Abstract
Trigone meningiomas are considered a surgical challenge, as they tend to be considerably large and hypervascularized at the time of presentation. We experienced a case of a large and very hard trigone meningioma that was effectively treated using initial microsurgical feeder occlusion followed by surgery in stages. A 19-year-old woman who presented with loss of consciousness was referred to our hospital for surgical treatment of a brain tumor. Radiological findings were compatible with a left ventricular trigone meningioma extending laterally in proximity to the Sylvian fissure. At initial surgery using the transsylvian approach, main feeders originating from the anterior and lateral posterior choroidal arteries were occluded at the inferior horn; however, only a small section of the tumor could initially be removed because of its firmness. Over time, feeder occlusion resulted in tumor necrosis and a 20% decrease in its diameter; the mass effect was alleviated within 1 year. The residual meningioma was then totally excised in staged surgical procedures after resection became more feasible owing to ischemia-induced partial softening of the tumor. When a trigone meningioma is large and very hard, initial microsurgical feeder occlusion in the inferior horn can be a safe and effective option, and can lead to necrosis, volume decrease, and partial softening of the residual tumor to allow for its staged surgical excision.
Keywords: feeder occlusion, lateral ventricle, meningioma, tumor volume
Introduction
Intraventricular meningiomas are rare, and correspond to 0.5–5% of all intracranial meningiomas. The most common location is the trigone,1,2) and most are considerably large and hypervascularized before they produce symptoms owing to their fluid ventricular cavities and slow growth rates.2) Therefore, trigone meningiomas normally present a surgical challenge.3) Many surgical strategies have been reported for this type of lesion,1,2,4–7) but the optimal procedure appears to depend on each tumor’s individual characteristics. Herein, we report a case of a large and very hard trigone meningioma in which initial microsurgical occlusion of the main feeders was effective for achieving staged excision following gradual necrosis, volume decrease, and partial softening of the residual tumor.
Case Report
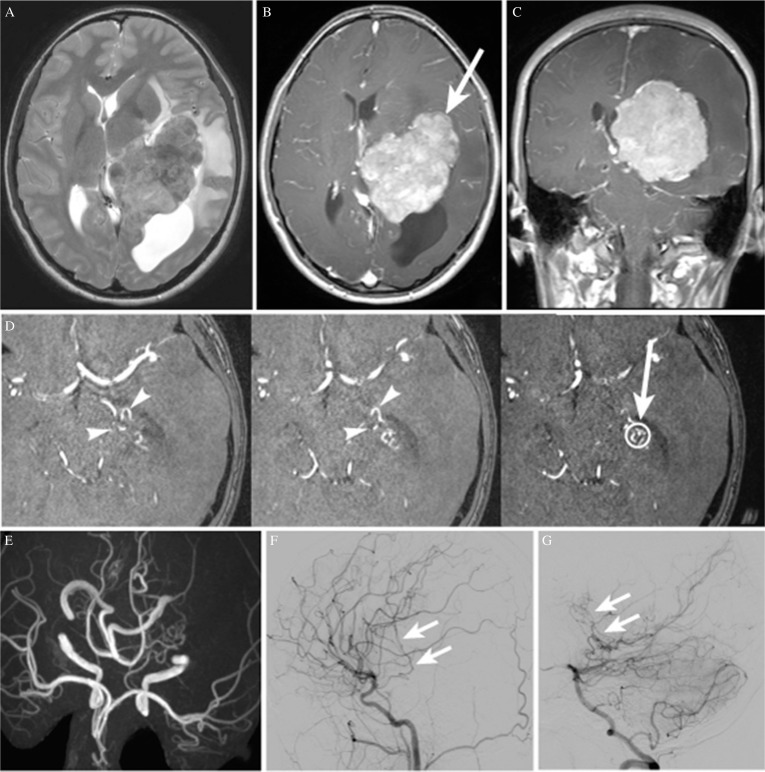
A 19-year-old, right-handed, previously healthy woman was referred to our hospital for surgical treatment of a large brain tumor originating from the left lateral ventricle. The tumor was found during examinations performed after she lost consciousness. On admission, she had no symptoms of increased intracranial pressure, and her neurological examination revealed no abnormal findings. The tumor was hyperdense on computed tomography (CT), indicating scattered calcification; the mass was hypointense on T1-weighted magnetic resonance images (MRI) and was iso- to hyperintense on T2-weighted images. There was marked peritumoral brain edema in the left temporal and parietal lobes associated with a midline shift to the right, and the posterior horn of the left lateral ventricle was entrapped (Fig. 1A). The tumor showed almost homogeneous enhancement with gadolinium (Figs. 1B and 1C). Raw magnetic resonance angiography (MRA) images showed the feeders ascending on the antero-inferior surface of the tumor (Fig. 1D). MRA images also showed that both the left anterior choroidal and left lateral posterior choroidal arteries were dilated (Fig. 1E). The radiological findings were consistent with a trigone meningioma extending laterally towards the Sylvian fissure. Cerebral angiograms revealed that the anterior quarter of the tumor was fed by the anterior choroidal artery, while the remaining three posterior quarters were fed by the lateral posterior choroidal artery (Figs. 1F and 1G).
Fig. 1.
(A) Axial T2-weighted magnetic resonance image (MRI) showing a large iso- to hyperintense tumor in the trigone of the left lateral ventricle, associated with peritumoral brain edema and a moderate midline shift to the right. The posterior horn of the lateral ventricle is entrapped. (B, C) Axial and coronal T1-weighted MRI with gadolinium, respectively, showing almost homogeneous tumor enhancement. The white arrow indicates the trajectory followed for tumor resection in the first and the second surgeries. (D) Raw magnetic resonance angiography (MRA) images showing feeders ascending to the antero-inferior surface of the tumor (arrowheads). The open circle indicates the target point for feeder occlusion, and the arrow indicates the trajectory followed to the target. (E) MRA image (axial view) showing the dilated left anterior choroidal and left lateral posterior choroidal arteries. (F) Left carotid angiogram (lateral view) showing feeders originating from the left anterior choroidal artery (arrow). (G) Left vertebral angiogram (lateral view) showing feeders originating from the left lateral posterior choroidal artery (arrow).
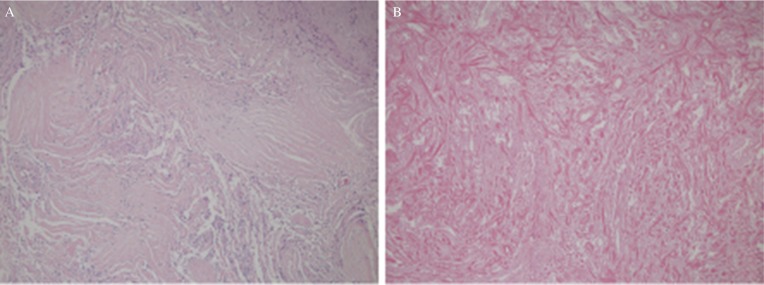
As endovascular feeder embolization was considered risky, we planned staged surgery consisting of initial microsurgical occlusion of the main feeders and removal of the lateral part of the tumor by the transsylvian approach, followed by medial part resection using the high parietal approach. Under general anesthesia, a left frontotemporal craniotomy was performed. The Sylvian fissure was exposed as widely as possible toward the proximal side. A cortical incision 2 cm in length was created on the anterior surface of the temporal lobe close to the middle cerebral artery (MCA) bifurcation, and a small trajectory into the inferior horn of the lateral ventricle was then followed with the aid of a neuronavigation system. Through the trajectory, the feeders originating from the anterior choroidal and lateral posterior choroidal arteries were identified on the antero-inferior surface of the tumor (Fig. 1D). After confirming that the transcranial motor-evoked potentials remained stable for 3 min after temporary occlusion of these feeders, they were coagulated. Next, the distal side of the Sylvian fissure was exposed and another 2 cm cortical incision was made on the insular cortex between the upper and the lower trunks of the MCA. At a depth of 1 cm, a white, bloodless and elastic hard tumor was exposed. As the tumor was too firm to allow internal decompression to be performed (even with a surgical ultrasonic aspirator) it was necessary to remove it in a piecemeal fashion by using microscissors. As this would have been time-consuming, we decided to wait for ischemia-induced tumor necrosis after removing a small anterolateral section of the tumor. The formalin-fixed tumor specimens showed mildly swollen and proliferating spindle-shaped tumor cells associated with abundant collagen fibers (Fig. 2A). The pathological diagnosis was Grade I transitional meningioma according to the World Health Organization guidelines. MRI with gadolinium acquired 3 days after the surgery showed enhancement only at the margin of the residual tumor, suggesting that tumor infarction was widely induced (Fig. 3A).
Fig. 2.
(A) Histologic features of the tumor observed at the first surgery, showing abundant collagen fibers and mildly swollen spindle-shaped tumor cells compatible with transitional meningioma (hematoxylin and eosin stain, ×100, original magnification). (B) Histologic features of the tumor observed at the second surgery, showing an ischemic scar consisting of extensive fibrosis, hyalinization, and few tumor cells (hematoxylin and eosin stain, ×100, original magnification).
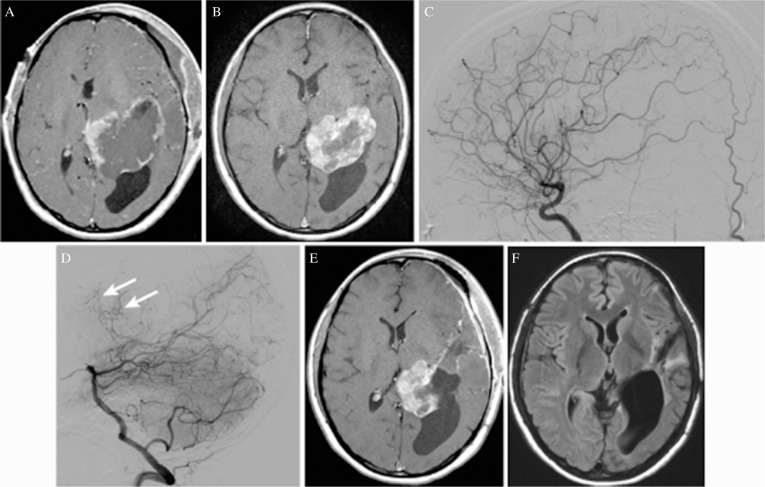
Fig. 3.
(A) Axial T1-weighted magnetic resonance image (MRI) with gadolinium acquired 3 days after the first surgery showing only marginal enhancement of the residual tumor, suggesting that tumor infarction has been induced widely. (B) Axial T1-weighted MRI with gadolinium acquired 1 year after the first surgery showing a remarkable decrease in the volume of the residual tumor (a 20% decrease in diameter) associated with resolution of the midline shift. An increased contrast enhancement area suggests revascularization of the residual tumor. (C) Left carotid angiogram (lateral view) just before the second surgery showing that feeders originating from the left anterior choroidal artery had been obstructed. (D) Left vertebral angiogram (lateral view) just before the second surgery showing remaining or newly developed small feeders originating from the left lateral posterior choroidal artery (arrow). (E) Axial T1-weighted MRI with gadolinium acquired 6 days after the second surgery, showing the remaining medial half of the tumor. (F) Axial fluid-attenuated inversion recovery image acquired 6 days after the third surgery performed by using the high parietal approach, showing complete resection of the tumor.
The second surgery was initially planned within six months, but the patient was admitted 13 months later owing to family obligations. MRI with gadolinium acquired 1 year after the first surgery showed a remarkable decrease in the volume of the residual tumor (i.e., a 20% decrease in its diameter) that was associated with resolution of the midline shift. The increased area of contrast enhancement suggested revascularization of the residual tumor (Fig. 3B). Cerebral angiograms taken just before the second surgery showed complete occlusion of feeders originating from the anterior choroidal artery and remaining or newly developed fine feeders originating from the lateral posterior choroidal artery. (Figs. 3C and 3D) As the tumor stain was weak, we thought that the residual tumor could be resected safely without additional feeder occlusion. At the second surgery, the lateral part of the tumor was resected without much difficulty by the same transsylvian approach because the residual tumor was softer than before. When the tumor close to the internal capsule was dissected, the motor evoked potential (MEP) decreased to 20% of the control, so further attempts at dissection were abandoned. The MEP returned to control levels at the time of wound closure, and the patient recovered from anesthesia with no neurological deficit. Blood loss as a result of the second surgery was 360 mL. MRI acquired 6 days after the second surgery showed that almost half of the tumor had been removed (Fig. 3E). Histological examination of the specimens in the second surgery showed extensive fibrosis and hyalinization without tumor cells, which were consistent with coagulation necrosis (Fig. 2B).
Five months later, the third surgery was performed using the high parietal approach. Follow up MRI images acquired between the second and the third surgery had shown no particular changes in enhancement pattern of the residual tumor. The intraparietal sulcus was carefully exposed and a cortical incision 3 cm in length was made at the bottom of the sulcus. The lateral ventricle was then penetrated and the residual tumor was totally removed in a piecemeal fashion with a total blood loss of 270 mL (Fig. 3F).
The patient developed a seizure disorder 10 months after the first surgery, which was controlled with an appropriate anticonvulsant. She had a right homonymous hemianopsia after the third surgery, which gradually improved to the level of the right homonymous inferior quadrantanopia. There has been no recurrence of the tumor during a follow-up period of 2 years. The patient provided informed consent for inclusion in this case report.
Discussion
Most trigone meningiomas grow quite large and are already hypervascularized by the time of diagnosis.2) Additionally, the calcification rates of intraventricular meningiomas are reported to be relatively higher than those of meningiomas of other locations.2) The present case describes our approach to excising a large and hard trigone meningioma. Simple microsurgical occlusion of the feeders in the inferior horn during the initial surgery changed the residual tumor to a bloodless white mass, resulting in its shrinkage and alleviating the mass effect. It also facilitated tumor resection during subsequent staged surgeries by partially softening the lesion via coagulation necrosis.
Preoperative endovascular feeder embolization is commonly used for meningiomas to reduce intra-operative blood loss and facilitate their rapid and complete resection.8,9) Embolization may also soften meningiomas.9–13) According to a review article by Shah et al.14), liquid embolic materials are preferably used because they penetrate deeper into the trunk and distal tumor vessels maximizing the effect of devascularization, while small particle agents are believed to carry a higher risk of postprocedural hemorrhage. However, it has been recognized that embolization of feeders originating from the choroidal arteries carries considerable risk of ischemic complications, and superselective embolization has been attempted only by some experts.4,15–17) In the present case, we thought that it would be possible to occlude most of feeders microsurgically with less risk than attempting endovascular feeder embolization.
In the present case, the transsylvian approach provided not only short and safe access to the laterally extended tumor, but also to the main feeders in the inferior horn of the lateral ventricle. As trigone meningiomas are supplied almost exclusively by feeders originating from the anterior and lateral posterior choroidal arteries, their simple microsurgical occlusion in the inferior horn may lead to sufficient devascularization of the tumor. To have accurate and safe access to relatively small feeders, it is necessary to incorporate radiological findings specific to each case and knowledge of microsurgical anatomy to a neuronavigation system assisted surgery. For this purpose raw images of MRA were useful to understand the course of feeders in relation to the tumor and ventricular structures. It is generally considered that branches of the anterior choroidal artery supplying eloquent territories arise from the cisternal segment.4,16,17) On the contrary, Fernandez-Miranda et al. have reported that 38% of the capsulothalamic artery originated from the first part of the plexal segment in a cadaveric study.18) To safely occlude feeding arteries originating from choroidal arteries, intraoperative MEP monitoring would be recommended even when such an attempt is carried out distal to the plexal point.
Many meningiomas are too rich in fibers to remove with an ultrasonic aspirator.3) The present case showed that microsurgical devascularization of the very firm meningioma led to an obvious decrease in volume during the follow-up period, and was accompanied by partial softening of the tumor. As for the optimal interval between feeder embolization and tumor resection, some recommend that surgery should be scheduled approximately 1 week after embolization because of greater softening effect of tumors,11,19) and others recommend tumors to be resected within 7 days after embolization to avoid revascularization of tumors.19,20) On the other hand, there are a few case reports which show long-term effects of feeder embolization for meningiomas. Koike et al. have reported a case in which feeder embolization resulted in the improvement of clinical symptoms and a decrease of the tumor volume as shown on CT 10 months later. In this case, tumor regrowth was detected 4 years after embolization.21) Terada et al. have reported a case of presumed intraventricular meningioma treated by feeder embolization followed by gamma knife radiosurgery. According to their report, the size of tumor continued to decrease for more than 8 months after embolization, although the tumor gradually became contrast enhancing again.22) In the present case, by the time of the second surgery performed 13 months after feeder occlusion, significant tumor shrinkage had been obtained, although revascularization of the tumor was suspected on MRI except for its core. If a patient’s neurological condition affords the time to wait, it may be reasonable to wait until substantial tumor shrinkage is obtained after feeder embolization in cases of large, firm and deep seated meningiomas.
The high parietal approach through the intraparietal sulcus is preferably used for trigone meningiomas especially when they are large and located in the dominant hemisphere because it has low risk of injury to the optic radiation and language cortex and fibers.1,2,7,23) Another useful approach for them is the interhemispheric parieto-occipital precuneus approach which also has a merit to avoid disturbance of the optic radiation and cortical functions. This approach is usually indicated for small or medium-size meningiomas with medial projection because of its narrow working angle and narrow surgical corridor.1,2,24) In the present case, the former approach was taken because we were more familiar with it. The visual field defect noted after the last surgery might be related to surgical manipulation near the left lateral geniculate body, although no apparent tissue damage was detected on post-operative MRI. Recommendation to improve radiological follow up plan in the present case was to take contrast enhanced MRI within 72 h after surgery to avoid any surgical trauma induced brain enhancement.25,26)
In conclusion, microsurgical occlusion of feeders originating from the choroidal arteries in the inferior horn of the lateral ventricle to treat large and hard trigone meningiomas is an effective option before performing staged surgery, and can lead to necrosis, volume decrease, and partial softening of the lesions.
Footnotes
Conflicts of Interest Disclosure
The authors have no personal, financial, or institutional interests in any of the drugs, materials, or devices presented in this article. All authors who are members of The Japan Neurosurgical Society (JNS) have completed the online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1).Zanini MA, Faleiros AT, Almeida CR, Clara CA, Gabarra RC: Trigone ventricular meningiomas: surgical approaches. Arq Neuropsiquiatr 69: 670–675, 2011 [DOI] [PubMed] [Google Scholar]
- 2).Ma J, Cheng L, Wang G, Lin S: Surgical management of meningioma of the trigone area of the lateral ventricle. World Neurosurg 82: 757–769, 2014 [DOI] [PubMed] [Google Scholar]
- 3).Schramm J: Meningiomas of the trigone. World Neurosurg 83: 486–487, 2015 [DOI] [PubMed] [Google Scholar]
- 4).Oyama H, Noda S, Negoro M, et al. : Giant meningioma fed by the anterior choroidal artery: successful removal following embolization—case report. Neurol Med Chir (Tokyo) 32: 839–841, 1992 [DOI] [PubMed] [Google Scholar]
- 5).Zhu W, Xie T, Zhang X, et al. : A solution to meningiomas at the trigone of the lateral ventricle using a contralateral transfalcine approach. World Neurosurg 80: 167–172, 2013 [DOI] [PubMed] [Google Scholar]
- 6).Oi S, Saito K, Ichikawa M, et al. : [Case of large sphenoid ridge meningioma treated by 2-stage surgery]. No Shinkei Geka 36: 885–890, 2008. (Japanese) [PubMed] [Google Scholar]
- 7).Nakamura M, Roser F, Bundschuh O, Vorkapic P, Samii M: Intraventricular meningiomas: a review of 16 cases with reference to the literature. Surg Neurol 59: 491–503; discussion 503–504, 2003 [DOI] [PubMed] [Google Scholar]
- 8).Yoon YS, Ahn JY, Chang JH, et al. : Pre-operative embolisation of internal carotid artery branches and pial vessels in hypervascular brain tumours. Acta Neurochir (Wien) 150: 447–452; discussion 452, 2008 [DOI] [PubMed] [Google Scholar]
- 9).Borg A, Ekanayake J, Mair R, et al. : Preoperative particle and glue embolization of meningiomas: indications, results, and lessons learned from 117 consecutive patients. Neurosurgery 73: ons244–ons251; discussion ons252, 2013 [DOI] [PubMed] [Google Scholar]
- 10).Chun JY, McDermott MW, Lamborn KR, Wilson CB, Higashida R, Berger MS: Delayed surgical resection reduces intraoperative blood loss for embolized meningiomas. Neurosurgery 50: 1231–1235; discussion 1235–1237, 2002 [DOI] [PubMed] [Google Scholar]
- 11).Kai Y, Hamada J, Morioka M, Yano S, Todaka T, Ushio Y: Appropriate interval between embolization and surgery in patients with meningioma. AJNR Am J Neuroradiol 23: 139–142, 2002 [PMC free article] [PubMed] [Google Scholar]
- 12).Nakase H, Ohnishi H, Matsuyama T, Morimoto T, Sakaki T: Two-stage skull base surgery for tumours extending to the sub- and epidural spaces. Acta Neurochir (Wien) 140: 891–898, 1998 [DOI] [PubMed] [Google Scholar]
- 13).Shah AH, Patel N, Raper DM, et al. : The role of preoperative embolization for intracranial meningiomas. J Neurosurg 119: 364–372, 2013 [DOI] [PubMed] [Google Scholar]
- 14).Shah A, Choudhri O, Jung H, Li G: Preoperative endovascular embolization of meningiomas: update on therapeutic options. Neurosurg Focus 38: E7, 2015 [DOI] [PubMed] [Google Scholar]
- 15).Fusco DJ, Spetzler RF: Surgical considerations for intraventricular meningiomas. World Neurosurg 83: 460–461, 2015 [DOI] [PubMed] [Google Scholar]
- 16).Jack AS, Lu JQ, Ashforth RA, Broad RW, Darsaut TE: Pre-operative embolization of an intraventricular meningioma using Onyx. Can J Neurol Sci 43: 206–209, 2016 [DOI] [PubMed] [Google Scholar]
- 17).Elkordy A, Endo H, Sato K, et al. : Embolization of the choroidal artery in the treatment of cerebral arteriovenous malformations. J Neurosurg 126: 1114–1122, 2017 [DOI] [PubMed] [Google Scholar]
- 18).Fernández-Miranda JC, de Oliveira E, Rubino PA, Wen HT, Rhoton AL: Microvascular anatomy of the medial temporal region: part 1: its application to arteriovenous malformation surgery. Neurosurgery 67: ons237–ons276; discussion ons276, 2010 [DOI] [PubMed] [Google Scholar]
- 19).Singla A, Deshaies EM, Melnyk V, et al. : Controversies in the role of preoperative embolization in meningioma management. Neurosurg Focus 35: E17, 2013 [DOI] [PubMed] [Google Scholar]
- 20).Probst EN, Grzyska U, Westphal M, Zeumer H: Preoperative embolization of intracranial meningiomas with a fibrin glue preparation. AJNR Am J Neuroradiol 20: 1695–1702, 1999 [PMC free article] [PubMed] [Google Scholar]
- 21).Koike T, Sasaki O, Tanaka R, Arai H: Long-term results in a case of meningioma treated by embolization alone—case report. Neurol Med Chir (Tokyo) 30: 173–177, 1990 [DOI] [PubMed] [Google Scholar]
- 22).Terada T, Yokote H, Tsuura M, et al. : Presumed intraventricular meningioma treated by embolisation and the gamma knife. Neuroradiology 41: 334–337, 1999 [DOI] [PubMed] [Google Scholar]
- 23).D’Angelo VA, Galarza M, Catapano D, Monte V, Bisceglia M, Carosi I: Lateral ventricle tumors: surgical strategies according to tumor origin and development—a series of 72 cases. Neurosurgery 56 (1 Suppl): 36–45, 2005 [DOI] [PubMed] [Google Scholar]
- 24).Faquini I, Fonseca RB, Vale de Melo SL, et al. : Trigone ventricular meningiomas: is it possible to achieve good results even in the absence of high tech tools?. Surg Neurol Int 6: 180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Albert FK, Forsting M, Sartor K, Adams HP, Kunze S: Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34: 45–60; discussion 60–61, 1994 [DOI] [PubMed] [Google Scholar]
- 26).Henegar MM, Moran CJ, Silbergeld DL: Early postoperative magnetic resonance imaging following nonneoplastic cortical resection. J Neurosurg 84: 174–179, 1996 [DOI] [PubMed] [Google Scholar]