Abstract
Objective
This study observed the effects of cooking method and final core temperature on cooking loss, lipid oxidation, aroma volatiles, nucleotide-related compounds and aroma volatiles of Hanwoo brisket (deep pectoralis).
Methods
Deep pectoralis muscles (8.65% of crude fat) were obtained from three Hanwoo steer carcasses with 1+ quality grade. Samples were either oven-roasted at 180°C (dry heat) or cooked in boiling water (moist heat) to final core temperature of 70°C (medium) or 77°C (well-done).
Results
Boiling method reduced more fat but retained more moisture than did the oven roasting method (p<0.001), thus no significant differences were found on cooking loss. However, samples lost more weight as final core temperature increased (p<0.01). Further, total saturated fatty acid increased (p = 0.02) while total monounsaturated fatty acid decreased (p = 0.03) as final core temperature increased. Regardless the method used for cooking, malondialdehyde (p<0.01) and free iron contents (p<0.001) were observed higher in samples cooked to 77°C. Oven roasting retained more inosinic acid, inosine and hypoxanthine in samples than did the boiling method (p<0.001), of which the concentration decreased as final core temperature increased except for hypoxanthine. Samples cooked to 77°C using oven roasting method released more intense aroma than did the others and the aroma pattern was discriminated based on the intensity. Most of aldehydes and pyrazines were more abundant in oven-roasted samples than in boiled samples. Among identified volatiles, hexanal had the highest area unit in both boiled and oven-roasted samples, of which the abundance increased as the final core temperature increased.
Conclusion
The boiling method extracted inosinic acid and rendered fat from beef brisket, whereas oven roasting intensified aroma derived from aldehydes and pyrazines and prevented the extreme loss of inosinic acid.
Keywords: Cooking Method, Core Temperature, Hanwoo, Inosinic Acid, Lipid Oxidation, Volatile
INTRODUCTION
In gastronomy, taste and aroma are two important traits that must be considered when preparing a meal. The preparation stage normally starts with the selection of ingredients and cooking method. Beef is generally cooked to both tenderize the meat and improve its taste and aroma. Alternative postmortem techniques and cooking methods have been suggested to improve the palatability of specific beef muscles [1]. Blackmon et al [2] showed that cooked ground beef patties prepared from brisket (deep pectoralis) released more pleasant aroma volatiles. In Korea, whole brisket is commonly cooked slowly (braised) in boiling water containing soy sauce to tenderize and preserve the beef. In Western countries, beef brisket is occasionally roasted after braising to enhance the flavor.
At the molecular level, moisture, fats, proteins, vitamins, minerals, reducing sugars and other trace compounds in food stuff are responsible for the pleasure found in eating. Some of those compounds are stable when heated, but others may break down into other compounds that may later affect the sensory acceptance of the cooked meal [3]. In the case of beef, fat content is positively associated with taste preferences [4]. Among five basic tastes, umami has been associated with the pleasure of eating meat and meat products [5]. Water-soluble compounds, such as nucleotides and peptides, are associated with umami taste [6]. Inosinic acid, or inosine-5′monophosphate (IMP), is a nucleotide that has been shown to enhance the umami intensity of glutamic acid [7]. Species, breed and diet influence the amount of intramuscular fat and water-soluble taste precursors in meat. Moreover, postmortem techniques, such as aging and processing, play a role in enhancing, reducing or converting umami-related taste precursors [8–10].
Grain-fed Hanwoo beef is renowned as the most exclusi ve type of beef available in Korean markets. A muscle profiling study of Hanwoo beef revealed that different retail cuts showed different trends in eating quality with respect to the fat content [11]. Furthermore, cooking to different end-point temperatures influenced the sensory acceptance of longissimus dorsi muscles from Hanwoo steers [12]. This study aimed to determine the effects of cooking method and final core temperature on aroma volatiles and inosinc acid content of Hanwoo brisket (deep pectoralis) to better understand how cooking method and final core temperature affect the taste and aroma of cooked deep pectoralis muscle (brisket) at molecular level.
MATERIALS AND METHODS
Sample and cooking
Vacuum-packed beef brisket (deep pectoralis) was purchased from a local market (Chuncheon, Korea) seven days post-slaughter. The brisket samples were obtained from three Hanwoo steer carcasses of quality grade 1+ that were finished on a grain-based diet and slaughtered within the same batch. Eight slices from one animal (24 total slices) were prepared (6×6×2.5 cm), and all external fat was trimmed. Sample weight was recorded, and the samples were subsequently cooked to final core temperatures of 70°C (medium) or 77°C (well-done) by either oven-roasting at 180°C (dry heat) in an electric oven (Hauzen, Samsung Electronics Co., Ltd., Suwon, Korea) or cooking in boiling water (moist heat). Prior to cooking, the oven was set to 180°C for 15 min, and water was brought to a boil. The following four cooking treatments were used: oven-roasted medium, oven-roasted well-done, boiled medium and boiled well-done. Final core temperature was monitored using a digital stainless cooking thermometer (HCP2, Habor Precise Industries Co., Ltd., Zhejiang, China). After reaching the desired core temperature, samples were removed, cooking time was recorded and samples were cooled for 15 min on aluminum trays. Paper towels were used to remove any remaining moisture on the surface of cooked samples, and the final weight was recorded. Cooking loss was expressed as the percentage of weight lost during cooking. For other analyses, samples were ground using a food blender (Hanil Electronics Co., Ltd., Seongnam, Korea) and immediately used to measure the pH, lipid oxidation, aroma volatiles, moisture and crude fat content. The remaining samples were frozen at −24°C and then stored at −71°C on the following day for determining the fatty acid composition, free iron, inosinic acid and hypoxanthine content. Chemical analyses were performed within two weeks after cooking. Prior to analyses, samples were defrosted overnight at 2°C±2°C.
pH, moisture and crude fat content measurement
Sample pH was measured using meat slurry prepared by homogenizing 5 g of sample with 45 mL of distilled water at 10,000 rpm for 60 s (PH91, SMT Co., Ltd., Chiba, Japan). The pH value of the slurry was recorded using a pH meter calibrated with acid (pH 4.01) and neutral (pH 7.00) technical buffer solutions at 22°C according to manufacturer’s instruction and automatic temperature compensation program was used (Seven Easy pH, Mettler-Toledo GmbH, Greifensee, Switzerland). Moisture and crude fat were determined in triplicate by AOAC official methods [13].
Lipid oxidation
Lipid oxidation was measured using the 2-thiobarbituric acid reactive substances (TBARS) method with slight modification [14]. Sample (0.5 g) was prepared in triplitcate in 25-mL test tube, vortex-mixed with 0.1 g of antioxidant mixture (consisting of 54% propylene glycol, 40% Tween 20, 3% butylated hydroxytoluene, and 3% butylated hydroxyanisole) and 3 mL of 1% thiobarbituric acid in 0.3% NaOH. Subsquently, 17 mL of 2.5% trichloroacetic acid in 36 mM HCl was added and the tube was closed with cap. The sample was heated in a water bath (BW-20G, Biotechnical Services, Inc., North Little Rock, AR, USA) that was set on at 100°C for 30 min and immersed in icy water for 15 min. About 5 mL of aqueous sample was mixed with 3 mL of chloroform and the mixture was centrifuged at 2,400×g for 30 min at 4°C (1248R, Labogene, Lynge, Denmark) to remove the dirt. The absorbance value of the upper layer was recorded at 532 nm (UV-mini 1240 PC, Shimadzu Corp., Kyoto, Japan) against blank (deionized water was used to replace sample). Data was expressed in mg of malondialdehyde per kg dry matter.
Free iron content
Free iron content was determined by a modified ferrozine method [15]. Sample (5 g) was homogenized with 15 mL of citrate phosphate buffer (pH 5.5) at 13,500 rpm for 15 s (Ultra-Turrax T25 basic, Ika Werke GmbH and Co., Staufen, Germany). The homogenate was centrifuged at 3,200×g for 30 min at 4°C (1248R, Labogene, Denmark). Subsequently, 1 mL of the supernatant was mixed with 0.5 mL of freshly prepared 2% ascorbic acid in 0.2 N HCl and incubated at room temperature for 5 min. The mixture was mixed thoroughly with 1.5 mL of 11.3% trichloroacetic acid and centrifuged at 10,000×g using a centrifuge (Eppendorf Micro 17R+, Hanil Science Industrial, Incheon, Korea) for 10 min. Supernatant (1 mL) was mixed with 0.4 mL of 10% ammonium acetate and 0.1 mL of ferroin indicator followed by 5 min incubation at room temperature. The absorbance was read at 562 nm against a blank. The free iron value was expressed as μg/g dry matter.
Nucleotide-related compound content
The method reported by Jayasena et al [16] using high-performance liquid chromatography (HPLC) was used with modification. A 5 g sample was prepared in triplicate and homogenized with 25 mL of 0.7 M perchloric acid at 13,000 rpm for 15 s (UltraTurrax T25 basic, IkaWerke GmbH and Co., Staufen, Germany). Samples were centrifuged at 2,090×g for 15 min at 2°C (1248R, Labogene, Denmark). The supernatant was filtered through a filter paper No. 1 (Whatman Ltd., Little Chalfont, UK) and adjusted to pH 7.0 with 5 N KOH. The pH-adjusted supernatant was placed into a volumetric flask and made to a volume of 100 mL with HPLC-grade distilled water. Approximately 25 mL of supernatant was centrifuged at 1,130×g for 15 min at 0°C (1248R, Labogene, Denmark) and then filtered through a 0.45-μm polyvinylidene fluoride syringe filter (Hyundai Micro Co., Ltd., Seoul, Korea). The filtrate was analyzed using an Agilent 1260 Infinity HPLC system equipped with an Agilent C18 column (4.6×250 mm, 5 μm particle size, Agilent Technologies, Santa Clara, CA, USA). Mobile phase A was 0.04% (v/v) triethylamine in phosphate buffer (58.72 mM Na2HPO4, 40 mM KH2PO4, pH 7.02 at 22°C), and mobile phase B was a mixture of HPLC-grade distilled water and acetonitrile (40:60 v/v). The flow rate was 1.0 mL/min, and the injection volume was 10 μL. The linear gradient was 0% to 15% mobile phase B for 17 min and 15% to 100% mobile phase B for 3 min (maintained for another 5 min) followed by re-equilibration with 100% mobile phase A for 10 min before the next injection. The column temperature was maintained at 35°C and the detection was monitored at a wavelength of 260 nm using diode array detectors. Peaks were identified using the retention time of known standards, IMP (or inosinic acid), inosine and hypoxanthine (Sigma-Aldrich Corp., LLC., St. Louis, MO, USA), and the concentrations were quantified according to the standard curve. Data are presented as mg/100 g dry matter.
Fatty acid composition
Fatty acid composition was determined using an Agilent gas chromatography system (6890N, Agilent Technologies, USA) with an auto sampler (7683, Agilent Technologies, USA). Meat fat was extracted according to Folch et al [17] with a chloroform-methanol (2:1 v/v) solution and prepared in duplicate. Fatty acids were converted into methyl esters as described by the AOAC method [18]. Fatty acid methyl esters were then dissolved in 1.5 mL of hexane. Sample (1 μL) was injected into the gas chromatography (GC) port by the auto sampler. The injector temperature was set at 250°C with a split ratio of 100:1. Fatty acid methyl esters were separated using a WCOT-fused silica capillary column (100 m×0.25 mm i.d., 0.20 μm film thickness; Varian Inc., Palo Alto, CA, USA) with a 1.0 mL/min helium flow. The oven was programmed as follows: 150°C/1 min, 150°C to 200°C at 7°C/min, 200°C/5 min, 200°C to 250°C at 5°C/min, and 250°C/10 min. The temperature of the detector was 275°C. The fatty acid peaks were identified by comparison with the retention time of fatty acid standards (47015-U, Sigma-Aldrich Corp., LLC., USA). The peak area of each identified fatty acid was used to calculate the proportion (%) against total identified peak area.
Aroma pattern discrimination
Samples (1 g) were put into 10 mL headspace vials and prepared in triplicate. Cooked samples were then heated to 60°C in an electronic nose-coupled oven and shaken at 500 rpm for 5 min to generate sufficient volatiles in the headspace. The headspace gas (2.5 mL) was extracted using an automatic sampler syringe at 65°C (HS 100, Alpha MOS, Toulouse, France) and injected into the electronic nose injection port. For raw samples, the incubation and syringe temperatures were set at 30°C and 35°C, respectively. Synthetic air (purity 99.9%) was used as the carrier gas with a flow rate of 150 mL/min. Volatiles were detected using a metal oxide sensor (MOS) array system (FOX 3000, Alpha MOS, France) that consisted of 6 sensors (T30/1, P10/1, P10/2, P40/1, T70/2, PA2) with an acquisition time of 150 s. The sensor resistance ratio was automatically calculated using the following formula: (R−R0)/R0, where R is the real-time resistance and R0 is the resistance baseline of the sensor. The time to return to baseline after acquisition was 18 min. The maximum resistance ratio represented the value for one measurement. Principal component analysis (PCA) was used for discrimination analysis using Alpha Soft package version 8.01.
Aroma volatiles identification
Aroma volatiles of cooked samples were separated and identified using a gas chromatography-mass spectrometry (GC-MS) according to Ba et al [19] with slight modification. Samples (3 g) were put into a 50 mL headspace vial and prepared in duplicate. The vials were heated to 60°C in a drying oven for 10 min and a carboxen/polydimethylsiloxane fiber (Sigma-Aldrich Corp., LLC., USA) with a 75 μm diameter was injected into the vial. Extraction was performed for 30 min after adjustment. Following extraction, the fiber was injected into the GC port, which was set at 250°C, and the volatiles were desorbed for 5 min at a 1:5 split ratio. Separation was performed using a DB5 fused silica column (30 m×0.25 mm i.d., 0.25 μm film thickness, J&W Scientific, Folcom, CA, USA) in a GC (7890A Agilent Technologies, USA). The GC oven was programmed as follows: 40°C/2 min, 40°C to 160°C at 5°C/min, 160°C to 180°C at 6°C/min, 180°C/5 min, 180°C to 200°C at 10°C/min, and 200°C/5 min. The interface and quadruple temperatures were 280°C and 150°C, respectively. Helium was used as the carrier gas at the constant flow of 1 mL/min. Volatile compound was detected by a mass spectrometer (5975C, Agilent Technologies, USA). The ion source temperature of the MS was set at 280°C with an electron impact of 70 eV. A scanning mass range of 50 to 450 m/z with a scan rate of 1 scan/s was used. Tentative identification was performed by comparing the mass spectra to National Institute of Standards and Technology (NIST) Mass Spectral Library. A series of n-alkanes (C8-C20) were analyzed under the same conditions to obtain the retention indices (RIs) for the identified volatiles. The RIs were then compared to the published database available at http://www.flavornet.org/. Data are presented as area unit×106/g dry matter.
Statistical analysis
Two-way analysis of variance (ANOVA) was applied to determine significant differences between treatment groups (cooking methods and final core temperatures) using general linear mixed model. The cooking method and final core temperature were treated as fixed terms. For proximate composition, pH, TBARS value, free iron, nucleotide-related compounds, fatty acid composition and sensor resistance ratio, data of cooked samples were pooled and compared with those of raw samples using one-way ANOVA. Significant differences between cooked and raw samples were observed on all measured parameters, except oleic acid and alpha linolenic acid. Analyses were performed using R version 3.3.2 with the “lme4” and “Agricolae” library (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS AND DISCUSSION
Cooking time, cooking loss and proximate composition
The effects of cooking method and final core temperature on cooking time and cooking loss are shown in Table 1. Samples that were oven-roasted at 180°C required a longer time to cook than did samples that were boiled (p<0.001), and a longer time was needed to reach higher final core temperature. The higher end-point cooking temperature resulted in greater cooking loss regardless of the method used for cooking. Samples lost more moisture by oven roasting than by boiling in water because the former prolonged the heat exposure duration. It is assumed that boiling slows the loss of moisture as it is used water as the medium of heat transfer. Moisture loss caused by dry heating increased the proportion of fat in the cooked beef samples. A lower fat content remained in boiled-beef due to its fat loss to the water. Similar results were also reported in previous cooking studies using different types of meat [20,21].
Table 1.
Effects of cooking method and final core-temperature on cooking time, cooking loss, proximate composition, pH, lipid oxidation, free iron content, and nucleotide-related compound content of Hanwoo brisket
| Item | Raw | CM | FT | SEM | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Boiled | Oven-roasted | 70°C | 77°C | CM | FT | CM×FT | |||
| Cooking time (min) | - | 19.7 | 44.8 | 27.7 | 36.6 | 1.16 | <0.001 | 0.02 | 0.47 |
| Cooking loss (%) | - | 33.4 | 35.9 | 32.6 | 36.7 | 4.78 | 0.11 | 0.01 | 0.72 |
| Moisture (%) | 71.5 | 56.7 | 51.4 | 56.7 | 51.4 | 1.88 | <0.001 | <0.001 | 0.78 |
| Crude fat (%) | 8.65 | 12.7 | 17.7 | 14.6 | 15.8 | 0.87 | <0.001 | 0.40 | 0.95 |
| pH | 5.37 | 5.50 | 5.66 | 5.60 | 5.56 | 0.03 | <0.001 | <0.01 | 0.11 |
| TBARS value (mg MDA/kg DM) | 0.24 | 1.35 | 1.46 | 1.30 | 1.51 | 0.09 | 0.09 | <0.01 | <0.001 |
| Free iron (mg/100 g DM) | 0.38 | 1.49 | 2.41 | 1.14 | 2.81 | 0.17 | 0.02 | <0.001 | 0.04 |
| Inosinic acid (mg/100 g DM) | 499 | 282 | 328 | 362 | 249 | 14.30 | <0.001 | <0.001 | <0.001 |
| Inosine (mg/100 g DM) | 115 | 46 | 58 | 57 | 47 | 4.00 | <0.001 | <0.001 | <0.001 |
| Hypoxanthine (mg/100 g DM) | 202 | 63 | 78 | 68 | 73 | 8.10 | <0.001 | <0.001 | <0.001 |
CM, cooking method; FT, final core temperature; SEM, standard error of the mean; CM×FT, interaction between cooking method and final core temperature; TBARS, 2-thiobarbituric acid reactive substances; MDA, malondialdehyde; DM, dry matter.
pH, TBARS value and free iron content
Table 1 also shows the effects of cooking method and final core temperature on the meat pH, TBARS value and free iron content. As expected, cooking increased the pH of the meat by 0.13 to 0.29 units. Thermal denaturation and aggregation of muscle proteins and lipid oxidation are assumed to increase the pH value. Samples cooked by oven roasting had higher pH values than did samples cooked in boiling water (p<0.001), it decreased as final core temperature increased (p<0.01). Because oven-roasting required longer cooking times to reach 70°C and 77°C, lipid oxidation tended to occurred higher (p = 0.09) in oven-roasted samples than in boiled samples without any significant effects. However, final core temperature influenced the TBARS value significantly, even when the same cooking method was used. Samples cooked to 77°C had higher TBARS values (p<0.01) than samples cooked to 70°C. The concentration of free iron in meat has a positive correlation with the TBARS value [22]. In this study, the free iron content of oven-roasted group was higher (p = 0.02) than that of the boiled group. Moreover, in both cooking methods, a higher final core temperature resulted in a higher content of free iron. This suggests that prolonged exposure to heat increases the release of free iron to accelerate lipid oxidation.
Nucleotide-related compound content
The following water-soluble taste precursors were analyzed in this study: inosinic acid, inosine and hypoxanthine. Previous studies have shown that the content of inosinic acid and its breakdown compounds, i.e., inosine and hypoxanthine, is higher in chicken breast meat (deep pectoralis muscle) than in chicken thigh meat [16,23]. In this study, the concentration of inosinic acid was found to be relatively high in the deep pectoralis muscle of Hanwoo steers. Inosinic acid is associated with umami taste, whereas hypoxanthine is associated with the bitterness of cured meat [24,10]. Free ribose (a reducing sugar) and phosphate are released from the degradation of the remaining inosinic acid into the purine base hypoxanthine. Prolonged aging and elevated pH levels decrease the level of inosinic acid but increase the level of hypoxanthine [25]. In this study, both cooking methods reduced the concentration of these water-soluble flavor precursors in beef (p<0.001). This agrees with the previous report by Vani et al [23]. The concentration of water-soluble flavor precursors retained higher in oven-roasted samples than in boiled samples. In the oven-roasted group, the hypoxanthine content was higher in samples that were cooked to 77°C than in samples cooked to 70°C (interaction effects, p<0.001). These results suggest that cooking beef brisket by using a moist heat cooking method, such as in boiling water, extracts water-soluble flavor precursors, which is advantageous in the preparation of beef stock. In oven-roasted beef, higher levels of hypoxanthine may contribute to a bitter taste.
Fatty acid composition
The fatty acid composition of the beef brisket was influenced by both the cooking method and the final core temperature (Table 2). No significant effects of both cooking methods on the proportion of total saturated fatty acids (SFA). Final core temperature had significant role in the increasing proportion of SFA among cooking methods (p = 0.02), the increasing trend was found in C18:0 (stearic acid) particularly (p = 0.01). Although the proportion of C14:0 (myristic acid) significantly increased by cooking, among cooked samples, its proportion decreased (p<0.01) in samples that were cooked to 77°C. The proportion of monounsaturated fatty acids (MUFA) increased as the samples were cooked. This was caused by the increasing proportion of C16:1n7 (palmitoleic acid) after cooking. Nevertheless, cooking to higher final core temperature decreased the proportion of MUFA significantly (p = 0.03). Among the MUFA, the proportion of C18:1n9 (oleic acid) decreased as final core temperature increased regardless of the cooking method used. Cooking significantly reduced the proportion of total polyunsaturated fatty acids (PUFA) without any significant differences were found between cooking methods and final core temperatures. Total n-6 fatty acid content decreased after cooking as a result of decreased C18:2n6 (linoleic acid) content. No significant changes to the proportion of C18:3n6 (gamma linolenic acid) and C20:4n6 (arachidonic acid) were found. Although the total proportion of n-3 fatty acids was not affected by cooking, C20:5n3 (eicosapentaenoic acid, EPA) decreased significantly. A higher proportion of C18:3n3 (alpha linolenic acid) and total n-6 fatty acid content seemed to compensate for the decrease in EPA. The proportion of n-6 to n-3 fatty acid content remained unchanged. Except for SFA, the results of this study are in accordance with previous finding [26]. The highly oxidative nature of PUFA is associated with its decreased proportion and these changes affect other individual fatty acid composition.
Table 2.
Effects of cooking method and final core-temperature on fatty acid composition (% of total identified fatty acids) of Hanwoo brisket
| Item | Raw | CM | FT | SEM | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Boiled | Oven-roasted | 70°C | 77°C | CM | FT | CM×FT | |||
| C14:0 | 2.39 | 2.79 | 2.69 | 2.83 | 2.65 | 0.04 | 0.07 | <0.01 | 0.64 |
| C16:0 | 25.8 | 25.8 | 25.5 | 25.5 | 25.7 | 0.09 | 0.19 | 0.41 | 0.12 |
| C16:1n7 | 5.50 | 7.05 | 7.06 | 7.03 | 7.08 | 0.18 | 0.95 | 0.80 | 0.90 |
| C18:0 | 8.88 | 7.91 | 8.27 | 7.68 | 8.50 | 0.17 | 0.22 | 0.01 | 0.47 |
| C18:1n9 | 54.4 | 53.5 | 53.6 | 54.0 | 53.2 | 0.12 | 0.72 | <0.01 | 0.07 |
| C18:2n6 | 2.51 | 2.22 | 2.11 | 2.21 | 2.13 | 0.05 | 0.10 | 0.24 | 0.82 |
| C18:3n6 | 0.06 | 0.07 | 0.07 | 0.06 | 0.08 | 0.004 | 0.64 | 0.13 | 0.08 |
| C18:3n3 | 0.55 | 0.55 | 0.54 | 0.55 | 0.55 | 0.01 | 0.72 | 0.98 | 0.49 |
| C20:4n6 | 0.13 | 0.08 | 0.09 | 0.08 | 0.09 | 0.01 | 0.79 | 0.84 | 0.60 |
| C20:5n3 | 0.06 | 0.03 | 0.03 | 0.03 | 0.03 | 0.003 | 0.62 | 0.63 | 0.52 |
| SFA | 37.0 | 36.5 | 36.5 | 36.1 | 36.9 | 0.19 | 0.98 | 0.02 | 0.10 |
| MUFA | 59.6 | 60.6 | 60.6 | 61.0 | 60.3 | 0.21 | 0.73 | 0.03 | 0.12 |
| PUFA | 3.30 | 2.95 | 2.84 | 2.93 | 2.87 | 0.05 | 0.17 | 0.44 | 0.49 |
| PUFA/SFA | 0.09 | 0.08 | 0.08 | 0.08 | 0.08 | 0.001 | 0.23 | 0.18 | 0.29 |
| n6 | 2.69 | 2.37 | 2.27 | 2.35 | 2.29 | 0.05 | 0.16 | 0.39 | 0.58 |
| n3 | 0.61 | 0.58 | 0.57 | 0.57 | 0.57 | 0.01 | 0.70 | 0.96 | 0.48 |
| n6/n3 | 4.42 | 4.12 | 4.02 | 4.10 | 4.03 | 0.08 | 0.72 | 0.98 | 0.49 |
CM, cooking method; FT, final core temperature; SEM, standard error of the mean; CM×FT, interaction between cooking method and final core temperature; SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid.
Aroma volatiles
To discriminate the aroma pattern between treatments and raw samples, an electronic nose was used. The maximum resistance ratio of its sensors and the results of a PCA are reported in Table 3 and Figure 1, respectively. Sensor response was used to determine aroma intensity, and sensor PA2 showed the highest response. Among 6 sensors used, no differences were found on the response of sensor P10/2 among cooked samples. Sensor P10/1 detected the aroma differently based on different final core temperature only (p = 0.02). The maximum resistance ratio of sensor T30/1 and T70/2 was influenced by both cooking method (p< 0.001, p = 0.02) and final core temperature (p = 0.01, p = 0.04), while the response of sensor P40/1 (p = 0.03) and PA2 (p = 0.01) was only affected by cooking method. The PCA successfully discriminated each treatment with a discrimination index of 86 and a total contribution rate higher than 85% (C1, 98.19% and C2, 0.99%), which corresponds with results from a previous study [27]. Discrimination pattern went to the right as aroma intensity increased.
Table 3.
The maximum resistance ratio of six metal oxide sensors (MOS) of electronic nose
| Six sensors | Raw | CM | FT | SEM | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Boiled | Oven-roasted | 70°C | 77°C | CM | FT | CM×FT | |||
| T30/1 | 0.020 | 0.085 | 0.099 | 0.085 | 0.098 | 0.004 | <0.01 | 0.01 | 0.49 |
| P10/1 | 0.019 | 0.063 | 0.067 | 0.060 | 0.070 | 0.003 | 0.34 | 0.02 | 0.12 |
| P10/2 | 0.022 | 0.075 | 0.079 | 0.074 | 0.080 | 0.004 | 0.51 | 0.31 | 0.35 |
| P40/1 | 0.022 | 0.077 | 0.088 | 0.078 | 0.087 | 0.004 | 0.03 | 0.08 | 0.41 |
| T70/2 | 0.017 | 0.066 | 0.077 | 0.067 | 0.076 | 0.003 | 0.02 | 0.04 | 0.76 |
| PA2 | 0.028 | 0.116 | 0.135 | 0.121 | 0.130 | 0.005 | 0.01 | 0.20 | 0.81 |
CM, cooking method; FT, final core temperature; SEM, standard error of the mean; CM×FT, interaction between cooking method and final core temperature.
Figure 1.
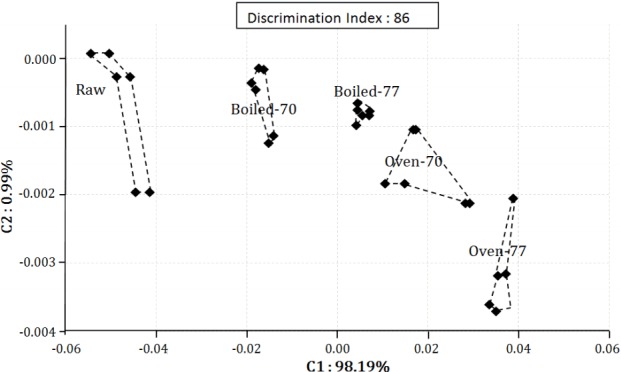
Effects of cooking method and final core-temperature on aroma pattern of Hanwoo brisket.
The results from the electronic nose are in accordance with the total peak area of identified volatile compounds that were analyzed using GC-MS. The effects of cooking method and final core temperature on aroma volatiles and their associated aroma descriptors are shown in Table 4. According to total peak area, samples oven-roasted to 77°C released the most intense aroma volatiles. Aldehydes derived from lipid oxidation (propanal, butanal, pentanal, hexanal, heptanal, octanal, and nonanal) and pyrazine (2-methyl and 2,5-dimethylpyrazine) were found to be predominant in this study. Among the aldehydes, hexanal dominated the other compounds with a peak area of 28.1 to 43.6 area unit×106/g dry matter. Cooking method had no effects on undecanone, 2-methyl-2-(methyldithio) propanal, nonanoic acid ester, toluene, octanal and nonanal, and final core temperature had no effects on 2-methyl butanal and nonanoic acid ester. The oven-roasted samples had higher abundance of 2-methyl butanal, pentanal, 1-pentanol, 2-methyl pyrazine, heptanal, 2(5H)-furanone, 2,5-dimethyl pyrazine and benzaldehyde, but less abundance of methylfuranthiol, 3-methyl butanal and 1-octen-3-ol than did the boiled samples. No interaction effects were observed on 2-methyl-2-(methyldithio) propanal, pentanal, nonanoic acid ester, hexanal, 1-octen-3-ol and total identified volatiles. The abundance of volatiles that was affected by final core temperature, increased as final core temperature increased except for 2-methyl pyrazine. According to published aroma descriptors, beef brisket that is cooked by oven roasting released more lipid oxidation-related volatiles such as hexanal and heptanal and roast beef-like volatiles (pyrazines), which were derived from the Maillard reaction [28]. In the oven-roasted samples, higher TBARS values and the loss of inosinic acid are attributed to the generation of aldehydes and pyrazines that are derived from lipid oxidation, Strecker degradation and Maillard reaction. The thermal degradation of inosinic acid and other proteins provides free ribose (reducing sugar) and amino acids that generate Maillard reaction products, such as benzaldehyde and pyrazines. The volatiles identified in this study are in line with previous reports using different types of muscles [29].
Table 4.
Effects of cooking method and final core-temperature on volatile compounds (area unit×106/g dry matter) of Hanwoo brisket
| Item | CM | FT | SEM | Aroma descriptor1) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Boiled | Oven-roasted | 70°C | 77°C | CM | FT | CM×FT | |||
| Undecanone | 3.54 | 3.63 | 2.93 | 4.23 | 0.41 | Green, fresh | 0.79 | <0.01 | 0.03 |
| Propanal, 2-methyl-2-(methyldithio) | 1.26 | 1.38 | 1.06 | 1.59 | 0.16 | Smoke, fat | 0.51 | <0.01 | 0.26 |
| Methylfuranthiol | 5.82 | 4.60 | 4.67 | 5.76 | 0.58 | Meat | 0.02 | 0.04 | 0.02 |
| Butanal, 3-methyl | 1.36 | 0.64 | 0.75 | 0.63 | 0.16 | Cocoa, almond | <0.001 | <0.001 | <0.001 |
| Butanal, 2-methyl | 0.50 | 0.87 | 0.75 | 0.63 | 0.11 | Malt | <0.01 | 0.24 | <0.01 |
| Pentanal | 14.3 | 21.7 | 16.8 | 19.1 | 1.90 | Almond, pungent | <0.001 | <0.01 | 0.78 |
| Nonanoic acid ester | 2.06 | 3.70 | 1.98 | 3.77 | 0.74 | Green, fat | 0.16 | 0.13 | 0.12 |
| Toluene | 0.67 | 0.77 | 0.65 | 0.79 | 0.07 | Paint | 0.06 | 0.01 | <0.01 |
| 1-Pentanol | 10.1 | 16.4 | 10.3 | 16.2 | 1.86 | Balsamic | <0.001 | <0.001 | <0.001 |
| Hexanal | 33.4 | 38.2 | 28.1 | 43.6 | 3.88 | Tallow, fat | <0.01 | <0.001 | 0.40 |
| Pyrazine, 2-methyl | 1.46 | 1.63 | 1.86 | 1.23 | 0.22 | Nut, cocoa, meat | 0.01 | <0.001 | <0.001 |
| Heptanal | 5.53 | 10.6 | 5.60 | 10.5 | 1.38 | Fat, rancid | <0.001 | <0.001 | 0.02 |
| 2(5H)-Furanone | 0.87 | 1.89 | 1.11 | 1.65 | 0.21 | Buttery | <0.001 | <0.001 | <0.001 |
| Pyrazine, 2,5-dimetyl | 0.85 | 1.51 | 0.96 | 1.40 | 0.15 | Roast beef | <0.001 | <0.001 | <0.001 |
| Benzaldehyde | 0.91 | 1.53 | 0.78 | 1.67 | 0.20 | Almond, burnt sugar | <0.001 | <0.001 | <0.001 |
| 1-octen-3-ol | 2.89 | 1.93 | 1.90 | 2.92 | 0.30 | Mushroom | <0.001 | <0.001 | 0.33 |
| Octanal | 4.20 | 4.62 | 2.57 | 6.24 | 0.69 | Fat | 0.07 | <0.001 | <0.001 |
| Nonanal | 1.90 | 2.14 | 1.14 | 2.91 | 0.36 | Fat | 0.33 | <0.001 | <0.01 |
| Total volatiles | 91.6 | 117.7 | 83.9 | 124.8 | 5.41 | - | <0.001 | <0.001 | 0.06 |
CM, cooking method; FT, final core temperature; SEM, standard error of the mean; CM×FT, interaction between cooking method and final core temperature.
Published aroma descriptor was adapted from http://www.flavornet.org/
CONCLUSION
Prolonged heat exposure (oven roasting to 77°C) increased the lipid oxidation, free iron content, aldehydes derived from lipid oxidation (pentanal, hexanal, heptanal, octanal, nonanal), Stecker aldehyde (benzaldehyde) and pyrazines (2-methylpyrazine and 2,5-dimethylpyrazine) but retained more hypoxanthine in the beef brisket than did cooking to medium or cooking in boiling water. Cooking to a higher final core temperature reduced the concentrations of inosinic acid, regardless of the cooking method used. However, boiling allowed more inosinic acid to be extracted by the water. This evidence shows that it is recommended to cook beef brisket slowly in boiling water to extract fat and water-soluble taste precursors and that this should be followed by roasting to enhance the aroma.
ACKNOWLEDGEMENTS
This research was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bioindustry Technology Development Program funded by Ministry of Agriculture, Food and Rural Affairs, Republic of Korea (Project No. 315017-05).
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Jeremiah LE, Gibson LL, Aalhus JL, Dugan MER. Assessment of palatability attributes of the major beef muscles. Meat Sci. 2003;65:949–58. doi: 10.1016/S0309-1740(02)00307-8. [DOI] [PubMed] [Google Scholar]
- 2.Blackmon T, Miller RK, Kerth C, Smith SB. Ground beef patties prepared from brisket, flank and plate have unique fatty acid and sensory characteristics. Meat Sci. 2015;103:46–53. doi: 10.1016/j.meatsci.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Barham P, Skibsted LH, Bredie WLP, et al. Molecular gastronomy: a new emerging scientific discipline. Chem Rev. 2010;110:2313–65. doi: 10.1021/cr900105w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank D, Joo S, Warner R. Consumer acceptability of intramuscular fat. Korean J Food Sci Anim Resour. 2016;36:699–708. doi: 10.5851/kosfa.2016.36.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahidi F. Flavor of meat and meat products. 1st ed. Salisbury, England: Springer-Science+Business Media; 1994. [Google Scholar]
- 6.Dashdorj D, Amna T, Hwang I. Influence of specific taste-active components on meat flavor as affected by intrinsic and extrinsic factors: an overview. Eur Food Res Technol. 2015;241:157–71. [Google Scholar]
- 7.Zhang F, Klebansky B, Fine RM, et al. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA. 2008;105:20930–4. doi: 10.1073/pnas.0810174106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank D, Ball A, Hughes J, et al. Sensory and flavor chemistry characteristics of Australian beef: influence of intramuscular fat, feed, and breed. J Agric Food Chem. 2016;64:4299–311. doi: 10.1021/acs.jafc.6b00160. [DOI] [PubMed] [Google Scholar]
- 9.Lim D, Cha J, Jo C, et al. Comparison of physicochemical and functional traits of Hanwoo steer beef by the quality grade. Korean J Food Anim Resour. 2014;34:287–96. doi: 10.5851/kosfa.2014.34.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichimura S, Nakamura Y, Yoshida Y, Hattori A. Hypoxanthine enhances the cured meat taste. Anim Sci J. 2016;88:379–85. doi: 10.1111/asj.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Jeong D, Na C, Hwang I. Eating qulity traits of Hanwoo longissimus dorsi muscle as a function of end-point cooking temperature. Korean J Food Sci Anim Resour. 2016;36:291–9. doi: 10.5851/kosfa.2016.36.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung E, Hwang Y, Joo S. The relationship between chemical compositions, meat quality, and palatability of the 10 primal cuts from Hanwoo steer. Korean J Food Anim Resour. 2016;36:145–51. doi: 10.5851/kosfa.2016.36.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AOAC . Official Methods of Analysis. 17th ed. Gaithersburg, MD, USA: AOAC International; 2002. [Google Scholar]
- 14.Sinnhuber RO, Yu TC. The 2-thiobarbituric acid reaction, an objective measure of the oxidative deterioration occurring in fat and oil. J Oleo Sci. 1977;26:259–67. [Google Scholar]
- 15.Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine) Anal Biochem. 1971;40:450–8. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- 16.Jayasena DD, Jung S, Kim HJ, et al. Comparison of quality traits of meat from Korean native chickens and broilers used in two different traditional Korean cuisines. Asian-Australas J Anim Sci. 2013;26:1038–46. doi: 10.5713/ajas.2012.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloaney Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.AOAC . Official Methods of Analysis. 16th ed. Gaithersburg, MD: AOAC International; 1995. [Google Scholar]
- 19.Ba HV, Oliveros MC, Ryu K, Hwang I. Development of analysis condition and detection of volatile compounds from cooked Hanwoo beef by SPME-GC/MS analysis. Korean J Food Sci Anim Resour. 2010;30:73–86. [Google Scholar]
- 20.Goñi SM, Salvadori VO. Prediction of cooking times and weight losses during meat roasting. J Food Eng. 2010;100:1–11. [Google Scholar]
- 21.Utama DT, Lee SG, Baek KH, et al. Correlation between antioxidant enzyme activity, free iron content and lipid oxidation in four lines of Korean native chicken meat. Korean J Food Sci Anim Resour. 2016;36:44–50. doi: 10.5851/kosfa.2016.36.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domínguez R, Gómez M, Fonseca S, Lorenzo JM. Effect of different cooking methods on lipid oxidation and formation of volatile compounds in foal meat. Meat Sci. 2014;97:223–30. doi: 10.1016/j.meatsci.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Vani ND, Modi VK, Kavitha S, Sachindra NM, Mahendrakar NS. Degradation of inosine-5′-monophosphate in aqueous and in laying chicken muscle fiber system: effect of pH and temperature. Lebenson Wiss Technol. 2006;39:627–32. [Google Scholar]
- 24.Nishimura T, Goto S, Miura K, et al. Umami compounds enhance the intensity of retronasal sensation of aromas from model chicken soups. Food Chem. 2016;196:577–83. doi: 10.1016/j.foodchem.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Tikk M, Tikk K, Tørngren MA, et al. Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J Agric Food Chem. 2006;54:7769–77. doi: 10.1021/jf060145a. [DOI] [PubMed] [Google Scholar]
- 26.Alfaia CMM, Alves SP, Lopes AF, et al. Effect of cooking methods on fatty acids, conjugated isomers of linoleic acid and nutritional quality of beef intramuscular fat. Meat Sci. 2010;84:769–77. doi: 10.1016/j.meatsci.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Seburg RA, Tsai E, Puech S, Mifsud JC. Flavor analysis in a pharmaceutical oral solution formulation using an electronic-nose. J Pharm Biomed Anal. 2004;34:453–61. doi: 10.1016/s0731-7085(03)00651-4. [DOI] [PubMed] [Google Scholar]
- 28.Back HH. Process flavors. In: Nollet LML, editor. Handbook of meat, poultry, and seafood quality. Ames, IA, USA: Blackwell Publishing; 2007. pp. 311–26. [Google Scholar]
- 29.Legako JF, Brooks JC, O’Quinn TG, et al. Consumer palatability scores and volatile beef flavor compounds of five USDA quality grades and four muscles. Meat Sci. 2015;100:291–300. doi: 10.1016/j.meatsci.2014.10.026. [DOI] [PubMed] [Google Scholar]


