Abstract
Appropriate patient selection for active surveillance is challenging. Our study of 217 patients demonstrated that the preoperative absolute neutrophil and lymphocyte counts were better predictors of aggressive oncologic features than were the neutrophil-to-lymphocyte ratio in the assessment of low-risk prostate cancer patients. Our findings suggest that routine hematologic workup could be used to further stratify low-risk prostate cancer patients.
Introduction
The neutrophil-to-lymphocyte ratio (NLR) has emerged as a ubiquitous prognostic biomarker in cancer-related inflammation, specifically in patients with metastatic castration-resistant prostate cancer (PCa). We evaluated the clinical utility of the preoperative NLR, absolute neutrophil count (ANC), and absolute lymphocyte count (ALC) as a risk stratification tool for patients with low-risk PCa.
Materials and Methods
We identified 217 low-risk PCa patients with preoperative hematologic data who had met the criteria for active surveillance but had undergone robot-assisted radical prostatectomy at our institution from 2006 to 2015. Logistic regression models were constructed to determine whether the baseline NLR, ANC, and ALC were associated with upstaging, upgrading, and biochemical recurrence (BCR). Survival analyses were performed using the Kaplan-Meier method.
Results
On multivariate analysis, a higher prostate-specific antigen level (odds ratio [OR], 1.554; 95% confidence interval [CI], 1.148–2.104), a greater number of positive cores (OR, 2.098; 95% CI, 1.043–2.104), and a higher ALC (OR, 4.311; 95% CI, 1.258–14.770) were associated with upstaging. More importantly, the 5-year biochemical recurrence-free survival was significantly lower in the high ANC group (ANC > 4.0 × 109/L) compared with that of the low ANC group (P = .011). The NLR was not associated with upstaging, upgrading, or BCR in our study cohort (P = .368, P = .573, and P = .504, respectively). The only significant association with upgrading was patient age (OR, 1.106; 95% CI, 1.043–1.173).
Conclusion
NLR was not useful in predicting adverse pathologic outcomes in our patients with low-risk PCa. However, relative neutrophilia and lymphocytosis might indicate an early manifestation of harboring a more aggressive PCa.
Keywords: Active surveillance, Neutrophil-to-lymphocyte ratio, Pathological outcome, Prostate cancer, Radical prostatectomy
Introduction
As a marker of cancer-related inflammation, the neutrophil-to-lymphocyte ratio (NLR) has become a clinically useful tool for predicting the response to therapy and prognosis in various types of malignancies.1 Although the precise mechanism of inflammatory response in the tumor microenvironment remains to be elucidated, many studies have revealed that inflammation plays a key role in cancer development and its progression.2–4 An elevated NLR indicates a high level of neutrophil-dependent inflammation with a concurrent reduction in the lymphocyte-mediated immune response, reflecting a carcinogenic milieu.5,6 However, studies have also shown that tumor-infiltrating lymphocytes (TILs) are recruited to eradicate cancer cells in the early stages.7 This would theoretically result in a lower NLR in the early stage of the disease. Adding further complexity, the temporal relationship between the increase in neutrophil and decrease in lymphocyte counts remains uncertain in the course of cancer progression.8
Despite our limited understanding of the mechanism, NLR has proved to be a valid prognostic biomarker in metastatic castration-resistant prostate cancer (mCRPC), with a higher NLR correlating with a worse prognosis.9 In the context of localized prostate cancer (PCa), however, the role of the NLR and leukocyte subgroups has not been investigated. Because the understaging of patients considering active surveillance (AS) is a major concern, additional biomarkers are necessary to allow clinicians to better stratify patients who are considering AS.10 Although the level of inflammation in low-risk PCa might not be as pronounced as that in mCRPC, we postulated that misclassified higher risk patients who meet the AS eligibility criteria could be detected using the aforementioned hematologic markers and that this information would supplement the current methods used in the risk stratification of patients with PCa.
The objective of our study was to evaluate the preoperative NLR, absolute neutrophil count (ANC), and absolute lymphocyte count (ALC) as independent predictors of adverse pathologic features, specifically upstaging, upgrading, and biochemical recurrence (BCR), among patients who would qualify for AS but who had undergone robot-assisted radical prostatectomy (RARP).
Materials and Methods
Patients
The present retrospective study was conducted using a prospectively maintained database of patients who had undergone RARP at the Rutgers Cancer Institute of New Jersey (RCINJ). Under an institutional review board-approved protocol, patients who met the AS criteria and had the complete blood count (CBC) with differential results available were selected for our analysis.
The RCINJ AS criteria are as follows: preoperative prostate-specific antigen (PSA) < 10 ng/mL, Gleason score ≤ 6 without grade 4 or 5, clinical stage ≤ T2a, ≤ 3 positive cores, and ≤ 50% cancer involvement in each positive core.11 A single preoperative CBC with differential was performed as part of the routine preoperative assessment testing. The white blood cell (WBC) count, ANC, and ALC and their relative percentages were used in the present study. Our study patients first underwent prostate biopsy, followed by preoperative blood testing and then RARP. The pre-operative hematologic workup was performed approximately 2 weeks before RARP. In accordance with our institutional policy, a minimum 6 weeks of wait time was required for all patients undergoing RARP from the most recent prostate biopsy date. Only the CBC without differential was routinely performed at the follow-up visits.
The demographic data (eg, age, race, body mass index), PSA level, and pertinent biopsy findings and postoperative assessments, including pathologic Gleason score (PGS), pathologic stage, prostate weight, and BCR, were studied. Medical conditions (hematologic malignancy and diabetes mellitus) and medications (steroid, aspirin, and statin) that might have influenced the level of NLR were also assessed from the electronic medical record.12–15
In the present study, clinically significant upstaging was defined as any change in T stage from clinical T1–T2a to pathologic T3–T4. A PGS of 7 (both 3 + 4 and 4 + 3) and higher was considered as clinically significant upgrading. BCR was defined as an increasing PSA level on 2 consecutive measurements after RARP, with the last PSA value > 0.2 ng/mL.
The cutoff value of the NLR used for present analysis was 2.6, the median NLR in our study cohort. Previous studies have used a cutoff value of 2 to 3 because no single optimal value for baseline NLR has been established.16
Statistical Analysis
All statistical analyses were performed using SPSS, version 21 (IBM Corp, Armonk, NY) and SAS, version 9.4 (SAS Institute, Cary, NC). P values < .05 were considered statistically significant. Comparisons between groups were performed using chi-square tests for categorical variables, and t tests and analysis of variance were used for continuous variables. Univariate and multivariate logistic regression analyses, adjusted for pertinent medical history and medications, were conducted to identify variables predictive of upgrading and upstaging. Survival analyses were performed using the Kaplan-Meier method with a log-rank test.
Results
Of the 1137 patients analyzed, 217 patients met our institution’s AS criteria but proceeded with RARP. With a median follow-up of 18.0 months, our study cohort was divided by the median NLR into high (≥ 2.6) and low (< 2.6) groups. Both groups had similar baseline demographic characteristics, except that the high NLR group had a greater proportion of white men (91.6% vs. 77.3%) and diabetes (10.3% vs. 2.7%) compared with the low NLR group (P = .012 and P = .028, respectively; Table 1). With the exception of the preoperative hemoglobin levels (P = .275), the preoperative NLR correlated with the various hematologic markers as expected, including the WBC counts, absolute and percentage of neutrophils, and absolute and percentage of lymphocytes (P < .0001 for all). Although the likelihood of upstaging (P = 573) and upgrading (P = .368) were comparable in the high and low NLR groups, the distribution of the PGS was significantly different between the 2 groups, with patients with a GS ≥ 8 were exclusively found in the high NLR group (P = .019).
Table 1.
Baseline Characteristics of Patients Who Met AS Criteria and Underwent RARP
| Characteristic | Total (n = 217) | NLR <2.6 (n = 110) | NLR ≥2.6 (n = 107) | P Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years) | .225 | |||
| Median | 59 | 58 | 60 | |
| IQR | 55–63 | 54–63 | 55–63 | |
| Race (n) | .012 | |||
| White | 183 (84.3) | 85 (77.3) | 98 (91.6) | |
| African American | 26 (12) | 20 (18.2) | 6 (5.6) | |
| Other | 8 (3.7) | 5 (4.5) | 3 (2.8) | |
| BMI (kg/m2) | .062 | |||
| Median | 27.3 | 27.5 | 27.1 | |
| IQR | 25.2–30.1 | 26.1–30.5 | 25.0–29.4 | |
| Preoperative PSA (ng/mL) | .935 | |||
| Median | 4.4 | 4.4 | 4.4 | |
| IQR | 3.6–5.9 | 3.7–5.7 | 3.6–6.2 | |
| Positive core (n) | .815 | |||
| 1 | 110 (50.7) | 54 (49.1) | 56 (52.3) | |
| 2 | 67 (30.9) | 34 (30.9) | 33 (30.8) | |
| 3 | 40 (18.4) | 22 (20.0) | 18 (16.8) | |
| Maximum cancer in core (%) | .630 | |||
| Mean | 17.1 | 16.7 | 17.6 | |
| Range | 1–50 | 1–50 | 1–50 | |
| Preoperative Gleason score | – | |||
| Median | 6 | 6 | 6 | |
| IQR | 6–6 | 6–6 | 6–6 | |
| Preoperative Hb (g/dL) | .275 | |||
| Median | 14.9 | 14.7 | 15.1 | |
| IQR | 14.2–15.6 | 14.2–15.3 | 14.1–15.7 | |
| WBC count (×109/L) | <.0001 | |||
| Median | 6.4 | 5.7 | 7.0 | |
| IQR | 5.4–7.6 | 4.9–6.8 | 6.2–8.0 | |
| Neutrophil (×109/L) | <.0001 | |||
| Median | 4.0 | 3.3 | 4.8 | |
| IQR | 3.2–5.0 | 2.6–3.9 | 4.1–5.7 | |
| Lymphocyte (×109/L) | <.0001 | |||
| Median | 1.52 | 1.8 | 1.3 | |
| IQR | 1.25–1.88 | 1.5–2.2 | 1.1–1.5 | |
| Neutrophil (%) | <.0001 | |||
| Median | 63.0 | 57.7 | 69.3 | |
| IQR | 57.5–69.23 | 52.3–60.7 | 67.1–72.8 | |
| Lymphocyte (%) | <.0001 | |||
| Median | 24.9 | 30.7 | 20.1 | |
| IQR | 20.2–30.8 | 27.6–34.8 | 17.2–22.3 | |
| Clinical stage (n) | .174 | |||
| T1 | 205 (94.5) | 106 (96.4) | 99 (92.5) | |
| T2a | 12 (5.5) | 4 (3.6) | 8 (7.5) | |
| Medication and medical history (n) | ||||
| Aspirin | .664 | |||
| No | 149 (68.7) | 74 (67.3) | 75 (70.1) | |
| Yes | 68 (31.3) | 36 (32.7) | 32 (29.9) | |
| Statin | 1.00 | |||
| No | 138 (63.6) | 70 (63.6) | 68 (63.6) | |
| Yes | 79 (36.4) | 40 (36.4) | 39 (36.4) | |
| Steroid | .618 | |||
| No | 214 (98.6) | 109 (99.1) | 105 (98.1) | |
| Yes | 3 (1.4) | 1 (0.9) | 2 (1.9) | |
| Diabetes | .028 | |||
| No | 203 (93.5) | 107 (97.3) | 96 (89.7) | |
| Yes | 14 (6.5) | 3 (2.7) | 11 (10.3) | |
| Hematologic malignancy | .984 | |||
| No | 215 (99.1) | 109 (99.1) | 106 (99.1) | |
| Yes | 2 (0.9) | 1 (0.9) | 1 (0.9) | |
| Pathologic characteristics | ||||
| Pathologic stage | .573 | |||
| T2 | 199 (91.7) | 101 (91.8) | 98 (91.6) | |
| T3a | 18 (8.3) | 9 (8.2) | 9 (8.4) | |
| Pathologic Gleason score | .019 | |||
| 6 | 159 (73.3) | 79 (71.8) | 80 (74.8) | |
| 7 | 52 (24.0) | 31 (28.2) | 21 (19.6) | |
| ≥8 | 6 (2.8) | 0 (0) | 6 (5.6) | |
| Upstaging (≥T3a) | .573 | |||
| No | 199 (91.7) | 101 (91.8) | 98 (91.6) | |
| Yes | 18 (8.3) | 9 (8.2) | 9 (8.4) | |
| Upgrading (PGS ≥7) | .368 | |||
| No | 159 (73.3) | 79 (71.8) | 80 (74.8) | |
| Yes | 58 (26.7) | 31 (28.2) | 27 (25.2) | |
| Prostate weight (g) | .420 | |||
| Median | 49.0 | 49.0 | 48.0 | |
| IQR | 40.0–58.5 | 40.0–61.3 | 39.0–58.0 | |
| Margin status | .549 | |||
| Negative | 189 (87.1) | 96 (87.3) | 93 (86.9) | |
| Positive | 28 (12.9) | 14 (12.7) | 14 (13.1) | |
| Biochemical recurrence | 6 (2.8) | 4 (3.6) | 2 (1.9) | .355 |
Data presented as n (%), unless otherwise noted.
Abbreviations: AS = active surveillance; BMI = body mass index; Hb = hemoglobin; IQR = interquartile range; NLR = neutrophil-to-lymphocyte ratio; PGS = pathologic Gleason score; PSA = preoperative prostate-specific antigen; RARP = robot-assisted radical prostatectomy; WBC = white blood cell.
In addition to the NLR, the ANC and ALC were assessed by dividing the study population at their median value of 4.0 × 109/L and 1.52 × 109/L, respectively (Table 2). On univariate analysis (Table 3), the common clinical parameters reported to be associated with upgrading and upstaging were selected and tested. The PSA level (95% confidence interval [CI], 1.044–1.739; P = .022) and ALC (95% CI, 1.171–11.570; P = .026) were significantly associated with upstaging. For upgrading, the only significant association was patient age (95% CI, 1.027–1.136; P = .003). No medical conditions or medications were predictive of upgrading or upstaging.
Table 2.
Comparison of Upstaging and Upgrading Between High and Low ANC and ALC
| Variable | Upstaging | Upgrading (≥3 + 4) |
Upgrading (≥4 +9 3) |
|---|---|---|---|
| ANC | |||
| <4.0 | 6 (5.6) | 27 (25.0) | 3 (2.8) |
| ≥4.0 | 12 (11.0) | 31 (28.4) | 8 (7.3) |
| P value | .145 | .567 | .126 |
| ALC | |||
| <1.52 | 4 (3.8) | 28 (26.4) | 6 (5.7) |
| ≥1.52 | 14 (12.6) | 30 (27.0) | 5 (4.5) |
| P value | .018 | .919 | .698 |
Data presented as n (%).
High and low groups divided by the median value.
Abbreviations: ALC = absolute lymphocyte count; ANC = absolute neutrophil count.
Table 3.
Univariate Logistic Regression Analysis of Preoperative Parameters Predicting Upstaging and Upgrading
| Parameter | Cutoff | Univariate P Value | |
|---|---|---|---|
| Upstaging | Upgrading | ||
| Age (years) | – | .468 | .003a |
| Race | White vs. nonwhite | .581 | .421 |
| BMI (kg/m2) | – | .212 | .795 |
| Preoperative PSA (ng/mL) | – | .022b | .102 |
| Positive core | 1 or 2 vs. 3 | .097 | .605 |
| Hb (g/dL) | – | .887 | .172 |
| WBC (×109/L) | – | .075 | .639 |
| ANCc (×109/L) | <4.0 vs. ≥4.0 | .132 | .902 |
| ALCc (×109/L) | <1.52 vs. ≥1.52 | .026d | .919 |
| NLRc | <2.6 vs. ≥2.6 | .951 | .624 |
| Aspirin | Yes vs. no | .734 | .546 |
| Statin | Yes vs. no | .777 | .500 |
| Diabetes | Yes vs. no | .872 | .872 |
Abbreviations: ALC = absolute lymphocyte count; ANC = absolute neutrophil count; BMI = body mass index; CI = confidence interval; Hb = hemoglobin; OR = odds ratio; NLR = neutrophil-to-lymphocyte ratio; PSA = prostate-specific antigen; WBC = white blood cell.
OR, 1.080; 95% CI 1.027–1.136.
OR, 1.347; 95% CI, 1.044–1.739.
Group divided at the median value.
OR, 3.680; 95% CI, 1.171–11.570.
A multivariate analysis model (Table 4) was constructed of the common demographic variables, pertinent medical conditions and medications, and variables with a P value < .05 on univariate analysis. Multivariate analysis showed that a higher PSA (odds ratio [OR], 1.554; 95% CI, 1.148–2.104; P = .004), a greater number of positive cores (OR, 2.098; 95% CI, 1.043–2.104; P = .038), and a higher ALC (OR, 4.311; 95% CI, 1.258–14.770; P = .020) were associated with upstaging. Patient age (OR, 1.106; 95% CI, 1.043–1.173; P = .001), adjusted for other variables, was the only significant predictor of upgrading.
Table 4.
Multivariate Logistic Regression Analysis of Preoperative Parameters Predicting Upstaging and Upgrading
| Parameter | Cutoff | Multivariate P Value | |
|---|---|---|---|
| Upstaging | Upgrading | ||
| Age (years)a | – | .168 | .001b |
| Race | White vs. nonwhite | .419 | .609 |
| BMI (kg/m2) | – | .120 | .229 |
| Preoperative PSA (ng/mL) | – | .004c | .080 |
| Positive core | 1 or 2 vs. 3 | .038d | .176 |
| ALC (×109/L)a | <1.52 vs. ≥1.52 | .020e | .660 |
| Aspirin | Yes vs. no | .342 | .574 |
| Statin | Yes vs. no | .584 | .255 |
| Diabetes | Yes vs. no | .762 | .752 |
Abbreviations: ALC = absolute lymphocyte count; BMI = body mass index; CI = confidence interval; OR = odds ratio; PSA = prostate-specific antigen.
Group divided at the median value.
OR, 1.106; 95% CI, 1.043–1.173.
OR, 1.554; 95% CI, 1.148–2.104.
OR, 2.098; 95% CI, 1.043–2.104.
OR 4.311; 95% CI, 1.258–14.770.
A total of 6 patients (2.8%) developed BCR in the course of their follow-up. The number of patients with BCR was not significantly different between the high NLR and low NLR groups (P = .504; Figure 1A). We then examined ANC and ALC individually. When our study cohort was divided into high and low ANC groups, the biochemical recurrence-free survival (bRFS) of the high ANC group (≥ 4.0 × 109/L) was significantly lower than that of the low ANC group (< 4.0 × 109/L; P = .011; Figure 1B). No significant difference was found in bRFS between the high (≥ 1.52 × 109/L) and low (< 1.52 × 109/L) ALC groups (P = .130; Figure 1C).
Figure 1.
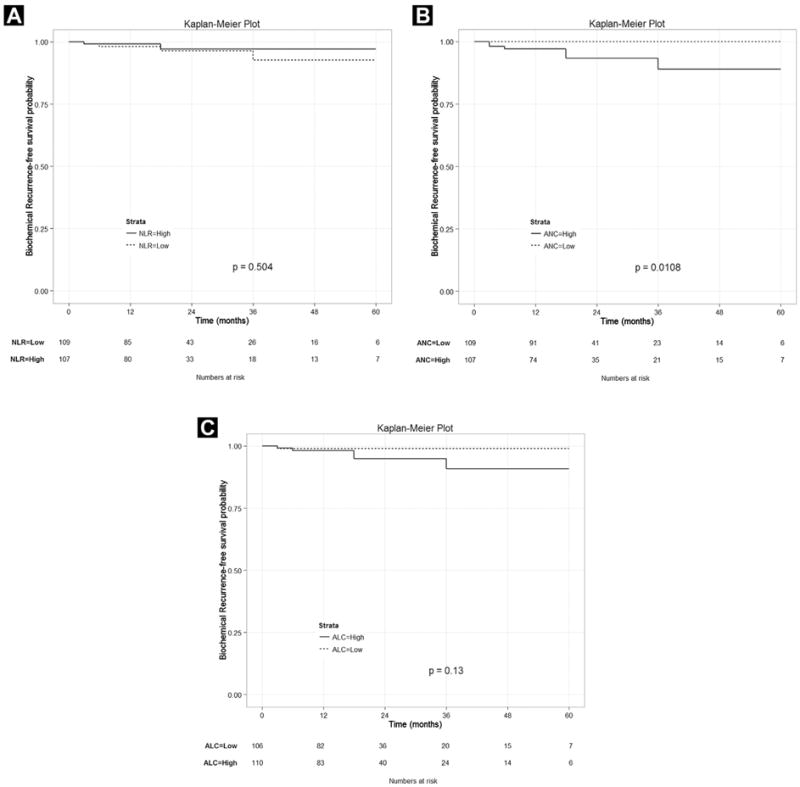
(A) Number of Patients With Biochemical Recurrence Was Not Significantly Different Between the High Neutrophil-to-Lymphocyte Ratio (NLR) and Low NLR Groups (P = .504). (B) Biochemical Recurrence-Free Survival (bRFS) of High Absolute Neutrophil Count (ANC) Group (≥ 4.0 × 109/L) Was Significantly Lower Than That of Low ANC Group (< 4.0 × 109/L; P = .011). (C) No Significant Difference Was Found in bRFS Between High (≥ 1.52 × 109/L) and Low (< 1.52 × 109/L) Absolute Lymphocyte Count (ALC) Groups (P = .130)
Discussion
In the present study, we investigated the potential role of NLR, ANC, and ALC as biomarkers to aid in identifying the few occult high-grade PCa cases in men who qualify for AS. Our analysis showed evidence that relative neutrophilia and lymphocytosis, albeit at a subclinical level, predict for more aggressive PCa.
Although only 6 patients (2.8%) experienced BCR in our cohort during the study period, the 5-year bRFS rates were significantly different when the median ANC of 4.0 × 109/L was used. The high ANC group included all 6 patients with BCR, and the low ANC group had none (P = .011). However, the patients in the low and high NLR groups were both found to have comparable BCR rates (3.6% vs. 1.9%; P = .504). Similarly, no statistically significant difference was found in bRFS with different ALC levels (P = .130; Figure 1). These findings suggest that a relatively high neutrophil level at an early stage of disease might be associated with more aggressive PCa. This hypothesis is consistent with findings from renal cell carcinoma for which neutrophilia is a validated independent risk factor for a poor prognosis.17 Neutrophil-derived cytokines and proteins stored within granules have also been studied to predict tumor progression and severity in other types of cancers.18 In the framework of prostate pathology, recent studies have linked elevated neutrophil counts in those with chronic prostatitis and other infectious diseases to an increased risk of developing PCa.19
In addition to the role of relative neutrophilia in tumor-related inflammation and the subsequently increased risk of BCR, our results revealed that relative lymphocytosis is associated with upstaging (OR, 4.872; 95% CI, 1.384–17.152; P = .014). High ALCs are typically observed in viral infections20; however, no known relationships have been established with PCa. According to immunoediting during cancer development, Kim et al21 described 3 distinct phases, the first of which involves complete elimination of tumor cells by T lymphocytes, explaining the relative lymphocytosis in the early-stage tumor. Moreover, in the study of prognostic significance of tumor-infiltrating lymphocytes (TILs) in localized PCa, Kärjä et al9 analyzed the immunohistochemical characteristics of lymphocytes and concluded that strong expression of TILs was linked to perineural and capsular invasion (P = .017 and P < .0001, respectively). Although the scope of our study precluded immunohistochemical analysis, we suspect that the increased levels of TILs in the high ALC group might have influenced the likelihood of upstaging. Notwithstanding these results, additional studies are necessary to further define the role of lymphocytosis in early-stage PCa, because the lymphocyte count was not associated with bRFS.
In contrast to the ANC and ALC, the NLR was not associated with upgrading, upstaging, or BCR (P = .368; P = .573; and P = .504, respectively). The univariate analysis constructed with NLR and other hematologic markers and other commonly associated clinical parameters also did not show a significant correlation of NLR with upgrading and upstaging. Although our small sample size might have contributed to this pattern, a possible explanation for this finding lies in the nature of our study cohort. Specifically, our patients had low-volume, low-grade cancer with a PSA upper limit of 10 ng/mL. The tumor burden of the patients in our study cohort might not have exceeded the threshold at which cancer-related inflammation becomes evident in the NLR. That most of our patients had an ANC and ALC within the reference ranges (92.6% and 98.6%, respectively) further suggests that only subclinical inflammatory responses, if any, were present in this cohort. The overall upgrading and upstaging rates using our AS criteria were 26.7% and 8.3%, respectively. These rates are slightly lower than those from other previous studies using AS cohorts.22,23
The 6 patients with a high PGS (≥ 8) were found exclusively in the high NLR group (P = .019), supporting the hypothesis that the NLR might be a potential inflammatory marker associated with more advanced cancer. Although multivariate and univariate analyses were not performed because of the small sample size, it should be noted that significant upgrading from a biopsy GS of 6 to a PGS of ≥ 8 is rare in men meeting the AS criteria.24 Aggarwal et al4 previously reported that a high NLR with high pro-inflammatory cytokines is more likely to occur in those with metastatic PCa. Although the NLR did not have a significant predictive value in our regression model, the association found between a high NLR and high-grade GS is consistent with the study by Ploussard et al25 and provides ample support for further investigation of NLR.
The present study showed that a higher preoperative ANC and ALC, compared with the conventional NLR, were better predictors for worse clinicopathologic features, demonstrating their role in stratifying AS patients. We believe that the routine hematologic workup is immensely important in the era of AS, because sampling error during biopsy could place the patient in AS, missing the therapeutic window of intervention.25 PSA remains the most widely used biomarker in predicting the aggressiveness of PCa. However, in an AS-eligible cohort, in which the variability of PSA is limited, leukocyte subgroups and NLR analysis might potentially offer additional parameters that can further stratify a seemingly homogenous group of patients. Although studies have also shown that the postoperative NLR is a prognostic marker for recurrence and measure of response to therapy in renal cell carcinoma,26,27 our analyses for this project were limited to the preoperative setting to assess the cancer-related inflammation manifested in the pretreatment hematologic parameters.
Similar to NLR, the Glasgow prognostic score (GPS) used the combination of serum C-reactive protein with albumin as a prognostic and therapeutic indicator and has a decade of use and validation data across many types of cancer.28 A study from a Scottish cancer registry, in particular, reported that the GPS is a prognostic indicator for the survival of patients with PCa.29 It remains to be determined how the prognostic value of NLR, ANC, and ALC will compare with that of the GPS; however, it is certain that all these systemic inflammatory markers should continue to be studied in various cancer types.
The present study is the first, to our knowledge, that has attempted to elucidate the role of NLR, ANC, and ALC in low-risk PCa patients with histopathologic and clinical outcomes. In patients with low-grade PCa, we have concluded that a high ANC and ALC, respectively, correlate with biochemical recurrence and upstaging. We postulated that, unlike the positive associations found between the NLR and disease severity in other cancer settings, our patients might not have reached the critical point in disease progression such that the NLR would be significant. Nevertheless, our findings support the utility of ANC and ALC and that NLR should continue to be assessed to precisely define their roles in stratifying the risk of patients in AS.
The present study had several limitations. First, the retrospective study design we used is susceptible to hidden confounding variables. Second, our findings were from a single surgeon database at a tertiary surgical center and might not be generalizable to the general population of AS candidates. Third, although we adjusted for some potential confounders that might influence the level of neutrophils and lymphocytes in our multivariate regression model, it is unlikely that the reported values were solely attributed to PCa-related inflammation. Fourth, we did not adjust for the racial variation in NLR, despite the potential ethnic trends.30 Fifth, the chance of a false-positive result (type I error) from multiple comparisons was not entirely negligible; thus, our results should be interpreted with caution. Sixth, the current usage of a single preoperative blood sample was less reliable and could be subject to fluctuation. Thus, our study findings could have been strengthened by different pre-operative sets of blood samples from each patient within a short period.
A multicenter prospective study would be ideal to further assess the utility of these hematologic markers. Also, as an extension to our research, these markers could be evaluated in the entire post-RARP cohort, stratified by relevant risk parameters, and a group of men actually undergoing AS. Finally, multiple postoperative CBCs with differential at different follow-up visits could potentially uncover important long-term patterns of oncologic control.
Conclusion
Our present study has demonstrated that the baseline ANCs and ALCs are superior predictors of biochemical recurrence and upstaging, respectively, than the NLR in the assessment of low-risk PCa patients. When considering patients for AS, the presence of relative neutrophilia and lymphocytosis could be associated with increased risk. From our findings, we believe that routine hematologic workup could offer clinicians a ubiquitous cost-effective tool for further stratifying low-risk PCa patients considering AS.
Clinical Practice Points.
The NLR has become a clinically useful marker for predicting the response to therapy and prognosis in different types of malignancies, including mCRPC.
Because AS has become a widely accepted management option for localized PCa, appropriate patient selection is imperative to the management of safe and effective AS.
Our study of 217 low-risk PCa patients who underwent RARP demonstrated that higher baseline ANCs and ALCs were associated with an increased risk of BCR and upstaging, respectively.
Our study is noteworthy, because it attempted to elucidate the role of NLR, ANC, and ALC in low-risk PCa patients with the histopathologic and clinical outcomes.
With further studies, a role might be shown for routine hematologic blood workup in assessing the level of cancer-related inflammation and stratifying low-risk PCa patients considering AS.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
References
- 1.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5:2024–36. doi: 10.1097/jto.0b013e3181f387e4. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–30. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 5.Brandau S, Dumitru CA, Lang S. Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol. 2013;35:163–76. doi: 10.1007/s00281-012-0344-6. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gooden MJM, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. doi: 10.1186/1755-7682-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kärjä V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: a prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25:4435–8. [PubMed] [Google Scholar]
- 10.Harlan SR, Cooperberg MR, Elkin E, et al. Time trends and characteristics of men choosing watchful waiting for initial treatment of localized prostate cancer: results from CaPSURE. J Urol. 2003;170:1804–7. doi: 10.1097/01.ju.0000091641.34674.11. [DOI] [PubMed] [Google Scholar]
- 11.Han CS, Parihar JS, Kim IY. Active surveillance in men with low-risk prostate cancer: current and future challenges. Am J Clin Exp Urol. 2013;1:72–82. [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Farre A, Caramelo C, Esteban A, et al. Effects of aspirin on platelet-neutrophil interactions: role of nitric oxide and endothelin-1. Circulation. 1995;91:2080–8. doi: 10.1161/01.cir.91.7.2080. [DOI] [PubMed] [Google Scholar]
- 13.Greenwood J, Mason JC. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007;28:88–98. doi: 10.1016/j.it.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alba-Loureiro TC, Munhoz CD, Martins JO, et al. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40:1037–44. doi: 10.1590/s0100-879x2006005000143. [DOI] [PubMed] [Google Scholar]
- 15.Deinard AS, Page AR. A study of steroid-induced granulocytosis in a patient with chronic benign neutropenia of childhood. Br J Haematol. 1974;28:333–45. doi: 10.1111/j.1365-2141.1974.tb00815.x. [DOI] [PubMed] [Google Scholar]
- 16.Lorente D, Mateo J, Templeton AJ, et al. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2014;26:750–5. doi: 10.1093/annonc/mdu587. [DOI] [PubMed] [Google Scholar]
- 17.Jensen HK, Donskov F, Marcussen N, Nordmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–17. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 18.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–7. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199–215. doi: 10.1111/j.1365-2559.2011.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCash SI, Marionneaux S. Epstein-Barr virus–associated lymphocytosis masquerading as lymphoma. Blood. 2014;123:1444. doi: 10.1182/blood-2013-12-542159. [DOI] [PubMed] [Google Scholar]
- 21.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iremashvili V, Pelaez L, Manoharan M, Jorda M, Rosenberg DL, Soloway MS. Pathologic prostate cancer characteristics in patients eligible for active surveillance: a head-to-head comparison of contemporary protocols. Eur Urol. 2012;62:462–8. doi: 10.1016/j.eururo.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 23.El Hajj A, Ploussard G, de la Taille A, et al. Analysis of outcomes after radical prostatectomy in patients eligible for active surveillance (PRIAS) BJU Int. 2013;111:53–9. doi: 10.1111/j.1464-410X.2012.11276.x. [DOI] [PubMed] [Google Scholar]
- 24.Jain S, Loblaw A, Vesprini D, et al. Gleason upgrading with time in a large prostate cancer active surveillance cohort. J Urol. 2015;194:79–84. doi: 10.1016/j.juro.2015.01.102. [DOI] [PubMed] [Google Scholar]
- 25.Ploussard G, Salomon L, Xylinas E, et al. Pathological findings and prostate specific antigen outcomes after radical prostatectomy in men eligible for active surveillance—does the risk of misclassification vary according to biopsy criteria? J Urol. 2010;183:539–44. doi: 10.1016/j.juro.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Ohno Y, Nakashima J, Ohori M, Gondo T, Hatano T, Tachibana M. Followup of neutrophil-to-lymphocyte ratio and recurrence of clear cell renal cell carcinoma. J Urol. 2012;187:411–7. doi: 10.1016/j.juro.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Park YH, Ku JH, Kwak C, Kim HK. Post-treatment neutrophil-to-lymphocyte ratio in predicting prognosis in patients with metastatic clear cell renal cell carcinoma receiving sunitinib as first line therapy. Springerplus. 2014;3:243. doi: 10.1186/2193-1801-3-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMillan DC. The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–40. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Shafique K, Proctor MJ, McMillan DD, et al. The modified Glasgow prognostic score in prostate cancer: results from a retrospective clinical series of 744 patients. BMC Cancer. 2013;13:292. doi: 10.1186/1471-2407-13-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadeghi N, Badalato GM, Hruby G, Grann V, McKiernan JM. Does absolute neutrophil count predict high tumor grade in African-American men with prostate cancer? Prostate. 2012;72:386–91. doi: 10.1002/pros.21440. [DOI] [PubMed] [Google Scholar]


