Abstract
Objective
Piglet pre-weaning mortality is an important variable indicating the efficacy of farrowing management and animal well-being during lactation. The present study determined the association of newborn traits measured soon after birth with piglet pre-weaning mortality and growth.
Methods
In total, 805 piglets born from 57 multiparous sows were investigated. Their blood oxygen saturation, blood glucose and rectal temperature at 24 h after birth (RT24h) were monitored. Birth order, sex, skin color, integrity of the umbilical cord, attempts to stand and birth intervention were monitored. Piglets were weighed at day 0, 7, and 21 to evaluate average daily gain (ADG).
Results
Piglet pre-weaning mortality for lactation period was 12.6% and cumulative mortality during the first 7 days of age was 8.6%. A higher proportion of piglets with pale skin color died compared to piglets with normal skin color (26.7% vs 7.7%, p<0.001). A higher (p<0.001) proportion of piglets that attempted to stand after 5 min (38.5%) died compared to piglets that attempted to stand within 1 min (6.3%) after birth. Piglet body weight at birth (BWB), blood glucose and the number of piglets born alive (BA) were correlated with ADG (p<0.05). Piglets with BWB <1.30 kg had higher (p<0.001) mortality rate than piglets with BWB≥1.80 kg (19.0% vs 3.3%) and piglets with BWB 1.30 to 1.79 kg (4.0%). Piglet with RT24h <37.0°C had higher (p<0.001) mortality rate (86.2%) than piglets with RT24h >38.5°C (3.9%).
Conclusion
Low BWB and low RT24h compromise piglet survival during the lactation period in the tropical conditions. Piglets in the litters with a high BA, low BWB and low blood glucose have reduced ADG.
Keywords: Average Daily Gain, Birth Weight, Mortality, Newborn Traits, Pig
INTRODUCTION
In modern swine industry, producers expect up to 30 pigs weaned per sow per year [1]. However, in practice, a high proportion of piglet mortality occur during pre-weaning period [2]. Thus, factors associated with piglet pre-weaning mortality are becoming a major concern in swine industry worldwide [3]. One of main reason for a high proportion of piglet pre-weaning mortality is the use of high prolific sow genetics, which improved the number of piglets born alive per litter among swine commercial herds worldwide during the past 10 years [1]. Litter size (LS) is an important sow factor related to piglet survival and growth [2]. Nuntapaitoon and Tummaruk [2] demonstrated that piglet pre-weaning mortality in the litter with 13 to 15 littermate pigs (24.1%) was significantly higher than the litter with 1 to 7 (11.9%), 8 to 10 (11.8%), and 11 to 12 (14.6%) littermate pigs, respectively. Large LS also increases the body weight at birth (BWB) variation among the piglets within litter [4]. Hence, the proportion of piglets with low BWB is also increased [5]. Vallet and Miles [6] observed that low BWB piglets had a higher risk of being crushed due to their slower speed of movement and reflexive actions. Intra-partum hypoxia is a main factor influencing piglet vitality as it damages the foetal central nervous system [7]. Thus, several indicators can be used in newborn piglets to evaluate the level of intra-partum hypoxia suffered during the birth process. In addition, piglet vitality determines their capacity to suckle and compete for a teat and is also positively correlated with piglet growth and survival until weaning [8]. One reason for reduced piglet vitality might be related to nutrients and oxygen supplies via umbilical cord from dam to fetus. Blood oxygen, blood glucose concentration, and time to stand after birth have been used as indirect measures of intra-partum hypoxia and neonatal piglet viability [9].
Factors influencing piglet pre-weaning mortality under field conditions include sow factors (e.g., breed, parity, nutritional status, farrowing duration, maternal behavior and LS), piglet factors (e.g., BWB, birth order and sex) and environmental factors (e.g., ambient temperature and stocking density) [2,9]. To our knowledge, most of the studies on risk factors associated with piglet pre-weaning mortality were performed in cold or moderate climates [10–12]. Additional information in hot and humid climate countries is required to improve farrowing management and care of newborn piglets. The objective of the present study was to determine the effects of piglet neonatal traits and physiological characteristics measured soon after birth on their pre-weaning survival and growth.
MATERIALS AND METHODS
Animal care
The experiment followed the guidelines documented in The Ethical Principles and Guidelines for the Use of Animals for Scientific Purposes edited by the National Research Council of Thailand, and was approved by the Institutional Animal Care and Use Committee (IACUC) in accordance with the university regulations and policies governing the care and use of experimental animals (approval no. 1431063).
Herd and management
The present study was carried out in a 3,500-sows commercial swine herd in the western part of Thailand between June and August 2013. The average ambient temperature during the experimental period ranged from 25.8°C to 30.0°C. The minimum and maximum temperature ranged from 21.1°C to 26.3°C and from 28.1°C to 37.6°C, respectively. The average relative humidity varied from 72.0% to 96.0%. Sows were kept in individual crates (1.2 m2) during gestation in a conventional open-housing system and were provided with fans and individual water sprinklers to reduce the impact of high ambient temperature. During gestation, sows were fed a commercial gestation diet that met or exceed their nutritional requirement estimates (19.7% crude protein, 5.8% fat, 3.8% fibre, and 14.0 metabolizable energy, MJ/kg) [13]. Feed was provided twice a day following a standardised feeding pattern, resulting in an average of 2.5 kg per sow per day. During lactation, sows were fed twice a day with a commercial lactation diet, increasing the daily amount of feed offered, until ad libitum feed was reached after one week of lactation. The animals were received water ad libitum in a continuous water channel. Pregnant sows were moved to the farrowing house about one week before their expected farrowing date. Sows were kept in individual farrowing crates (1.2 m2) placed at the centre of the pens with a space allowance of 4.2 m2. The pens were fully slatted with concrete at the centre for sows and with steel slats at both sides of the farrowing crate for piglets. Each pen was provided with a creep area for piglets (0.60 m2) placed on the floor on one side, covered by a plastic plate and heating lamp during the first week after farrowing. The heating lamp was usually turned on during the night or when the environment temperature fell below 30°C. The temperature in the creep area was between 30°C to 36°C.
Supervision of parturition process
The parturition process was carefully supervised by the first author (M. Nuntapaitoon). Briefly, sows were interfered with as little as possible during parturition, and birth intervention was performed only when dystocia was clearly identified. Dystocia was considered when an interval of >30 min elapsed from the birth of the last piglet, and when the sow showed intermittent straining accompanied by paddling of the legs or when the sow expelled small quantities of foetal fluid together with marked tail switching for >30 min without any piglet being born. Routine procedures performed on piglets included weighing, tail docking, tooth clipping and 1 mL (200 mg) iron supplement administered intramuscularly (Gleptosil, Alstoe Ltd. Animal Health, Leicestershire, England) on the day of birth. Piglets were orally administered a coccidiocide of 20 mg/kg body weight (Baycox [Toltrazuril 5.0% oral suspension], Bayer Inc., Mississauga, ON, Canada) to control neonatal coccidiosis at 3 days of age. In total, 805 piglets born from 57 Landrace×Yorkshire crossbred sows were included. The mean parity was 4.0±1.6 (ranged 2 to 7). Lactation length was on an average of 23.0±2.0 days.
Data collection
The following reproductive variables of the sows were recorded: farrowing duration (i.e., time between the first and last born piglets), total number of piglets born per litter (TB), number of piglets born alive per litter (BA), and mummified foetuses, stillbirths and number of piglets at weaning per litter. The piglets’ heart rate and blood oxygen saturation (SatO2) were monitored within 5 min after birth by using a veterinary pulse oximetry (EDAN VE-H100B Pulse Oximeter, Edan Instrument Inc., San Diego, CA, USA). Thereafter, blood samples for glucose analysis were collected from piglets within 5 to 10 min after birth (i.e., before first suckling). A small amount of blood sample (a mixture of venous and arterial blood) was obtained from the cut umbilical cord. Few drops of blood sample were used to determined blood glucose concentration by using a portable test kit of human glucometer (Accu-Chek Performa, Roche, Mannheim, Germany). Birth order, birth interval (the time elapsed between each piglet born), sex, and time elapsed from birth until first attempts to stand (3 groups: <1 min, 1 to 5 min, and >5 min) were recorded for each piglet. Also, birth assistance was recorded (yes or no) if required by piglets. Skin color of the piglets was recorded at birth and was classified into 2 groups (normal or pale). Integrity of the umbilical cord of each piglet was examined and classified into two groups (intact or broken). Rectal temperature was measured at 24 h after birth (RT24h) with a digital thermometer (Microlife, Microlife AG Swiss Corporation, Widnau, Switzerland, with a display resolution of 0.01°C and ±0.1°C accuracy). All piglets were individually identified by an ear tattoo. Body weight of the piglets was measured immediately at birth. Litters were equalized to 13 or 14 piglets per litter after 24 h and within 48 h after birth. The LS was defined as the number of piglets after cross-fostering. Piglets were weighed again at day 7 and 21 after birth. Mortality rate of the piglets was also determined at day 7 and 21 of lactation. Creep feeding and drinking water were provided to the piglets from 7 days of age until weaning.
Statistical analysis
All statistical analyses were performed using SAS 9.0 (SAS Inst. Inc., Cary, NC, USA) [14]. Descriptive analysis (mean±standard deviation [SD] median and range) and frequency analysis were obtained for all reproductive parameters. To identify the potential indicators for piglet mortality and piglet average daily weight gain (ADG) at days 7 and 21 of lactation, each recorded factor was individually tested using univariate analyses. Continuous variables indicating the neonatal piglet characters (i.e., TB, BA, LS, birth order, BWB, birth interval, heart rate, SatO2 and blood glucose concentration) were compared between piglets dying and surviving at days 7 and 21 of lactation by using Student’s t test (PROC TTEST). The association between categorical variables (i.e., sex, skin color, attempts to stand, umbilical cord integrity and birth assistance) and the piglet mortality (dead or alive) were analyzed using Chi-square test. The effect of these categorical variables on ADG at days 7 and 21 of lactation was analyzed using the PROC GLM of SAS. Pearson’s correlation was performed to study collinearity among the continuous variables in the univariate models.
For multivariate analyses, generalised linear mixed models (GLMMIX macro) with the dependent variable (mortality) modeled as a binary outcome (dead or alive) was conducted. Factors with significant levels of p<0.10 were included in the final models. Highly correlated variables were not included in the same multivariate analyses models. Then, the final multivariate GLMMIX models for piglet mortality at days 7 and 21 of lactation included BWB of the piglet classes (<1.30, 1.30 to 1.79, and ≥1.80 kg) and RT24h classes (<37.0°C, 37.0°C to 38.5°C, and >38.5°C). The classification of the independent variables was made according to frequency distribution with some minor adjustment based on biological reliability. Similarly, general linear mixed models (PROC MIXED) were conducted to analyze piglet ADG at days 7 and 21 of lactation. The final multivariate MIXED models for piglet ADG at day 7 and 21 included BWB classes (<1.30, 1.30 to 1.79, and ≥1.80 kg), blood glucose concentration classes (≤24 and >24 mg/dL), BA classes (<12, 12 to 14, and ≥15 piglets), sex and attempts to stand (<1, 1 to 5, and >5 min). In all models, sow or litter were introduced as a random effect. Least squares means were obtained from each class of the variables and were compared using Tukey–Kramer adjustment for multiple comparisons. p< 0.05 was regarded to be statistically significance and 0.05<p<0.10 was considered as a tendency for statistically significance. Piglets were defined as experimental units.
RESULTS
Of the 805 piglets, 81 were stillborn (10.1%), 34 were mummified fetuses (4.2%) and 690 were BA (85.7%). Descriptive statistics of newborn piglet traits and piglet performance are presented in Table 1. On average (means±SD), TB, BA, and the number of piglets at weaning were 14.2±3.7, 12.1±3.4, and 11.7±1.7 piglets, respectively. The duration of farrowing averaged 217.8±83.7 min, and the average birth interval between piglets was 16.4±24.1 min. Overall, piglet pre-weaning mortality was 12.6% and cumulative mortality at day 7 was 8.6%.
Table 1.
Descriptive statistics of 690 piglets evaluated at birth and during the lactation period
| Variables | N | Mean±SD | Median | Range |
|---|---|---|---|---|
| Birth weight (kg) | 690 | 1.49±0.38 | 1.48 | 0.46 to 2.71 |
| Heart rate (bpm) | 685 | 66.2±33.20 | 57.0 | 23.0 to 250 |
| Glucose (mg/dL) | 687 | 49.1±17.72 | 47.0 | 11.0 to 159 |
| Oxygen saturation (%) | 685 | 91.2±8.85 | 92.0 | 10.0 to 100 |
| Rectal temperature at 24 h (°C) | 670 | 38.7±0.57 | 38.7 | 35.5 to 40.3 |
| Weight at day 7 (kg) | 632 | 2.67±0.71 | 2.7 | 0.75 to 4.64 |
| Weight at day 21 (kg) | 602 | 6.09±1.51 | 6.1 | 1.88 to 10.07 |
| ADG at day 7 (g/d) | 632 | 163.7±67.32 | 164.6 | −7.9 to 340.7 |
| ADG at day 21 (g/d) | 602 | 216.8±63.05 | 213.3 | 24.0 to 376.9 |
SD, standard deviation; ADG, average daily gain.
Univariate analyses
Factors associated with piglet pre-weaning mortality until day 7 after birth
Factors influencing piglet pre-weaning mortality rate until day 7 after birth are presented in Tables 2, 3. Both BWB and RT24h influenced piglet mortality until day 7 after birth (p< 0.001). Piglets that died before day 7 of life had a lower birth interval (9.5 vs 14.9 min, p = 0.005) and were reared in litters with a higher LS (13.7 vs 13.2 piglets, p = 0.038). The total number of piglets born per litter, BA, birth order, heart rate, SatO2, and blood glucose concentration did not influence piglet survival (p>0.05, Table 2). A higher proportion of piglets with pale skin color died compared to piglets with normal skin color (26.7% vs 7.7%; p<0.001, Table 3). A higher proportion of piglets that attempted to stand after 5 min (38.5%) died compared to piglets that attempted to stand within 1 min (6.3%, p<0.001) and within 1 to 5 min (9.2%, p<0.001, Table 3). Birth intervention, sex and umbilical cord integrity had no effect on piglet mortality until day 7 after birth (p>0.05, Table 3).
Table 2.
Potential indicators for piglet pre-weaning mortality (means±standard error of the mean) comparing surviving piglets from birth to day 7 of lactation (n = 631) with dying piglets (n = 59)
| Variables | Surviving | Dying | p-value |
|---|---|---|---|
| Total number of piglets born per litter | 14.9±0.14 | 15.6±0.43 | 0.171 |
| Number of piglets born alive per litter | 13.0±0.12 | 13.3±0.45 | 0.491 |
| Litter size after cross-fostering | 13.2±0.07 | 13.7±0.22 | 0.038 |
| Birth interval (min) | 14.9±0.80 | 9.5±1.72 | 0.005 |
| Birth weight (kg) | 1.52±0.01 | 1.11±0.05 | <0.001 |
| Birth order | 7.5±0.18 | 8.5±0.59 | 0.130 |
| Heart rate (bpm) | 66.5±1.34 | 62.5±3.68 | 0.381 |
| Glucose (mg/dL) | 49.3±0.70 | 47.8±2.69 | 0.605 |
| Oxygen saturation (%) | 91.3±0.36 | 90.4±0.99 | 0.458 |
| Rectal temperature at 24 h (°C) | 38.7±0.02 | 38.2±0.14 | <0.001 |
Table 3.
Categorical variables influencing piglet pre-weaning mortality at day 7 and 21 after birth
| Variables | Piglet pre-weaning mortality (%) | |
|---|---|---|
|
| ||
| Day 7 | Day 21 | |
| Skin color | ||
| Normal | 7.7a | 11.5a |
| Pale | 26.7b | 36.7b |
| Time attempted to stand | ||
| <1 min | 6.3a | 10.8a |
| 1 to 5 min | 9.2a | 10.8a |
| >5 min | 38.5b | 41.0b |
| Birth intervention | ||
| No | 9.0a | 13.4a |
| Yes | 5.1a | 6.3a |
| Sex | ||
| Male | 8.0a | 13.6a |
| Female | 9.1a | 11.6a |
| Umbilical cord integrity | ||
| Intact | 8.9a | 13.0a |
| Broken | 7.8a | 11.9a |
Values with different superscripts within the same column within variable differ significantly (p<0.05).
Factors associated with piglet pre-weaning mortality until day 21 after birth
Factors influencing piglet pre-weaning mortality rate until day 21 after birth are presented in Table 3, 4. Both BWB and RT24h influenced piglet mortality until day 21 after birth (p<0.001). Piglets that died before day 21 of life were born from sows with a higher TB, were reared in litters with a higher LS, and had lower birth intervals (p<0.05, Table 4). Birth order, heart rate, SatO2 and blood glucose concentration did not influence piglet survival (p>0.05, Table 4). A higher proportion of piglets with pale skin color died compared to piglets with normal skin color (36.7% vs 11.5%; p<0.001, Table 3). A higher proportion of piglets that attempted to stand after 5 min (41.0%) died compared to piglets that attempted to stand within 1 min (10.8%, p<0.001) and within 1 to 5 min (10.8%, p<0.001, Table 3). Birth intervention, sex and umbilical cord integrity had no effect on piglet mortality until day 21 after birth (p>0.05, Table 3).
Table 4.
Potential indicators for piglet pre-weaning mortality (means±standard error of the mean) comparing surviving piglets from birth to day 21 of lactation (n = 603) with dying piglets (n = 87)
| Variables | Surviving | Dying | p-value |
|---|---|---|---|
| Total number of piglets born per litter | 14.9±0.14 | 15.9±0.33 | 0.010 |
| Number of piglets born alive per litter | 12.9±0.12 | 13.7±0.32 | 0.028 |
| Litter size after cross-fostering | 13.2±0.07 | 13.6±0.18 | 0.042 |
| Birth interval (min) | 15.2±0.83 | 9.3±1.37 | <0.001 |
| Birth weight (kg) | 1.53±0.01 | 1.16±0.05 | <0.001 |
| Birth order | 7.5±0.19 | 8.1±0.45 | 0.319 |
| Heart rate (bpm) | 66.9±1.39 | 61.4±2.71 | 0.076 |
| Glucose (mg/dL) | 49.5±0.71 | 46.6±2.13 | 0.156 |
| Oxygen saturation (%) | 91.2±0.37 | 91.3±0.80 | 0.872 |
| Rectal temperature at 24 h (°C) | 38.7±0.02 | 38.3±0.10 | <0.001 |
Factors associated with average daily gain until day 7 after birth
Piglet BWB (p<0.001) and blood glucose concentration (p<0.001) positively influenced piglet ADG at day 7 (r = 0.172, p<0.001, Table 5). Piglets born from sows with higher TB and higher BA had a lower ADG at day 7 (p<0.001, Table 5). Piglets without birth intervention had lower growth than piglets with birth intervention (161.1±2.8 vs 182.4±7.7 g/d, p = 0.010, Table 6). Male piglets had higher growth than female piglets (169.1±3.7 vs 157.6±3.9 g/d, p = 0.032, Table 6). Piglets that attempted to stand after 5 min (127.1±13.6 g/d) had lower growth than piglets that attempted to stand within 1 min (165.9±2.9 g/d, p = 0.006) or within 1 to 5 min (156.3±13.6 g/d, p = 0.071, Table 6).
Table 5.
Pearson’s correlations among the most significant potential predictor measured during farrowing and soon after birth and average daily gain (ADG) of the piglets from birth until 7 and 21 days of age
| TB | BA | BI | BWB | HR | GLU | |
|---|---|---|---|---|---|---|
| BA | 0.798*** | - | - | - | - | - |
| BI | −0.138*** | −0.128*** | - | - | - | - |
| BWB | −0.309*** | −0.348*** | 0.180*** | - | - | - |
| HR | −0.092* | −0.077* | - | - | - | - |
| SatO2 | - | - | - | - | −0.179*** | - |
| GLU | −0.118** | −0.136*** | 0.146*** | 0.187*** | - | - |
| RT24h | - | - | - | 0.128*** | - | - |
| ADG7 | −0.272*** | −0.283*** | - | 0.430*** | - | 0.172*** |
| ADG21 | −0.197*** | −0.284*** | - | 0.431*** | - | 0.106** |
TB, total number of piglets born per litter; BA, number of piglets born alive per litter; BI, birth interval; BWB, birth weight; HR, heart rate; GLU, blood glucose concentration; SatO2, blood oxygen saturation; RT24h, rectal temperature at 24 h after birth; ADG7, average daily gain from birth until day 7; ADG21, average daily gain from birth until day 21.
Significance levels:
p<0.05,
p<0.01,
p<0.001.
Table 6.
Categorical variables influencing average daily gain (means±standard error of the mean) at day 7 and 21 after birth from univariate analyses
| Variables | Average daily gain (g/d) | |
|---|---|---|
|
| ||
| Day 7 | Day 21 | |
| Skin color | ||
| Normal | 163.6±2.7a | 217.0±2.6a |
| Pale | 164.4±14.4a | 210.6±14.5a |
| Time attempted to stand | ||
| <1 min | 165.9±2.8a | 218.0±2.8a |
| 1 to 5 min | 156.3±8.7ab | 215.9±8.3ab |
| >5 min | 127.1±13.7b | 190.3±13.1b |
| Birth intervention | ||
| No | 161.1±2.8a | 214.3±2.7a |
| Yes | 182.4±7.7b | 235.1±7.3b |
| Sex | ||
| Male | 169.1±3.7a | 219.1±3.6a |
| Female | 157.6±3.9b | 214.4±3.7a |
| Umbilical cord integrity | ||
| Intact | 160.4±3.2a | 215.6±3.1a |
| Broken | 170.6±4.7a | 219.4±4.5a |
Values with different superscripts within the same column within variable differ significantly (p<0.05).
Factors associated with average daily gain until day 21 after birth
Piglet BWB (p<0.001) and blood glucose concentration (p = 0.009) positively influenced piglet ADG at day 21 (r = 0.106, p<0.01, Table 5). Piglets born from sows with higher TB and BA had lower ADG at day 21 (Table 5, p<0.001). Piglets without birth intervention had lower growth than piglets with birth intervention (214.3±2.7 vs 235.1±7.3 g/d, p = 0.008, Table 6). Piglets that attempted to stand after 5 min (190.3±13.1 g/d) had lower growth than piglets that attempted to stand within 1 min (218.0±2.8 g/d, p = 0.039, Table 6).
Multivariate analyses
Average daily gain
The final multi-covariate model for ADG at day 7 included BWB (p<0.001), blood glucose concentration (p< 0.001), BA (p = 0.025), sex (p = 0.087), and attempts to stand (p = 0.093) (Table 7). At day 7, the piglets with BWB<1.30 kg had a lower ADG than the piglets with BWB≥1.80 kg (p<0.001) and the piglets with BWB 1.30 to 1.79 kg (p<0.001, Table 7). The piglets with a blood glucose concentration of ≤24 mg/dL had a lower ADG at day 7 than the piglets with a blood glucose concentration of >24 mg/dL (p<0.001, Table 7). Furthermore, the piglets with a low BA (<12 piglet) had a higher ADG at day 7 than the piglets with 12 to 14 BA (p = 0.087) and the piglets with ≥15 BA (p = 0.030, Table 7). The male piglets tended to have a higher ADG at day 7 than the female piglets (p = 0.087, Table 7). The piglets that stood within 1 min tended to have a higher ADG at day 7 than the piglets that stood after 5 min (p = 0.075, Table 7).
Table 7.
Predictive factors included in the final models for average daily gain (least square means±standard error of the mean) from birth until 7 and 21 days of life from multivariate analyses
| Variables | Average daily gain (g/d) | |
|---|---|---|
|
| ||
| Day 7 | Day 21 | |
| Number of piglets born alive per litter, piglets | ||
| <12 | 150.6±11.09aA | 226.8±9.88a |
| 12 to 14 | 126.4±11.39abB | 190.6±9.60b |
| ≥15 | 121.3±11.16b | 192.8±9.94b |
| Birth weight (kg) | ||
| <1.30 | 88.9±9.72c | 165.5±9.01c |
| 1.30 to 1.79 | 145.3±9.52b | 210.5±8.45b |
| ≥1.80 | 164.2±10.59a | 234.2±9.40a |
| Glucose (mg/dL) | ||
| ≤24 | 107.8±15.32b | 190.3±15.95b |
| >24 | 157.8±6.16a | 220.6±4.99a |
| Sex | ||
| Male | 136.5±9.45A | NS |
| Female | 129.1±9.53B | NS |
| Attempt to stand (min) | ||
| <1 | 141.8±8.27A | NS |
| 1 to 5 | 140.2±10.78AB | NS |
| >5 | 116.4±13.87B | NS |
Values with different small superscripts within the same column differ significantly (p<0.001).
Values with different capital superscripts within the same column tended to be differences (0.05<p<0.10).
NS, the variables were not significant (p>0.01) and were not included in the final models.
Based on a multivariate statistical model, factors significantly influencing ADG of the piglets at day 21 in the final model included BWB (p<0.001), BA (p<0.001), and blood glucose concentration (p = 0.042). The statistical model revealed that, at day 21, the piglets with a BWB of <1.30 kg had a lower ADG than the piglets with a BWB of ≥1.80 kg (p<0.001) and the piglets with a BWB 1.30 to 1.79 kg (p<0.001) (Table 7). The piglets from litters with a low BA (<12 piglets) had a higher ADG at day 21 than those from litters with a BA of 12 to 14 (p<0.001) and ≥15 (p<0.001). The piglets with a blood glucose concentration of ≤24 mg/dL had a lower ADG at day 21 than the piglets with a blood glucose concentration of >24 mg/dL (p = 0.042). Sex and attempts to stand did not influence ADG of the piglet at day 21 (p>0.10).
Piglet pre-weaning mortality
The final multivariate model for mortality at day 7 included BWB and RT24h (p<0.001). At day 7, the piglets with BWB<1.30 kg had a higher (p<0.001) mortality rate (19.0%) than the piglets with BWB≥1.80 kg (3.3%) and piglets with BWB 1.30 to 1.79 kg (4.0%, Figure 1). The piglets with RT24h < 37.0°C had a higher (p<0.001) mortality rate (86.2%) at day 7 than the piglets with RT24h 37.0°C to 38.5°C (7.3%) and RT24h >38.5°C (3.9%, Figure 2).
Figure 1.
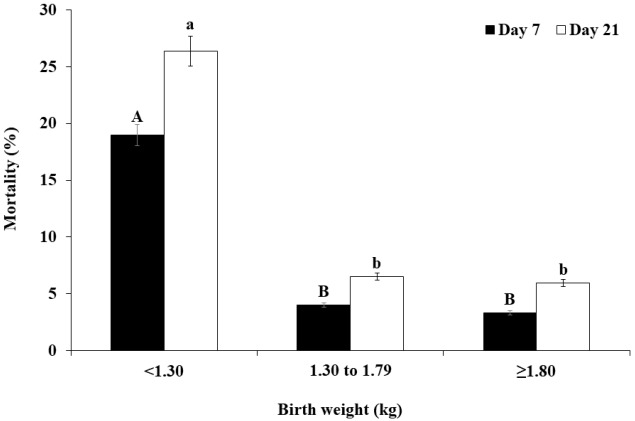
Percentage of mortality at days 7 and 21 for all piglets classified into low (<1.30 kg, n = 216), medium (1.30 to 1.79 kg, n = 323) and high (≥1.80 kg, n = 151) birth weight categories. A,B,a,b Values with different superscripts within the same day differ significantly (p<0.05).
Figure 2.
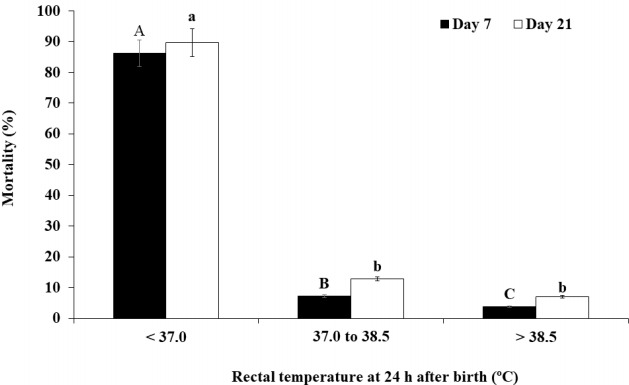
Percentage of mortality at days 7 and 21 for all piglets classified into low (<37.0°C, n = 29), medium (37.0°C to 38.5°C, n = 248) and high (>38.5°C, n = 413) rectal temperature categories at 24 h after birth. A,B,C,a,b,c Values with different superscripts within the same day differ significantly (p<0.05).
Based on a multivariate statistical model, factors influencing piglet mortality at day 21 included BWB (p<0.001) and RT24h (p<0.001). At day 21, the piglets with BWB<1.30 kg had a higher (p<0.001) mortality rate (26.4%) than the piglets with BWB≥1.80 kg (6.0%) and the piglets with BWB 1.30 to 1.79 kg (6.5%, Figure 1). The piglets with RT24h <37.0°C had a higher (p<0.001) mortality rate (89.7%) at day 21 than the piglets with RT24h 37.0°C to 38.5°C (12.9%) and RT24h >38.5°C (7.0%, Figure 2).
DISCUSSION
The main objective of this study was to determine the effect of certain newborn piglet traits measured soon after birth on piglet pre-weaning mortality and growth. As expected, BWB and RT24h were the most influential postnatal survival factors at both day 7 and at weaning. Furthermore, BWB, BA and blood glucose concentration significantly influenced piglet growth during the whole lactation period, while sex and time spent attempting to stand had some impact on their growth up to day 7.
In agreement with our results, many studies have identified piglet BWB as the main predictor for both survival and growth during lactation [9,15]. Piglet BWB is positively associated with their physiological maturity and, in turn, correlates with different physical and physiological parameters such as colostrum intake capacity and thermoregulation ability [3]. Small piglets usually have decreased viability and a lower capacity to compete for a teat [16]. Johansen et al [17] found that piglets with a low BWB often have lower ADG in the suckling period. In addition, low BWB piglets are less able to maintain body temperature [18] which leads to starvation, lethargy and increased risk of crushing by the sow. Therefore, lower BWB piglets may also show a low nutritional status and poor passive immunity [19]. In agreement with our results, rectal temperature after birth was identified as an important indicator for piglet survival [9,16], indicating that piglets with low rectal temperature after 24 h might have lower thermoregulation abilities. Thermoregulation is a crucial physiological event for all newborn piglets. The piglets that die during the first days of life are not able to maintain optimal rectal temperature during the first 24 h of life [10]. In the present study, RT24h was significantly associated with mortality rate but was not related with ADG. In contrast, Panzardi et al [9] identified RT24h as an indicator for piglet growth at weaning. Likewise, Pedersen et al [20] found that piglets with low RT24h had low ADG from birth to weaning. One possible explanation might be due to that the piglets with low RT24h may consume less colostrum than the piglets with high RT24h. Thus, growth rate of these piglets might be compromise.
In the current study, BA was observed to influence piglet growth. Likewise, other studies found that BA negatively correlated with the piglet weaning weight [20]. This might be due to the fact that there is also an increase in the number of small piglets in the litter with an increased BA [21]. The present study also demonstrated that BWB of the piglets was significantly decreased when TB increased (Table 5). Competition between littermates might have a negative impact on piglet colostrum intake, especially in the small piglets, resulting in reduced growth during lactation [3]. Moreover, BA was not related with mortality in the present study, whereas in the previous study, there was a positive relationship between the number of piglets in the litter and piglet mortality [2]. Nonetheless, Muns et al [8] and Pedersen et al [20] found a relationship between BA and piglet growth, but not between BA and mortality rate.
In the present study, blood glucose concentration at birth was a significant predictor for piglet ADG at 7 and 21 days of life but not for piglet mortality. Accordingly, studies failed to demonstrate a correlation between blood glucose concentration in newborn piglet and their survival [10,15,16]. However, Panzadi et al [9] found that either too low (24 to 30 mg/dL) or too high (45 to 162 mg/dL) levels of blood glucose in neonatal piglets were associated with an increased pre-weaning mortality. In the present study, piglets with high glucose concentrations at birth might have high energy reserves and, subsequently, enhanced capacity for suckling and growth. This implies that some source of glucose or energy supplementation in neonatal piglets maybe needed to improve the suckling capacity and growth performance in the neonatal piglets.
In the multivariate models for ADG at day 7, time from birth to first attempts to stand was also identified as a predictive factor. In the present study, piglets spending >5 min from birth to first attempts to stand had low ADG. Decaluwé et al [12] also found that piglets spending a long time from birth to suckling had a lower ADG during the lactation period than those spending a short time from birth to suckling. Moreover, neonatal piglets spending a short time from birth to first attempts to stand resulted in a low pre-weaning mortality [9,10]. However, Leenhouwers et al [22] found no relationship between time from birth to first attempts to stand and piglet mortality rate during the first week of lactation. Nonetheless, time elapsed from birth to first suckling significantly influences colostrum intake of the piglets [23]. Therefore, “the time elapsed from birth to first suckling” has been included in a formula for estimating colostrum consumption in piglets [23]. In the present study, piglets spending a short time period from birth to first attempts to stand are probably faster at first suckling, thus increasing their colostrum consumption. The colostrum consumption of the neonatal piglets significantly influence the piglet survival and passive immunity [24]. Therefore, in practice, newborn piglets spending more than 5 min to first attempt to stand need special cares.
In conclusion, low BW B and low RT24h compromise piglet survival during the lactation period in the tropical conditions. In addition, piglets in the litters with a high number of BA and piglets with low BWB and/or low blood glucose concentration have reduced body weight growth during lactation.
ACKNOWLEDGMENTS
Financial support for the present study was provided by a grant for International Research Integration: Chula Research Scholar, Ratchadaphiseksomphot Endowment Fund. M. Nuntapaitoon is a grantee of the Research and Researchers for Industries (RRI) Ph.D. program and the Thailand Research Fund, and part of the research grant was supported by the Rachadapisek Sompote Endowment Fund for the 90th Anniversary of Chulalongkorn University. Ramon Muns was supported by the Rachadapisek Sompote Endowment Fund for Postdoctoral Fellowship by Chulalongkorn University.
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Knox R. Getting to 30 pigs weaned/sow/year. Proceedings of London Swine Conference; 2005; London, UK. pp. 47–59. [Google Scholar]
- 2.Nuntapaitoon M, Tummaruk P. Piglets pre-weaning mortality in a commercial swine herd in Thailand. Trop Anim Health Prod. 2015;47:1539–46. doi: 10.1007/s11250-015-0895-3. [DOI] [PubMed] [Google Scholar]
- 3.Muns R, Nuntapaitoon M, Tummaruk P. Non-infectious causes of pre-weaning mortality in piglets. Livest Sci. 2016;184:46–57. [Google Scholar]
- 4.Shankar BP, Madhusudhan HS, Harish DB. Pre-weaning mortality in pig-causes and management. Vet World. 2009;2:236–9. [Google Scholar]
- 5.Campos PHRF, Silva BAN, Donzele JL, Oliveira RFM, Knol EF. Effects of sow nutrition during gestation on within-litter birth weight variation: a review. Animal. 2012;6:797–806. doi: 10.1017/S1751731111002242. [DOI] [PubMed] [Google Scholar]
- 6.Vallet JL, Miles JR. Comparison of myelination between large and small pig fetuses during late gestation. Anim Reprod Sci. 2012;132:50–7. doi: 10.1016/j.anireprosci.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Herpin P, Le Dividich J, Hulin JC, et al. Effect of the level of asphyxia during delivery on viability at birth and early postnatal vitality of newborn pigs. J Anim Sci. 1996;74:2067–75. doi: 10.2527/1996.7492067x. [DOI] [PubMed] [Google Scholar]
- 8.Muns R, Manzanilla EG, Sol C, Manteca X, Gasa J. Piglet behaviour as a measure of vitality and its influence on piglet survival and growth during lactation. J Anim Sci. 2013;91:1838–43. doi: 10.2527/jas.2012-5501. [DOI] [PubMed] [Google Scholar]
- 9.Panzardi A, Bernardi ML, Mellagi AP, et al. Newborn piglet traits associated with survival and growth performance until weaning. Prev Vet Med. 2013;110:206–13. doi: 10.1016/j.prevetmed.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Baxter EM, Jarvis S, D’Eath RB, et al. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology. 2008;69:773–83. doi: 10.1016/j.theriogenology.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen LJ, Malmkvist J, Kammersgaard T, Jørgensen E. Avoiding hypothermia in neonatal pigs: effect of duration of floor heating at different room temperatures. J Anim Sci. 2013;91:425–32. doi: 10.2527/jas.2011-4534. [DOI] [PubMed] [Google Scholar]
- 12.Decaluwé R, Maes D, Wuyts B, et al. Piglets’colostrum intake associates with daily weight gain and survival until weaning. Livest Sci. 2014;162:185–92. [Google Scholar]
- 13.Committee on Nutrient Requirements of Swine, National Research Council . Nutrient requirements of swine. 11th ed. Washington, DC, USA: National Academy Press; 2012. [Google Scholar]
- 14.SAS Institute Inc . Statistics version 9.0. Cary, NC, USA: SAS Institute Inc; 1996. SAS User’s guide. [Google Scholar]
- 15.Rootwelt V, Reksen O, Farstad W, Framstad T. Postpartum deaths: piglet, placental, and umbilical characteristics. J Anim Sci. 2013;91:2647–56. doi: 10.2527/jas.2012-5531. [DOI] [PubMed] [Google Scholar]
- 16.Tuchscherer M, Puppe B, Tuchscherer A, Tiemann U. Early identification of neonates at risk: traits of newborn piglets with respect to survival. Theriogenology. 2000;54:371–88. doi: 10.1016/S0093-691X(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 17.Johansen M, Alban L, Kjærsgård HD, Bæbo P. Factors associated with sucking piglet average daily gain. Prev Vet Med. 2004;63:91–102. doi: 10.1016/j.prevetmed.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Theil PK, Nielsen MO, Sørensen MT, Lauridsen C. Lactation, milk and suckling. In: Bach KKE, Kjeldsen NJ, Poulsen HD, Jensen BB, editors. Nutritional physiology of pigs. Copenhagen, Denmark: Pig Research Centre; 2012. p. 49. [Google Scholar]
- 19.Quiniou N, Dagorn J, Gaudré D. Variation of piglets’ birth weight and consequences on subsequent performance. Livest Prod Sci. 2002;78:63–70. [Google Scholar]
- 20.Pedersen LJ, Schild SLA, Malmkvist J. The influence of the thermal environment and other early life events on growth rate of piglets during lactation. Animal. 2015;9:1529–35. doi: 10.1017/S1751731115001007. [DOI] [PubMed] [Google Scholar]
- 21.Quesnel H, Brossard L, Valancogne A, Quiniou N. Influence of some sow characteristics on within-litter variation of piglet birth weight. Animal. 2008;2:1842–9. doi: 10.1017/S175173110800308X. [DOI] [PubMed] [Google Scholar]
- 22.Leenhouwers JI, de Almeida CA, Jr, Knol EF, van der Lende T. Progress of farrowing and early postnatal pig behavior in relation to genetic merit for pig survival. J Anim Sci. 2001;79:1416–22. doi: 10.2527/2001.7961416x. [DOI] [PubMed] [Google Scholar]
- 23.Devillers N, van Milgen J, Prunier A, Le Dividich J. Estimation of colostrum intake in the neonatal pig. Anim Sci. 2004;78:305–13. [Google Scholar]
- 24.Devillers N, Le Dividich J, Prunier A. Influence of colostrum intake on piglet survival and immunity. Animal. 2011;5:1605–12. doi: 10.1017/S175173111100067X. [DOI] [PubMed] [Google Scholar]


