (delay in deciding to seek care and delay in reaching appropriate care) relate directly to the issue of access to care, including factors in the family and the community, such as transportation. The third delay (delay in receiving care at health facilities) relates to factors specifically at the health facility, including quality of care. Ironically, it is crucial to address the third delay first because it would be of limited value to facilitate access to a health facility if it was not well staffed, well equipped, and provided good quality care. In spite of programs to improve access, these obstacles act as bottlenecks that prevent better provision of safe anesthesia.13
Minimum Requirements for Safe Anesthesia
The international standards for safe practice of anesthesia were recommended by WFSA for anesthesia professionals throughout the world.5 In resource-limited settings, minimum, mandatory standards are often not met. Provision of anesthesia under such circumstances should be restricted to procedures that are essential for the urgent or emergency saving of life or limb. In spite of this, hospitals continue to conduct elective as well as emergency surgeries. Every effort should be made by those responsible for the provision of health care in these areas and settings to ensure that the standards are met.
The national referral hospitals in East Africa sometimes lacked essential drugs such as antihypertensives (Kenya, Tanzania, and Rwanda) (Table 1). Although piped-in oxygen and cylinders were the major sources of oxygen in the theaters, not all hospitals had reserve cylinders (Table 3). Although availability of a fully trained anesthetist throughout surgery is a basic requirement in the WFSA standards, 93% of the anesthetists identified a shortage of trained anesthesia personnel as a major challenge at the national referral hospitals.
The challenges facing anesthetists and surgeons in many low-income countries include a critical shortage of adequately trained providers and poor facilities and infrastructure, combined with a lack of basic drugs, equipment, and supplies.14 In East Africa, anesthesia as a specialty has been under-represented for many years, resulting in few physician anesthetists. Therefore, nonphysicians deliver the majority of anesthetics. Most surgical and anesthesia services in Mozambique, Tanzania, and Uganda were provided by generalist doctors, mid-level providers, and nurses.15
A systematic review of barriers to surgical care in low-and middle-income countries showed substantial evidence that one of the main barriers is lack of facilities, equipment, and expertise, including anesthesia and critical care provision.14,16 Inadequate access to appropriate health care means that many women still do not survive pregnancy and childbirth.17 This is most visible in sub-Saharan Africa, where the overwhelming majority of maternal deaths in the world take place. In Kenya, the referral system has 3 levels, with 2 national hospitals, 8 provincial hospitals, and 70 district hospitals, with only 30% of the rural population having access to health facilities within 4 km. The quality of health services is low because of inadequate supplies, equipment, and lack of personnel.18
It was noted that out of the 300 registered clinical officer anesthetists in Kenya, only 200 are in public hospitals, mainly in the Nairobi metropolitan area, with the rural areas falling short.f Of the 120 specialist physician anesthetists, 17 work in public hospitals against an overall population of about 40 million Kenyans. The shortage of human resources for health in Tanzania is one of the most severe in Africa, and the available skilled workforce is only 32% of that recommended.19,20 In Rwanda, a comprehensive countrywide assessment of surgical capacity found severe shortages in available resources. There were only 13 trained physician anesthetists, and anesthesia care was provided mainly by anesthesia technicians. Six of the 44 hospitals had no trained anesthesia provider, and one hospital reported that general doctors provided anesthesia care.21
Although high levels of morbidity and mortality are found across the entire region, they are especially prevalent in rural areas, which tend to be underprovisioned in both infrastructure and workforce. In general, as shown in Figure 5, very few qualified anesthesiologists choose to work in rural areas. Nonphysician anesthesia providers, performing unsupervised anesthetics, do have a role to play in obstetric anesthesia in low-resource settings,22 particularly in remote parts of Africa, where there is no possibility of adequate staffing by medical practitioners.23 However, initial training as well as continuing medical education are considered critically important, especially in this group, because poor training has been implicated in mortality rates as high as 1 in 150 anesthetics in one recent report (and half of these reported deaths were in obstetric cases).24 A similar trend is seen in East Africa, where anesthesiologists are concentrated in urban areas (Figure 5).
Although further studies are needed to evaluate the extent of these issues in all other hospitals in the region, policy changes and implementation of basic protocols are urgently needed to rectify many of the issues identified in this study, thereby allowing the provision of safe and effective care across the region. There is need to strengthen health systems in the region to support the effective delivery of safe surgery and safe anesthesia. Governments should ensure that the basic equipment required for safe anesthesia is available as a first step toward improving access to safe surgery.
We identified significant shortages of both personnel and equipment needed to provide safe anesthetic care at the main referral and teaching hospitals in East Africa. We conclude that more funding is required for training of physician anesthetists if developing countries are to reach the targeted specialist workforce density of the Lancet Commission on Global Surgery of 20 surgical, anesthetic, and obstetric physicians per 100,000 population by 2030. Ministries of health need to acknowledge the work done by anesthesia providers in the region and improve working conditions, including remuneration. Seventy-five percent of anesthetists work in the private sector to supplement their income from their public hospital positions. More refresher courses on management of obstetric and neonatal emergencies are required. Anesthesia is an indispensible component of emergency obstetric care, and there is urgent need to strengthen this service in developing countries. Supervision of nonphysician anesthesia providers by anesthesiologists is essential to safe practice and requires investment in the training of anesthesiologists.
Supplementary Material
Table 2.
Operating Table and Suction Machines
| State of Operating Table and Suction Machine
|
|||||
|---|---|---|---|---|---|
| Hospital
|
|||||
| Variable | Mulago, Uganda | CHU Kamenge, Burundi | Kenyatta, Kenya | Muhimbili, Tanzania | CHU Kigali, Rwanda |
| Operating table | |||||
| Operating table | Present | Present | Present | Present | Present |
| Number of operating tables | Two | Two | Two | Two | Two |
| Tiltable operating tables | Yes | Yes | Yes | Yes | Yes |
| Serviced | No | No | No | Yes | Yes |
| Suction machine | |||||
| Suction machine | Absent | Present | Present | Present | Present |
| Functionality | Unknown | Functional | Functional | Functional | Functional |
| Serviced | Unknown | No | No | Yes | Yes |
Abbreviation: CHU, Centre Hospitalier Universite.
Acknowledgments
The Government of Uganda and the Association of Anaesthetists of Great Britain and Ireland (AAGBI) sponsored the first author’s Masters of Medicine in Anaesthesiology and Critical Care. We also acknowledge support from the World Federation of Societies of Anaesthesiologists (WFSA), the World Bank, WFSA Subcommittee (University of California, San Francisco), Global Partners in Anesthesia and Surgery, and the Government of Uganda, for funding this research. The first author’s 2014 Fellowship in Global Health was sponsored by the National Institutes of Health (NIH research training grant No. R25TW009343). We are grateful to Dr Wanzira Humphrey, the epidemiologist who analyzed our data. Special recognition and thanks also go to Harriet K. Mayanja and Dr Isabeau Walker for their contribution to this final report.
Funding: World Federation of Societies of Anesthesiologists (WFSA) 2013 Research Award, WFSA Safety Subcommittee, Global Partners in Anesthesia and Surgery, World Bank, and Government of Uganda, none of which influenced manuscript writing or decision to submit for publication.
Footnotes
The authors declare no conflicts of interest.
WHO. Maternal mortality. 2014 [cited 2014 16/December/2014]; Available at: http://www.who.int/mediacentre/factsheets/fs348/en/.
UNFPA. Sub-Saharan Africa’s maternal death rate down 41 per cent. 2012 17/May/2012 [cited 2014; 1/May/2014]. Available at: http://africa.unfpa.org/public/cache/offonce/news/pid/10767.
Khan K, Wojdyla D, Say L, Gülmezoglu A, Van Look P: WHO analysis of causes of maternal death: a systematic review. Lancet. 2006, 367:1066–1074.
Li XF, Fortney JA, Koltelchuck M, Glover LH: The postpartum period: the key to maternal mortality. Int J Gynaecol Obstet. 1996, 54:1–10.
UNFPA. Emergency obstetric care. 2014 [cited 2014 25/4/2014]; Available at: http://www.unfpa.org/public/home/mothers/pid/4385.
Minister H. Kenya decries shortage of anesthetists. 2009 15/September/2009 [cited 2014 28/April/2014]; Available at: http://www.capitalfm.co.ke/news/2009/09/kenya-decries-shortage-of-anesthetists/?wpmp_switcher=mobile.
DISCLOSURES
Name: Isabella Epiu, MBChB.
Contribution: The principal investigator designed the research, sought ethical approval, and conducted the interviews. She also entered the results and wrote the report.
Name: Jossy Verel Bahe Tindimwebwa, MBChB.
Contribution: This author was a co-investigator and participated in designing the research paper and obtaining ethical approval and writing the report.
Name: Cephas Mijumbi, MBChB.
Contribution: This author was a co-investigator and participated in designing the research paper and obtaining ethical approval and writing the report.
Name: Thomas M. Chokwe, MBChB.
Contribution: This author collected the data and participated in writing and editing the report.
Name: Edwin Lugazia, MBChB.
Contribution: This author collected the data and participated in writing and editing the report.
Name: Francois Ndarugirire, PhD.
Contribution: This author collected the data and participated in writing and editing the report.
Name: Theogene Twagirumugabe, MBChB.
Contribution: This author collected the data and participated in writing and editing the report.
Name: Gerald Dubowitz, MBChB.
Contribution: This author was a co-investigator and participated in designing the research paper and obtaining ethical approval, and writing the report.
This manuscript was handled by: Angela Enright, MD.
References
- 1.Prata N, Passano P, Sreenivas A, Gerdts CE. Maternal mortality in developing countries: challenges in scaling-up priority interventions. Womens Health (Lond) 2010;6:311–327. doi: 10.2217/whe.10.8. [DOI] [PubMed] [Google Scholar]
- 2.Ronsmans C, Graham WJ, Lancet Maternal Survival Series Steering Group Maternal mortality: who, when, where, and why. Lancet. 2006;368:1189–1200. doi: 10.1016/S0140-6736(06)69380-X. [DOI] [PubMed] [Google Scholar]
- 3.OAA/AAGBI Guidelines for Obstetric Anaesthesia Services. London: Association of Anaesthetists of Great Britain and Ireland; 1998. revised 2005. [Google Scholar]
- 4.Dyer RA, Reed AR, James MF. Obstetric anaesthesia in low-resource settings. Best Pract Res Clin Obstet Gynaecol. 2010;24:401–412. doi: 10.1016/j.bpobgyn.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Merry AF, Cooper JB, Soyannwo O, Wilson IH, Eichhorn JH. International standards for a safe practice of anesthesia 2010. Can J Anaesth. 2010;57:1027–1034. doi: 10.1007/s12630-010-9381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman LP, Waldman RJ, de Pinho H, Wirth ME, Chowdhury AM, Rosenfield A. Transforming health systems to improve the lives of women and children. Lancet. 2005;365:997–1000. doi: 10.1016/S0140-6736(05)71090-4. [DOI] [PubMed] [Google Scholar]
- 7.Tanzania MoH. Health Management Information Systems report. 2012 [Google Scholar]
- 8.National Institute of Statistics Rwanda. National Population Projection, 2007–2022. Kigali: National Institute of Rwanda; 2009. [Google Scholar]
- 9.National Institute of Statistics Rwanda. Rwanda Demographic and Health Survey Report Kigali. Rwanda: National Institute of Statistics of Rwanda; 2010. [Google Scholar]
- 10.Costello A, Azad K, Barnett S. An alternative strategy to reduce maternal mortality. Lancet. 2006;368:1477–1479. doi: 10.1016/S0140-6736(06)69388-4. [DOI] [PubMed] [Google Scholar]
- 11.Clyburn P, Morris S, Hall J. Anaesthesia and safe motherhood. Anaesthesia. 2007;62(Suppl 1):21–25. doi: 10.1111/j.1365-2044.2007.05293.x. [DOI] [PubMed] [Google Scholar]
- 12.Walker IA, Newton M, Bosenberg AT. Improving surgical safety globally: pulse oximetry and the WHO Guidelines for Safe Surgery. Paediatr Anaesth. 2011;21:825–828. doi: 10.1111/j.1460-9592.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 13.Barnes-Josiah D, Myntti C, Augustin A. The “three delays” as a framework for examining maternal mortality in Haiti. Soc Sci Med. 1998;46:981–993. doi: 10.1016/s0277-9536(97)10018-1. [DOI] [PubMed] [Google Scholar]
- 14.Grimes CE, Bowman KG, Dodgion CM, Lavy CB. Systematic review of barriers to surgical care in low-income and middle-income countries. World J Surg. 2011;35:941–950. doi: 10.1007/s00268-011-1010-1. [DOI] [PubMed] [Google Scholar]
- 15.Kruk ME, Wladis A, Mbembati N, et al. Human resource and funding constraints for essential surgery in district hospitals in Africa: a retrospective cross-sectional survey. PLoS Med. 2010;7:e1000242. doi: 10.1371/journal.pmed.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kushner AL, Cherian MN, Noel L, Spiegel DA, Groth S, Etienne C. Addressing the millennium development goals from a surgical perspective: essential surgery and anesthesia in 8 low- and middle-income countries. Arch Surg. 2010;145:154–159. doi: 10.1001/archsurg.2009.263. [DOI] [PubMed] [Google Scholar]
- 17.Chambers V, Booth D. Delivering Maternal Health: Why Is Rwanda Doing Better Than Malawi, Niger And Uganda? 2012 Available at http://www.institutions-africa.org/filestream/20120611-odi-briefing-paper-74-delivering-maternal-health-why-is-rwanda-doing-better-than-malawi-niger-and-uganda-v-chambers-and-d-booth-may-2012. Accessed November 2016.
- 18.WHO, Health KMo. WHO Integrated Management for Emergency and Essential Surgical Care. WHO and Ministry of Health Kenya; Aug 8–10, 2006. 2006. Available at http://apps.who.int/medicinedocs/documents/s15341e/s15341e.pdf. Accessed November 2016. [Google Scholar]
- 19.Paxton A, Bailey P, Lobis S. The United Nations Process Indicators for emergency obstetric care: reflections based on a decade of experience. Int J Gynaecol Obstet. 2006;95:192–208. doi: 10.1016/j.ijgo.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Tanzania M. Ministry of Health & Social Welfare: Primary Health Services Development Program 2007–2017. 2007 Available at http://www.tzdpg.or.tz/fileadmin/documents/dpg_internal/dpg_working_groups_clusters/cluster_2/health/Key_Sector_Documents/Induction_Pack/2007-2017__MMAM_-_PRIMARY_HEALTH_SERVICES.pdf. Accessed November 2016.
- 21.Petroze RT, Nzayisenga A, Rusanganwa V, Ntakiyiruta G, Calland JF. Comprehensive national analysis of emergency and essential surgical capacity in Rwanda. Br J Surg. 2012;99:436–443. doi: 10.1002/bjs.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman LP. Shifting visions: “delegation” policies and the building of a “rights-based” approach to maternal mortality. J Am Med Womens Assoc. 2002;57:154–158. [PubMed] [Google Scholar]
- 23.Morris S, Clyburn P, Harries S, Rees L, Sewell J, Hall J. Mothers of Africa–an anaesthesia charity. Anaesthesia. 2007;62(Suppl 1):108–112. doi: 10.1111/j.1365-2044.2007.05312.x. [DOI] [PubMed] [Google Scholar]
- 24.Ouro-Bang’na Maman AF, Tomta K, Ahouangbévi S, Chobli M. Deaths associated with anaesthesia in Togo, West Africa. Trop Doct. 2005;35:220–222. doi: 10.1258/004947505774938666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


