Abstract
After approval of the first plant-made biopharmaceutical by FDA for human use, many protein drugs are now in clinical development. Within the last decade, significant advances have been made in expression of heterologous complex/large proteins in chloroplasts of edible plants using codon optimized human or viral genes. Furthermore, advances in quantification enable determination of in-planta drug dosage. Oral delivery of plastid-made biopharmaceuticals (PMB) is affordable because it eliminates prohibitively expensive fermentation, purification processes addressing major challenges of short shelf-life after cold storage. In this review, we discuss recent advances in PMBs against metabolic, inherited or infectious diseases, and also mechanisms of post-translational modifications (PTM) in order to increase our understanding of functional PMBs.
Introduction
Biopharmaceuticals play a major role in treating various human diseases. However, they are produced in prohibitively expensive systems that require high purity and cold storage, further limited by short shelf-life. The high cost of protein drugs makes them unaffordable for most of the global population. These problems could be addressed by developing a cost-effective expression platform for production of recombinant proteins in large scale [1]. The production of pharmaceutical proteins from nuclear transgenic plants was largely abandoned by the biotech industry, mainly due to its low yield and structural heterogeneity of final products [2]. In contrast, expression of transgenes in plant chloroplast, also known as transplastomic expression, has several advantages including higher yield and more stable level of gene expression via the ability to direct transgene integration to precise sites within the chloroplast genome. Also, maternal inheritance of transgenes eliminates the escape of transgenes via pollen, offering total transgene containment when biopharmaceuticals are made in leaves [3]. Recently significant progress has been made in therapeutic protein production in chloroplasts of edible plant cells. Biopharmaceuticals produced in edible plants eliminate expensive fermentation, purification processes and cold storage/transportation [1,3]. Bioencapsulated protein drugs in freeze-dried plant cells are stable after several years of storage at ambient temperature [4••].
Oral Drug Delivery
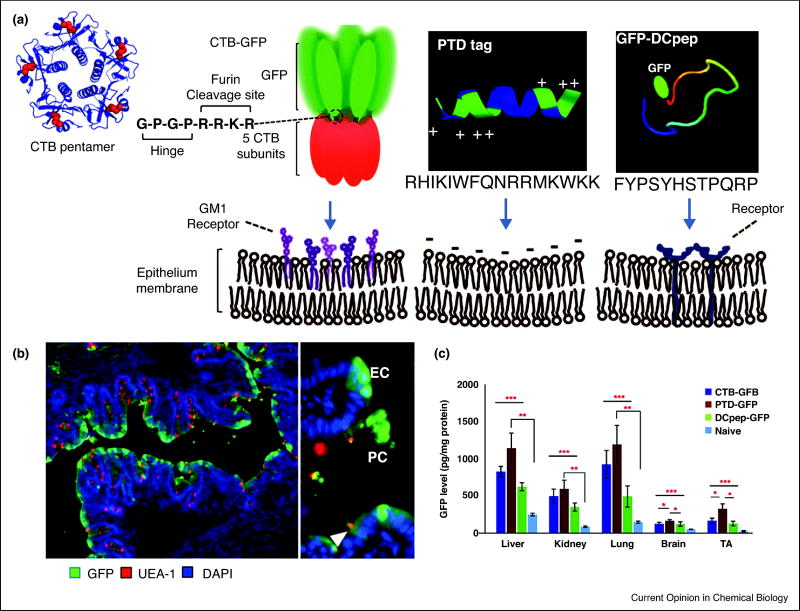
The recombinant proteins encapsulated within the plant cell wall cannot be hydrolyzed by stomach acids or other digestive enzymes and this confers a natural barrier to protect encapsulated protein drugs from degradation in the human digestive system. Once intact plant cells reach the small intestine, commensal microbes digest plant cell wall glycans, facilitating the release of expressed protein drugs. When fused with suitable tags, protein drugs cross gut epithelial cells and enter into the circulatory or immune system [5–8]. Recent studies show that oral delivery of protein to different cell types can be achieved using different transmucosal peptides fused to the N- or C-terminus of recombinant proteins [7••, Figure 1]. When cholera non-toxic B subunit (CNTB), protein transduction domain (PTD) or dendritic cell peptide (DCpep) were fused with GFP, fluorescence was widely distributed in mouse respiratory, digestive and skeletal muscle or other tissues or organs. Delivery of CTB fusion proteins across the blood-brain barrier or blood-retinal barrier was also observed in recent studies performed in mice [9••,10••]. Once delivered to the small intestine, proteins are taken up by the gut epithelial or microfold cells (M cells) before they enter the circulation system or protein drugs enter via the gut-liver axis [7••]. CTB fusion protein was detected in both human non-immune and immune modulatory cells [7••]. Recent reports also show that antimicrobial peptide (AMP), Protegrin-1 (PG-1) enters various human cell types including periodontal ligament stem cells, head and neck squamous cell carcinoma cells, Gingiva-derived mesenchymal stromal cells and adult gingival keratinocytes more efficiently than any other known cell penetrating peptides [11••].
Figure 1.
Folding and assembly of receptor-binding protein or cell-penetrating peptides expressed in plant chloroplasts for targeted drug delivery. (a) Assembly of CTB pentamer with disulfide bonds that bind to GM1 receptors and proper folding of protein transduction domain (PTD) and dendritic cell peptide (DCpep) fused to protein drugs are shown. The predicted 3D structure of CTB pentamer, PTD and Dcpep are shown. Disulfide bonds in CTB pentamer are presented as red spheres. The green color in PTD tag shows charged amino acids. (b) Transmucosal delivery of CTB-GFP through the gut epithelial cells in mouse small intestine. Small intestine sections of mice fed with lyophilized CTB-GFP cells were stained with Alexa 488 labeled anti-GFP antibody (green signal), rhodamine labeled M-cell stain (UEA-1, red signal), and nuclear stain (DAPI, blue signal). Solid arrow shows GFP+ M cells; EC, epithelial cells; PC, plant cells. (c) Biodistribution of GFP signal in different tissues of mice fed with lyophilized leaves expressing GFP fused with PTD, CTB and Dcpep tags. The figure was modified from previous publication from our lab [7••, 8].
In the last two decades, an increasing number of protective antigens against human and animal pathogens were expressed in plants [12–14]. Glucocerebrosidase made in carrot cells is the first plant-made pharmaceutical approved by U.S. Food and Drug Administration [15] to treat Gaucher’s Disease. In this review, we highlight recent advances in plastid-made biopharmaceuticals (PMB), post-translational modifications (PTM) in chloroplasts and future challenges in advancing this field.
Recombinant protein production in plant chloroplasts
Prevention and treatment of infectious diseases
This concept involves the cloning of various vaccine antigens from bacteria, viruses, fungi or protozoa into chloroplast expression vectors and transforming them into the chloroplast genome via gene gun bombardment [3]. Once homoplasmy (transformation of all copies of chloroplast genome) is achieved, recombinant proteins are expressed at high levels - up to 70% of total leaf protein [16••,17••]. Several subunit vaccine candidates have been expressed in plant chloroplasts against various infectious pathogens [13,18]. PMBs produced against global infectious diseases include several Mycobacterium tuberculosis vaccine antigens, Vibrio cholerae non-toxin B subunit, Bacillus anthracis protective antigen (PA), Yersinia pestis F1-V antigen and Poliomyelitis (polio VP1) vaccine antigen with expression levels ranging from 4% to 18% of the total soluble protein [19••–23•]. Chloroplast-derived vaccine antigens generated neutralizing antibodies upon oral boosting and conferred protection against toxin or pathogen challenges. However, vaccine antigens made in chloroplasts require injectable priming with adjuvants and can only serve as booster vaccines [19••]. Without priming, antigens can suppress immunity as described below in the immune modulation section.
In addition to subunit vaccine antigens, antimicrobial peptides have also been expressed in chloroplasts. AMPs, also called peptide antibiotics [24] are a growing class of low molecular mass oligopeptides found in many organisms with a broad range of antimicrobial activity against viruses, fungi and bacteria. Unlike antibiotics, AMPs targets lipopolysaccharides in the microbial cell membrane and induce rapid killing of pathogens. In addition to anti-bacterial effects, AMPs have been shown to possess immunomodulatory and wound healing properties [25,26••]. Both natural and synthetic antimicrobial agents have been reported so far, of which MSI-99 [26••], an analog of magainin 2, PG1 and Retrocyclin (RC101) [27•] were expressed in tobacco chloroplasts. Many human infections are biofilm associated including dental caries caused by S. mutans. The topical application of plant produced AMPs (PMAMP) PG1 and RC101 on tooth mimetic surface effectively inhibited biofilm formation [11••]. In addition, PMAMPs when combined with exopolysaccharide degrading enzymes (dextranase/mutanase) disrupted mature biofilms and killed S. mutans [11••].
Treatment of metabolic disorders
Protein therapeutics in diabetes mellitus treatment has the highest demand as it affects over one third of adult population nationwide [28]. By utilizing transplastomic technology, expression level of complete proinsulin in plant plastids reached 50% of the total soluble protein [16••]. The massive production level in transplastomic plants facilitates scale-up of 20 million doses of insulin per acre of plant/year. Chloroplast expressed CTB-proinsulin fusion protein was efficiently assembled in plant chloroplasts, which was fully functional in animal model evaluation. Treatment of type II diabetes is also achieved using glucagon like peptide (GLP-1) that stimulates the secretion of insulin from the pancreas. Due to the extremely short half-life of GLP-1 in serum (<2 min), type II diabetes treatment has focused on dipeptidyl peptidase IV (DPP-IV) resistant analogs like exendin-4 (EX4), which has significantly longer serum half-life (~4 h). Oral delivery of plant derived EX4 fused with human transporter protein (transferrin) to mice exerted a glucose-lowering effect by stimulating insulin secretion, as well as promoting differentiation and proliferation of pancreatic β-cells [29]. Moreover, oral administration of plant made EX4-CTB fusion protein significantly improved glucose regulation in mice. Intriguingly, chloroplast expressed CTB-EX4 exhibited insulin stimulating effect upon oral administration without causing undesired hypoglycemia side effect [30•]. Exceptional high production level of CTB-EX4 fusion protein in transplastomic plants makes this an ideal cost-effective delivery system for type II diabetes treatment.
Immune modulation
Hemophilia is a bleeding disorder caused by the X-linked mutation in coagulation factors. Hemophilia A and B are the most common forms of the disease affecting one among every 5,000 and 30,000 males, respectively [31]. Currently, life-time infusion of coagulation factor VIII (FVIII, for hemophilia A) or factor IX (FIX, for hemophilia B) is the standard treatment for hemophilia patients. One of the major challenges in hemophilia therapy is development of inhibitory antibodies after continuous protein replacement. This immune response to protein antigens can cause life-threatening anaphylaxis in severe cases [32]. Current clinical protocols of immune tolerance induction (ITI) require administration of high doses of blood clotting factor over a long period of time [33]. However, this clinical protocol is highly expensive and it does not guarantee the effective immune tolerance [1]. Oral delivery of plant cells expressing FIX was reported to induce immune tolerance during protein replacement therapy [34••]. Oral administration of lyophilized transplastomic plant cells expressing domains of blood coagulation factor VIII or full length FIX in chloroplasts successfully eliminated inhibitor formation in mice and dog models [4••,35•,36]. Besides hemophilia, the oral administration of the acid alpha glucosidase (GAA) epitopes expressed in plant chloroplasts [37] also eliminated antibody response in Pompe disease mouse model. Therefore, the concept of immune modulation using antigens expressed in chloroplasts has been demonstrated in several disease models.
Post-translational modifications (PTM) in chloroplasts
PTM play an important role in maintaining the stability and bioactivity of therapeutic proteins [38]. Although PTM pathways in plant cytosol and secretory pathway have been largely elucidated, mechanisms of protein modification in chloroplasts are still poorly understood. Nevertheless, the recent proteomic studies provide further insights into chloroplast PTM [39]. Reversible phosphorylation tightly regulates the activity of protein functions in almost all aspects of cellular processes; therefore, abnormal phosphorylation is one of the major causes for a number of diseases including cancers, neurodegeneration and chronic inflammation [40,41]. Many native therapeutic proteins are phosphorylated and the phosphorylation states highly regulate protein functions [42]. Previous studies suggested that the phosphorylation of the FVa heavy chain coagulation factor increases its susceptibility to activate protein C (APC) inactivation and thereby decreases the coagulation efficiency [43]. Similarly, hyper-phosphorylation of tissue factor prevents its interaction with other hemostatic proteins and interferes with the thrombin formation [44]. The extent of phosphorylation of the vaccine carrier, monophosphoryl lipid A also affects the toxicity and immunological activity [45]. The presence of phosphorylation activity in plant chloroplasts is evidenced by the identification of phosphor-proteins localized to the chloroplast and the corresponding kinases that perform phosphorylation functions.
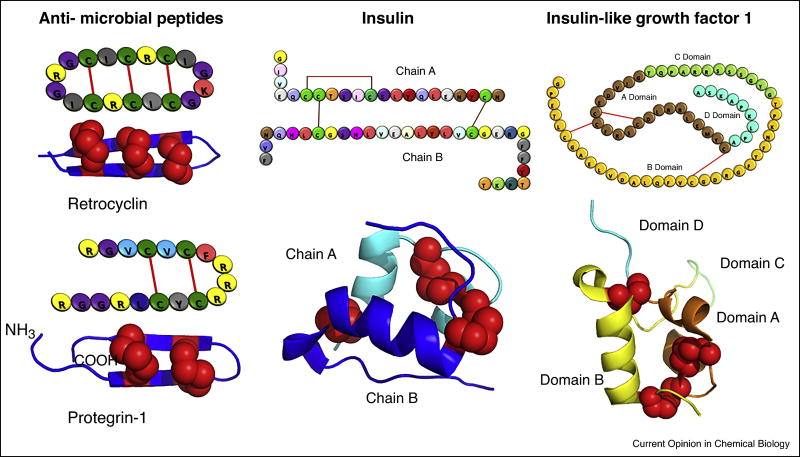
The sophisticated machinery of redox regulation in plant chloroplasts suggests the possibility of multimeric protein assembly in chloroplast systems via disulfide bond formation [46]. Disulfide bond formation is important to stabilize the tertiary structure and to facilitate the function of many different recombinant therapeutic proteins. The success in producing functional pharmaceutical proteins for the treatment of diabetes, blood diseases, metabolic disorders and infectious diseases in plant chloroplasts demonstrates the presence of endogenous protein disulfide transferase in chloroplasts for maintaining proper protein confirmation [16••,27•,47•–49•]. RC101 expressed in chloroplast was found to be cyclized by disulfide bonds. Moreover, efficient binding of chloroplast-derived CTB fusion proteins to the GM1 receptor requires the formation of a pentameric structure via disulfide bonds (Figure 2). In addition to the endogenous enzymes, co-expression of exogenous protein disulfide isomerase and thioredoxin showed an increase in protein folding efficiency of human serum albumin in chloroplasts [50•].
Figure 2.
Examples of therapeutic protein folding and modifications reported in plant chloroplasts. The predicted 3D structure of Retrocyclin, Protegrin, Insulin and Insulin-like growth factor 1 (IGF-1) are shown. Disulfide bonds are presented as red spheres. Different protein domains are labeled with different colors.
Lipidation, which is another type of PTM, refers to the covalent attachment of lipids to certain amino acids in the proteins. Lipidation of proteins facilitates subcellular targeting and protein-protein interactions [51]. The outer surface protein A (OspA) of Borrelia burgdorferi is an effective vaccine candidate to treat Lyme disease [52•]. Lipidation of OspA is essential for its immunogenicity and non-lipidated form did not induce protective immunity in mice. The chloroplast-derived recombinant OspA was fully functional at eliciting an immune response via the generation of protective antibodies against burgdorferi in mice.
Future prospective and conclusions
Although many foreign proteins are expressed in chloroplasts, development of fully functional proteins is a significant challenge. For example, very high level expression of prokaryotic genes of bacterial origin is feasible but expression of large human or viral genes is a major challenge. Optimization of human or viral gene sequences based on the codon preference of psbA genes, from 133 sequenced chloroplast genomes, significantly increased their expression (up to 125-fold) in plant chloroplasts [53••] by improving the ribosome read-through during translation and eliminating rare codons. Drug dosage determination is currently feasible only in purified biopharmaceuticals. However, drug dosage determination in-planta has been developed for the first time using targeted proteomic quantitation by parallel reaction monitoring; when normalized with stable isotope labeled standard peptides or housekeeping proteins, targeted proteomic quantitation yielded more accurate results than quantitation by western blots, eliminating the need for protein purification before quantitation [53••]. This should facilitate further advancement of oral drug delivery of biopharmaceuticals made in plant cells. Furthermore, marker free transplastomic plants must be developed in edible crops to advance the chloroplast made biopharmaceuticals for clinical use [54]. More importantly, further studies are required to understand the post-translational modifications of foreign proteins expressed in plant chloroplasts.
Highlights.
Chloroplasts are ideal bioreactors for low cost production of biopharmaceuticals.
Tags fused with protein drugs facilitate their delivery to different cell types.
Chloroplast-derived biopharmaceuticals are properly folded and fully functional.
Advances in drug dosage determination in-planta without purification are discussed
Recent advances in chloroplast-made biopharmaceuticals are discussed.
Acknowledgments
Research reported from the Daniell lab was supported by the Bill and Melinda Gates Foundation (OPP1031406), Novo Nordisk, Bayer and NIH grants R01 HL107904, R01 HL109442 to Henry Daniell. He thanks all coauthors and collaborators from his laboratory for their valuable contributions to advance this field.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
•of special interest
•• of outstanding interest
- 1.Kwon K-C, Daniell H. Low-cost oral delivery of protein drugs bioencapsulated in plant cells. Plant Biotechnol J. 2015;13:1017–1022. doi: 10.1111/pbi.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabalza M, Christou P, Capell T. Recombinant plant-derived pharmaceutical proteins: current technical and economic bottlenecks. Biotechnol Lett. 2014;36:2367–2379. doi: 10.1007/s10529-014-1621-3. [DOI] [PubMed] [Google Scholar]
- 3.Daniell H, Chan H-T, Pasoreck EK. Vaccination via Chloroplast Genetics: Affordable Protein Drugs for the Prevention and Treatment of Inherited or Infectious Human Diseases. Annu Rev Genet. 2016;50:595–618. doi: 10.1146/annurev-genet-120215-035349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Su J, Zhu L, Sherman A, Wang X, Lin S, Kamesh A, Norikane JH, Streatfield SJ, Herzog RW, Daniell H. Low cost industrial production of coagulation factor IX bioencapsulated in lettuce cells for oral tolerance induction in hemophilia B. Biomaterials. 2015;70:84–93. doi: 10.1016/j.biomaterials.2015.08.004. This is the first commercial scale production of a biopharmaceutical protein in a cGMP facility in an edible plant cell, facilitating further clinical studies. This study also reported that biopharmaceutical proteins are stable at ambient temperature for several years maintaining their folding, assembly and functionality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martens EC, Lowe EC, Chiang H, Pudlo NA, Wu M, McNulty NP, Abbott DW, Henrissat B, Gilbert HJ, Bolam DN, et al. Recognition and Degradation of Plant Cell Wall Polysaccharides by Two Human Gut Symbionts. PLoS Biol. 2011;9:e1001221. doi: 10.1371/journal.pbio.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flint HJ, Scott KP, Duncan SH, Louis P, Forano E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes. 2012;3:289–306. doi: 10.4161/gmic.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7••.Xiao Y, Kwon K-C, Hoffman BE, Kamesh A, Jones NT, Herzog RW, Daniell H. Low cost delivery of proteins bioencapsulated in plant cells to human non-immune or immune modulatory cells. Biomaterials. 2016;80:68–79. doi: 10.1016/j.biomaterials.2015.11.051. Several cell penetrating peptides suitable for protein drug delivery to different human cell types including non-immune and immune modulatory cells are investigated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon K-C, Daniell H. Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells. Mol Ther. 2016;24:1342–1350. doi: 10.1038/mt.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Kohli N, Westerveld DR, Ayache AC, Verma A, Shil P, Prasad T, Zhu P, Chan SL, Li Q, Daniell H. Oral Delivery of Bioencapsulated Proteins Across Blood-Brain and Blood-Retinal Barriers. Mol Ther. 2014;22:535–546. doi: 10.1038/mt.2013.273. First protein drug delivery across the Blood-Brain Barrier, which is a major challenge in treatment of brain disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Shil PK, Kwon K-C, Zhu P, Verma A, Daniell H, Li Q. Oral Delivery of ACE2/Ang-(1-7) Bioencapsulated in Plant Cells Protects against Experimental Uveitis and Autoimmune Uveoretinitis. Mol Ther. 2014;22:2069–2082. doi: 10.1038/mt.2014.179. First protein drug delivery across Blood-Retinal Barrier, which is a major challenge in treatment of eye diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Liu Y, Kamesh AC, Xiao Y, Sun V, Hayes M, Daniell H, Koo H. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials. 2016;105:156–166. doi: 10.1016/j.biomaterials.2016.07.042. First example of topical drug delivery; biofilm causing dental caries was blocked from development using anti-microbial peptides (AMP); mature biofilms were disrupted my matrix degrading enzymes; AMPs also efficiently delivered protein drugs to different oral tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rybicki EP. Plant-made vaccines for humans and animals. Plant Biotechnology Journal. 2010;8:620–637. doi: 10.1111/j.1467-7652.2010.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan H-T, Daniell H. Plant-made oral vaccines against human infectious diseases—Are we there yet? Plant Biotechnol J. 2015;13:1056–1070. doi: 10.1111/pbi.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahid N, Daniell H. Plant-based oral vaccines against zoonotic and non-zoonotic diseases. Plant Biotechnology Journal. 2016;14:2079–2099. doi: 10.1111/pbi.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastores GM, Petakov M, Giraldo P, Rosenbaum H, Szer J, Deegan PB, Amato DJ, Mengel E, Tan ES, Chertkoff R, et al. A Phase 3, multicenter, open-label, switchover trial to assess the safety and efficacy of taliglucerase alfa, a plant cell-expressed recombinant human glucocerebrosidase, in adult and pediatric patients with Gaucher disease previously treated with imiglucerase. Blood Cells, Mol, Dis. 2014;53:253–260. doi: 10.1016/j.bcmd.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 16••.Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant biotechnol J. 2011;9:585–598. doi: 10.1111/j.1467-7652.2010.00582.x. First report of functional oral drug delivery using a suitable animal model and insulin expressed in edible plant chloroplasts. Both the CTB and proinsulin require disulfide bonds for functionality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Ruhlman T, Verma D, Samson N, Daniell H. The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol. 2010;152:2088–2104. doi: 10.1104/pp.109.152017. This study laid the foundation for expression of biopharmaceuticals in edible chloroplasts by developing species-specific chloroplast vectors and fully optimizing the lettuce transplastomic system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waheed MT, Ismail H, Gottschamel J, Mirza B, Lössl AG. Plastids: The Green Frontiers for Vaccine Production. Front Plant Sci. 2015;6 doi: 10.3389/fpls.2015.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Chan HT, Xiao Y, Weldon WC, Oberste SM, Chumakov K, Daniell H. Cold chain and virus-free chloroplast-made booster vaccine to confer immunity against different poliovirus serotypes. Plant Biotechnol J. 2016;14:2190–2200. doi: 10.1111/pbi.12575. This study conducted at the FDA, CDC and Daniell laboratories, funded by the Gates Foundation laid the foundation for developing booster vaccines against global infectious diseases; withdrawal of the oral polio vaccine 2 makes this an urgent unmet medical need for eradication of polio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lakshmi PS, Verma D, Yang X, Lloyd B, Daniell H. Low Cost Tuberculosis Vaccine Antigens in Capsules: Expression in Chloroplasts, Bio-Encapsulation, Stability and Functional Evaluation In Vitro. PLoS ONE. 2013;8:e54708. doi: 10.1371/journal.pone.0054708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Davoodi-Semiromi A, Schreiber M, Nallapali S, Verma D, Singh ND, Banks RK, Chakrabarti D, Daniell H. Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J. 2010;8:223–242. doi: 10.1111/j.1467-7652.2009.00479.x. This study provides detailed mechanistic aspects of immunity and protection conferred by vaccine antigens orally delivered to suitable animal models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Koya V, Moayeri M, Leppla SH, Daniell H. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. Animals were fully protected against anthrax toxin challenge when subcutaneouly immunized with anthrax protective antigen made in chloroplasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective Plague Vaccination via Oral Delivery of Plant Cells Expressing F1-V Antigens in Chloroplasts. Infect Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. Animals were fully protected against plague aerosol challenge when orally immunized with plant cells expressing the vaccine antigen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holaskova E, Galuszka P, Frebort I, Oz MT. Antimicrobial peptide production and plant-based expression systems for medical and agricultural biotechnology. Biotechnol Adv. 2015;33:1005–1023. doi: 10.1016/j.biotechadv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Gupta K, Kotian A, Subramanian H, Daniell H, Ali H. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget. 2015;6:28573–28587. doi: 10.18632/oncotarget.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.DeGray G, Rajasekaran K, Smith F, Sanford J, Daniell H. Expression of an Antimicrobial Peptide via the Chloroplast Genome to Control Phytopathogenic Bacteria and Fungi. Plant Physiol. 2001;127:852–862. First report of an antimicrobial peptide made in plant chloroplasts. [PMC free article] [PubMed] [Google Scholar]
- 27•.Lee S-B, Li B, Jin S, Daniell H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J. 2011;9:100–115. doi: 10.1111/j.1467-7652.2010.00538.x. Both antimicrobial peptides require three disufide bonds for functionality in addition, RC101 requires cyclization for antimicrobial activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Google Scholar]
- 29.Choi J, Diao H, Feng Z-C, Lau A, Wang R, Jevnikar AM, Ma S. A fusion protein derived from plants holds promising potential as a new oral therapy for type 2 diabetes. Plant Biotechnol J. 2014;12:425–435. doi: 10.1111/pbi.12149. [DOI] [PubMed] [Google Scholar]
- 30•.Kwon K-C, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2013;11:77–86. doi: 10.1111/pbi.12008. An ideal candidate for clinical translation because exendin is functional in a broad dose range and prevents hypoglycemia in 5,000 excess dose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr ME, Tortella BJ. Emerging and future therapies for hemophilia. J Blood Med. 2015;6:245–255. doi: 10.2147/JBM.S42669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiMichele DM. Immune tolerance in haemophilia: the long journey to the fork in the road. Br J Haematol. 2012;159:123–134. doi: 10.1111/bjh.12028. [DOI] [PubMed] [Google Scholar]
- 33.Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Blood. 2014;124:3365–3372. doi: 10.1182/blood-2014-05-577643. [DOI] [PubMed] [Google Scholar]
- 34••.Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA. 2010;107:7101–7106. doi: 10.1073/pnas.0912181107. This study laid the foundation for induction of oral tolerance in mice using biopharmaceuticals expressed in chloroplasts, in sharp contrast to previous reports on induction of immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H. Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood. 2014;124:1659–1668. doi: 10.1182/blood-2013-10-528737. Reports an ideal candidate for clinical translation because antibody formation is a major challenge among hemophilia A patients; this is an unmet medical need because of lack of any other suitable treatments to address this concern. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36••.Herzog RW, Nichols TC, Su J, Zhang B, Sherman A, Merricks EP, Raymer R, Perrin GQ, Hager M, Wiinberg B, et al. Oral Tolerance Induction in Hemophilia B Dogs Fed with Transplastomic Lettuce. Mol Ther. 2016 doi: 10.1016/j.ymthe.2016.11.009. http://dx.doi.org/10.1016/j.ymthe.2016.11.009. This study is the first demonstration of oral tolerance in a non-rodent, large animal model using proteins produced in edible chloroplasts, paving the road for human clinical studies. [DOI] [PMC free article] [PubMed]
- 37.Su J, Sherman A, Doerfler PA, Byrne BJ, Herzog RW, Daniell H. Oral delivery of Acid Alpha Glucosidase epitopes expressed in plant chloroplasts suppresses antibody formation in treatment of Pompe mice. Plant biotechnol J. 2015;13:1023–1032. doi: 10.1111/pbi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu J, Dolan MC, Medrano G, Cramer CL, Weathers PJ. Green factory: Plants as bioproduction platforms for recombinant proteins. Biotechnol Adv. 2012;30:1171–1184. doi: 10.1016/j.biotechadv.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 39.Lehtimäki N, Koskela MM, Mulo P. Posttranslational Modifications of Chloroplast Proteins: An Emerging Field. Plant Physiol. 2015;168:768–775. doi: 10.1104/pp.15.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen P. The role of protein phosphorylation in human health and disease. Eur J Biochem. 2001;268:5001–5010. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- 41.Tan CSH, Bodenmiller B, Pasculescu A, Jovanovic M, Hengartner MO, Jørgensen C, Bader GD, Aebersold R, Pawson T, Linding R. Comparative Analysis Reveals Conserved Protein Phosphorylation Networks Implicated in Multiple Diseases. Sci Signal. 2009;2:ra39–ra39. doi: 10.1126/scisignal.2000316. [DOI] [PubMed] [Google Scholar]
- 42.Zhong X, Wright JF. Biological Insights into Therapeutic Protein Modifications throughout Trafficking and Their Biopharmaceutical Applications. Int J Cell Biol. 2013;2013:19. doi: 10.1155/2013/273086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalafatis M. Identification and Partial Characterization of Factor Va Heavy Chain Kinase from Human Platelets. J Biol Chem. 1998;273:8459–8466. doi: 10.1074/jbc.273.14.8459. [DOI] [PubMed] [Google Scholar]
- 44.Mori Y, Hamuro T, Nakashima T, Hamamoto T, Natsuka S, Hase S, Iwanaga S. Biochemical characterization of plasma-derived tissue factor pathway inhibitor: post-translational modification of free, full-length form with particular reference to the sugar chain. J Thromb Haemost. 2009;7:111–120. doi: 10.1111/j.1538-7836.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Z, Mondal M, Liao G, Guo Z. Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self-adjuvanting glycoconjugate cancer vaccine carriers. Org.Biomol Chem. 2014;12:3238–3245. doi: 10.1039/c4ob00390j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wittenberg G, Danon A. Disulfide bond formation in chloroplasts: Formation of disulfide bonds in signaling chloroplast proteins. Plant Sci. 2008;175:459–466. [Google Scholar]
- 47•.Daniell H, Ruiz G, Denes B, Sandberg L, Langridge W. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009;9:33–33. doi: 10.1186/1472-6750-9-33. Insulin like growth factor requires disufide bonds for functionality. Functional IGF-1 was produced in chloroplasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Staub JM, Garcia B, Graves J, Hajdukiewicz PTJ, Hunter P, Nehra N, Paradkar V, Schlittler M, Carroll JA, Spatola L, et al. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat Biotech. 2000;18:333–338. doi: 10.1038/73796. Somatotropin requires disufide bonds for functionality. Functional somatotropin was produced in chloroplasts. [DOI] [PubMed] [Google Scholar]
- 49•.Arlen PA, Falconer R, Cherukumilli S, Cole A, Cole AM, Oishi KK, Daniell H. Field production and functional evaluation of chloroplast-derived interferon-α2b. Plant Biotechnol J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. Interferon α2b requires disufide bonds for functionality. Functional Interferon α2b was produced in chloroplasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Sanz-Barrio R, Millán AF-S, Corral-Martínez P, Seguí-Simarro JM, Farran I. Tobacco plastidial thioredoxins as modulators of recombinant protein production in transgenic chloroplasts. Plant Biotechnol J. 2011;9:639–650. doi: 10.1111/j.1467-7652.2011.00608.x. Human serum albumin requires 17 disufide bonds for functionality. Functional HSA was produced in chloroplasts. [DOI] [PubMed] [Google Scholar]
- 51.Meinnel T, Giglione C. Protein lipidation meets proteomics. Front Biosci. 2008;13:6326–6340. doi: 10.2741/3157. [DOI] [PubMed] [Google Scholar]
- 52•.Glenz K, Bouchon B, Stehle T, Wallich R, Simon MM, Warzecha H. Production of a recombinant bacterial lipoprotein in higher plant chloroplasts. Nat Biotechnol. 2006;24:76–77. doi: 10.1038/nbt1170. The outer surface protein A requires lipidation to confer immunity. Functional vaccine antigen was produced in chloroplasts. [DOI] [PubMed] [Google Scholar]
- 53••.Kwon K-C, Chan H-T, León IR, Williams-Carrier R, Barkan A, Daniell H. Codon Optimization to Enhance Expression Yields Insights into Chloroplast Translation. Plant Physiol. 2016;172:62–77. doi: 10.1104/pp.16.00981. This is the first report of drug dose determination in planta. In addition, authors provide insight into chloroplast translation and address challenges in expression of large human or viral genes in chloroplasts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Day A, Goldschmidt-Clermont M. The chloroplast transformation toolbox: selectable markers and marker removal. Plant Biotechnol. J. 2011;9:540–53. doi: 10.1111/j.1467-7652.2011.00604.x. [DOI] [PubMed] [Google Scholar]