Abstract
It was recently shown that fasting alters the composition of microbial communities residing in the distal intestinal tract of animals representing five classes of vertebrates [i.e., fishes (tilapia), amphibians (toads), reptiles (leopard geckos), birds (quail), and mammals (mice)]. In the current study, we tested the hypothesis that the extent of tissue reorganization in the fasted distal intestine was correlated with the observed changes in enteric microbial diversity. Segments of intestine adjacent to those used for the microbiota study were examined histologically to quantify cross-sectional and mucosal surface areas as well as thicknesses of mucosa, submucosa, and tunica muscularis. We found no fasting-induced differences in the morphology of distal intestines of the mice (3d), quail (7d), or geckos (28d). The toads, which exhibited a general increase in phylogenetic diversity of their enteric microbiota with fasting, also exhibited reduced mucosal circumference at 14 and 21 days of fasting. Tilapia showed increased phylogenetic diversity of their enteric microbiota, and showed a thickened tunica muscularis at 21 days fasting; but this morphological change was not related to microbial diversity or absorptive surface area, and thus, is unlikely to functionally match the changes in their microbiome. Given that fasting caused significant increases and reductions in the enteric microbial diversity of mice and quail, respectively, but no detectable changes in distal intestine morphology, we conclude that reorganization is not the primary factor shaping changes in microbial diversity within the fasted colon, and the observed modest structural changes are more related to the fasted state.
Keywords: starvation, nutrition, morphology, hindgut, malnutrition, microbiome
1. Introduction
The form and function of the digestive tract is evolutionarily tuned to an animal’s diet and feeding habits. Diet is well known to differ widely among taxa (Stevens and Hume, 1995; Horn et al., 2006; German and Miles, 2010; Karasov and Douglas, 2013), and feeding frequency may also vary with some species eating nearly constantly and others experiencing long bouts between meals (Wang et al., 2006; McCue, 2010). Because the digestive tract is expensive to maintain—accounting for 20–25% of an animal’s resting metabolic rate (Cant et al., 1996; Croom et al., 1999; Secor et al., 2012)—it is expected to be tightly regulated in the face of varying nutrient availability in response to varying diets and feeding schedules (Karasov and Diamond, 1988; Secor, 2001; Starck, 2005; Karasov and Douglas, 2013; Price et al., 2015).
Fasting tolerance is highly variable among animals, with some endothermic species expiring in less than two days without food and some ectothermic species lasting months to years without eating (Castellini and Rea, 1992; Navarro and Gutierrez, 1995; Wang et al., 2006; McCue, 2012). Despite various mechanisms to reduce energy expenditure (Secor and Carey, 2016), the fasted gut must be maintained in a state such that it retains the ability to quickly restore functionality in the event food is ingested.
The ‘small’ or ‘proximal’ intestine (hereafter: proximal intestine) is the most active region of the intestine, with high cellular turnover and activity, and it exhibits several adaptive responses to downregulate and conserve energy during times of fasting. Some of these changes include decreases in villi (or intestinal fold) size and number, a decrease in enterocyte cytoplasmic activity, and a decrease in microvilli length (Starck, 2003; Secor and Lignot, 2010; Lignot, 2012; Zaldua and Naya, 2014). The mechanisms of these reductions (e.g., changes in cell turnover versus cell volume) can vary among endothermic and ectothermic taxa (Starck, 2003, 2005).
The reduction in the surface area of the proximal intestine is concomitantly accompanied by decreases in membrane-bound enzyme activities (e.g., aminopeptidase, maltase) and nutrient transport rates (Secor et al., 1994; Ott and Secor, 2007; German et al., 2010). Although the mechanism of change varies among endothermic and ectothermic taxa, the general patterns of reduced activity and surface area in the proximal intestine in response to fasting are common among vertebrates having been documented in fishes (Gas and Noailliac-Depeyre, 1976; German et al., 2010; Zaldua and Naya, 2014), amphibians (Perez-Gonzalez and Robinson, 1971; Cramp and Franklin, 2003; Cramp et al., 2005), reptiles (Secor and Diamond, 2000; Starck and Beese, 2002; Secor and Lignot, 2010), birds (Hume and Biebach, 1996; Karasov et al., 2004; Smirnov et al., 2004), and mammals (Dunel-Erb et al., 2001; Habold et al., 2004; Habold et al., 2007).
The colons, hindguts, or distal intestines (hereafter: distal intestine) of vertebrate animals have received considerably less attention than the proximal intestine in terms of structural and functional responses to fasting and starvation (Gas and Noailliac-Depeyre, 1976; Baeverfjord and Krogdahl, 1996; German et al., 2010). Given that the distal intestine is responsible for absorbing nutrients, vitamins, electrolytes, and water (Savage, 1986; Stevens and Hume, 1995; Clements and Raubenheimer, 2006) a complete understanding of how fasting affects the gastrointestinal tract must consider the distal intestine (Okada et al., 2013).
The distal intestine is home to the largest enteric microbial population outside of the caeca in animals that possess them (Roediger, 1990; Caporaso et al., 2011; Kohl et al., 2014). These microbial communities subsist on nutritive digesta periodically pulsing through the gut, as well as host-produced glycans and mucins on the gut lining (Derrien et al., 2004; Sonnenburg et al., 2005). However, in fasted animals the nutrients in the distal intestine may become scarce (Okada et al., 2013) creating an ‘energy crisis’ for the intestinal microbes (McCue, 2012). This increase in phylogenetic diversity, appears to be driven by crashing populations of the species that predominate the gut in times of high nutrient availability when the hosts are nourished {Kohl, 2014 #4713}. Many of the enterocytes lining the distal intestine also obtain a significant proportion of their energy needs from the short chain fatty acids produced via microbial fermentation (Bjorndal, 1979; Troyer, 1984; Roediger, 1990; Bugaut and Bentejac, 1993; Crawford et al., 2009), and may require microbial metabolites (e.g., lactate) for proliferation (Okada et al., 2013).
Microbial communities often exhibit typical species-area relationships, such that larger habitats contain higher diversity (Bell et al., 2005; Godon et al., 2016), and this relationship has been demonstrated in vertebrate gut microbial communities (Bell et al., 2005; Godon et al., 2016). In times of fasting the enterocytes may also experience reduced nutrient uptake that may drive the atrophy of the proximal intestine thereby creating a ‘housing crisis’ for the microbiome (McCue, 2012). Thus, changes in the structure of the distal intestine, such as decreased volume or available membrane space, may result in changes to microbial diversity (Secor and Carey, 2016). However, as noted above, morphological changes to the distal intestine as a result of fasting have been largely overlooked, with one previous mammalian example showing no change in mucosal area of the distal intestine following fasting (Okada et al., 2013).
Recent studies of microbial diversity in the distal intestine demonstrate varying responses to fasting. Fasted animals show shifts in their microbial communities that range from increases, to no changes, to decreases in microbial diversity (Crawford et al., 2009; Sonoyama et al., 2009; Costello et al., 2010; Kohl et al., 2014; Xia et al., 2014). Although these changes are statistically significant there do not yet seem to be systematic changes that are generalizable among different groups of vertebrate animals (Kohl et al., 2014). Given the importance of the enteric microbiota in overall animal health (Jabbar et al., 2003; Backhed et al., 2004; Turnbaugh and Gordon, 2009; Caporaso et al., 2011; Mischke and Plosch, 2013), it is crucial to understand if and how fasting-induced structural changes in the distal intestine may drive the observed changes in the microbial community that it supports.
A recent study demonstrated that the diversity of the microbiomes within the distal intestines of animals representing five vertebrate groups (classes): a teleost fish (tilapia), an amphibian (toads), a reptile (leopard geckos), a bird (quail), and a eutherian mammal (mice) (Kohl et al. 2014; Table 1). Thus, within these organisms, we are presented with the unique opportunity to answer the following questions regarding the distal intestine in the face of fasting: 1) How do the distal intestines of disparate endothermic and ectothermic lineages respond to fasting, and, despite likely mechanistic differences, is there a common, generalizable response (e.g., reduction in surface area)? 2) How are fasting-induced structural changes of the distal intestine correlated with previously documented changes in enteric microbial diversity within this gut region?
Table 1.
Phylogenetic diversity (PD) of microbial communities at various fasting time points. Numbers represent PD means ± standard error. Diversity was compared within a species using Tukey’s HSD test. Values not sharing superscripted letters are significantly different. The last column presents the two most dominant microbial phyla in each species. More detailed analysis can be found in Kohl et al. (2014).
| Taxon | Fed | Early-fasting | Mid-fasting | Late-fasting | Major Phyla |
|---|---|---|---|---|---|
| Mouse | 24.49 ± 1.16a | 28.30 ± 1.09b | 29.97 ± 1.53b | 29.06 ± 1.20b | Bacteroidetes Firmicutes |
| Quail | 22.82 ± 1.69a | 16.21 ± 2.42bc | 21.65 ± 2.14ab | 14.31 ± 1.33c | Proteobacteria Firmicutes |
| Tilapia | 6.51 ± 1.48a | 8.81 ± 1.49a | 15.60 ± 1.22b | 18.95 ± 1.54b | Fusobacteria Firmicutes |
| Toad | 27.07 ± 2.92a | 36.25 ± 3.69b | 33.98 ± 2.71ab | 40.95 ± 1.83b | Firmicutes Bacteroidetes |
| Gecko | 26.43 ± 1.01 | 29.01 ± 0.97 | 24.74 ± 2.02 | 26.26 ± 0.97 | Bacteroidetes Verrucomicrobia |
In order to answer these questions, we examined the distal intestines of tilapia, toad, leopard gecko, quail, and mouse subjected to different levels of fasting. The samples we examined were collected from adjacent regions of the distal intestine that we previously used to characterize fasting-induced changes in the microbiome. We prepared histological cross sections and measured several characteristics including the surface area of the mucosa and serosa, as well as thickness of the tunica muscularis, submucosa, and mucosa to characterize the effect of fasting on the structure of the distal intestine.
2. Materials and Methods
Animals
All experiments involving vertebrates were conducted at St. Mary’s University (StMU) under the auspices of the StMU Institutional Animal Care and Use Committee (StMU #2010–4; 2011–5, 2012–1; 2012–2; 2012–3). The laboratory temperature (28±1°C) and photoperiod (14L:10D) remained constant during the experiments, and all animals experienced these temperatures whether in air or water. The only caveat is that the gecko cages were lit in one corner by an incandescent lightbulb, giving them one warm microclimate (~30°C) in which to bask.
Nile tilapia, Oreochromis niloticus, (males and females; fry ~1.5cm total length; TL) were obtained from a commercial breeder (Tilapia Depot, Saint Augustine, FL) and raised on the Tilapia Depot Standard High-Protein Pelleted Diet (>50% protein, >16% fat) for approximately 4 months in a 400-gallon aquarium until reaching ~20cm TL. Wild captured adult and subadult southern toads, Anaxyrus terrestris, (males and females; ~15–30g) were purchased from a commercial distributor (Gulf Hammock Herps, Dade City, FL) and maintained for 60 days on a diet of live crickets (Fluker Farms, Port Allen, LA). Captive bred, adult leopard geckos, Eublepharis macularius, (males and females; ~50–60g) were obtained from Leopardgecko.com (Boerne, TX) and maintained in the lab for approximately 9 months on a diet of live tenebrionid beetle larvae raised in the laboratory. Geckos were given supplementary heat lamps to permit voluntary basking. Common quail, Coturnix coturnix, hatchlings (males and females; 2 days old) were obtained from a commercial breeder (Diamond H Ranch, Bandera, TX) and raised in the lab for approximately 3 months on a standard quail diet (Nature Wise, Nutrena) until adulthood (~250g). Weanling mice, Mus musculus, (males; ~10–12g) were obtained from a commercial breeder (Alamo Aquatic Pets, San Antonio, TX) and raised in the lab on a standard rodent diet (Teklad, Harlan Laboratories) for approximately 2 months until adulthood (~26–30g).
Fasting and tissue sampling
On the first day of the fasting experiment, the toads and geckos were held without food for 24 hours, at which time they were sampled (e.g., fasting day 0; Table 2). Similarly, food was removed from the tilapia, quail, and mice 6 hours prior to the first sampling time point (e.g., fasting day 0). All other animals were maintained without access to food for prolonged periods of time (Table 2). Water was available at all times to fasting animals. Sample sizes are presented in Table 2.
Table 2.
Animals used in the fasting experiments. The first number is the number of days that postabsorptive animals were fasting. The value in parentheses refers to the sample size at each time point.
| Taxon | Scientific name | nourished | early-fasting | mid-fasting | late-fasting |
|---|---|---|---|---|---|
| Mouse | Mus musculus | 0 (7) | 1 (6) | 2 (5) | 3 (8) |
| Quail | Coturnix coturnix | 0 (7) | 2 (7) | 4 (7) | 7 (4) |
| Tilapia | Oreochromis niloticus | 0 (7) | 7 (5) | 14 (6) | 21 (6) |
| Toad | Anaxyrus terrestris | 0 (7) | 7 (5) | 14 (6) | 21 (7) |
| Gecko | Eublepharis macularius | 0 (7) | 7 (7) | 14 (4) | 28 (5) |
At predetermined time points, animals were euthanized (Table 2) in accordance with standard guidelines for euthanasia (AVMA, 2013). The ‘late-fasting’ time point was determined using preliminary data with the goal of achieving a 20–30% loss of body mass in the endotherms (Toth and Gardiner, 2000; Rowland, 2007), and a sufficient period (≥three weeks) in the ectotherms, which show variable fasting times. The fasting time frame was divided in thirds to determine the ‘early-fasting’ and ‘mid-fasting’ time points.
Within 10 minutes of euthanasia, the gastrointestinal tract from the distal esophagus to the rectum was removed intact. A central section (approximately 1cm in length) of large intestine was removed from each animal, placed in a tissue cassette, and fixed in Carnoy’s solution (v/v; 60% anhydrous methanol, 30% chloroform, and 10% glacial acetic acid) (Johansson and Hansson, 2012). Each sample was embedded in paraffin, sectioned and fixed onto a (1”×3”) slide with 6 to 9 tissue sections per slide (Texas A&M Department of Veterinary Pathobiology) and later stained with hematoxylin and eosin using standard protocols (Meyerholz et al., 2009).
Morphometrics
The stained slides were digitized into .tiff files using PathScan Enabler IV (Meyer Instruments, Houston, TX). The highest quality cross-section from each animal was selected for morphological measurements. We used Image J (NIH.gov) software to measure distances and areas. When measuring short distances (e.g., thickness of tunica muscularis or mucosal thickness) we made four measurements (randomly selected from each of the four quadrants of the cross-section) and averaged them (n=16 measures for each value). For more complex distances and areas (e.g., basement membrane length or lumen area) the values were based on polygons usually exceeding 200 landmarks – an approach used in other studies (Meyerholz et al., 2010; Risse et al., 2012). Cross section was not calculated for the toads because the sections were not circular.
In order to adjust for small differences in individual body size we divided the mucosal area by the total cross-sectional area to create a unitless relativized mucosal length for the endotherms. We used a similar approach for the relativized mucosal length in the ectotherms by dividing the mucosa length by the serosa perimeter. We used serosa perimeter in the ectotherms because we were less confident that the apparent cross sectional area of the ectotherm sections accurately represented the caliber of the intestine. All values were examined using the same statistical approaches for the other metrics (see next section).
Statistics
After confirming normality with a Shapiro-Wilk test, and homogeneity of variance with Levene’s test, we used a parametric one-way analysis of variance (ANOVA) to compare measured variables within a species across the four time points. We corrected p-values using the Benjamini-Hochberg False Discovery Rate correction (Benjamini and Hochberg, 1995), and a critical α of 0.05 to determine significance. In cases where the ANOVAs were significant (Bold values in Table 3 and 4) we ran Holm-Sidak post hoc tests comparing measurements at each fasting treatment (early, mid, late) with the control (nourished) values. ANOVAs were executed using JMP 12.0, while Holm-Sidak post hoc tests were executed using SigmaPlot 12.
Table 3.
Measurements of the distal intestine in fasting endotherms. Values are means followed by standard deviations in parentheses. Mucosa areas are presented in absolute terms (i.e. mm2) or in a unitless value (denoted by ‘–‘) that adjusts for body size by dividing the mucosa area by the cross sectional area. P-values were corrected using the Benjamini-Hochberg False Discovery Rate correction. When correlating large intestinal measurements with microbial diversity, fasting state and interaction terms were included in the model, though these were not significant in any models. Here, we only present the P-values for correlation of anatomical measurements with microbial diversity.
| Fasting day | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mouse | metric | units | 0 | 1 | 2 | 3 | Effect of Fasting P-value | Correlation with Microbial Diversity P-value |
| Cross section | area | mm2 | 6.45 (4.86) | 9.14 (4.48) | 5.43 (1.64) | 4.39 (0.45) | 0.32 | 0.87 |
| Serosa | perimeter | mm | 8.47 (2.80) | 10.58 (2.56) | 8.58 (1.47) | 7.23 (0.35) | 0.32 | 0.87 |
| Tunica muscularis | thickness | mm | 0.161 (0.052) | 0.12 (0.032) | 0.143(0.072) | 0.134 (0.032) | 0.69 | 0.87 |
| Basement | perimeter | mm | 13.698 (4.472) | 17.668 (3.829) | 15.38 (5.618) | 11 (4.037) | 0.32 | 0.87 |
| Lumen | perimeter | mm | 13.367 (4.855) | 17.365 (3.28) | 15.668 (5.485) | 11.16 (5.084) | 0.32 | 0.87 |
| Lumen | area | mm2 | 1.93 (1.29) | 3.83 (1.60) | 1.26 (0.56) | 0.88 (0.21) | 0.47 | 0.91 |
| Mucosa | thickness | mm | 0.24 (0.072) | 0.212 (0.045) | 0.234 (0.085) | 0.266 (0.052) | 0.70 | 0.87 |
| Mucosa | area | mm2 | 2.759 (0.725) | 3.528 (0.856) | 2.737 (0.533) | 2.258 (0.354) | 0.30 | 0.87 |
| Mucosa | area | – | 0.511 (.136) | 0.447 (.177) | 0.520 (0.090) | 0.519 (.097) | 0.74 | 0.87 |
|
| ||||||||
| Fasting day | ||||||||
| Quail | metric | units | 0 | 2 | 4 | 7 | Effect of Fasting P-value | Correlation with Microbial Diversity P-value |
|
| ||||||||
| Cross section | area | mm2 | 6.020 (1.269) | 6.638 (0.649) | 6.329 (2.989) | 6.650 (1.736) | 0.95 | 0.95 |
| Serosa | perimeter | mm | 8.520 (0.801) | 8.601 (0.440) | 8.270 (1.753) | 8.822 (1.017) | 0.95 | 0.95 |
| Tunica muscularis | thickness | mm | 0.283 (0.056) | 0.29 (0.061) | 0.254 (0.059) | 0.235 (0.031) | 0.71 | 0.95 |
| Basement | perimeter | mm | 8.518 (1.284) | 8.801 (0.536) | 8.987 (2.073) | 8.609 (1.103) | 0.95 | 0.95 |
| Lumen | perimeter | mm | 5.346 (0.941) | 5 (0.855) | 5.977 (2.551) | 5.961 (1.637) | 0.95 | 0.95 |
| Lumen | area | mm2 | 0.820 (0.402) | 0.989 (0.402) | 1.391 (1.950) | 1.989 (1.593) | 0.80 | 0.95 |
| Mucosa | thickness | mm | 0.384 (0.040) | 0.403 (0.08) | 0.359 (0.086) | 0.316 (0.043) | 0.71 | 0.95 |
| Mucosa | area | mm2 | 2.626 (0.534) | 2.786 (0.210) | 2.534 (0.655) | 2.346 (0.502) | 0.80 | 0.95 |
| Mucosa | area | – | 0.438 (0.032) | 0.421 (0.035) | 0.435 (0.104) | 0.365 (0.066) | 0.71 | 0.95 |
Table 4.
Measurements of the distal intestine in fasting ectotherms. Values are means followed by standard deviations in parentheses. The lengths of the mucosa lengths are presented in absolute terms (i.e., mm) or in a unitless value (denoted by ‘–‘) that adjusts for body size by dividing the mucosa length by the serosa perimeter. P-values were corrected using the Benjamini-Hochberg False Discovery Rate correction. Unique subscripts indicate significant differences between fasting day 0 and other time points according to Holm-Sidak pairwise tests. When correlating large intestinal measurements with microbial diversity, fasting state and interaction terms were included in the model, though these were not significant in any models. Here, we only present the P-values for correlation of anatomical measurements with microbial diversity.
| Fasting day | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tilapia | metric | units | 0 | 7 | 14 | 21 | Effect of Fasting P-value | Correlation with Microbial Diversity P-value |
| Cross section | area | mm2 | 2.912 (1.168)a | 4.411 (2.482)a | 1.883 (0.891)a | 2.063 (0.771)a | 0.025 | 0.71 |
| Serosa | perimeter | Mm | 6.31 (1.14)a | 7.51 (1.09)a | 5.04 (1.37)a | 5.26 (0.89)a | 0.025 | 0.71 |
| Tunica muscularis | thickness | Mm | 0.030 (0.007)a | 0.037 (0.009)a | 0.031 (0.010)a | 0.051 (0.011)b | 0.019 | 0.71 |
| Submucosa | thickness | Mm | 0.039 (0.014)a | 0.055 (0.009)b | 0.044 (0.007)a | 0.049 (0.007)a | 0.028 | 0.71 |
| Mucosa | length | mm | 11.49 (2.51)a | 9.22 (2.69)a | 7.34 (2.03)b | 9.33 (1.45)a | 0.025 | 0.79 |
| Mucosa | length | – | 1.87 (0.50)a | 1.25 (0.25)b | 1.49 (0.41)a | 1.79 (0.24)a | 0.025 | 0.79 |
| Fasting day | ||||||||
| Toad | metric | units | 0 | 7 | 14 | 21 | Effect of Fasting P-value | Correlation with Microbial Diversity P-value |
|
| ||||||||
| Serosa | perimeter | mm | 11.0 (4.4)a | 17.2 (4.8)b | 12.9 (3.0)a | 15.9 (2.0)b | 0.044 | 0.87 |
| Tunica muscularis | thickness | mm | 0.086 (0.034) | 0.113 (0.057) | 0.143 (0.054) | 0.118 (0.044) | 0.26 | 0.82 |
| Submucosa | thickness | mm | 0.12 (0.04)a | 0.26 (0.11)b | 0.20 (0.10)a | 0.17 (0.04)a | 0.014 | 0.82 |
| Mucosa | length | mm | 19.548 (10.051) | 24.830 (13.149) | 15.621 (2.875) | 17.024 (2.557) | 0.26 | 0.87 |
| Mucosa | length | – | 1.73 (0.37)a | 1.41 (0.40)a | 1.25 (0.29)b | 1.08 (0.11)b | 0.014 | 0.82 |
| Fasting day | ||||||||
| Gecko | metric | units | 0 | 7 | 14 | 28 | Effect of Fasting P-value | Correlation with Microbial Diversity P-value |
|
| ||||||||
| Cross section | area | mm2 | 12.7 (5.9) | 14.1 (1.91) | 16.0 (4.9) | 13.2 (6.7) | 0.86 | 0.97 |
| Serosa | perimeter | mm | 13.0 (3.2) | 14.2 (0.2) | 14.4 (2.2) | 13.1 (3.3) | 0.86 | 0.97 |
| Tunica muscularis | thickness | mm | 0.17 (0.05) | 0.13 (0.03) | 0.18 (0.06) | 0.21 (0.06) | 0.84 | 0.97 |
| Submucosa | thickness | mm | 0.175 (0.119) | 0.148 (0.027) | 0.103 (0.037) | 0.140 (0.068) | 0.86 | 0.97 |
| Mucosa | length | mm | 16.8 (6.9) | 16.4 (1.7) | 15.3 (2.5) | 18.1 (3.0) | 0.86 | 0.97 |
| Mucosa | length | – | 1.27 (0.07) | 1.16 (0.11) | 1.07 (0.14) | 1.44 (0.37) | 0.84 | 0.97 |
Additionally, we were interested whether changes in gut morphology might correlate with previously documented changes in luminal microbial diversity. For each species, we tested for interactions between fasting state, anatomical measurements, and measurements of Faith’s phylogenetic diversity, and relative abundances of microbial phyla, as determined by Kohl et al. (2014). Faith’s phylogenetic diversity is determined using a phylogenetic tree of all microbial members, and is calculated as the cumulative branch lengths from a random sampling of many microbial members from a particular sample (Faith, 1992). For each species, we used the Response Screening function in JMP 12.0 to test for correlations between anatomical measurements and either Faith’s phylogenetic diversity or relative abundances of microbial phyla while incorporating fasting state as an additional independent variable. Resulting P-values were corrected using the Benjamini-Hochberg False Discovery Rate correction (Benjamini and Hochberg, 1995), and a critical α of 0.05 to determine significance.
3. Results
None of the measured variables in the distal intestine of the endotherms (i.e., mouse or quail) changed in response to prolonged fasting (Table 3). Because the duration of fasting of the mice and quail was sufficient to elicit ~25% reductions in body mass (McCue et al., 2013; McCue and Pollock, 2013) any fasting-induced morphological changes in the distal intestines should have occurred within the observation periods.
The only significant fasting-induced changes that we observed in the distal intestines of the ectotherms occurred in the tilapia and the toads (Table 4). In tilapia, all six measured variables differed significantly among the fasting time points. The perimeter of the serosa significantly differed (ANOVA, df=3, F=4.09, FDR-corrected p=0.025), however post hoc comparisons did not identify any particular time points where the fasted values differed from the initial values. The thickness of the tunica muscularis differed across treatments (ANOVA, df=3, F=6.18, FDR-corrected p=0.019). Post hoc comparisons revealed that the tunica muscularis was significantly thicker at day 21 (Holm-Sidak0–21; t=3.759, p<0.001), and this represents a 70% increase in thickness in comparison to day 0. The tunica muscularis is composed of an outer, longitudinal layer of muscle and an inner, circular muscle with fibers that run perpendicular to the longitudinal layer. We reexamined the slides of the tilapia from day 0 and day 21 and concluded that this difference appears to be driven by a relative enlargement of the inner, circular muscle layer (Fig. 1). Interestingly, each of the ectothermic taxa increased the thickness of the tunica muscularis by the end of the experiment (toad by 37%, gecko by 25%), but it was only a significant increase in tilapia (Table 4).
Figure 1.
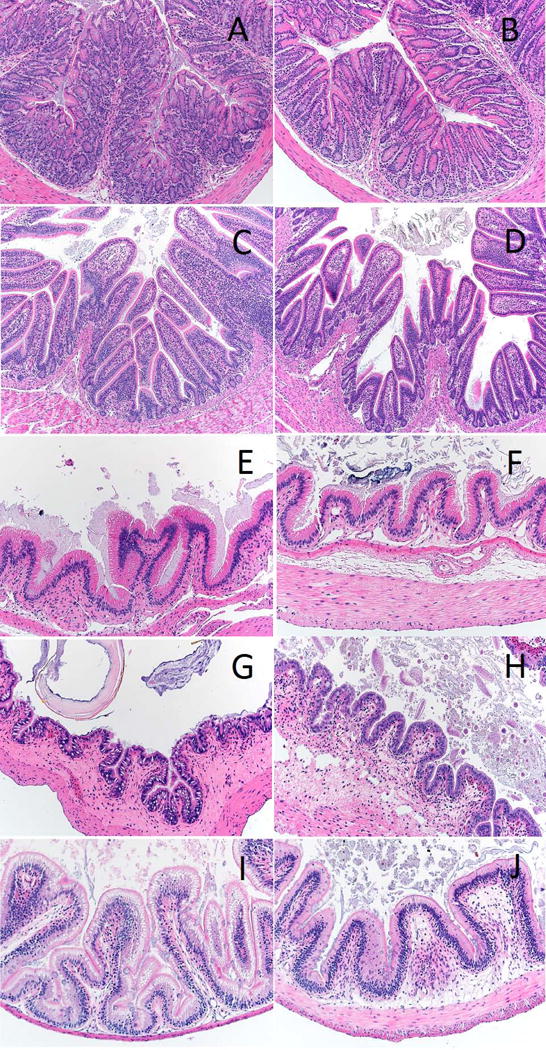
Representative light micrographs of distal intestine cross sections of each taxon at the beginning and end of the fasting trials, respectively. (A-B, mouse; C-D, quail; E-F, gecko; G-H, toad; I-J, tilapia)
The total cross-sectional areas of the tilapia distal intestines differed across time in comparison to the time 0 sections (ANOVA, df=3, F=3.96, FDR-corrected p=0.025), but post hoc tests did not show that the values on day 0 significantly differed from any of those at other fasting time points. The mean thickness of the submucosa of the fish also significantly differed among the fasting treatments (ANOVA, df=3, F=3.63, FDR-corrected p=0.028). Comparisons among the time points revealed the submucosa was significantly thicker on day 7 (Holm-Sidak0–7; t=3.150, p=0.004), but not at the later time points. Both the actual length of the mucosa (ANOVA, df=3, F=4.00, FDR-corrected p=0.025) and the relativized mucosa lengths (ANOVA, df=3, F=4.09, FDR-corrected p=0.025) differed among the fasting treatments. Post hoc comparisons revealed that the mucosa length significantly decreased by day 14 (Holm-Sidak0–14; t=3.458, p=0.002), but that the relativized mucosa length was decreased only at day 7 (Holm-Sidak0–7; t=3.148, p=0.005).
Three of the five variables in the toads exhibited significant differences among the fasting treatments (Table 4). Like the tilapia, the perimeter of the serosa differed (ANOVA, df=3, F=3.72, FDR-corrected p=0.044) and values were significantly greater on day 7 (Holm-Sidak0–7; t=2.983, p=0.007) and day 21 (Holm-Sidak0–21; t=2.449, p=0.023). The submucosa thickness also differed (ANOVA, df=3, F=5.53, FDR-corrected p=0.014) and was significantly greater at day 7 (Holm-Sidak0–7; t=3.221, p=0.004).
The toads exhibited shorter relative mucosal lengths as a result of fasting. In particular, these lengths were shorter at day 14 (Holm-Sidak0–14; t=2.810, p=0.010) and day 21 (Holm-Sidak0–21; t=3.693, p<0.001) of fasting. This trend was not apparent in the absolute mucosal lengths likely because of variation in the body sizes of the toads. Of the species we studied the toads had the largest variation in body size (~2-fold range), and so the index values (see Materials and Methods) provide a better proxy for variation caused by fasting. We reexamined the slides of the toads from day 0 and day 21 of fasting and it appears that the basement membrane had contracted or otherwise become less convoluted during fasting. The mucosa thickness also appeared to be reduced at day 21 (Fig. 1).
Last, we investigated whether anatomical changes in the distal intestine correlated with previously documented changes in microbial diversity (Kohl et al., 2014). We did not detect any significant correlations in any host species and or any morphological measurement with measurements of Faith’s phylogenetic diversity (Tables 3 and 4). Plots of microbial phylogenetic diversity as a function of mucosal area (endotherms) or mucosal length (ectotherms) can be seen (Fig. 2). To test whether the abundances of specific bacterial phyla (e.g., Bacteroidetes, Proteobacteria, and Fusbacteria; Table 1) may have varied with mucosal area or length, we also generated plots to test those relationships (Fig. 3). No significant relationships among the abundance of specific bacterial phyla and mucosal area or length were detected.
Figure 2.
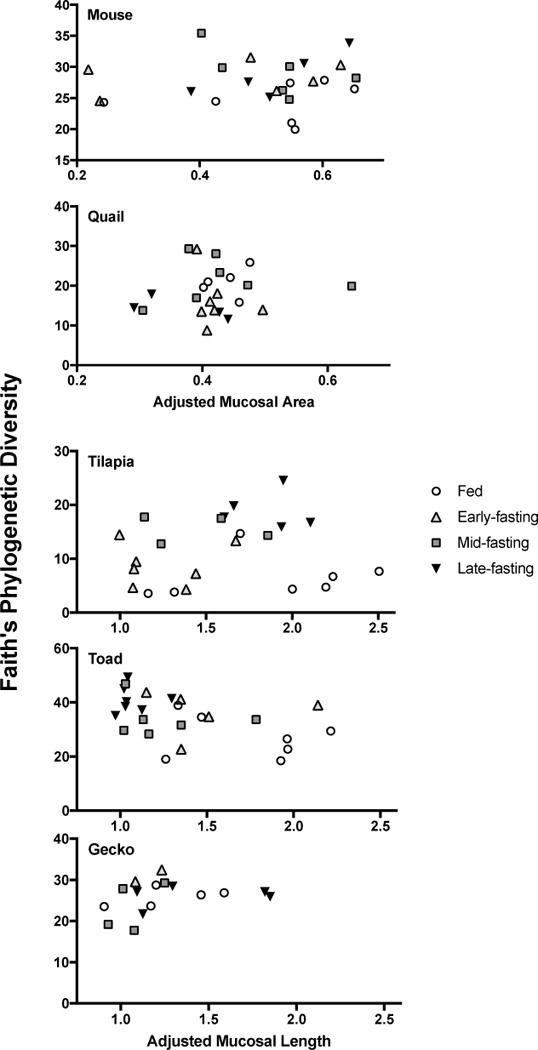
Relationships between Faith’s phylogenetic diversity and either adjusted mucosal area (for endotherms) or adjusted mucosal length (for ectotherms). No relationships were statistically significant.
Figure 3.
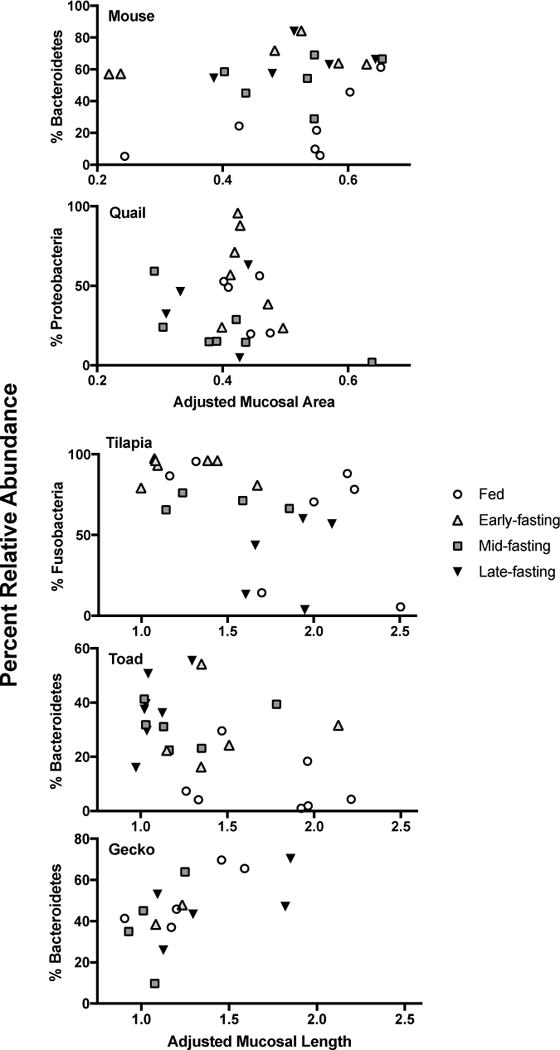
Relationships between the relative abundances of dominant microbial phyla and either adjusted mucosal area (for endotherms) or adjusted mucosal length (for ectotherms). No relationships of any microbial phyla were statistically significant.
4. Discussion
In this study we asked two questions: 1) How the distal intestine of disparate endothermic and ectothermic species respond to fasting, and, despite likely mechanistic differences, is there a common, generalizable response (e.g., reduction in surface area)? And, 2) How are fasting-induced structural changes of the distal intestine correlated with previously documented changes in enteric microbial diversity within this gut region? The answers to the first question are “variable responses”, and “no, there is not a common response”. We observed no significant changes in the endothermic mouse and quail. Among the ectotherms the results were mixed with changes in the tilapia and the toads but no changes in the geckos. Thus, there is no common, generalizable response of the distal intestine to fasting. Several studies in fishes have observed changes in the distal intestine structure following extended periods of fasting. For instance, after ≥ six months of fasting, (Gas and Noailliac-Depeyre, 1976) and (German et al., 2010) showed decreased mucosal surface area in the distal intestines of Cyprinus carpio and Pterygoplichthys disjunctivus, respectively, whereas (Baeverfjord and Krogdahl, 1996) observed decreased surface area in salmon distal intestine after 21 days of fasting. The mucosal surface area reduction is more pronounced after six months (Gas and Noailliac-Depeyre, 1976; German et al., 2010), as would be expected, but the differences in 21 days (Baeverfjord and Krogdahl, 1996) are still measurable, and are similar to what we observed in tilapia in this study.
Difficult to explain is the apparent increase in the thickness of the tunica muscularis in the ectothermic organisms. Although this could be an artifact of decreased overall gut length in response to fasting (Weaver et al., 1991) making the muscle appear proportionally larger, correlations of muscularis thickness and serosal circumference are not strong (R2 = 0.0117), suggesting this is not the case (we would expect a large negative correlation if this were true). Alternatively, muscle thickness may be maintained or even show a trophic increase in response to fasting in the ectothermic taxa [e.g., (do Nascimento et al. 2016)]. For example, it is clear that each of the specimens still had material in their distal intestine at the end of the food deprivation (Fig. 1), and fecal material was noted at time of dissection. This is consistent with an animal with low-intake (i.e., in a fasted state). For example, digestion in animals tends to operate in the rate vs yield continuum (Sibly, 1981; Hume, 2005; German et al., 2015). On one end, a rate maximizer has high-intake, rapid movement of digesta through the gastrointestinal tract, and little reliance on microbial digestion in the distal intestine. Rate maximizers tend to digest more of the soluble components of their ingested food, and have lower digestibilities overall, and especially of more fibrous material. On the other end, a yield maximizer has lower-intake, slower movement of digesta through the alimentary canal, and more of a reliance on microbial digestion in the distal intestine to result in higher overall digestibilities, especially of fibrous material (Sibly, 1981; Hume, 2005; German et al., 2015).
Some animals [e.g., rate-maximizing loricariid catfishes (German and Bittong, 2009); yield-maximizing ruminants (Hofmann, 1989)] can largely exist near extreme ends of the spectrum, whereas many animals likely switch back and forth depending on intake (e.g., (Klumpp and Nichols, 1983; Connor et al., 2016) because the presence of new ingesta in the stomach is a promoter of gut motility (Olsson and Holmgren, 2001). Because the animals in this study were deprived of food, we forced them into a yield maximizing situation (i.e., no intake means no new ingesta promoting more rapid gut motility). With reduced extraction rates, each of the animals, therefore, maintained material in their distal intestines to obtain as many nutrients as possible from what remained. This could include microbial metabolism of any nutrients (carbohydrates, proteins, lipids) in addition to fibrous components of ingesta remaining in the distal intestine. Not all distal intestine fermentation is of carbohydrates (Bergman, 1990; Clements et al., 2016). This microbial activity would require muscular contractions (e.g., segmental contractions; (Olsson and Holmgren, 2001)) of the distal intestine to mix the digesta, especially to allow contact of potentially fermentable substrates with microbial symbionts (Stevens and Hume, 1995). Indeed, all of the ectothermic animals apparently increased the thickness of the tunica muscularis, albeit only significantly so in tilapia. Interestingly, (do Nascimento et al., 2016) also observed a thickening of proximal intestine muscularis thickness in juvenile tegus (Tupinambis merianae), which they attributed to a potential anticipatory response for refeeding.
Tilapia are known to have active microbial fermentation and absorption of short chain fatty acids in their distal intestine (Titus and Ahearn, 1988, 1991), and intestinal microbes secrete more essential amino acids when tilapia are fed low-protein diets (Newsome et al., 2011). Hence, the alleged thickening of the muscle may be a trophic increase in response to a yield maximizing strategy imposed by fasting. Another possibility is that the thickening of the muscle could represent a pathology resulting from a change in the expression of specific genes (e.g., malfunctioning Cystic Fibrosis Transmembrane-conductance Regulator (CFTR) leads to muscle thickening in the intestine of pigs; Meyerholz et al. 2010) or an immune response (Sekirov et al., 2010) to fasting. For instance, an absence of resources on which enteric microbes can metabolize leads them to forage on host mucus, which causes inflammatory responses in the gut, and can allow for pathogen invasion (Desai et al., 2016). Either way, why a thickening of the smooth muscle doesn’t also occur in the endothermic mouse and quail is unknown. (Okada et al., 2013) clearly showed that colonic cellular turnover explains the lack of changes in the mucosal thickness of mice: cell proliferation arrests during fasting, whereas elevated cell proliferation observed upon refeeding is matched by cellular shedding. The consistent mucosal thickness we observed in our mice matches this finding. Quail may also show similar alterations in cellular turnover, and thus, show little change in their distal intestine mucosal thickness.
With regards to changes in gut structure matching with changes in the microbiome, the answer is also no. Again, we predicted that decreases in microbial diversity would be matched by decreases in the size of the distal intestine, given that microbial communities (including those of the vertebrate gut) exhibit traditional species-area relationships (Bell et al., 2005; Godon et al., 2016). Tilapia and toad generally showed an increase in intestinal microbe diversity with fasting (Kohl et al., 2014), and here, we see different responses of the gut tissue to a lack of food (Table 4). For comparison, the Burmese python shows a decrease in distal intestine microbial diversity (Costello et al., 2010) with a decrease in distal intestine mass (Secor et al., 1994). The gecko showed no change in its enteric microbial diversity or distal intestine structure with fasting. The endothermic quail (decrease in microbiome diversity) and mouse (increase in intestinal microbial diversity) showed contrasting patterns (Kohl et al., 2014) with little change in their distal intestine structures. Thus, although we expected to see a decrease in microbial diversity match with a decrease in distal intestine structure [e.g., (Ott and Secor, 2007; Costello et al., 2010)], we did not observe such a pattern. We also did not observe any correlations between anatomical measurements and the relative abundances of microbial phyla, suggesting that changes in intestinal structure did not drive the community shifts observed in the studied taxa (Kohl et al., 2014). Though, it should be noted that our studies only conducted 16S rRNA sequencing, which only inventories the relative abundances of microbial taxa. It is likely that fasting alters absolute microbial abundance or microbial load, but additional techniques are required to determine these measurements (Stammler et al., 2016). Overall, changes in distal intestine microbial diversity and community structure as a result of fasting do not seem linked to structural changes in the distal intestine, and thus the notion of a ‘housing crisis’ is not supported. Rather, the changes in diversity may be driven by an ‘energy crisis’ as nutrients are no longer entering the system, and microbial metabolites likely play a key role in cellular proliferation of the distal intestine upon re-feeding (Okada et al., 2013). In vitro culture systems may be useful for testing the ‘energy crisis’ hypothesis in the future.
The next logical steps are to evaluate such changes over longer time scales, [sensu (Gas and Noailliac-Depeyre, 1976; German et al., 2010) (e.g., 6 months of food deprivation in fishes)], to observe whether starker changes in distal intestine morphology to prolonged fasting do indeed match with changes in microbiome diversity. Alternatively, there may be smaller-scale changes in host physiology that underlie these changes in diversity, such as alterations in host immune physiology (Fukatsu and Kudsk, 2011; Hodin et al., 2011) or mucus dynamics (Thompson and Applegate, 2006). This should then be followed by investigations of the potential roles played by microbes in the restructuring of distal intestine morphology (Scheppach, 1994; Sharma et al., 1995; Scheppach et al., 1997; Okada et al., 2013) in preparation for digestion after the resumption of feeding (Secor et al., 1994; Costello et al., 2010).
Acknowledgments
We are grateful for extensive feedback and suggestions from Donovan German and Kevin Kohl throughout the development of this manuscript. We thank Kelly Pruitt for mounting the slide sections. We thank James Amaya, Joey Sandoval, Susana Lara, Miranda Rodriguez, Evelyn Oliva, Agnelio Cardentey, Alice Yang, Marena Guzman, Brandon Ruiz, and Jasmine Brown for helping to raise the animals and harvest tissues.
Literature cited
- A. V. M. Association, editor. AVMA. The AVMA Guidelines for the Euthanasia of Animals. Schaumburg, IL: 2013. p. 102. [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factors that regulates fat storage. Proc Nat Acad Sci. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeverfjord G, Krogdahl A. Development and regression of soybean meal induced enteritus in Atlantic salmon, Salmo salar L., distal intestine: a comparison with the intestines of fasted fish. J Fish Dis. 1996;19:375–387. [Google Scholar]
- Bell T, Ager D, Song J-I, Newman JA, Thompson IP, Lilley AK, van der Gast CJ. Larger islands house more bacterial taxa. Science. 2005;308:1884. doi: 10.1126/science.1111318. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling for the false discovery rate: a proctical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57B:289–300. [Google Scholar]
- Bergman E. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Binner M, Kloas W, Hardewig I. Energy allocation in juvenile roach and burbot under different temperature and feeding regimes. Fish Physiol Biochem. 2008;34:103–116. doi: 10.1007/s10695-007-9151-8. [DOI] [PubMed] [Google Scholar]
- Bjorndal KA. Cellulose digestion and the volatile fatty acid production in the green turtle, Chelonia mydas. Comp Biochem Physiol. 1979;63A:127–133. [Google Scholar]
- Black EC. Combined effect of starvation and severe exercise on glycogen metabolism of rainbow trout, Salmogairdneri. J Fisheries Research Board of Canada. 1966;23:1461–1463. [Google Scholar]
- Brown RP, Griffin S. Lower selected body temperatures after food deprivation in the lizard Anolis carolinensis. J Thermal Biology. 2005;30:79–83. [Google Scholar]
- Bugaut M, Bentejac M. Biological effects of short-chain fatty acids in nonuminant mammals. Ann Rev Nutr. 1993;13:217–241. doi: 10.1146/annurev.nu.13.070193.001245. [DOI] [PubMed] [Google Scholar]
- Cant JP, McBride BW, Croom WJ. The regulation of intestinal metabolism and its impact on whole animal energetics. J Animal Sci. 1996;74:2541–2553. doi: 10.2527/1996.74102541x. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombauch J, Knights D, Gajer P, Ravel J, Fierer N, Gordon JI, Knight R. Moving pictures of the human microbiome. Genome Biology. 2011;12:R50. doi: 10.1186/gb-2011-12-5-r50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellini MA, Rea LD. The biochemistry of natural fasting at its limits. Experientia. 1992;48:575–582. doi: 10.1007/BF01920242. [DOI] [PubMed] [Google Scholar]
- Clements KD, Raubenheimer D. Feeding and nutrition. In: Evans DH, editor. The physiology of fishes In The Physiology of Fishes. Boca Raton, FL: CRC Press; 2006. pp. 47–82. (ed D H Evans) [Google Scholar]
- Clements KD, German DP, Piche J, Tribollet A, Choat JH. Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc. 2016 in press. [Google Scholar]
- Connor KM, Sung A, Garcia NS, Gracey AY, German DP. Modulation of digestive Physiol Biochem in Mytilus californianus in response to feeding level acclimation and microhabitat. Biology Open. 2016;5 doi: 10.1242/bio.019430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JT, Sutterlin AM, McNiven MA. Effect of food deprivation on oxygen consumption and body composition of growth-enhanced transgenic Atlantic salmon (Salmo salar) Aquaculture. 2000;188:47–63. [Google Scholar]
- Costello EK, Gordon JI, Secor SM, Knight R. Postprandial remodeling of the gut microbiota in Burmese pythons. ISME Journal. 2010;4:1375–1385. doi: 10.1038/ismej.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson RA, Hernandez T. Amino acid metabolism in chameleons. Comp Biochem Physiol. 1968;25:861–872. doi: 10.1016/0010-406x(68)90574-4. [DOI] [PubMed] [Google Scholar]
- Cramp RL, Franklin CE. Is re-feeding efficiency compromised by prolonged starvation during aestivation in the green striped burrowing frog, Cycloranaalboguttata? J Experimental Zoology. 2003;300A:126–132. doi: 10.1002/jez.a.10272. [DOI] [PubMed] [Google Scholar]
- Cramp RL, Franklin CE, Meyer EA. The impact of prolonged fasting during aestivation on the structure of the small intestine in the green-striped burrowing frog, Cyclorana alboguttata. Acta Zoologica. 2005;86:13–24. [Google Scholar]
- Crawford PA, Crowley JR, Sambandam N, Muegge BD, Costello EK, Hamady M, Knight R, Gordon JI. Regulation of myocardia ketone body metabolism by the gut microbiota during nutrient deprivation. Proc Nat Acad Sci. 2009;106:11276–11281. doi: 10.1073/pnas.0902366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croom WJ, Brake J, Coles BA, Havenstein GB, Christensen VL. Is intestinal absorption capacity rate-limiting for performance in poultry? J Appl Poultry Res. 1999;8:242–252. [Google Scholar]
- Csengeri I. Dietary effects on fatty acid metabolism of common carp. Archives of Animal Nutrition. 1996;49:73–92. doi: 10.1080/17450399609381866. [DOI] [PubMed] [Google Scholar]
- Czesny S, Richard J, Garcia Abiado MA, Dabowski K. The effect of fasting, prolonged swimming, and predator presence on energy utilization and stress in juvenile walleye (Stizostedion vitreum) Physiology and Behavior. 2003;79:597–603. doi: 10.1016/s0031-9384(03)00124-0. [DOI] [PubMed] [Google Scholar]
- Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Internat J Systematic Evol Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Nunez G, Martins EC. A dietary fiber-deprived gu microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167:1339–1353. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Nascimento LFR, da Silveira LC, Nisembaum LG, Colquhoun A, Abe AS, Mandarim-de-Lacerda CA, de Sousa SCR. Morphological and metabolic adjustments in the small intestine to energy demands of growth, storage, and fasting in the first Ann cycle of a hibernating lizard (Tupinambis merianae) Comp Biochem Physiol. 2016;195A:55–64. doi: 10.1016/j.cbpa.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Dunel-Erb S, Chevalier C, Laurent P, Bach A, Decrock F, Le Maho Y. Restoration of the jejunal mucosa in rats refed after prolonged fasting. Comp Biochem Physiol. 2001;129A:933–947. doi: 10.1016/s1095-6433(01)00360-9. [DOI] [PubMed] [Google Scholar]
- Dunlap KD. Hormonal and behavioral responses to food and water deprivation in a lizard (Sceloporus occidentalis): implications for assessing stress in a natural population. J Herpetology. 1995;29:345–351. [Google Scholar]
- Faith DP. Conservation evaluation and phylogenetic diversity. Biological Conservation. 1992;61:1–10. [Google Scholar]
- Falahatkar B. The metabolic effects of feeding and fasting in beluga Huso huso. Marine Environmental Research. 2012;82:69–75. doi: 10.1016/j.marenvres.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Foster GD, Moon TW. Hypometabolism with fasting in the yellow perch (Perca flavescens): a study of enzymes, hepatocyte metabolism, and tissue size. Physiol Zool. 1991;64:259–275. [Google Scholar]
- French SS, Johnston GIH, Moore MC. Immune activity suppresses reproduction in food-limited female tree lizards Urosaurus ornatus. Functional Ecology. 2007;21:1115–1122. [Google Scholar]
- Fukatsu K, Kudsk KA. Nutrition and gut immunity. Surg Clin North Am. 2011;91:755–770. doi: 10.1016/j.suc.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gas N, Noailliac-Depeyre J. Studies on intestinal epithelium involution during prolonged fasting. J Ultrastruct Res. 1976;56:137–151. doi: 10.1016/s0022-5320(76)80161-x. [DOI] [PubMed] [Google Scholar]
- Gaucher L, Vidal N, D’Anatro A, Naya DE. Digestive flexibility during fasting in the characid fish Hyphessobrycon luetkenii. J Morphol. 2012;273:49–56. doi: 10.1002/jmor.11005. [DOI] [PubMed] [Google Scholar]
- German DP, Bittong RA. Digestive enzyme activities and gastrointestinal fermentation in wood-eating catfishes. J Comp Physiol. 2009;179B:1025–1042. doi: 10.1007/s00360-009-0383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DP, Miles RD. Stable carbon and nitrogen incorporation in blood and fin tissue of the catfish Pterygoplichthys disjunctivus (Siluriformes, Loricariidae) Environ Biol Fishes. 2010;89:117–133. [Google Scholar]
- German DP, Sung A, Jhaveri PK, Agnihotri A. More than one way to be an herbivore: convergent evolution of herbivory using different digestive strategies in prickleback fishes (family Stichaeidae) Zoology. 2015;118:161–170. doi: 10.1016/j.zool.2014.12.002. [DOI] [PubMed] [Google Scholar]
- German DP, Neuberger DT, Callahan MN, Lizardo NR, Evans DH. Feast to famine: the effects of food quality and quantity on the gut structure and function of a detritivorous catfish (Teleosti: Loricariidae) Comp Biochem Physiol. 2010;155A:281–293. doi: 10.1016/j.cbpa.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Gilbert AL, Miles DB. Food, temperature and endurance: effects of food deprivation on the thermal sensitivity of physiological performance. Functional Ecology. 2016 in press. [Google Scholar]
- Gillis TE, Ballantyne JS. The effects of starvation on plasma free amino acid and glucose concentrations in lake sturgeon. J Fish Biol. 1996;49:1306–1316. [Google Scholar]
- Gist DH. The effects of starvation and refeeding on carbohydrate and lipid reserves of Anolis carolinensis. Comp Biochem Physiol. 1972;43A:771–780. [Google Scholar]
- Glass NR. The effect of time of food deprivation on the routine oxygen consumption of largemouth black bass (Micropterussalmonoides) Ecology. 1968;49:340–343. [Google Scholar]
- Godet R, Mattei X, Dupe-Godet M. Alterations of endocrine pancreas B cells in a Sahelian reptile (Varanus exanthematicus) during starvation. J Morphology. 1984;180:173–180. doi: 10.1002/jmor.1051800302. [DOI] [PubMed] [Google Scholar]
- Godon J-J, Arulazhahan P, Steyer J-P, Hamlin J. Vertebrate bacterial gut diversity: size also matters. BMC Ecology. 2016;16:12. doi: 10.1186/s12898-016-0071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossling J, Loesche WL, Nace GW. Response of intestinal flora of laboratory-reared leopard frogs (Rana pipiens) to cold and fasting. Appl Environ Microbiol. 1982a;44:67–71. doi: 10.1128/aem.44.1.67-71.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossling J, Loesche WL, Nace GW. Large intestine bacterial flora of nonhibernating and hibernating leopard frogs (Rana pipiens) Appl Environ Microbiol. 1982b;44:59–66. doi: 10.1128/aem.44.1.59-66.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habold C, Reichardt F, Foltzer-Jourdainne C, Lignot JH. Morphological changes of the rat intestinal lining in relation to body stores depletion during fasting and after refeeding. Pflugers, Arch Eur J Physiol. 2007;455:323–332. doi: 10.1007/s00424-007-0289-0. [DOI] [PubMed] [Google Scholar]
- Habold CH, Chevalier C, Dunel-Erb S, Foltzer-Jourdainne C, Le Maho Y, Lignot J-H. Effects of fasting and refeeding on jejunal morphology and cellular activity in rats in relation to depletion of body stores. Scand J Gastroenterol. 2004;39:531–539. doi: 10.1080/00365520410004514. [DOI] [PubMed] [Google Scholar]
- Hervant F, Meathieu J, Durand J. Behavioural, physiological and metabolic responses to long-term starvation and refeeding in a blind cave-dwelling (Proteus anguinus) and a surface-dwelling (Euproctus asper) salamander. J Experimental Biology. 2001;204:269–281. doi: 10.1242/jeb.204.2.269. [DOI] [PubMed] [Google Scholar]
- Hodin CM, Lenaerts K, Grootjans J, de Haan JJ, Hadfoune M, Verheyen FK, Kiyama H, Heineman E, Burrman WA. Starvation compromises paneth cells. Am J Pathol. 2011;179:2285–2893. doi: 10.1016/j.ajpath.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann R. Evolutionary steps of ecophysiological adaptation and diversifi-cation of ruminants – a comparative view of their digestive system. Oecologica. 1989;78:443–457. doi: 10.1007/BF00378733. [DOI] [PubMed] [Google Scholar]
- Horn MH, Gawlicka A, German DP, Logothetis EA, Cavanagh JW, Boyle KS. Structure and function of the stomachless digestive system in three related species of New World silverside fishes (Atherinopsidae) representing herbivory, omnivory, and carnivory. Marine Biology. 2006;149:1237–1245. [Google Scholar]
- Hume ID. Concepts of digestive efficiency. In: Starck J, Wang T, editors. Physiological and Ecological Adaptations to Feeding in Vertebrates. Enfield, NH: Science Publishers; 2005. [Google Scholar]
- Hume ID, Biebach H. Digestive tract function in the long-distance migratory garden warbler, Sylvia borin. J Comp Physiol. 1996;166b:388–395. [Google Scholar]
- Hur JW, Jo JH, Park I. Effects of long-term starvation on hepatocyte ultrastructure of olive flounder Paralichthys olivaceus. Ichthyological Research. 2006;53:306–310. [Google Scholar]
- Jabbar A, Chang W-K, Dryden GW, McClave SA. Gut immunology and the differential response to feeding and starvation. Nutrition in Clinical Practice. 2003;18:461–482. doi: 10.1177/0115426503018006461. [DOI] [PubMed] [Google Scholar]
- Jena BS, Patnaik BK. Age-related responses of hepatic ATPases to starvation and cold stress in male garden lizards. Comp Biochem Physiol. 1995;111B:545–552. doi: 10.1159/000213210. [DOI] [PubMed] [Google Scholar]
- Jezierska B, Hazel JR, Gerking SD. Lipid mobilization during starvation in the rainbow trout, Salmo gairdneri Richardson, with attention to fatty acids. J Fish Biol. 1982;21:681–692. [Google Scholar]
- Johansson L, Kiessling A. Effects of starvation on rainbow trout. Acta Agric Scand. 1991;41:207–216. [Google Scholar]
- Johansson MEV, Hansson GC. Preservation of mucus in histological sections, immunostaining of mucins in fixed tissue, and localization of bacteria with FISH. In: McGuckin MA, Thornton DJ, editors. Mucins: Methods and Protocols. Vol. 842. Springer; 2012. pp. 229–235. [DOI] [PubMed] [Google Scholar]
- Jungries AM. The effects of long-term starvation and acclimation temperature on glucose regulation and nitrogen anabolism in the frog, Rana pipiens—II. Summer animals. Comp Biochem Physiol. 1970;32:433–444. [Google Scholar]
- Jungries AM, Hooper AB. The effects of long-term starvation and acclimation temperature on glucose regulation and nitrogen anabolism in the frog, Rana pipiens—I. Winter animals. Comp Biochem Physiol. 1970;32:417–432. [Google Scholar]
- Kamra SK. Effect of starvation and refeeding on some liver and blood constituents of Atlantic cod. J Fisheries Research Board of Canada. 1966;23:975–982. [Google Scholar]
- Karasov WH, Diamond J. Interplay between physiology and ecology in digestion. BioScience. 1988;38:602–611. [Google Scholar]
- Karasov WH, Douglas AE. Comparative digestive physiology. Comprehensive Physiology. 2013;3:741–783. doi: 10.1002/cphy.c110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasov WH, Pinshow B, Starck JM, Afik D. Anatomical and histological changes in the alimentary tract of migrating blackcaps (Sylvia atricapilla): a comparison among fed, fasted, food-restricted, and refed birds. Physiol Biochem Zool. 2004;77:149–160. doi: 10.1086/381465. [DOI] [PubMed] [Google Scholar]
- Khalil F, Yanni M. Studies on carbohydrates in reptiles I. glucose in body fluids of Uromastyx aegyptia. Zeitschrift fur Vergleichendi Physiologie. 1959;42:192–198. [Google Scholar]
- Kieffer JD, Tufts BL. Effects of food deprivation on white muscle energy reserves in rainbow trout (Oncorhynchus mykiss): the relationships with body size and temperature. Fish Physiol Biochem. 1998;19:239–245. [Google Scholar]
- Klumpp DW, Nichols PD. Nutrition of the southern sea garfish Hyporhamphus melanochir: gut passage rate and daily consumption of two food types and assimilation of seagrass components. Marine Ecol Prog Series. 1983;12:207–216. [Google Scholar]
- Kluytmans JHFM, Zandee DI. Lipid metabolism in the northern pike (Esox lucius L.)-I. the fatty acid composition of the northern pike. Comp Biochem Physiol. 1973;44B:451–458. doi: 10.1016/0305-0491(73)90018-7. [DOI] [PubMed] [Google Scholar]
- Kohl KD, Amaya JA, Passement CA, Dearing MD, McCue MD. Unique and shared responses of the gut microbiota to prolonged fasting: a comparative study across five classes of vertebrate hosts. FEMS Microbiology Ecology. 2014;90:883–894. doi: 10.1111/1574-6941.12442. [DOI] [PubMed] [Google Scholar]
- Krogdahl A, Bakke-McKellep AM. Fasting and refeeding cause rapid changes in intestinal tissue mass and digestive enzyme capacities of Atlantic salmon (Salmo salar L.) Comp Biochem Physiol. 2005;141A:450–460. doi: 10.1016/j.cbpb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Leggatt RA, Devlin RH, Farrell AP, Randall DJ. Oxygen uptake of growth hormone transgenic coho salmon during starvation and feeding. J Fish Biol. 2003;62:1053–1066. [Google Scholar]
- Lignot JH. Changes in form and function of the gastrointestinal tract during starvation: from pythons to rats. In: McCue MD, editor. Comparative Physiology of Fasting, Starvation, and Food Limitation. New York: Springer-Verlag; 2012. pp. 217–236. [Google Scholar]
- Lillywhite HB, Licht P, Chelgren P. The role of behavioral thermoregulation in the growth energetics of the toad, Bufo boreas. Ecology. 1973;54:375–383. [Google Scholar]
- Machado CR, Garofalo MAR, Roselino JES, Kettelhut IC, Migliorini RH. Effects of starvation, refeeding, and insulin on energy-linked metabolic processes in catfish (Rhamdia hilarii) adapted to a carbohydrate-rich diet. General and Comparative Endocrinology. 1988;71:429–437. doi: 10.1016/0016-6480(88)90272-9. [DOI] [PubMed] [Google Scholar]
- McCue MD. Fatty acid analyses may provide insight into the progression of starvation among squamate reptiles. Comp Biochem Physiol. 2008;151A:239–246. doi: 10.1016/j.cbpa.2008.06.034. [DOI] [PubMed] [Google Scholar]
- McCue MD. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp Biochem Physiol. 2010;156A:1–18. doi: 10.1016/j.cbpa.2010.01.002. [DOI] [PubMed] [Google Scholar]
- McCue MD. Horizons in starvation research. In: McCue MD, editor. Comparative Physiology of Fasting, Starvation, and Food Limitation. New York: SpringerVerlag; 2012. pp. 409–420. [Google Scholar]
- McCue MD, Pollock ED. Measurements of substrate oxidation using 13CO2 breath testing reveals shifts in fuel mix during prolonged fasting. J Comp Physiol. 2013;183B:1039–1052. doi: 10.1007/s00360-013-0774-z. [DOI] [PubMed] [Google Scholar]
- McCue MD, Amaya JA, Yang AS, Erhardt EB, Wolf BO, Hanson DT. Targeted 13C enrichment of lipid and protein pools in the body reveals circadian changes in oxidative fuel mixture during prolonged fasting: a case study using Japanese quail. Comp Biochem Physiol. 2013;166A:546–554. doi: 10.1016/j.cbpa.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Mehner T, Wieser W. Energetics and metabolic correlates of starvation in juvenile perch (Perca fluviatilis) J Fish Biol. 1994;45:325–333. [Google Scholar]
- Mendez G, Wieser W. Metabolic responses to food deprivation and refeeding in juveniles of Rutilus rutilusi (Teleostie: Cyprinidae) Environ Biology Fishes. 1993;36:73–81. [Google Scholar]
- Merkle S, Hanke W. Long-term starvation in Xenopus laevis Daudin-II. effects on several organs. Comp Biochem Physiol. 1988;90A:491–495. doi: 10.1016/0300-9629(88)90225-3. [DOI] [PubMed] [Google Scholar]
- Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in a RSV vaccine-enhanced inflammation model. Toxicol Pathol. 2009;37:249–255. doi: 10.1177/0192623308329342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischke M, Plosch T. More than just a gut instinct - the potential interplay between a baby’s nutrition, its gut microbiome, and the epigenome. Am J Physiol. 2013;304:R1065–R1069. doi: 10.1152/ajpregu.00551.2012. [DOI] [PubMed] [Google Scholar]
- Molony BW. Effects of feeding history on mobilisation and deposition of body constutuents and on growth in juvenile Ambassis vachelli (Pisces: Chandidae) Marine Biology. 1993;116:389–397. [Google Scholar]
- Nagai M, Ikeda S. Carbohydrate metabolism in fish-I. effects of starvation and dietary composition on the blood glucose level and the hepatopancreatic glycogen and lipid contents in carp. Bulletin of the Japanese Society of Scientific Fisheries. 1971;37:404–409. [Google Scholar]
- Navarro I, Gutierrez J. Fasting and starvation. In: Hochachka PW, Mommsen TP, editors. Biochemistry and molecular biology of fishes. Vol. 4. New York: Elsevier; 1995. pp. 393–434. [Google Scholar]
- Newsome SD, Fogel ML, Kelly LJ, Martinez del Rio C. Contributions of direct incorporation from diet and microbial amino acids to protein synthesis in Nile tilapia. Funct Ecol. 2011;25:1051–1062. [Google Scholar]
- Okada T, Fukuda S, Hase K, Nishiumi S, Izumi Y, Yoshida M, Hagiwara T, Kawashima R, Yamazaki M, Oshio T, Otsubo T, Inagaki-Ohara K, Kakimoto K, Higuchi K, Kawamura YI, Ohno H, Dohi T. Microbiota-derived lactate accelerates colon epithelial cell turnover in starvation-refed mice. Nature Communications. 2013;4:1654. doi: 10.1038/ncomms2668. [DOI] [PubMed] [Google Scholar]
- Olsson C, Holmgren S. The control of gut motility. Comp Biochem Physiol. 2001;128A:479–501. doi: 10.1016/s1095-6433(00)00330-5. [DOI] [PubMed] [Google Scholar]
- Ott BD, Secor SM. Adaptive regulation of digestive performance in the genus Python. J Exp Biol. 2007;210:340–356. doi: 10.1242/jeb.02626. [DOI] [PubMed] [Google Scholar]
- Pastoureaud A. Influence of starvation at low temperatures on utilization of energy reserves, appetite recovery and growth character in sea bass, Dicentrarchus labrax. Aquaculture. 1991;99:167–178. [Google Scholar]
- Paul AJ, Paul JM. Comparisons of whole body energy content of captive age zero Alaskan Pacific herring (Clupea pallasi Valenciennes) and cohorts over-wintering in nature. J Experimental Marine Biology and Ecology. 1998;226:76–86. [Google Scholar]
- Perez-Gonzalez M, Robinson JWL. The response of the toad intestine to prolonged starvation: a morphological, functional and metabolic study. Eur J Clin Invest. 1971;2:15–22. doi: 10.1111/j.1365-2362.1971.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Perez-Jimenez A, Guedes MJ, Morales AE, Oliva-Teles A. Metabolic responses to short starvation and refeeding in Dicentrarchus labrax. effect of dietary composition. Aquaculture. 2007;265:325–335. [Google Scholar]
- Phillips AM, Livingston DL, Dumas RF. Effect of starvation and feeding on the chemical composition of brook trout. The Progressive Fish-Culturist. 1960;22:147–154. [Google Scholar]
- Pinto JF, Nunes ML, Cardoso C. Feeding interruption and quality of cultured gilthead sea bream. Food Chemistry. 2007;100:1504–1510. [Google Scholar]
- Pontes RDQ, Cartaxo ACL, Jonas R. Concentrations of ketone bodies in the blood of the green lizard Ameiva ameiva (Teiidae) in different physiological situations. Comp Biochem Physiol. 1988;89A:309–311. doi: 10.1016/0300-9629(88)91030-4. [DOI] [PubMed] [Google Scholar]
- Price ER, Brun A, Caviedes-Vidal E, Karasov WH. Digestive adaptations of aerial lifestyles. Physiology. 2015;30:69–78. doi: 10.1152/physiol.00020.2014. [DOI] [PubMed] [Google Scholar]
- Pusey BJ. The effect of starvation on oxygen consumption and nitrogen excretion in Lepidogalaxias salamandroides (Mees) J Comp Physiol. 1986;156B:701–705. [Google Scholar]
- Risse PA, Kachmar L, Matusovsky OS, Novali M, Gil FR, Javeshghani S, Keary R, Haston CK, Michoud MC, Martin JG, Lauzon AM. Ileal smooth muscle dysfunction and remodeling in cystic fibrosis. Am J Physiol. 2012;303:G1–G8. doi: 10.1152/ajpgi.00356.2011. [DOI] [PubMed] [Google Scholar]
- Roediger WEW. The starved colon - diminished mucosal nutrition, diminished absorption, and colitis. Dis Col Rect October. 1990:858–862. doi: 10.1007/BF02051922. [DOI] [PubMed] [Google Scholar]
- Rowland NE. Food or fluid restriction in common laboratory animals: balancing welfare considerations with scientific inquiry. Comp Med. 2007;57:149–160. [PubMed] [Google Scholar]
- Rueda FM, Martinez FJ, Zamora S, Kentouri M, Divanach P. Effect of fasting and refeeding on growth and body composition of red porgy, Pagrus pagrus L. Aquaculture Research. 1998;29:447–452. [Google Scholar]
- Russell NR, Wootton RJ. Appetite and growth compensation in the European minnow, Phoxinus phoxinus (Cyprinidae), following short periods of food restriction. Environ Biology Fishes. 1992;34:277–285. [Google Scholar]
- Sakamoto S, Yone Y. Effect of starvation on hematological characteristics, and the contents of chemical components and activities of enzymes in blood serum of red sea bream. J the Faculty of Agriculture, Kyushu University. 1978;23:63–69. [Google Scholar]
- Savage DC. Gastrointestinal microflora in mammalian nutrition. Ann Rev Nutr. 1986;6:155–178. doi: 10.1146/annurev.nu.06.070186.001103. [DOI] [PubMed] [Google Scholar]
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994;35:S35–S38. doi: 10.1136/gut.35.1_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppach W, Muller JG, Boxberger F, Dusel G, Richter F, Bartram HP, Christi SU, Demfle CE, Kasper H. Histological changes in the colonic mucosa following irrigation with short-chain fatty acids. Eur J Gastroenterol Hepatol. 1997;9:163–168. doi: 10.1097/00042737-199702000-00010. [DOI] [PubMed] [Google Scholar]
- Secor SM. Regulation of digestive performance: a proposed adaptive response. Comp Biochem Physiol. 2001;128A:565–577. doi: 10.1016/s1095-6433(00)00325-1. [DOI] [PubMed] [Google Scholar]
- Secor SM, Diamond J. Evolution of regulatory responses to feeding in snakes. Physiol Biochem Zool. 2000;73:123–141. doi: 10.1086/316734. [DOI] [PubMed] [Google Scholar]
- Secor SM, Lignot JH. Morphological plasticity of vertebrate aestivation. Prog Mol Subcell Biol. 2010;49:183–208. doi: 10.1007/978-3-642-02421-4_9. [DOI] [PubMed] [Google Scholar]
- Secor SM, Carey C. Integrative physiology of fasting. Comprehensive Physiology. 2016;6:773–825. doi: 10.1002/cphy.c150013. [DOI] [PubMed] [Google Scholar]
- Secor SM, Stein ED, Diamond J. Rapid upregulation of snake intestine in response to feeding: a new model of intestinal adaptation. American J Physiology. 1994;266:G695–G705. doi: 10.1152/ajpgi.1994.266.4.G695. [DOI] [PubMed] [Google Scholar]
- Secor SM, Taylor JR, Grosell M. Selected regulation of gastrointestinal acid-base secretion and tissue metabolism for the diamondback water snake and Burmese python. J Exp Biol. 2012;215:185–196. doi: 10.1242/jeb.056218. [DOI] [PubMed] [Google Scholar]
- Segner H, Dolle A, Bohm R. Ketone body metabolism in the carp Cyprinus carpio: biochemical and 1H NMR spectroscopical analysis. Comp Biochem Physiol. 1997;116B:257–262. doi: 10.1016/s0305-0491(96)00213-1. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LM, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Sharma R, Schumacher U, Ronaasen V, Coates M. Rat intestinal mucosal responses to a microbial flora and different diets. Gut. 1995;36:209–214. doi: 10.1136/gut.36.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeno S, Kheyyali D, Takeda M. Metabolic adaptation to prolonged starvation in carp. Nippon Suisan Gakkaishi. 1990;56:35–41. [Google Scholar]
- Sibly RM. Strategies of digestion and defecation. In: Townsend CR, Calow P, editors. Physiological Ecology: an Evolutionary Approach to Resource Use. Boston: Blackwell Scientific; 1981. [Google Scholar]
- Smirnov A, Sklan D, Uni Z. Mucin dynamics in the chick small intestine are altered by starvation. J Nutr. 2004;134:736–742. doi: 10.1093/jn/134.4.736. [DOI] [PubMed] [Google Scholar]
- Soengas JL, Strong EF, Fuentes J, Veira JAR, Andres MD. Food deprivation and refeeding in Atlantic salmon, Salmo salar: effects on brain and liver carbohydrate and ketone bodies metabolism. Fish Physiol Biochem. 1996;15:491–511. doi: 10.1007/BF01874923. [DOI] [PubMed] [Google Scholar]
- Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- Sonoyama K, Fujiwara R, Takemura N, Ogasawara T, Watanabe J, Ito H, Morita T. Response of gut microbiota to fasting and hibernation in Syrian hamsters. Appl Environ Microbiol. 2009;75:6451–6456. doi: 10.1128/AEM.00692-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammler F, Glasner J, Hiergiest A, Holler E, Weber DC, Oefner PJ, Gessner A, Spang R. Adjusting microbiome profiles for differences in microlbial load by spike-in bacteria. Microbiome. 2016;4:28. doi: 10.1186/s40168-016-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck JM. Shaping up: how vertebrates adjust their digestive system to changing environmental conditions. Animal Biology. 2003;53:245–257. [Google Scholar]
- Starck JM. Structural flexibility of the digestive system of tetrapods: patterns and processes at the cellular and tissue level. In: Starck JM, Wang T, editors. Physiological and Ecological Adaptations to Feeding in Vertebrates. Enfield, NH: Science Publishers, Inc.; 2005. pp. 175–200. [Google Scholar]
- Starck JM, Beese K. Structural flexibility of the small intestine and liver of garter snakes in response to feeding and fasting. J Experimental Biology. 2002;205:1377–1388. doi: 10.1242/jeb.205.10.1377. [DOI] [PubMed] [Google Scholar]
- Stevens CE, Hume ID. Comparative Physiology of the Vertebrate Digestive System. New York: Cambridge University Press; 1995. [Google Scholar]
- Stirling HP. Effects of experimental feeding and starvation on the proximate composition of the European sea bass Dicentrarchus labrax. Marine Biology. 1976;34:85–91. [Google Scholar]
- Tandler A, Watanabe T, Satoh S, Fukusho K. The effect of food deprivation on the fatty acid and lipid profile of red seabream (Pagrus major) larvae. British J Nutrition. 1989;62:349–361. doi: 10.1079/bjn19890036. [DOI] [PubMed] [Google Scholar]
- Thompson KL, Applegate TJ. Feed withdrawal alters small-intestinal morphology and mucus of broilers. Poultry Sci. 2006;85:1535–1540. doi: 10.1093/ps/85.9.1535. [DOI] [PubMed] [Google Scholar]
- Tidwell JH, Webster CD, Clark JA. Effects of feeding, starvation, and refeeding on the fatty acid composition of channel catfish, Ictalurus punctatus, tissues. Comp Biochem Physiol. 1992;103A:365–368. [Google Scholar]
- Titus E, Ahearn GA. Short-chain fatty acid transport in the intestine of a herbivorous teleost. J Exp Biol. 1988;135:77–94. doi: 10.1242/jeb.135.1.77. [DOI] [PubMed] [Google Scholar]
- Titus E, Ahearn GA. Transintestinal acetate transport in a herbivorous teleost: anion exchange at the basolateral membrane. J Exp Biol. 1991;156:41–61. [Google Scholar]
- Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. Contemp Top Lab Anim Sci. 2000;39:9–17. [PubMed] [Google Scholar]
- Tripathi G, Verma P. Starvation-induced impairment of metabolism in a freshwater catfish. Zeitschrift fur Naturforshung. 2003;58C:446–451. doi: 10.1515/znc-2003-5-626. [DOI] [PubMed] [Google Scholar]
- Troyer K. Structure and function of the digestive tract of a herbivorous lizard Iguana iguana. Physiol Zool. 1984;57:1–8. [Google Scholar]
- Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk PLM, Staaks G, Hardewig I. The effect of fasting and refeeding on temperature preference, activity and growth of roach, Rutilus rutilus. Oecologia. 2002;130:496–504. doi: 10.1007/s00442-001-0830-3. [DOI] [PubMed] [Google Scholar]
- Wang T, Hung CCY, Randall DJ. The comparative physiology of food deprivation: from feast to famine. Ann Rev Physiol. 2006;68:223–251. doi: 10.1146/annurev.physiol.68.040104.105739. [DOI] [PubMed] [Google Scholar]
- Weaver LT, Austin S, Cole TJ. Small intestinal length: a factor essential for gut adaptation. Gut. 1991;32:1321–1323. doi: 10.1136/gut.32.11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser W, Krumschnabel G, Ojwang-Okwor JP. The energetics of starvation and growth after refeeding in juveniles of three cyprinid species. Environ Biology Fishes. 1992;33:63–71. [Google Scholar]
- Woo NYS, Murat JC. Studies on the biology of the red sea bream Chrysophrys major III. metabolic response to starvation in different salinities. Marine Biology. 1981;61:255–260. [Google Scholar]
- Wood CM, Walsh PJ, Kajimura M, McClelland GB, Chew SF. The influence of feeding and fasting on plasma metabolites in the dogfish shark (Squalus acanthias) Comp Biochem Physiol. 2009;155A:435–444. doi: 10.1016/j.cbpa.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Xia JH, Lin G, Fu GH, Wan ZY, Lee M, Wang L, Liu XJ, Yue GH. The intestinal microbiome of fish under starvation. BMC Genomics. 2014;15:266. doi: 10.1186/1471-2164-15-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi YH, Chang YJ. Physiological effects of seamustard supplement diet on the growth and body composition of young rockfish, Sebastes schlegeli. Bulletin of the Korean Fisheries Society. 1994;27:69–82. [Google Scholar]
- Zaldua N, Naya DE. Digestive flexibility during fasting in fish: a review. Comp Biochem Physiol. 2014;169A:7–14. doi: 10.1016/j.cbpa.2013.12.006. [DOI] [PubMed] [Google Scholar]
- Zeng L-Q, Li F-J, Li X-M, Cao Z-D, Fu S-J, Zhang Y-G. The effects of starvation on digestive tract function and structure in juvenile southern catfish (Silurus meridionalis Chen) Comp Biochem Physiol. 2012;162A:200–211. doi: 10.1016/j.cbpa.2012.02.022. [DOI] [PubMed] [Google Scholar]


