Abstract
Aβ (amyloid β-peptide) is believed to cause AD (Alzheimer’s disease). Aβ42 (Aβ comprising 42 amino acids) is substantially more neurotoxic than Aβ40 (Aβ comprising 40 amino acids), and this increased toxicity correlates with the existence of unique Aβ42 oligomers. Met35 oxidation to sulfoxide or sulfone eliminates the differences in early oligomerization between Aβ40 and Aβ42. Met35 oxidation to sulfoxide has been reported to decrease Aβ assembly kinetics and neurotoxicity, whereas oxidation to sulfone has rarely been studied. Based on these data, we expected that oxidation of Aβ to sulfone would also decrease its toxicity and assembly kinetics. To test this hypothesis, we compared systematically the effect of the wild-type, sulfoxide and sulfone forms of Aβ40 and Aβ42 on neuronal viability, dendritic spine morphology and macroscopic Ca2+ currents in primary neurons, and correlated the data with assembly kinetics. Surprisingly, we found that, in contrast with Aβ-sulfoxide, Aβ-sulfone was as toxic and aggregated as fast, as wild-type Aβ. Thus, although Aβ-sulfone is similar to Aβ-sulfoxide in its dipole moment and oligomer size distribution, it behaves similarly to wild-type Aβ in its aggregation kinetics and neurotoxicity. These surprising data decouple the toxicity of oxidized Aβ from its initial oligomerization, and suggest that our current understanding of the effect of methionine oxidation in Aβ is limited.
Keywords: Alzheimer’s disease, amyloid β-protein, methionine, oxidation, oligomer
INTRODUCTION
AD (Alzheimer’s disease) is characterized by memory loss and progressive cognitive decline [1]. Extracellular amyloid plaques comprising predominantly fibrillar Aβ (amyloid β-protein) and intracellular neurofibrillary tangles made of hyperphosphorylated tau are neuropathological hallmarks of AD [2]. Abundant evidence suggests that assembly of Aβ into pre-fibrillar neurotoxic oligomers initiates the disease process in AD, and formation of amyloid plaques and neurofibrillary tangles follow neuronal injury by Aβ oligomers [3].
Aβ exists predominantly in two major forms comprising 40 (Aβ40) or 42 (Aβ42) amino acid residues. Genetic, physiological and biochemical evidence indicates that Aβ42 plays a predominant role in the pathogenesis of AD [4], although it is ~10-fold less abundant than Aβ40 [5]. In the AD brain, Aβ42 is the main component of parenchymal plaques, whereas Aβ40 is the main component of vascular deposits [6]. Aβ42 aggregates faster and is substantially more neurotoxic than Aβ40 [7].
Study of Aβ oligomerization in vitro using PICUP (photo-induced cross-linking of unmodified proteins) and ion-mobility MS has revealed that the oligomer size distributions of Aβ40 and Aβ42 are distinct [8,9]. Aβ40 exists mainly as a mixture of monomer, dimer, trimer and tetramer units, whereas Aβ42 preferentially forms pentamer and hexamer units, termed paranuclei, which self-associate into higher-order oligomers [8,9]. The distinct oligomerization patterns of Aβ40 and Aβ42 correlate with the difference in the toxicity of the two Aβ alloforms and may explain these differences. This suggests that the C-terminal region of Aβ is a crucial factor in Aβ assembly and toxicity. Supporting this hypothesis, structural studies using experimental and computational methods have identified substantial differences in the flexibility and conformational preferences of the C-terminal region of Aβ40 and Aβ42 [10–12].
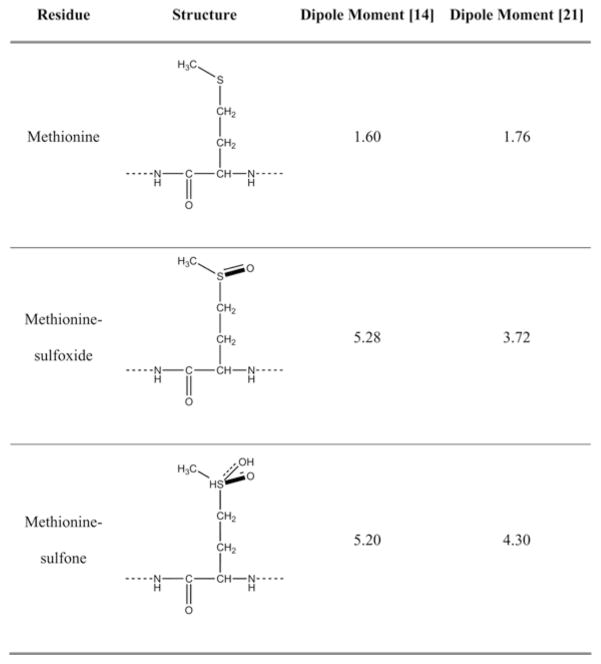
The single methionine residue in Aβ, Met35, is located in the middle of the hydrophobic C-terminal region of Aβ and is readily oxidized in vivo [13]. The dramatic increase in the polarity of the methionine side chain that occurs upon oxidation (Figure 1) has a profound effect on the hydropathy of the entire region [14]. Aβ in which Met35 is oxidized to sulfoxide (Aβ-sulfoxide) has been found in amyloid plaques of AD brain [13], and consequently has been the subject of many studies [15]. Despite the abundance of literature on the subject, it is not clear whether Aβ-sulfoxide contributes to AD aetiology or results from the highly oxidative environment around amyloid plaques where fibrillar Aβ may be trapped for long periods and get oxidized.
Figure 1.
Structure and calculated dipole moments (in Debye) of methionine and its oxidized forms
In addition to oxidation to sulfoxide, methionine can undergo a second oxidation reaction yielding methionine-sulfone (Figure 1). This reaction is less common than oxidation of methionine to methionine-sulfoxide in vivo, yet methionine-sulfone has been observed in the brain of patients with AD or Parkinson’s disease [16] in the antioxidant protein DJ-1. Ali et al. [17] have proposed that Aβ-sulfone also might form in vivo and this has not so far been observed, simply because it has not been looked for [17].
Oxidation of Met35 to sulfoxide has been reported to decrease formation of Aβ40 trimers and tetramers [18], lower Aβ assembly kinetics [11,19] and reduce Aβ neurotoxicity [15,20]. Solution-state NMR studies have shown that the lower assembly kinetics and toxicity correlated with higher conformational flexibility in the C-terminal region of Aβ-sulfoxide relative to WT (wild-type) Aβ [19]. The assembly kinetics and toxicity of Aβ-sulfone have not been reported previously.
A PICUP study of the effect of Met35 oxidation in Aβ revealed striking differences between Aβ40 and Aβ42 [14]. The oligomer size distribution of Aβ40 was unaffected by oxidation of Met35. In contrast, oxidation of Met35 in Aβ42 to either sulfoxide or sulfone was found to abolish paranucleus formation and produce oligomer size distributions indistinguishable from those of Aβ40 [14]. In agreement with these observations, molecular dynamics simulations of the WT, sulfoxide and sulfone forms of Aβ42 monomers showed that the two oxidized forms adopted conformations that were distinct from that of WT Aβ42, both in secondary structure and in global fold [21]. In particular, long-range interactions between the C-terminal region and the CHC (central hydrophobic cluster), which were prominent in WT Aβ42 and have been observed in other modelling studies of Aβ42 monomers [22] and oligomers [10,23], were absent in the simulations of the two oxidized forms [21]. Rather, the sulfoxide and sulfone groups, which are hydrogen-bond acceptors, formed hydrogen bonds with backbone amide groups in the C-terminal regions stabilizing short-range turn conformations. In modelling studies comparing Aβ40 and Aβ42 oligomerization [10,23] the interaction between the C-terminus and the CHC regions was substantially less prominent in Aβ40 compared with Aβ42, suggesting that this structure may be necessary for paranucleus formation.
Attempting to correlate the structural and functional data, we hypothesized that the Aβ oligomer size distributions observed using PICUP correlated with toxicity. If this hypothesis were correct, oxidation of Aβ40 would be predicted to have little effect on toxicity, whereas oxidation of Aβ42 to sulfoxide or sulfone would result in toxicity lower than that of WT Aβ42 and similar to that of Aβ40. The lower toxicity of Aβ42-sulfoxide reported in the literature [15,20,24] supported this hypothesis, yet we found a dearth of information regarding the effect of oxidation of methionine to methionine-sulfoxide in Aβ40 or oxidation of either Aβ alloforms to sulfone.
Because methionine-sulfoxide can be reduced to methionine in vivo by methionine-sulfoxide reductases [25], when cells are treated with Aβ-sulfoxide, they also are treated with unoxidized Aβ. The ratio of Aβ-sulfoxide to WT Aβ depends on the kinetics of methionine-sulfoxide reduction relative to peptide degradation. Additional factors determining the observed toxicity are the assembly state of each Aβ form in the context of the cellular environment [26] and the mechanisms by which each form causes toxicity, all of which are poorly understood. Because methionine-sulfone is not reduced in vivo [27], studying the effect of Aβ-sulfone on cultured neurons is a ‘clean’ system compared with Aβ-sulfoxide. Thus, to test our hypothesis of the effect of methionine oxidation on Aβ assembly and toxicity, we compared the WT, sulfoxide and sulfone forms of Aβ40 and Aβ42 using assays that measure assembly kinetics, cell viability and cellular function in primary cortical or hippocampal neurons. Surprisingly, we found that, contrary to our prediction, Aβ-sulfone behaved similarly to unoxidized Aβ and distinctly from Aβ-sulfoxide, raising new questions regarding the mechanisms by which each Aβ form causes toxicity.
EXPERIMENTAL
Peptide synthesis
Aβ40, [Met(O)35]Aβ40, [Met(O2)35]Aβ40, Aβ42, [Met(O)35]-Aβ42 and [Met(O2)35]Aβ42 were synthesized by incorporating Fmoc (fluoren-9-ylmethoxycarbonyl)–Met(O) or Fmoc-Met(O2) (EMD Biosciences) in position 35 where appropriate. They were then purified and characterized in the UCLA Biopolymers Laboratory. Quantitative amino acid analysis and MS were used to characterize the expected compositions and molecular masses respectively, for each peptide.
Preparation of peptide solutions
Purified peptides were stored as freeze-dried powders at − 20°C. Before use, peptides were treated with HFIP (1,1,1,3,3,3-hexafluoroisopropanol; TCI America) to disassemble pre-formed aggregates, and stored as dry films at − 20°C as described previously [28]. Immediately before use, films were dissolved in 60 mM NaOH at 10% of the desired volume. For biophysical measurements, the solution was then diluted to 50% of the desired volume with deionized water (18.2 MΩ produced by a Milli-Q system, Millipore) and sonicated for 1 min. Then, the solution was diluted with 20 mM sodium phosphate (pH 7.4), to 10 μM. The pH was adjusted, if necessary, with H3PO4 or 1 M NaOH. Alternatively, peptides were prepared by dilution into cell culture medium, similar to the preparation for toxicity experiments. For toxicity experiments, peptides were diluted with cell culture medium after initial dissolution in 60 mM NaOH, and then sonicated for 1 min. The peptides were then diluted in culture medium to the final concentration, which was 10 μM unless otherwise stated.
Animals
Experiments performed in UCLA were compliant with the National Research Council Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Animal Research Council and the Ethics Committee. Experiments performed in the Catholic University of Rome complied with Italian Ministry of Health guidelines, with national laws (Legislative decree 116/1992) and with European Union guidelines on animal research (No. 86/609/EEC). For experiments in UCLA, pregnant [E (embryonic day) 18] Sprague–Dawley rats were purchased from Charles River Laboratory (Wilmington, MA). In the Catholic University of Rome, Sprague–Dawley rats were bred in-house.
Cell culture
For TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick-end labelling) assay and dendritic spine morphology experiments, primary cortical or hippocampal neurons respectively, were prepared as described previously by Segal and Manor [29]. Briefly, E18 pregnant rats were killed with CO2 and the pups were collected immediately. The brains were dissected in chilled Leibovitz’s L-15 medium (A.T.C.C.) in the presence of 1 μg/ml penicillin/streptomycin (Invitrogen). The tissue was incubated with 0.25% trypsin/EDTA (A.T.C.C.) for 30 min and then mechanically dissociated in a small volume of Leibovitz’s L-15 medium using a fire-polished Pasteur pipette. Cortical neurons were used for cell-viability assays. They were suspended in DMEM (Dulbeco modified Eagle’s medium, obtained from A.T.C.C.) containing 10% heat-inactivated FBS (fetal bovine serum, A.T.C.C.) and 1 μg/ml penicillin/streptomycin, and plated in poly-D-lysine (0.1 mg, Sigma)-coated coverslips (Fisher Scientific) at a density of 3×105 cells/ml. Hippocampal neurons were used for dendritic spine studies. They were suspended in neurobasal media (Gibco) containing B-27 growth factor and 20 mM glutamine and plated on poly-D-lysine (0.1 mg%, Sigma)-coated coverslips. The cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 for 6 days for cell viability experiments and for 21 days for dendritic spine experiments. At 24 h after plating, the medium was replaced with fresh DMEM/neurobasal medium supplemented with 5 μM cytosine β-D-arabinofuranoside (Sigma) to inhibit the proliferation of glial cells.
For measurements of Ca2+ currents, primary cortical neurons were obtained from E17–E19 rat embryos as described previously by Dravid and Murray [30] with minor modifications. Briefly, cortices were dissected and incubated for 10 min at 37°C in PBS containing 0.025% trypsin/0.01% EDTA (Biochrom AG). The tissue was then mechanically dissociated at room temperature (23–25°C) using a fire-polished Pasteur pipette and the cell suspension was harvested and centrifuged at 235 g for 8 min. The pellet was suspended in 88.8% minimum essential medium (Biochrom), 5%FBS, 5%horse serum, 1%glutamine (2 mM), 0.2% gentamicin (0.1 mg/ml) and glucose (25 mM). Cells were plated on to 20-mm coverslips pre-coated with poly-L-lysine (0.1 mg/ml, Sigma) at a density of 1×105 cells/well. At 24 h after plating, the culture medium was replaced with a medium containing 97.3% neurobasal medium (Invitrogen), 2% B-27 (Invitrogen), 0.5% glutamine (2 mM) and 0.2% gentamicin (0.1 mg/ml). Finally, after 72 h, the culture medium was replaced with a similar medium lacking glutamine and cells were grown for 4–7 more days before experiments.
TUNEL staining
Cortical neurons were treated with Aβ analogues for 48 h and then stained using a TUNEL assay kit (APO-BrdU apoptosis detection kit, Invitrogen) as described previously [31]. Fluorescent signals were visualized using a Nikon Eclipse E400 microscope (Nikon Instruments) at λex = 480 nm and λem = 530 nm. Images were merged using the bundled software ‘Picture Frame’ (Optronics). Images were taken from multiple fields in three independent experiments and the number of TUNEL-positive cells divided by the total number of counted cells was expressed as the percentage of apoptotic death (means ± S.E.M.).
Patch-clamp recording of Ca2+ currents
According to standard protocols, Ba2+ was used as a charge carrier rather than Ca2+ [32]. Macroscopic Ba2+ currents flowing through VGCCs (voltage-gated calcium channels) were recorded by patch-clamp in whole-cell configuration [33] using an Axopatch 200B amplifier (Molecular Devices). Stimulation and data acquisition were performed using the Digidata 1200 series interface and pCLAMP 9.2 software (Molecular Devices), as described previously [20,33]. The external solution contained 125 mM NaCl, 10 mM BaCl2, 1 mM MgCl2, 10 mM Hepes and 2×10− 4 mM tetrodotoxin to block Na+ channels. The pH was adjusted to 7.3 with NaOH. The standard internal solution consisted of 110 mM CsCl, 10 mM tetraethylammonium chloride, 2 mM MgCl2, 10 mM EGTA, 8 mM glucose and 10 mM Hepes. To minimize current run-down during experiments, 4.0 mM ATP magnesium salt, 0.25 mM cAMP sodium salt and 4.0 mM phos-phocreatine disodium salt were added to this solution. The pH was adjusted to 7.3 with CsOH. The cell membrane was depolarized every 6 s (pulse duration 200 ms) to voltages ranging from − 50 to +50 mV from the holding potential of −80 mV. Compensation for capacitative transients and leakage currents was achieved online with the clamp-amplifier settings and off-line by subtraction of Cd2+-insensitive currents (200 μM Cd2+). Current density (pA/pF) was calculated by dividing current amplitude by cell membrane capacitance, which was measured with the membrane test feature of the pCLAMP 9.2 software. Cells were treated with 10 μM of each Aβ analogue for 12 h before experiments. All recordings were made at 23–25°C.
Dendritic spine morphology
Primary hippocampal neurons were used to study the dendritic spine morphology as described previously [34]. Briefly, neurons were treated with 3 μM of each Aβ analogue for 72 h. The cells were then fixed with 4% paraformaldehyde and individual neurons were stained with DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Sigma) as described previously [34] using a Model 5070 micromanipulator (Eppendorf). The DiI-labelled neurons were imaged at 100× magnification (oil-immersion objective) using a confocal laser-scanning microscope (Leica). Images were magnified further using a 3× zoom so the morphology of individual spines could be determined and subsequently quantified. The Z-stack images were collected at 0.3 μm intervals to cover the full depth of the dendritic arbors (20–30 μm) and compressed into a single JPEG image. The numbers of dendritic spines were counted per 100 μm length of dendritic branches using ImageJ. The dendritic segments were selected randomly among secondary dendrites from apical branches. At least 50 dendritic segments were used for morphometic analysis. In total, 10–15 individual neurons were analysed for each experimental group.
CD spectroscopy
Samples were incubated at 25°C with continuous agitation at 200 rev./min using an orbital shaker. Spectra were recorded every 2 h during the first 12 h, and then at 24, 48 and 72 h, using a J-810 spectropolarimeter (Jasco) equipped with a thermostable sample cell at 25°C using 1-mm path-length cuvettes. Spectra were collected from 190 to 260 nm with a 1 s response time, 50 nm/min scan speed, 0.2 nm resolution and 2 nm bandwidth, and averaged after background subtraction. The data are representative of three independent experiments.
Electron microscopy
Samples were incubated at 25°C with continuous agitation using an orbital shaker at 200 rev./min. Aliquots (8 μl) were applied to glow-discharged carbon-coated Formvar grids (Electron Microscopy Science) for 20 min, fixed with 5 μl of 2.5% glutaraldehyde (Sigma) for 4 min, and stained with 5 μl of 1% uranyl acetate (Sigma) for 3 min. The solution was wicked off and the grids were air-dried. The morphology was visualized using a CM120 transmission electron microscope (FEI-Philips).
RESULTS
Effect of WT and oxidized Aβ on neuronal viability
To investigate the structure–activity relationship of native and oxidized Aβ variants and gain insight into the mechanism of toxicity, we used TUNEL staining in rat primary cortical neurons (Figure 2). The TUNEL assay indicates DNA fragmentation as a measurement of apoptosis [35].
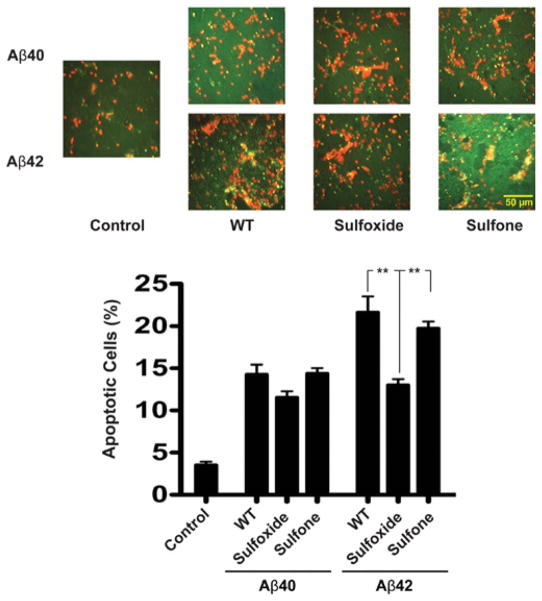
Figure 2. Native and oxidized Aβ-induced apoptosis.
Rat primary cortical neurons were cultured for 6 days on poly-D-lysine-coated coverslips and treated with 10 μM of each Aβ analogue for 48 h. The cells were then probed with the APO-BrdU kit for analysis of DNA fragmentation. (A) Representative micrographs of multiple fields. Red colour indicates normal cells. Green/yellow colour indicates TUNEL-positive cells. (B) The number of apoptotic cells was divided by the total number of cells and expressed as a percentage of apoptotic death. The results are means ± S.E.M. for three independent experiments. **P < 0.01.
Aβ40 was found to cause 14 ± 1%apoptosis (Figure 2). Aβ40-sulfoxide caused 11.5 ± 0.7% apoptosis, whereas Aβ40-sulfone had a similar effect to WT Aβ40, 14.3 ± 0.6%apoptosis. Overall, the differences observed among the Aβ40 analogues were not statistically significant. Under the same conditions, Aβ42 caused 20.5 ± 0.5%apoptosis (Figure 2). Similar to previously described data [20], Aβ42-sulfoxide was significantly less toxic, causing 13.0 ± 0.7%apoptosis, i.e. a similar level of toxicity to Aβ40, as predicted. Surprisingly, however, Aβ42-sulfone showed similar toxicity to WT Aβ42, causing 19.7 ± 0.8% apoptosis. These results were unexpected in view of the oligomer size distribution of Aβ42-sulfone, which was similar to that of Aβ42-sulfoxide and distinct from that of WT Aβ42 [14]. Similar trends were observed in both cortical and hippocampal neurons using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide] reduction and LDH (lactate dehydrogenase) release assays (results not shown). Thus three different assays showed that despite the similar increase in dipole moment upon oxidation of Met35 to sulfoxide or sulfone [14,21], and despite the fact that oxidation had a similar effect on both the sulfoxide and sulfone forms of Aβ42 [14], only the sulfoxide form behaved as we predicted and showed similar toxicity to WT Aβ40.
Effect of WT and oxidized Aβ on macroscopic Ca2+ currents
Next we asked whether the behaviour of the oxidized forms of Aβ correlated with more subtle toxic effects. Disruption of Ca2+ homoeostasis and excitotoxicity are thought to be important mechanisms by which Aβ disrupts synaptic activity and eventually lead to neuronal death [36]. Disruption of intracellular Ca2+ homoeostasis has long been implicated both in normal brain aging and in the pathogenesis of AD. Both conditions are characterized by impairment of the neurons’ ability to control Ca2+ fluxes and recover from Ca2+ loads [37]. To assess the effect on Ca2+ currents, we measured Aβ-induced changes in currents flowing through VGCCs in primary cortical neurons.
In whole-cell patch-clamp recordings, we measured macroscopic Ba2+ currents after treatment with the different Aβ40 or Aβ42 analogues. Previously, treatment of IMR32 cells with 10 μM Aβ42 for 6–24 h was found to yield maximal increase in depolarization-induced Ca2+ influx at 12 h [20]. Therefore we measured Ba2+ currents in the experiments described here at 12 h post-treatment.
Both Aβ40 and Aβ42 caused a significant increase (P < 0.001) in total Ba2+ current densities in cortical neurons (Figures 3A and 3B), similar to the previous results obtained using IMR32 cells [20], suggesting that each of them triggered dysregulation of Ca2+ channels. The effect of Aβ42 was stronger than that of Aβ40 (+69%compared with +55%respectively), although the difference was not statistically significant. In contrast, treatment of the cells with Aβ40- or Aβ42-sulfoxide had no effect on total Ba2+ currents. In agreement with the measurements of cell viability, the sulfone form of Aβ40 and Aβ42 caused an increase in Ba2+ current densities to the same magnitude as the corresponding WT Aβ form (Figures 3A and 3B). The data suggest that in contrast with the WT and sulfone forms of Aβ, under the experimental conditions used in the present study, the weak toxicity afflicted by Aβ-sulfoxide was not associated with significant changes in VGCC signals.
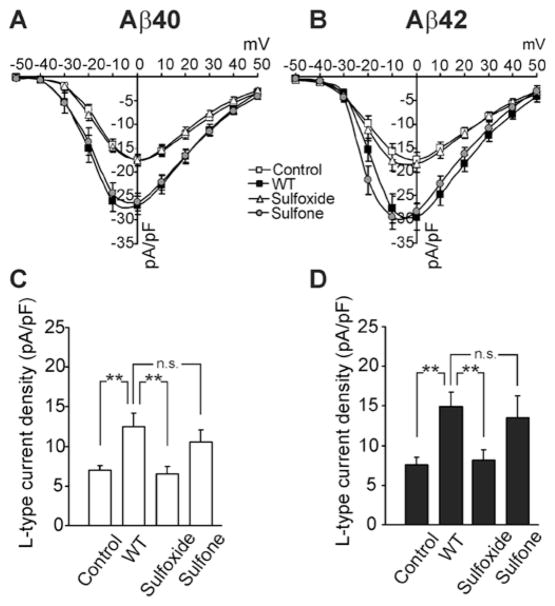
Figure 3. Effect of native and oxidized Aβ analogues on calcium currents.
Rat primary cortical neurons were treated with 10 μM of each Aβ40 or Aβ42 analogue for 12 h. Macroscopic Ba2+ currents flowing through VGCCs were recorded using patch-clamp in a whole-cell configuration. Current density (pA/pF) was calculated by dividing current amplitude by cell membrane capacitance. (A and B) Current-to-voltage relationships in neurons treated with Aβ40 and Aβ42 analogues respectively (n = 15 for each group). (C and D) Peak current densities of L-type currents in neurons treated with Aβ40 analogues or Aβ42 analogues respectively. **P < 0.01; n.s., not significant.
Previously, L-type Ca2+ (Cav1) channels were found to contribute significantly to the current increase induced by Aβ42 in IMR32 cells, and Ca2+ influx through these channels was found to be responsible for the calcium-mediated activation of pro-apoptotic pathways underlying the stronger neurotoxicity of WT Aβ42 relative to Aβ42-sulfoxide [20]. To determine whether a similar correlation exists between L-current increases and Aβ neurotoxicity in cortical neurons, and to what extent it is activated by each of the oxidized forms of Aβ, we studied the Aβ-induced modulation of current amplitudes before and after applying 5 μM of the selective L-channel blocker nifedipine. Analysis of the data revealed that peak Ba2+ currents flowing through L-channels significantly after treatment with WT Aβ40 or Aβ42 (+80% and +98% respectively; P < 0.01), or their respective sulfone forms (+51% and +80% respectively; P < 0.01; Figures 3C and 3D), whereas Aβ40- or Aβ42-sulfoxide did not produce significant changes.
Aβ-induced perturbation of dendritic spines
Dendritic spines are semi-autonomous compartments containing molecular machinery important for synaptic transmission and plasticity [38]. The number of dendritic spines per unit length of the dendrite in the adult brain is indicative of the number of excitatory synapses present. Examination of post-mortem brains from patients with AD or Down syndrome dementia has shown substantial loss of dendritic spines [39].
We analysed dendritic spines from apical branches of hippocampal pyramidal neurons after treatment with Aβ42 or its oxidized analogues (Figure 4). In initial experiments, we found that 3 μM Aβ42 induced a robust effect on dendritic spine number and morphology in this assay, whereas 10 μM led to overt cell death that precluded observation of the effect on dendritic spines. Therefore we used 3 μM for comparison of WT and oxidized Aβ42 analogues. Because the toxicity of Aβ40 was substantially lower than that of Aβ42 in cell viability assays (Figure 2, and data not shown for MTT and LDH assays), we did not use Aβ40 in this assay.
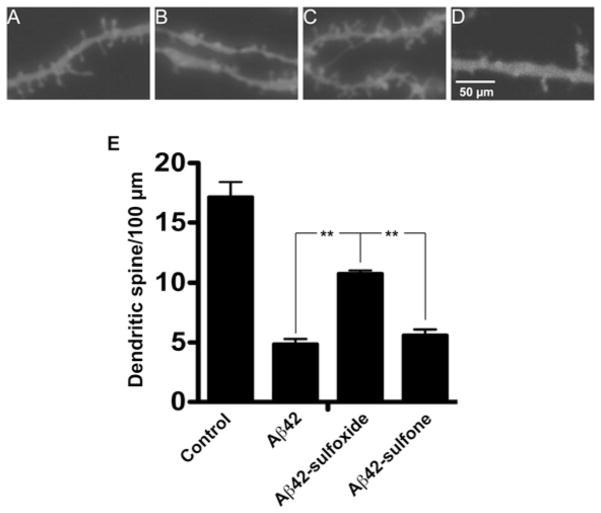
Figure 4. Native and oxidized Aβ42-induced changes in dendritic spine morphology.
Rat primary hippocampal neurons were grown for 21 days, treated with 3 μM of each Aβ42 analogue for 72 h, and stained with DiI. Micrographs are representative of secondary apical branches from multiple neurons. (A) Medium alone, (B) WT Aβ42, (C) Aβ42-sulfoxide and (D) Aβ42-sulfone. (E) The total number of spines in three independent experiments was counted and expressed as means ± S.E.M. **P < 0.01.
After treatment with 3 μM Aβ42, the dendrites showed abundant varicosities and the number of dendritic spines per 100 μm decreased to <30% of that in cells treated with medium (Figures 4B and 4E). Consistent with the cell viability assays described above, Aβ42-sulfoxide showed little induction of varicosities and had a significantly weaker effect on dendritic spine density compared with WT Aβ42, decreasing the number of dendritic spines per 100 μm to ~70%of medium-treated cells (Figures 4C and 4E). In contrast, and consistent with the other assay results, Aβ42-sulfone induced abundant varicosities and decreased the dendritic spine density to the same level as WT Aβ42 (Figures 4D and 4E).
Assembly kinetics of WT and oxidized Aβ
Because the biological activity of Aβ42-sulfone contradicted our predictions, which were based on the initial oligomer size distributions for the WT and oxidized Aβ42 analogues, we asked whether the bioactivity correlated with aggregation kinetics. Early studies of the effect of methionine oxidation to sulfoxide on Aβ assembly have led to mixed results [40,41], whereas most of the later studies reported that assembly kinetics of Aβ-sulfoxide was decreased relative to that of WT Aβ.
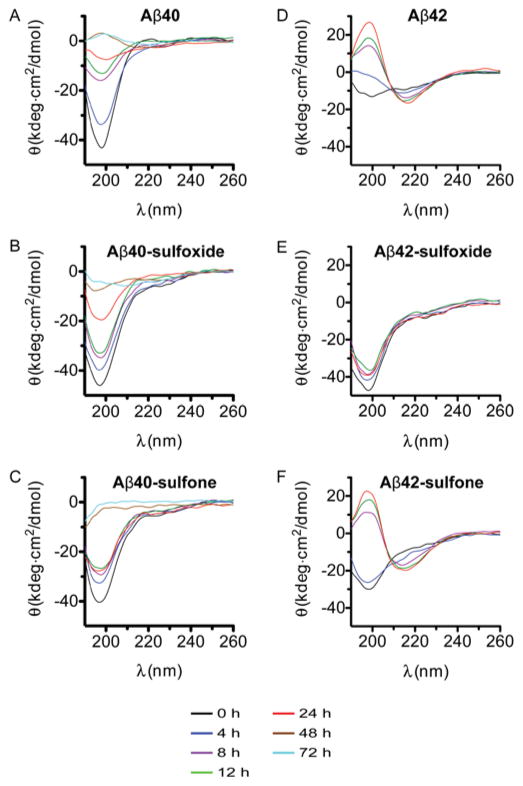
We used CD spectroscopy to study the effect of Met35 oxidation on β-sheet formation during Aβ fibrillogenesis at the same concentration as used for cell viability measurements (Figure 5). All of the peptides showed an initial CD spectrum characterized by a minimum at 196–200 nm, suggesting that the conformation was predominantly statistical coil. Aβ40 showed a gradual decrease in the magnitude of this minimum over 72 h, at which point a maximum at 197–198 nm appeared (Figure 5A), suggesting an increase in β-sheet content. Observation of the β-sheet-characteristic minimum at 215–218 nm required a higher concentration of Aβ40 (results not shown). For consistency with other measurements, we kept the concentration at 10 μM in the presented experiments. Aβ40-sulfoxide (Figure 5B) and Aβ40-sulfone (Figure 5C) showed somewhat slower conformational conversion during the first 24 h, although the overall rate of conformational change over 72 h was similar to that of WT Aβ40. In both cases, at 48–72 h the spectrum did not show the expected maximum at 197–198 nm and instead appeared nearly flat. However, in each case the solution remained free of particulate material or precipitates, suggesting that the spectrum reflected bona fide conformational change rather than precipitation of aggregated material.
Figure 5. Time-dependent conformational transition in native and oxidized Aβ analogues.
Time-dependent conformational changes in Aβ40, Aβ42 and their respective oxidized analogues were recorded using CD spectroscopy. HFIP-treated peptides were incubated in 10 mM sodium phosphate (pH 7.4). The spectra are representative of at least three independent experiments. (A) WT Aβ40, (B) Aβ40-sulfoxide, (C) Aβ40-sulfone, (D) WT Aβ42, (E) Aβ42-sulfoxide and (F) Aβ42-sulfone.
The spectrum of Aβ42 showed a conversion from a minimum at 197 nm into a maximum at 198 nm within 24 h, with concomitant appearance of a minimum at 217–218 nm, indicating conversion from statistical coil into a β-sheet-rich conformation (Figure 5D). The spectra showed an isodichroic point at 210 nm, suggesting that other conformations did not accumulate to a significant extent. In contrast, the spectrum of Aβ42-sulfoxide did not change during the first 24 h of measurement (Figure 5E), consistent with previous observations of delayed aggregation and β-sheet formation of this peptide relative to WT Aβ42 [42]. At later time points (48–72 h), Aβ42-sulfoxide did show slow conversion into a typical β-sheet-rich conformation (results not shown). The slow conformational transition of Aβ42-sulfoxide correlated with the lack of paranucleus formation by this peptide [14] and with the low toxicity described above. Also in correlation with its biological activity, Aβ42-sulfone behaved similarly to WT Aβ42. After a short lag-phase, the CD spectrum changed from predominantly statistical coil to β-sheet-rich, with an isodichroic point at 210 nm.
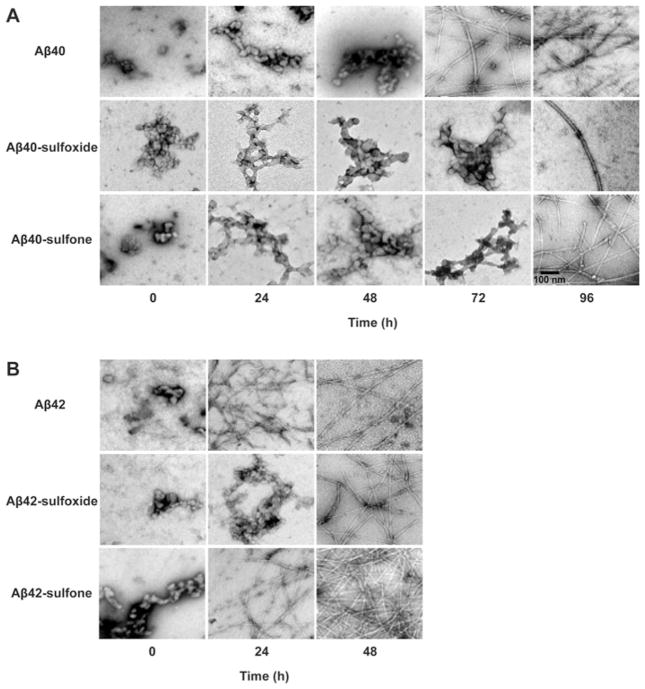
To test whether the conformational transition observed by CD spectroscopy correlated with fibril formation, we examined morphological changes in Aβ40 and Aβ42 in their native, sulfoxide or sulfone forms by electron microscopy (Figure 6). All of the peptides initially had non-fibrillar morphology and showed structures consistent with oligomers. The morphology of Aβ40 remained non-fibrillar for 48 h. At 72 h of incubation, typical amyloid fibrils were present together with remaining non-fibrillar structures, and by 96 h, fibrils largely predominated the morphology of Aβ40. Aβ40-sulfoxide and Aβ40-sulfone both followed similar, albeit slower, morphological changes. Both peptides were still non-fibrillar at 72 h and formed fibrils by 96 h. Thus fibril formation was delayed by ~24 h relative to WT Aβ40. The fibrils of Aβ40-sulfoxide were scarce relative to those of WT Aβ40 or Aβ40-sulfone. These data were consistent with the kinetic trends observed by CD.
Figure 6. Morphology of native and oxidized Aβ analogues.
Aβ analogues were incubated in sodium phosphate (pH 7.4), and aliquots were spotted on glow-discharged, carbon-coated grids, stained with 1 % uranyl acetate, and examined by electron microscopy. Each image is representative of three independent experiments.
As expected, fibril formation by Aβ42 analogues was faster than by Aβ40 analogues. Correlating with the conformational transition observed by CD and with the toxicity data, Aβ42 and Aβ42-sulfone had similar kinetics and showed abundant fibrils following 24 h of incubation, whereas Aβ42-sulfoxide was non-fibrillar at this time point and showed fibril formation only after 48 h.
The environment the peptides experience in cell culture experiments is different from the hypotonic buffer used in the CD and electron microscopy experiments in the present study and may affect peptide assembly. Detailed structural investigation of peptide conformation and morphology using CD and electron microscopy respectively, is not possible in the cell culture environment, which includes medium, serum and the cells themselves. However, investigating the structures in the cell culture medium (without serum or cells) may provide clues to the behaviour of the peptides in this environment. Therefore we incubated the peptides at the same concentration (10 μM) in DMEM at 37°C and investigated their conformation and morphology periodically for several days. The results are shown in Supplementary Figures S1 and S2 (at http://www.BiochemJ.org/bj/433/bj4330323add.htm).
CD spectra could be obtained in medium only down to 203 nm because at lower wavelengths the dynode voltage exceeded the instrument’s recommended limits. Thus the minimum and maximum molar ellipticity at 195–198 that characterize statistical coil and β-strand respectively, could not be observed. In addition, the noise level was higher than in the spectra obtained in buffer. Nevertheless, temporal changes were observed consistent with conformational transition. Under these conditions, the conformational transition happened in all cases with faster kinetics than in buffer and appeared to be complete or nearly complete by 24 h (Supplementary Figure S1). Aβ40 (Supplementary Figure S1A) and Aβ40-sulfone (Supplementary Figure S1C) appeared to have similar kinetics of conformational transition, whereas the kinetics of Aβ40-sulfoxide was somewhat slower (Supplementary Figure S1B). A similar trend was observed for the Aβ42 analogues (Supplementary Figure S1D–S1F), although the high noise levels made it difficult to draw unambiguous conclusions.
Consistent with the CD data, all of the peptides showed amorphous structures at t = 0 h, although not in all cases could these be distinguished from structures observed in the medium itself (Supplementary Figure S2). All of the peptides formed fibrils within 24 h of incubation in medium. Aβ40 formed abundant short fibrils that were mixed with amorphous structures at 24 and 48 h. At 24 h, the fibrils of Aβ40-sulfoxide and Aβ40-sulfone appeared longer than those of WT Aβ40 and looked ribbon-like, comprising up to seven protofilaments. Amorphous structures were observed together with the fibrils at this time point in Aβ40-sulfoxide, but not Aβ40-sulfone samples. Interestingly, by 48 h, the fibrils of both oxidized forms appeared shorter than at 24 h. Aβ42 analogues behaved similarly and were assembled into fibrils by 24 h. At this time point, amorphous aggregates were observed together with fibrils for Aβ42-sulfoxide, but not for WT Aβ42 or Aβ42-sulfone. The fibrillation process for the latter two peptides appeared to be complete by 24 h.
DISCUSSION
The high toxicity we found for Aβ42-sulfone (Figures 2–4) contradicted our expectations, which were based on the difference between the oligomer size distributions of Aβ42-sulfoxide and Aβ42-sulfone, and that of WT Aβ42 [14], the calculated dipole moments of methionine, methionine-sulfoxide and methionine-sulfone (Figure 1), and structures predicted in several modelling studies [10,21–23]. Despite the similarity between the behaviours of the sulfoxide and sulfone forms of Aβ42, and the difference between the two oxidized forms and WT Aβ42, Aβ42-sulfone was as toxic as WT Aβ42. Several mechanisms may explain these unexpected observations. In the present study we tested the hypothesis that the toxicity correlated with aggregation rather than with early oligomerization.
The toxicity levels of the WT and oxidized Aβ42 analogues correlated with their kinetics of β-sheet and fibril formation (Figures 5 and 6). One interpretation of the data is that the toxic effect may be mediated by later-forming large oligomers or aggregates rather than by initial small oligomers. Currently, there is no consensus regarding the size and type of Aβ oligomers that contribute the most to toxicity, or even concerning whether certain oligomers are more important than others for AD pathology. Side-by-side comparison of small and large oligomer populations made of synthetic Aβ and fractionated from the same initial source has suggested that larger structures were more toxic than small ones [43]. A recent examination of individual Aβ40 oligomers stabilized by PICUP has shown a correlation between increased oligomer size and toxicity [44], yet the study found substantial toxicity already for dimers, trimers and tetramers. Comparison of memory impairment in rats induced by Aβ oligomers of different sizes from synthetic, cell culture or transgenic animal brain sources has suggested that small oligomers were more toxic than larger ones [45].
Although high-resolution structures for Aβ oligomers are beginning to emerge [46], direct structure–activity comparisons among small and large oligomers are difficult. Thus whether or not the trends we observed among assembly kinetics and toxicity of the different Aβ analogues represent cause and effect relationship or a mere correlation is an open question. An answer to this question may be provided in future studies by electrophysiological experiments comparing the sulfoxide and sulfone forms of Aβ42 because such experiments can measure toxic effects induced by Aβ oligomers within minutes, probably before substantial amounts of larger oligomers form [47].
Alternatively, we may explain our findings by questioning our fundamental assumptions. Based on the similarity in the dipole moments of the sulfoxide and sulfone groups (Figure 1) and the similarity between the oligomer size distributions of Aβ42-sulfoxide and Aβ42-sulfone, we assumed that the three-dimensional structure of the oligomers formed by the two oxidized Aβ42 analogues was similar (and distinct from that of WT Aβ42). We postulated that because of the large increase in dipole moment upon oxidation, the hydrophobic methionine side chain, which presumably is shielded from the aqueous solvent in Aβ42 oligomers, would partition preferably into the solvent once the sulfide group is oxidized to sulfoxide or sulfone [14]. If such a structural change occurs in Aβ42-sulfoxide, but not in Aβ42-sulfone, it would explain why the sulfone form is as toxic as WT Aβ42. This explanation is unlikely and does not account for the similarity in the oligomer size distribution between Aβ42-sulfoxide and Aβ42-sulfone.
Yet another alternative explanation of the data is that the intrinsic toxicity of the WT, sulfoxide and sulfone forms of Aβ is similar and, in the case of Aβ42, is unrelated to the oligomer size distributions observed using PICUP, but a cellular response unique to the sulfoxide form reduces its toxicity relative to the WT and sulfone forms. One such cellular response might be an increase in expression levels of MsrA (methionine-sulfoxide reductase A), which was reported recently to increase in cells treated by Aβ42-sulfoxide, but not WT Aβ42 [48], and may provide a protective effect to the cells. Our data suggest that such protection probably is not entirely responsible for the lower toxicity of Aβ-sulfoxide because disruption of Ca2+ current through VGCC, an important mechanism contributing to Aβ toxicity, was substantially perturbed by WT Aβ and Aβ-sulfone and not at all by Aβ-sulfoxide. These data suggest that regardless of putative induction of a protective MsrA response, the toxicity of WT Aβ and Aβ-sulfone is mediated by at least one mechanism not shared by Aβ-sulfoxide. Our data also suggest that a substantial portion of Aβ-sulfoxide remains oxidized and does not get reduced by MsrA during the assay period.
The majority of the studies addressing Met35 oxidation in full-length Aβ have focused on Aβ42, whereas information about Aβ40 has been scarce. We found that Aβ40 analogues behaved generally similarly to Aβ42 analogues, although the differences in toxicity and assembly kinetics were smaller between the WT and oxidized Aβ40 peptides. In addition, Aβ40-sulfone behaved somewhat more similarly to Aβ40-sulfoxide than the counterpart Aβ42 analogues. These observations are in line with the lack of change in the oligomer size distribution of WT Aβ40 upon oxidation to sulfoxide or sulfone [14] and suggest that, in Aβ40, the relative contribution of the C-terminal region to the assembly and toxicity behaviour is lower than in Aβ42, consistent with previous reports [8,49].
The present study suggests that formation of methionyl radicals and participation of methionine in Fenton chemistry [17] probably do not contribute significantly to Aβ-induced toxicity because Aβ-sulfone is less likely than Aβ-sulfoxide to participate in Fenton chemistry or contribute to production of ROS (reactive oxygen species), yet it is as toxic as WT Aβ. Supporting this view, recently, substitution of Met35 by valine or norleucine in Aβ40 or Aβ42 was reported to yield analogues that were as toxic as the WT peptides [31], although in other experimental systems substitution of Met35 by norleucine abolished Aβ42 toxicity [15,20]. Interestingly, a recent study in a transgenic mouse model of AD found that substitution of Met35 by leucine had no effect on learning and memory deficits even though markers of oxidative stress were significantly decreased [50].
In summary, the results of the present study suggest a possible correlation between assembly kinetics and toxicity of native and oxidized Aβ, but demonstrate that the relationships among primary structure, quaternary structure and toxic activity are complex. Further study will be required to understand why the sulfoxide and sulfone forms of Aβ42 have similar electronic characteristics and produce similar oligomer size distributions in PICUP experiments, yet behave distinctly in terms of their toxicity and aggregation kinetics.
Supplementary Material
Acknowledgments
We thank Margaret M. Condron for peptide synthesis and amino acid analysis, Dr Huiyuan Li for valuable advice and critical discussion of the manuscript, and Dr David Teplow for the use of his CD spectrometer and plate reader.
FUNDING
This work was supported by the Alzheimer’s Association [grant number IIRG-07–5833]; and National Institutes of Health/National Institute of Aging [grant number AG027818 (to G.B.)]; and by MIUR (Italian Ministry of University and Research) and UCSC (Università Cattolica del Sacro Cuore) grants (to C.G.).
Abbreviations used
- Aβ
amyloid β-protein
- Aβ40
Aβ comprising 40 amino acids
- Aβ42
Aβ comprising 42 amino acids
- AD
Alzheimer’s disease
- CHC
central hydrophobic cluster
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DMEM
Dulbeco modified Eagle’s medium
- E
embryonic day
- FBS
fetal bovine serum
- Fmoc
fluoren-9-ylmethoxycarbonyl
- HFIP
1,1,1,3,3,3-hexafluoroisopropanol
- LDH
lactate dehydrogenase
- MsrA
methionine–sulfoxide reductase A
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide
- PICUP
photo-induced cross-linking of unmodified proteins
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP nick-end labelling
- VGCC
voltage-gated calcium channel
- WT
wild-type
Footnotes
AUTHOR CONTRIBUTION
Panchanan Maiti, Roberto Piacentini and Cristian Ripoli performed the research and analysed the data. Claudio Grassi and Gal Bitan designed the research and analysed the data. Panchanan Maiti and Gal Bitan wrote the manuscript.
References
- 1.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 3.Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567–577. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 4.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 5.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the Presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 6.Suo ZM, Humphrey J, Kundtz A, Sethi F, Placzek A, Crawford F, Mullan M. Soluble Alzheimers β-amyloid constricts the cerebral vasculature in vivo. Neurosci Lett. 1998;257:77–80. doi: 10.1016/s0304-3940(98)00814-3. [DOI] [PubMed] [Google Scholar]
- 7.Jarrett JT, Berger EP, Lansbury PT., Jr The C-terminus of the β protein is critical in amyloidogenesis. Ann N Y Acad Sci. 1993;695:144–148. doi: 10.1111/j.1749-6632.1993.tb23043.x. [DOI] [PubMed] [Google Scholar]
- 8.Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc Natl Acad Sci USA. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein SL, Dupuis NF, Lazo ND, Wyttenbach T, Condron MM, Bitan G, Teplow DB, Shea JE, Ruotolo BT, Robinson CV, Bowers MT. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat Chem. 2009;1:326–331. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urbanc B, Cruz L, Yun S, Buldyrev SV, Bitan G, Teplow DB, Stanley HE. In silico study of amyloid β-protein folding and oligomerization. Proc Natl Acad Sci USA. 2004;101:17345–17350. doi: 10.1073/pnas.0408153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan Y, Wang C. Aβ42 is more rigid than Aβ40 at the C terminus: implications for Aβ aggregation and toxicity. J Mol Biol. 2006;364:853–862. doi: 10.1016/j.jmb.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Lazo ND, Grant MA, Condron MC, Rigby AC, Teplow DB. On the nucleation of amyloid β-protein monomer folding. Protein Sci. 2005;14:1581–1596. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Näslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, Silberring J, Gandy SE, Winblad B, Greengard P, et al. Relative abundance of Alzheimer Aβ amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci USA. 1994;91:8378–8382. doi: 10.1073/pnas.91.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bitan G, Tarus B, Vollers SS, Lashuel HA, Condron MM, Straub JE, Teplow DB. A molecular switch in amyloid assembly: Met35 and amyloid β -protein oligomerization. J Am Chem Soc. 2003;125:15359–15365. doi: 10.1021/ja0349296. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield DA, Boyd-Kimball D. The critical role of methionine 35 in Alzheimer’s amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim Biophys Acta. 2005;1703:149–156. doi: 10.1016/j.bbapap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Choi J, Sullards MC, Olzmann JA, Rees HD, Weintraub ST, Bostwick DE, Gearing M, Levey AI, Chin LS, Li L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281:10816–10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali FE, Separovic F, Barrow CJ, Cherny RA, Fraser F, Bush AI, Masters CL, Barnham KJ. Methionine regulates copper/hydrogen peroxide oxidation products of Aβ. J Pept Sci. 2005;11:353–360. doi: 10.1002/psc.626. [DOI] [PubMed] [Google Scholar]
- 18.Palmblad M, Westlind-Danielsson A, Bergquist J. Oxidation of methionine 35 attenuates formation of amyloid β -peptide 1–40 oligomers. J Biol Chem. 2002;277:19506–19510. doi: 10.1074/jbc.M112218200. [DOI] [PubMed] [Google Scholar]
- 19.Hou L, Shao H, Zhang Y, Li H, Menon NK, Neuhaus EB, Brewer JM, Byeon IJ, Ray DG, Vitek MP, et al. Solution NMR studies of the Aβ(1–40) and Aβ(1–42) peptides establish that the Met35 oxidation state affects the mechanism of amyloid formation. J Am Chem Soc. 2004;126:1992–2005. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- 20.Piacentini R, Ripoli C, Leone L, Misiti F, Clementi ME, D’Ascenzo M, Giardina B, Azzena GB, Grassi C. Role of methionine 35 in the intracellular Ca2+ homeostasis dysregulation and Ca2+-dependent apoptosis induced by amyloid β -peptide in human neuroblastoma IMR32 cells. J Neurochem. 2008;107:1070–1082. doi: 10.1111/j.1471-4159.2008.05680.x. [DOI] [PubMed] [Google Scholar]
- 21.Triguero L, Singh R, Prabhakar R. Comparative molecular dynamics studies of wild-type and oxidized forms of full-length Alzheimer amyloid β-peptides Aβ(1–40) and Aβ(1–42) J Phys Chem B. 2008;112:7123–7131. doi: 10.1021/jp801168v. [DOI] [PubMed] [Google Scholar]
- 22.Yang M, Teplow DB. Amyloid β-protein monomer folding: free-energy surfaces reveal alloform-specific differences. J Mol Biol. 2008;384:450–464. doi: 10.1016/j.jmb.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urbanc B, Betnel M, Cruz L, Bitan G, Teplow DB. Elucidation of amyloid β -protein oligomerization mechanisms: discrete molecular dynamics study. J Am Chem Soc. 2010;132:4266–4280. doi: 10.1021/ja9096303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer’s Aβ(1–42) and Aβ(25–35) J Am Chem Soc. 2001;123:5625–5631. doi: 10.1021/ja010452r. [DOI] [PubMed] [Google Scholar]
- 25.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Binger KJ, Griffin MD, Heinemann SH, Howlett GJ. Methionine-oxidized amyloid fibrils are poor substrates for human methionine sulfoxide reductases A and B2. Biochemistry. 2010;49:2981–2983. doi: 10.1021/bi902203m. [DOI] [PubMed] [Google Scholar]
- 27.Ejiri SI, Weissbach H, Brot N. Reduction of methionine sulfoxide to methionine by Escherichia coli. J Bacteriol. 1979;139:161–164. doi: 10.1128/jb.139.1.161-164.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahimi F, Maiti P, Bitan G. Photo-induced cross-linking of unmodified proteins (PICUP) applied to amyloidogenic peptides. J Vis Exp. 2009;23 doi: 10.3791/1071. http://www.jove.com/index/details.stp?id=1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Segal M, Manor D. Confocal microscopic imaging of [Ca2+]i in cultured rat hippocampal neurons following exposure to N-methyl-D-aspartate. J Physiol. 1992;448:655–676. doi: 10.1113/jphysiol.1992.sp019063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dravid SM, Murray TF. Spontaneous synchronized calcium oscillations in neocortical neurons in the presence of physiological [Mg2+]: involvement of AMPA/kainate and metabotropic glutamate receptors. Brain Res. 2004;1006:8–17. doi: 10.1016/j.brainres.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 31.Maiti P, Lomakin A, Benedek GB, Bitan G. Despite its role in assembly, methionine 35 is not necessary for amyloid β-protein toxicity. J Neurochem. 2010;113:1252–1262. doi: 10.1111/j.1471-4159.2010.06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 33.Piacentini R, Ripoli C, Mezzogori D, Azzena GB, Grassi C. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Cav1-channel activity. J Cell Physiol. 2008;215:129–139. doi: 10.1002/jcp.21293. [DOI] [PubMed] [Google Scholar]
- 34.Papa M, Bundman MC, Greenberger V, Segal M. Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smale G, Nichols NR, Brady DR, Finch CE, Horton WE., Jr Evidence for apoptotic cell death in Alzheimer’s disease. Exp Neurol. 1995;133:225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 36.Barger SW. An unconventional hypothesis of oxidation in Alzheimer’s disease: intersections with excitotoxicity. Front Biosci. 2004;9:3286–3295. doi: 10.2741/1481. [DOI] [PubMed] [Google Scholar]
- 37.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 39.Takashima S, Ieshima A, Nakamura H, Becker LE. Dendrites, dementia and the Down syndrome. Brain Dev. 1989;11:131–133. doi: 10.1016/s0387-7604(89)80082-8. [DOI] [PubMed] [Google Scholar]
- 40.Snyder SW, Ladror US, Wade WS, Wang GT, Barrett LW, Matayoshi ED, Huffaker HJ, Krafft GA, Holzman TF. Amyloid-β aggregation: selective inhibition of aggregation in mixtures of amyloid with different chain lengths. Biophys J. 1994;67:1216–1228. doi: 10.1016/S0006-3495(94)80591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson AA, Fairlie DP, Craik DJ. Solution structure of methionine-oxidized amyloid β-peptide (1–40): does oxidation affect conformational switching? Biochemistry. 1998;37:12700–12706. doi: 10.1021/bi9810757. [DOI] [PubMed] [Google Scholar]
- 42.Hou L, Kang I, Marchant RE, Zagorski MG. Methionine 35 oxidation reduces fibril assembly of the amyloid β-(1–42) peptide of Alzheimer’s disease. J Biol Chem. 2002;277:40173–40176. doi: 10.1074/jbc.C200338200. [DOI] [PubMed] [Google Scholar]
- 43.Hepler RW, Grimm KM, Nahas DD, Breese R, Dodson EC, Acton P, Keller PM, Yeager M, Wang H, Shughrue P, et al. Solution state characterization of amyloid β-derived diffusible ligands. Biochemistry. 2006;45:15157–15167. doi: 10.1021/bi061850f. [DOI] [PubMed] [Google Scholar]
- 44.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid β-protein oligomers. Proc Natl Acad Sci USA. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed MN, Hofmeister JJ, Jungbauer L, Welzel AT, Yu C, Sherman MA, Lesne S, Ladu MJ, Walsh DM, Ashe KH, Cleary JP. Cognitive effects of cell-derived and synthetically derived Aβ oligomers. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, et al. Structural characterization of a soluble amyloid β-peptide oligomer. Biochemistry. 2009;48:1870–1877. doi: 10.1021/bi802046n. [DOI] [PubMed] [Google Scholar]
- 47.Fradinger EA, Monien BH, Urbanc B, Lomakin A, Tan M, Li H, Spring SM, Condron MM, Cruz L, Xie CW, et al. C-terminal peptides coassemble into Aβ42 oligomers and protect neurons against Aβ42-induced neurotoxicity. Proc Natl Acad Sci USA. 2008;105:14175–14180. doi: 10.1073/pnas.0807163105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misiti F, Clementi ME, Giardina B. Oxidation of methionine 35 reduces toxicity of the amyloid β-peptide(1–42) in neuroblastoma cells (IMR-32) via enzyme methionine sulfoxide reductase A expression and function. Neurochem Int. 2010;56:597–602. doi: 10.1016/j.neuint.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Bitan G, Vollers SS, Teplow DB. Elucidation of primary structure elements controlling early amyloid β-protein oligomerization. J Biol Chem. 2003;278:34882–34889. doi: 10.1074/jbc.M300825200. [DOI] [PubMed] [Google Scholar]
- 50.Butterfield DA, Galvan V, Lange MB, Tang H, Sowell RA, Spilman P, Fombonne J, Gorostiza O, Zhang J, Sultana R, Bredesen DE. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: requirement for methionine 35 in amyloid β-peptide of APP. Free Radical Biol Med. 2010;48:136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.