Abstract
Explosives create shockwaves that cause blast-induced neurotrauma, one of the most common types of traumatic brain injury (TBI) linked to military service. Blast-induced TBIs are often associated with reduced cognitive and behavioral functions due to a variety of factors. To study the direct effects of military explosive blasts on brain tissue, we removed systemic factors by utilizing rat hippocampal slice cultures. The long-term slice cultures were briefly sealed air-tight in serum-free medium, lowered into a 37 °C water-filled tank, and small 1.7-gram assemblies of cyclotrimethylene trinitramine (RDX) were detonated 15 cm outside the tank, creating a distinct shockwave recorded at the culture plate position. Compared to control mock-treated groups of slices that received equal submerge time, 1–3 blast impacts caused a dose-dependent reduction in the AMPA receptor subunit GluR1. While only a small reduction was found in hippocampal slices exposed to a single RDX blast and harvested 1–2 days later, slices that received two consecutive RDX blasts 4 min apart exhibited a 26–40% reduction in GluR1, and the receptor subunit was further reduced by 64–72% after three consecutive blasts. Such loss correlated with increased levels of HDAC2, a histone deacetylase implicated in stress-induced reduction of glutamatergic transmission. No evidence of synaptic marker recovery was found at 72 h post-blast. The presynaptic marker synaptophysin was found to have similar susceptibility as GluR1 to the multiple explosive detonations. In contrast to the synaptic protein reductions, actin levels were unchanged, spectrin breakdown was not detected, and Fluoro-Jade B staining found no indication of degenerating neurons in slices exposed to three RDX blasts, suggesting that small, sub-lethal explosives are capable of producing selective alterations to synaptic integrity. Together, these results indicate that blast waves from military explosive cause signs of synaptic compromise without producing severe neurodegeneration, perhaps explaining the cognitive and behavioral changes in those blast-induced TBI sufferers that have no detectable neuropathology.
Keywords: Blast-induced injury, Military explosive, GluR1, RDX, Shockwave, Synaptic decline, Synaptophysin, TBI, Traumatic brain injury
1. Introduction
Traumatic brain injury (TBI) and the related neurodegenerative complications of chronic traumatic encephalopathy (CTE) represent serious public health problems, contributing to a substantial number of deaths and cases of permanent disability. Nearly 2% of the population in the United States lives with disabilities caused by a TBI, among the approximately 2 million people each year that experience different types of traumatic injuries (Centers for Disease Control and Prevention. Traumatic brain injury in the United States: A Report to Congress, 1990). TBI and mild TBI can cause varying levels of CNS deterioration and dysfunction, leading to neurological and psychiatric problems (see Schwarzbold et al., 2008; Orlovska et al., 2014). This serious public health issue has been enhanced by the increased frequency of sports-related head injuries and battlefield exposures to anti-personnel mines and other explosive devices, as well as by improved detection of injuries.
TBI is a common injury among veterans returning from Iraqi and Afghan theaters. According to the Brain Trauma Foundation, 10–20% of Iraqi veterans suffer from the traumatic brain disorder, and a major problem exists due to the number of soldiers from the two wars with the signature injury (Williamson and Mulhall, 2009). The physical injury and psychological trauma have overlapping symptoms that may involve overlapping mechanisms in the brain (Miller, 2011). A wide variety of explosive devices cause primary blast-induced neurotrauma, one of the most common types of TBI associated with military service. A primary blast injury occurs due to changes in transient overpressure waves as a result of the detonation. Explosives create a characteristic shockwave consisting of an initial positive-pressure blast wave followed by a negative-pressure phase. The latter produces a distinct cavitation event in the brain that is thought to be a key factor underlying blast-induced injury (Moore and Jaffee, 2010; Goeller et al., 2012). Molecular and cellular changes associated with TBIs often result in the disruption of cognition, behavioral functions, and cell viability. While trying to understand the pathogenic mechanisms involved, most clinical data on blast-induced TBI include secondary traumatic events, thus limiting useful information regarding the direct effects of a primary blast (Elder et al., 2010). Accordingly, several in vitro studies have used shock tube systems to model injury mediated by transient overpressure waves in neuroblastoma cells (Arun et al., 2011), endothelial cells of the blood-brain barrier (Hue et al., 2013, 2014), and brain slices (Effgen et al., 2012, 2014). In order to study the specific blast waves generated by military explosives, a novel procedure was recently developed that generates reproducible shockwaves from a detonated explosive (Zander et al., 2015). The procedure uses a highly controlled construct of research department explosives (RDX), the major component of C-4 explosive and one of the most powerful military explosives.
In order to examine the direct effects of military explosives on brain tissue, the present study utilized rat hippocampal slice cultures that stably maintain the distinctive subfields of the hippocampus, as well as native neuronal morphology and connectivity (Muller et al., 1993; Bahr, 1995; Bahr et al., 1995a). The hippocampus is the main focus of this study not only due to it being distinctly vulnerable to traumatic and excitotoxic injuries, but also because it is a region that is important for higher order brain functions, that expresses synaptic plasticity to compute diverse information, and that is involved in routing encoded spatial, emotional, and reward information to other brain areas (see Bahr et al., 1998; Szinyei et al., 1999; Pelletier and Lacaille, 2008; Schober et al., 2014; Carlson et al., 2015; Ciocchi et al., 2015). The organotypic cultures allow studies to address specific issues in the absence of systemic variables, thus avoiding interpretation issues in the present study regarding whether subtle vs. severe neuronal changes are produced by single or multiple RDX blasts. Such slice cultures have proved valuable for determining pathways of neuropathology because of their similar cellular and genetic responses to various insults as compared to in vivo studies describing the unique pathogenic responsiveness of the hippocampus (Vornov et al., 1994; Bahr et al., 2002; Bendiske et al., 2002; Caba and Bahr, 2004; Bonde et al., 2005; Karanian et al., 2005; Noraberg et al., 2005; Wisniewski et al., 2011). In the current report, cultured hippocampal slices were placed in a specialized chamber in which defined assemblies of RDX were detonated outside the chamber to generate blast shockwaves from the military charge. Of particular interest were the negative effects of the explosive nitroamine on indicators of synaptic integrity.
2. Materials and methods
Sprague-Dawley rat litters (Charles River Laboratories, Wilmington, MA) were housed in accordance with guidelines from the National Institutes of Health. Hippocampal slice cultures were prepared from 12-day-old rats as previously described (Bendiske et al., 2002; Wisniewski et al., 2011). Slices were cut from pre-cooled hippocampi and briefly placed in ice-cold buffer containing 124 mM NaCl, 3 mM KCl, 2 mM CaCl2, 4 mM MgSO4, 1.25 mM KH2PO4, 26 mM NaHCO3, 10 mM d-glucose, and 2 mM ascorbic acid. Groups of 8–9 slices were quickly distributed on the Biopore PTFE membrane of each Millicell-CM culture insert (Fisher Scientific; Pittsburgh, PA) which was in contact with culture medium containing 50% Basal Medium Eagle, 25% Earl's balanced salt solution, 25% regular horse serum, and the following concentrations of supplements: 136 mM glutamine, 40 mM glucose, 0.5 mM ascorbic acid, 20 mM HEPES buffer (pH 7.3) 1 mg/l insulin, 5 units/ml of penicillin, and 5 mg/l streptomycin. Medium was changed every 2–3 days and the surface of the slices exposed to humidified air plus 5% CO2 at 37 °C. The slices consistently display characteristics of the adult hippocampus by two weeks in culture, including the distinct change in glycosylation state of adhesion molecules (Bahr, 1995; Bahr et al., 1995a; Hoffman et al., 2001), and such synaptic maturation markers are routinely confirmed in each preparation of slice cultures.
The blast protocol was conducted at culture day 22–28, in which hippocampal slices in a 6-well plate were sealed within an air-tight containment filled with serum-free media (horse serum was replaced with HEPES-buffered saline). Next, the plate was clamped in a vertical position using a rig that was then secured within a 37 °C water tank, 18 cm from the front inner wall. The vertical position directs the military blast wave to the surface of the brain slices, thus minimizing lateral stress on adhesion between the tissue and culture insert membranes. Reproducible assemblies of RDX (1.7 g) were positioned to produce an explosive blast 15 cm outside the tank's 2-cm wall, creating a well-defined air shockwave that propagates into and through the volume of water to the culture plate (total standoff distance = 35 cm). The specific setup distances took into consideration 1) the small explosive charge size that was accessible for the study and its short duration, 2) the charge size and number of consecutive charges that could be detonated in the Aberdeen Proving Ground facility by Army Research Laboratory personnel, and 3) the desired pressure impact for tests on cellular/synaptic integrity while avoiding damage to the culture insert Biopore support membrane or the air-tight containment. Control slices in a similar 6-well plate received mock treatment of equal submerge time in the containment with serum-free media. The slice cultures (50–60 per condition) were exposed to 1–3 RDX blasts approximately 4 min apart, and then quickly returned to normal culture conditions. One to two days after the blast event(s), slices were gently harvested into groups of 7– 9 each using ice-cold isosmotic buffer containing protease inhibitors, then homogenized in lysis buffer (Karanian et al., 2005). Some slice cultures were first infused with 100 μM N-methyl-d-aspartate (NMDA) in serum-free media, and incubated for 5 min at 37 °C before being rapidly harvested in isosmotic buffer containing phosphatase inhibitors. For a positive control of excitotoxicity, other slices were 1) treated with 100 μM α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) for 15 min at 37 °C, 2) given three media changes to washout the AMPA, 3) infused with CNQX and MK801 during the media changes in order to quench further glutamatergic activity, and 4) harvested 30– 48 h later in ice-cold buffer containing protease inhibitors. A subset of slices were fixed and subjected to Fluoro-Jade B staining (Neuroscience Associates; Knoxville, Tennessee) or anti-synaptophysin immunocyto-chemistry. Images were acquired using a Nikon Ti-E microscope linked to the C2 confocal system and software, as well as with Zeiss 710 and Zeiss 780 confocal systems.
Protein content of the slice samples was determined with a BSA standard, and equal protein aliquots were assessed by immunoblot for synaptic markers. Blots were incubated overnight at 4 °C with anti-synaptophysin (Boehringer Mannheim, Indianapolis, Indiana), anti-GluR1 (Millipore, Billerica, Massachusetts), anti-HDAC2 (Santa Cruz Biotechnology, Santa Cruz, California), anti-pCofilin-s3 (Abcam Inc., Cambridge, Massachusetts), anti-active MAPK (pERK1/pERK2; Cell Signaling, Beverly, Massachusetts), and antibodies that selectively recognize calpain-mediated spectrin breakdown product (Bahr et al., 1995b). Anti-IgG-alkaline phosphatase and anti-IgG-horse radish per-oxidase conjugates were used for the secondary antibody step, and development of antigen staining used either the 5 bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium substrate system or a chemiluminescence protocol (GE Amersham Imager AI600RGB). Each immunoblot was also stained with anti-actin (Sigma, St. Louis, MO) to provide a protein load control. Stained antigens were analyzed to determine integrated optical density (IOD) with BIOQUANT software (R & M Biometrics, Nashville, Tennessee). Within blots, each IOD value was normalized to percent of mock treatment control samples, and the means ± SEM were compared using unpaired, two-tailed t-tests or ANOVA.
3. Results
In order to examine the direct effects of military explosive blast waves on brain tissue, rat hippocampal slice cultures were utilized. The slices are typically maintained for 1–2 months living on culture insert membranes (Fig. 1A), displaying many characteristics of the adult hippocampus including distinct features of synaptic maturation by culture day 10–14 (Muller et al., 1993, 1996; Bahr et al., 1995a; Hoffman et al., 2001). Confirmed by routine histology, the long-term slice cultures retain the characteristic neuronal density and subfields of the hippocampus (Fig. 1B). For the RDX blast protocol, a six-well plate containing 50–60 hippocampal slices was sealed in serum-free medium and clamped in position within a warmed, water-filled tank (Fig. 1C). A spherical assembly of RDX explosive (Fig. 1D) was then quickly detonated outside the tank, producing an air blast and propagating pressure wave. The shockwave traveled through the poly(methyl methacrylate) tank wall into the water medium, and sensors located immediately above the culture plate measured a fast onset pressure profile followed by rapid decay and a negative region of the pressure history (Fig. 1E). Across blast experiments, the sensors recorded similar pressure-time profiles, with mean peak pressures of 110–115 psi found for the three sensor positions (see Table 1). Within each individual detonation, the average variance from the three peak pressure measures was 10.3 psi, thus 9.34% of the typical peak pressure.
Fig. 1.
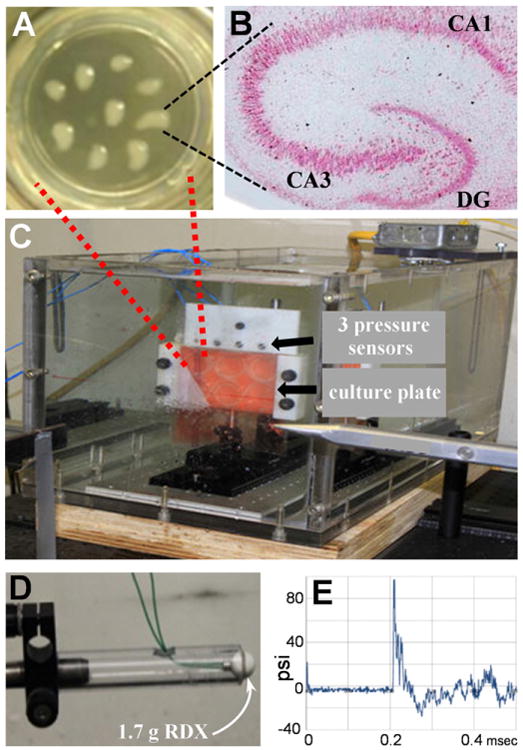
Hippocampal slice cultures were used to study the direct effects of blast waves from military explosive. Slices at culture day 4 are shown distributed on a 3-cm culture insert membrane (A). A hippocampal slice at culture day 28 displays features of the adult brain including intact hippocampal subfields visualized by H and E staining (B; view-field width: 3.5 mm). Slice cultures in a six-well plate were sealed in serum-free media then positioned within a warmed, water-filled tank (C). Three piezoelectric, high frequency pressure sensors are located directly above the clamped culture plate. A spherical assembly of RDX explosive (D) was detonated outside the water tank and the generated blast wave traveled through the wall of the tank, into the water medium, and was measured as a pressure history profile (E). DG, dentate gyrus.
Table 1.
Three sensors measured similar peak pressures across RDX detonations.
| Sensor position | Peak pressure, psi |
|---|---|
| Left | 109.5 ± 5.82 |
| Middle | 114.9 ± 7.40 |
| Right | 114.5 ± 8.38 |
Hippocampal slice cultures were sealed in serum-free medium and clamped within a water-filled tank with three high frequency pressure sensors positioned directly above the tissue culture plate. Upon detonation of live explosive outside the tank (1.7 g RDX), peak pressures resulting from the propagating pressure wave were measured by the three sensors (mean psi ± SEM, n = 12).
The first tests subjected hippocampal slice cultures to a single RDX blast or to 2–3 consecutive RDX blasts 4 min apart in order to examine the effects on the AMPA receptor subunit GluR1, also called GluA1 (Fig. 2A). The anti-GluR1 immunoblots were subsequently assessed for actin levels, confirming that equal protein was loaded for the different slice samples. In Fig. 2B, the explosive blasts are shown causing a dose-dependent reduction in GluR1 (ANOVA: p < 0.0001). The single RDX detonation was associated with a small 18.3 ± 12% reduction in GluR1 (n = 6 groups of 7–8 slices each) at 24–48 h post-blast, with wide variance and not significant compared to control hippocampal slices exposed to equal submerge time in the tank setup. A decline of 33.1 ± 7.5% (t-test: p = 0.011) was found in slice samples receiving two consecutive RDX blasts 4 min apart, and after three consecutive blasts GluR1 levels were reduced by 67.7 ± 4.5% (n= 7). The reduction in the triple-blast slice groups was significant in post hoc tests compared to both mock treatment control slices (p < 0.0001) and slices that received a single RDX blast (p = 0.001). The blast-induced loss of GluR1 was rapid, with much of the decline evident 24 h after the multiple detonations (Fig. 2C). The initial 50% reduction in GluR1 was followed by an additional 10–15% loss at 45 h post-blast, and no indication of recovery of the synaptic marker was found at 72 h. During the post-blast period with marked synaptic decline, actin levels were unchanged (Fig. 2D).
Fig. 2.
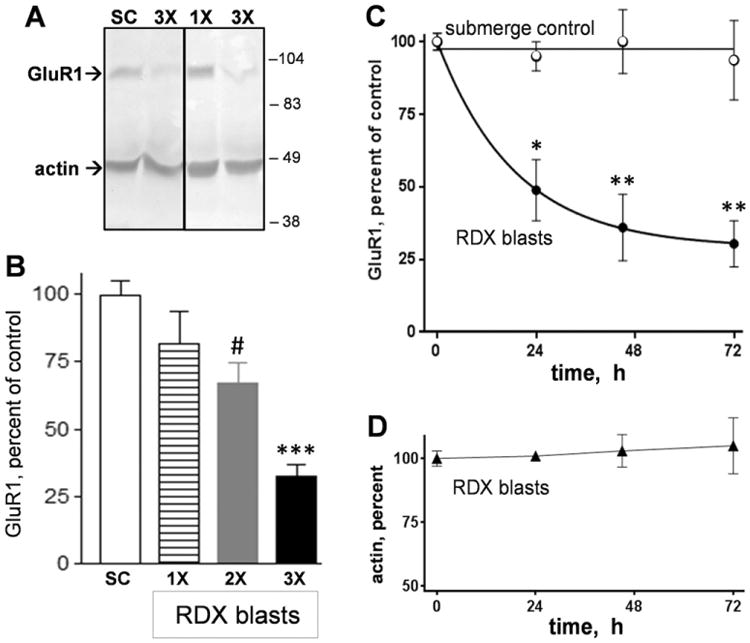
Military explosive blasts from 1.7-gram RDX assemblies cause a dose-dependent and time-dependent reduction in the AMPA receptor subunit GluR1. Hippocampal slice cultures exposed to 1–3 RDX blasts (1×–3×) were assessed together with mock submerge control slices (SC) by anti-GluR1 immunoblotting (A). Each blot was subsequently stained for actin for a protein load control, and positions for molecular weight standards are shown for 38–104 kDa.GluR1 immunoreactivities (means±SEM) are shown as percent to levels found in submerge control groups of slices (B) (ANOVA: p < 0.0001; compared to submerge control slices: #p = 0.011, ***p < 0.0001). Other slice cultures were subjected to RDX detonations or mock treatment, then harvested to assess GluR1 levels across post-blast times (C) (two-way ANOVA: p < 0.0001; comparison between control and RDX blast groups at each time point: *p < 0.05; **p < 0.01). The blast samples were also assessed for actin levels (D).
It is of interest that the military blast-induced synaptic decline corresponds with increases in HDAC2, a protein whose upregulation has been linked to GluR1 degradation (Wei et al., 2016). As shown in Fig. 3, the loss of GluR1 immunoreactivity correlates with increased HDAC2 levels within multiple-blast slice samples that were harvested across post-blast times. In the plotted immunoreactivity levels from individual slice samples, linear regression analysis found a significant correlation between indications of GluR1 removal and HDAC2 enhancement (R = −0.611, p < 0.01).
Fig. 3.
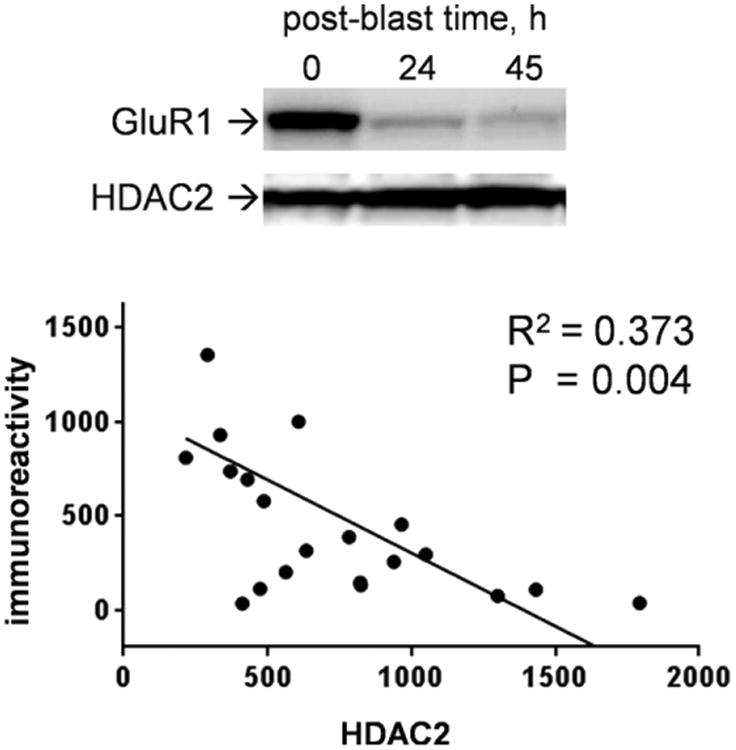
RDX blast-induced loss of GluR1 immunoreactivity in brain tissue samples corresponds with increased levels of the HDAC2 protein. Hippocampal slice cultures were subjected to three consecutive detonations of the military explosive then harvested over time in order to assess GluR1 and HDAC2 in the same immunoblot lanes (top). The immunoreactivity levels of the synaptic marker and HDAC2 across individual slice samples were plotted again each other and linear regression resulted in a significant correlation (R2 = 0.373; two-tailed, one-sample t-test on resulting slope: p = 0.004).
To determine if a presynaptic protein is susceptible to the explosive blasts, a subset of hippocampal slice samples were also assessed for synaptophysin (Fig. 4A). In the triple-blast slice groups that were assessed for both pre- and postsynaptic markers, synaptophysin was reduced by 50% (p < 0.0001; n = 10) while GluR1 levels were reduced by 57% (p < 0.0001; n = 13) as compared to mock treatment submerge control slices (Fig. 4B). Thus, the two synaptic markers exhibit similar susceptibility to consecutive RDX detonations in the cultured slice model. Synaptophysin also exhibited susceptibility following a single RDX blast, being reduced by 40% (Fig. 4B; p < 0.01). Thus, disruption of synaptic integrity is indicated by two important components of hippocampal synapses.
Fig. 4.
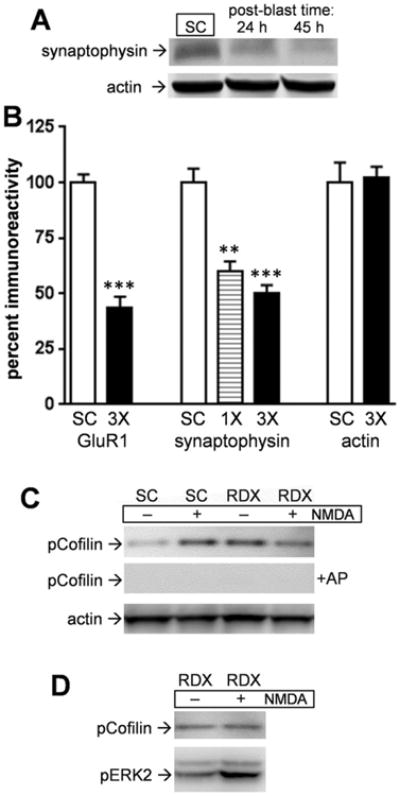
Pre- and postsynaptic markers exhibit similar susceptibility to military RDX blasts. Hippocampal slice cultures were subjected to 3 consecutive RDX detonations then harvested over time in order to assess synaptophysin and actin levels by immunoblot (A). Slices from single (1×) and 3 consecutive RDX blasts (3×) were assessed for levels of the two proteins, as well as for GluR1 for comparison, which were normalized within-blot to respective measures from submerge control slices (SC) and the data sets shown (B) (means ± SEM; unpaired t tests compared to controls: **p < 0.01, ***p < 0.0001). At 24 h post-blast, slice cultures treated with 2 RDX blasts and control slices were infused with 100 μM NMDA for 5 min and harvested in buffer containing phosphatase inhibitors. The identical samples on two nitrocellulose strips were incubated with or without alkaline phosphatase (AP) for 2 h at 37 °C before phospho-cofilin immunostaining (C). Each blot was subsequently stained for actin. Another set of double-blast slices were subjected to the NMDA treatment to assess pCofilin and pERK2 in the same slice samples (D).
Another test of synaptic integrity involved slice cultures subjected to two RDX detonations to assess for NMDA-mediated cofilin phosphorylation, a pathway component initiated by long-term potentiation and functional dynamics in the hippocampus (Rex et al., 2010). The RDX-treated and submerge control slices were infused with NMDA for 5 min followed by rapid harvesting, showing that only control slices exhibited increased phospho-cofilin after the brief NMDA receptor stimulation (Fig. 4C). High levels of phospho-cofilin were evident in the RDX-treated slices and NMDA infusion was unable to enhance such levels. Phosphorylation-specific antibody recognition was confirmed by pre-treating the same nitrocellulose-bound slice samples for 2 h with alkaline phosphatase. While the two consecutive RDX blasts altered NMDA receptor-cofilin signaling, they did not disrupt all neuronal pathways since NMDA was able to induce phosphorylation of the ERK2 isoform of MAPK (Fig. 4D).
The triple-blast slice groups were also tested for degenerative loss of the housekeeping protein actin. In the same slice groups exhibiting pre-and postsynaptic marker declines, actin levels were unchanged (102.3 ± 4.7% of the level found in control slices) (Fig. 4B). This lack of change in a key cytoskeletal protein suggests that the shockwaves produced by 1.7-g RDX explosives, while able to disrupt synaptic integrity, do not elicit overt cellular deterioration. To further address this issue, submerge control and multiple-blast slice groups were subjected to synaptophysin immunohistochemistry, DAPI counterstain, and Fluoro-Jade B staining (Fig. 5A). The punctate synaptophysin immuno-staining was reduced >90% (area fraction and intensity) and was disorganized in the stratum radiatum after the blast events, whereas the same image areas exhibited no reductions in DAPI staining and no evidence of nuclear shrinkage. Fluoro-Jade B staining also found no indication of degenerating neurons in the pyramidal fields of hippocampal slices exposed to single or triple RDX blast treatment (Fig. 5A and B). In contrast, extensive Fluoro-Jade B staining was found in slice cultures that were subjected to a 15-min excitotoxic insult using AMPA (Fig. 5B). The AMPA exposure also induced calpain-mediated spectrin breakdown product (BDP), an indicator of cellular damage (see Bahr et al., 1995b, 2002), whereas triple RDX blast slices with significantly-reduced GluR1 had no indication of BDP generation (Fig. 5C). While spectrin breakdown has long been a common factor in studies of brain injury, excitotoxicity, and other models of neuronal over-activation (see review: Vanderklish and Bahr, 2000), the 1.7-g RDX blast protocol appears to produce marked synaptic decline without the buildup of stable BDPs.
Fig. 5.
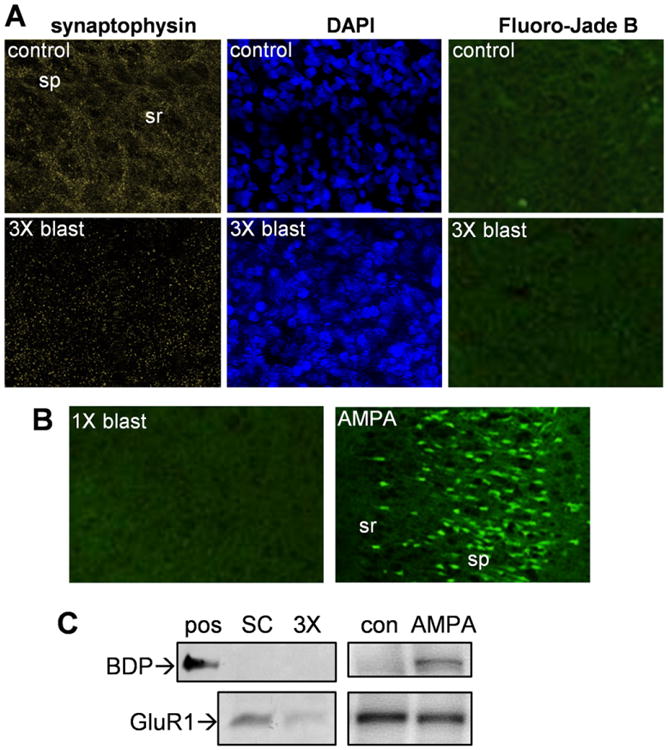
Military explosive detonations of 1.7-gram RDX assemblies produced synaptic marker decline but no evidence of cellular damage. Hippocampal slice cultures from a control mock treatment group and a triple-blast group were fixed 45 h after the detonation events and assessed for anti-synaptophysin immunostaining and DAPI counterstaining (A), and images from the same CA1 area are shown (view-field width: 180 μm). The same slices were also subjected to Fluoro-Jade B staining and a larger area imaged due to no cellular staining evident (A; view-field width: 400 μm). A positive control for the Fluoro-Jade stain consisted of slice cultures treated with 100 μM AMPA for 15 min, the excitotoxic insult then quenched with washes containing CNQX and MK-801, and the tissue fixed 48 h post-insult for CA1 imaging (B). In the same area of a slice subjected to a single RDX blast, Fluoro-Jade B did not stain any degenerating pyramidal neurons (view-field width: 450 μm). sp, stratum pyramidale; sr, stratum radiatum. Spectrin breakdown product (BDP) was assessed by immunoblot in a positive control sample of calpain-mediated BDP (pos) and in hippocampal slices from the submerge control group (SC), a triple-blast group (3×), untreated control group (con), and AMPA-insult group (D). The same blots were assessed for GluR1 immunostaining.
Another test was done to address whether the RDX blast insult is related to excitotoxicity with regards to synaptic marker reductions. In Fig. 6, the synaptic decline profile induced by RDX detonations was compared to 1) synaptic declines reported in excitotoxicity studies using hippocampal slice cultures (Bahr et al., 2002; Karanian et al., 2005; Naidoo et al., 2012) vs. 2) hippocampal slice synaptic declines from protein accumulation stress studies (Bendiske and Bahr, 2003; Butler et al., 2007; Wisniewski et al., 2011). As shown, the synaptic marker loss profile for multiple RDX blasts (solid black line) aligns much closer to the excitotoxic profile than the protein accumulation stress profile.
Fig. 6.
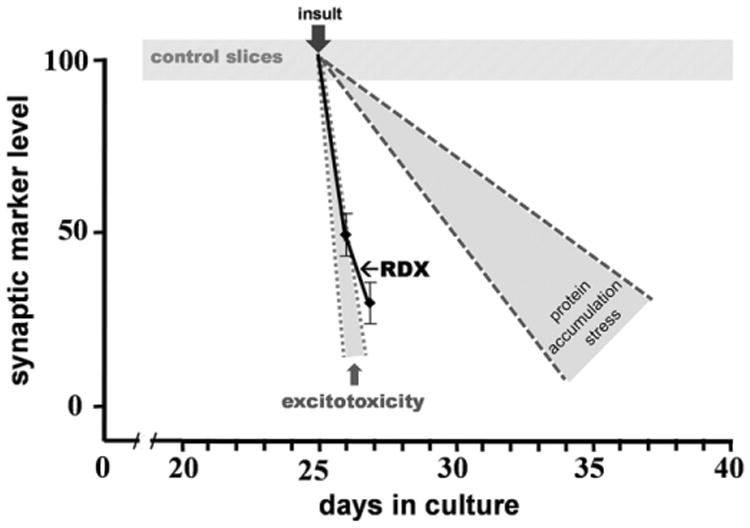
The blast-induced synaptic decline profile was compared to synaptic decline profiles related to excitotoxicity (small gray zone) and protein accumulation stress (large gray zone) summarized from previous hippocampal slice studies. Synaptic marker results normalized to control slices were compiled from reports on excitotoxic over-activation of glutamate receptors (Bahr et al., 2002; Karanian et al., 2005; Naidoo et al, 2012) and protein accumulation stress (Bendiske and Bahr, 2003; Butler et al., 2007; Wisniewski et al., 2011) to generate the most common “zone” for the two types of synaptic decline, the disparate insults being initiated at the typical time of culture day 25. Control slices from the current study as well as from several previous studies together showed that synaptic marker levels were stable across the period of 10–40 days in culture (hatched area). Triple RDX blast-induced synaptic marker loss is shown with the solid black line.
4. Discussion
Explosive blasts produce one of the most common types of TBI associated with the military, and increased use of improvised explosive devices warrants the study of blast shockwaves and their direct effects on the brain and brain function. Described here is the first study that subjects stable brain tissue explants to detonations of military explosives, finding that multiple blasts alter the levels of important synaptic proteins in hippocampus. The small, reproducible assemblies of RDX, a major component of C-4 plastic explosive, produced a shockwave that propagated extremely rapidly into the tissue holding tank, creating a positive-pressure blast wave followed by a negative-pressure phase which is characteristic of military explosions. Across number of consecutive blasts, a dose-dependent decline in synaptic proteins was found. Surprising, the study indicates that certain explosive intensities produce synaptic alterations independent of cellular degeneration. These results suggest the possibility that a seemingly innocuous blast wave can produce distinct changes in the brain. The blast-induced synaptic compromise may be the key event that leads to cognitive and behavioral dysfunctions before obvious neuropathology is detectable.
The in vitro military explosive blast paradigm provides a unique model to study military explosives and the direct effects of their specific blast waves (Piehler et al., 2016). Other models have used shock tubes, fluid percussion, hydrostatic pressure, and controlled impact devices to produce different types of traumatic injuries. However, the mechanisms of such injuries are very difficult to compare across the different models and many parameters involved (Svetlov et al., 2009). The pressure profile generated from such devices also may not match the specific profile produced by military explosives (see Chen and Constantini, 2013). Accordingly, precise RDX assemblies and their localized detonations were a key addition to the new blast paradigm, and they were shown to alter membrane permeability and induce axonal beading in PC12 cells and human neuroblastoma cells (Zander et al., 2015, 2016). While these two previous reports utilized dissociated cells, the current study made use of hippocampal slice cultures. The slice cultures are well known for their three-dimensional, native organization with a maintained neuronal network consisting of 10–15 neuron-thick tissue, complex arborization in the neuropil, and possessing similar synapse density and synaptic plasticity processes as found in the adult brain (e.g., see Vanderklish et al., 1992; Muller et al., 1993, 1996; Bahr et al., 1995a, 1998). Applying RDX detonations towards the intact brain tissue led to a compromise in synaptic integrity, evident through the assessment of pre- and postsynaptic markers and histological staining.
The hippocampal slice cultures that were subjected to a single RDX blast exhibited a small and varied reduction in GluR1, a subunit of post-synaptic AMPA receptors that are involved in neurotransmission, hippocampal plasticity (long-term potentiation), and memory (Vanderklish et al., 1992; Schmitt et al., 2004; Kopec et al., 2007). When exposed to consecutive RDX detonations, GluR1 levels were further reduced by as much as 72% after three blasts. A correlational analysis indicates that such synaptic decline may be mediated in part by HDAC2, a histone deacetylase whose upregulation has been associated with stress-induced GluR1 degradation, loss of glutamatergic transmission, and disrupted recognition memory (Wei et al., 2016). The link to HDAC2 also implicates alterations to AMPA receptor sorting pathways in the blast-induced synaptic decline, since HDAC2-related pathways have been found to play a role in regulating AMPA receptor trafficking (Schwarz et al., 2010; Hou et al., 2011) and ubiquitin/proteasome-mediated degradation of GluR1 which can lead to the deficit of certain cognitive processes (Yuen et al., 2012).
Similar to the RDX blast model, loss of hippocampal GluR1 was also found after controlled cortical impact TBI (Kharlamov et al., 2011), the in vivo study reporting a 51% loss in rats that exhibited posttraumatic epilepsy and a 34% loss in those that did not. The altered electrophysiology may be related to composition changes in glutamatergic synapses, and such changes may explain the impaired axonal conduction and disrupted long-term potentiation after insult paradigms using compressed gas blast tubes (Goldstein et al., 2012; Effgen et al., 2014). In addition to the loss of the postsynaptic GluR1 protein after multiple RDX detonations, the slice cultures also exhibited a similar decline in the pre-synaptic protein synaptophysin as compared to levels in mock-treated control slices. The level and patterned distribution of synaptophysin in dendritic fields was severely disrupted. Perhaps the vulnerability of the vesicular protein is related to the synaptic vesicle distribution change that is induced by TBI (Carlson et al., 2015).
Hippocampal pre- and postsynaptic proteins were also found reduced after unilateral cortical impact using a controlled pneumatic impact device (Ansari et al., 2008). A second study reported similar or further declines in pre- and postsynaptic proteins 96 h after a cortical impact depth of 2 mm was produced by a 5-mm impactor tip (Ansari et al., 2013). In contrast, it is noteworthy that a more moderate level of cortical impact injury (0.5-mm impact depth with a 3-mm impactor) resulted in 40–50% increases in synaptophysin in the ipsilateral hippocampus 48–72 h post-impact (Thompson et al., 2006). Enhanced synaptophysin levels are likely a result of compensatory signaling, and such restorative responses are apparently disrupted by the more damaging impactor. The fact that 1–3 RDX blasts caused decreases rather than increases in synaptophysin and GluR1 in the current study suggests that even a single blast wave from a detonated charge can disrupt synaptic maintenance pathways. The multiple RDX blasts also disrupted the ability of NMDA receptor stimulation to induce downstream phosphorylation of cofilin, a pathway implicated in cytoskeletal dynamics for modifying synapses and their functional plasticity (Rex et al., 2010). While excitability may explain the compromised NMDA-activated signaling pathway, non-specific damage to experimental setup or broad neuronal mechanisms does not appear to occur after RDX blasts since brief NMDA infusion still exhibited activation of pERK2, perhaps related to a glutamatergic pathway acting through MAPK kinase (Perkinton et al., 1999; Bahr et al., 2002). Subtle changes to neuronal function may explain blast-induced changes in cognition and behavior. For example, a study using fairly rapid blast overpressure loading in mice reported changes in hippocampus-dependent behaviors corresponding with electrophysiological changes in hippocampal subfield CA1 (Beamer et al., 2016). In addition, a recent study showed that multiple primary blasts significantly reduced long-term potentiation in the absence of altered basal neuronal responses (Effgen et al., 2016). This study used hippocampal slice cultures and suggests a specific effect on synaptic plasticity before overt damage to axons, dendrites, or cells.
It is also interesting that RDX blast-induced synaptic marker declines occurred without evidence of cellular degeneration in slice cultures. The absence of Fluoro-Jade B staining 2 days after a triple blast insult indicates that the shockwaves produced by 1.7-g RDX assemblies do not lead to neuronal damage in hippocampal subfields. Note that neurodegeneration occurred after different levels of controlled cortical impact injury, with most of the degenerating neurons identified within 24 h and additional numbers observed 48 h post-insult (Ansari et al., 2008; Zhou et al., 2012). Additionally, while the temporal profile of RDX blast-induced synaptic decline is similar to the rapid loss of GluR1 and other synaptic markers in the excitotoxic hippocampus (see Fig. 6), multiple RDX blasts do not produce the typical neurodegeneration commonly linked to excitotoxicity. In the current study, RDX detonations did not produce the cytoskeletal damage and cellular degeneration found subsequent to AMPA-mediated excitotoxicity, and a similar AMPA insult in hippocampal slices resulted in a tight association between GluR1 loss, generated spectrin breakdown products, and neuronal death (Bahr et al., 2002). Since RDX blasts do not exhibit calpain-mediated spectrin breakdown and do not align well with the slow synaptic decline profile linked to protein accumulation stress, it is of particular interest that calpain, with the use of inhibitors in Alzheimer's disease models, has been implicated in Alzheimer-type protein accumulation pathology (Trinchese et al., 2008; Hook et al., 2011; Rao et al., 2014). It should be noted that protein accumulation events involving Aβ and tau have been linked to brain trauma including a TBI model with a compressed-gas blast tube (see Goldstein et al., 2012; Du et al., 2016; Scott et al., 2016). On the other hand, the RDX synaptotoxic effect appears to occur earlier than the synaptic decline reported in hippocampal slice studies of protein accumulation stress related to Aβ and tau (Bendiske and Bahr, 2003; Butler et al., 2007; Wisniewski et al., 2011). Thus, from the above discussion, the level of detonated RDX used in the present study appears to produce a unique type of pathology comprised of altered synaptic integrity in the absence of cellular deterioration.
These findings add to the challenge in understanding TBIs resulting from explosives, and they point to the need for improved detection and treatment of military blast-induced changes in the brain. Exposures to unexpected explosions can have a huge range of blast intensities and a frequency ranging from a single detonation to a barrage of mortar shelling or artillery. In addition, military personnel routinely conduct explosive breaching, often with limited barrier protection. Thus, the monitoring of individuals' cumulative exposures to blast shockwaves is also a critical issue in light of the current study.
Acknowledgments
This material is based upon work supported primarily by the U.S. Army Research Laboratory and the U.S. Army Research Office under grant number W911NF-14-2-0087 (BAB). University of North Carolina-Pembroke researchers were also supported in part by grant W911NF-15-1-0432 from the Department of Defense Research and Education Program (BAB) and NIH RISE grant 5R25GM077634-04 to University of North Carolina-Pembroke. The funding agencies had no role in study design, data collection and analysis, or decision to publish. We thank Heather Romine, Manager of the William C. Friday Laboratory, for excellent technical assistance, and Greg Georgevitch (retired U.S. Army career NCO) and Nicole Zander and Rohan Banton (U.S. Army Research Laboratory, Aberdeen Proving Ground, Maryland) for helpful discussions.
Footnotes
Grant sponsor: U.S. Army Research Office, grant number W911NF-14-2-0087.
References
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Dose- and time-dependent neuroprotective effects of Pycnogenol® following traumatic brain injury. J Neurotrauma. 2013;30:1542–1549. doi: 10.1089/neu.2013.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun P, Spadaro J, John J, Gharavi RB, Bentley TB, Nambiar MP. Studies on blast traumatic brain injury using in-vitro model with shock tube. Neurol Rep. 2011;22:379–384. doi: 10.1097/WNR.0b013e328346b138. [DOI] [PubMed] [Google Scholar]
- Bahr BA. Long-term hippocampal slices: a model system for investigating synaptic mechanisms and pathologic processes. J Neurosci Res. 1995;42:294–305. doi: 10.1002/jnr.490420303. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Kessler M, Rivera S, Vanderklish PW, Hall RA, Singh Mutneja M, Gall C, Hoffman KB. Stable maintenance of glutamate receptors and other synaptic components in long-term hippocampal slices. Hippocampus. 1995a;5:425–439. doi: 10.1002/hipo.450050505. [DOI] [PubMed] [Google Scholar]
- Bahr BA, Tiriveedhi S, Park GY, Lynch G. Induction of calpain-mediated spectrin fragments by pathogenic treatments in long-term hippocampal slices. J Pharmacol Exp Ther. 1995b;273:902–908. [PubMed] [Google Scholar]
- Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G. Amyloid β protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. J Comp Neurol. 1998;397:139–147. [PubMed] [Google Scholar]
- Bahr BA, Bendiske J, Brown QB, Munirathinam S, Caba E, Rudin M, Urwyler S, Sauter A, Rogers G. Survival signaling and selective neuroprotection through glutamatergic transmission. Exp Neurol. 2002;174:37–47. doi: 10.1006/exnr.2001.7852. [DOI] [PubMed] [Google Scholar]
- Beamer M, Tummala SR, Gullotti D, Kopil C, Gorka S, Abel T, Bass CR, Morrison B, 3rd, Cohen AS, Meaney DF. Primary blast injury causes cognitive impairments and hippocampal circuit alterations. Exp Neurol. 2016;283:16–28. doi: 10.1016/j.expneurol.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendiske J, Bahr BA. Lysosomal activation is a compensatory response against protein accumulation and associated synaptopathogenesis – an approach for slowing Alzheimer disease? J Neuropathol Exp Neurol. 2003;62:451–463. doi: 10.1093/jnen/62.5.451. [DOI] [PubMed] [Google Scholar]
- Bendiske J, Caba E, Brown QB, Bahr BA. Intracellular deposition, microtubule destabilization, and transport failure: an “early” pathogenic cascade leading to synaptic decline. J Neuropathol Exp Neurol. 2002;61:640–650. doi: 10.1093/jnen/61.7.640. [DOI] [PubMed] [Google Scholar]
- Bonde C, Noraberg J, Noer H, Zimmer J. Ionotropic glutamate receptors and glutamate transporters are involved in necrotic neuronal cell death induced by oxygen-glucose deprivation of hippocampal slice cultures. Neurosci. 2005;136:779–794. doi: 10.1016/j.neuroscience.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Butler D, Bendiske J, Michaelis ML, Karanian DA, Bahr BA. Microtubule-stabilizing agent prevents protein accumulation-induced loss of synaptic markers. Eur J Pharmacol. 2007;562:20–27. doi: 10.1016/j.ejphar.2007.01.053. [DOI] [PubMed] [Google Scholar]
- Caba E, Bahr BA. Biphasic NF-κB activation in the excitotoxic hippocampus. Acta Neuropathol. 2004;108:173–182. doi: 10.1007/s00401-004-0876-5. [DOI] [PubMed] [Google Scholar]
- Carlson SW, Yan HQ, Ma X, Li Y, Henchir JJ, Dixon CE. Traumatic brain injury impairs SNARE complex formation and alters synaptic vesicle distribution in the hippocampus. J Neurotrauma. 2015;33:113–121. doi: 10.1089/neu.2014.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Traumatic Brain Injury in the United States: A Report to Congress. National Center for Injury Prevention and Control; Atlanta: 1990. [Google Scholar]
- Chen Y, Constantini S. Caveats for using shock tube in blast-induced traumatic brain injury research. Front Neurol. 2013;4:117. doi: 10.3389/fneur.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Passecker J, Malagon-Vina H, Mikus N, Klausberger T. Brain computation. Selective information routing by ventral hippocampal CA1 projection neurons. Science. 2015;348:560–563. doi: 10.1126/science.aaa3245. [DOI] [PubMed] [Google Scholar]
- Du X, West MB, Cheng W, Ewert DL, Li W, Saunders D, Towner RA, Floyd RA, Kopke RD. Ameliorative effects of antioxidants on the hippocampal accumulation of pathologic tau in a rat model of blast-induced traumatic brain injury. Oxidative Med Cell Longev. 2016;2016:4159357. doi: 10.1155/2016/4159357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effgen GB, Hue CD, Vogel E, 3rd, Panzer MB, Meaney DF, Bass CR, Morrison B., 3rd A multiscale approach to blast neurotrauma modeling: part II: methodology for inducing blast injury to in vitro models. Front Neurol. 2012;3:23. doi: 10.3389/fneur.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effgen GB, Vogel EW, Lynch KA, Lobel A, Hue CD, Meaney DF, Bass CR, Morrison B., 3rd Isolated primary blast alters neuronal function with minimal cell death in organotypic hippocampal slice cultures. J Neurotrauma. 2014;31:1202–1210. doi: 10.1089/neu.2013.3227. [DOI] [PubMed] [Google Scholar]
- Effgen GB, Ong T, Nammalwar S, Ortuño AI, Meaney DF, Bass CR, Morrison B., 3rd Primary blast exposure increases hippocampal vulnerability to subsequent exposure: reducing long-term potentiation. J Neurotrauma. 2016 doi: 10.1089/neu.2015.4327. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Mitsis EM, Ahlers ST, Cristian A. Blast-induced mild traumatic brain injury. Psychiatr Clin North Am. 2010;33:757–781. doi: 10.1016/j.psc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Goeller J, Wardlaw A, Treichler D, O'Bruba J, Weiss G. Investigation of cavitation as a possible damage mechanism in blast-induced traumatic brain injury. J Neurotrauma. 2012;29:1970–1981. doi: 10.1089/neu.2011.2224. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, Upreti C, Kracht JM, Ericsson M, Wojnarowicz MW, Goletiani CJ, Maglakelidze GM, Casey N, Moncaster JA, Minaeva O, Moir RD, Nowinski CJ, Stern RA, Cantu RC, Geiling J, Blusztajn JK, Wolozin BL, Ikezu T, Stein TD, Budson AE, Kowall NW, Chargin D, Sharon A, Saman S, Hall GF, Moss WC, Cleveland RO, Tanzi RE, Stanton PK, McKee AC. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. 2012;4:134. doi: 10.1126/scitranslmed.3003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KB, Murray BA, Lynch G, Munirathinam S, Bahr BA. Delayed and isoform-specific effect of NMDA exposure on neural cell adhesion molecules in hippocampus. Neurosci Res. 2001;39:167–173. doi: 10.1016/s0168-0102(00)00214-5. [DOI] [PubMed] [Google Scholar]
- Hook G, Hook V, Kindy M. The cysteine protease inhibitor, E64d, reduces brain amyloid-β and improves memory deficits in Alzheimer's disease animal models by inhibiting cathepsin B, but not BACE1, β -secretase activity. J Alzheimers Dis. 2011;26:387–408. doi: 10.3233/JAD-2011-110101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Gilbert J, Man HY. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72:806–818. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue CD, Cao S, Haider SF, Vo KV, Effgen GB, Vogel E, 3rd, Panzer MB, Bass CR, Meaney DF, Morrison B., 3rd Blood-brain barrier dysfunction after primary blast injury in vitro. J Neurotrauma. 2013;30:1652–1663. doi: 10.1089/neu.2012.2773. [DOI] [PubMed] [Google Scholar]
- Hue CD, Cao S, Dale Bass CR, Meaney DF, Morrison B., 3rd Repeated primary blast injury causes delayed recovery, but not additive disruption, in an in vitro blood-brain barrier model. J Neurotrauma. 2014;31:951–960. doi: 10.1089/neu.2013.3149. [DOI] [PubMed] [Google Scholar]
- Karanian DA, Brown QB, Makriyannis A, Kosten TA, Bahr BA. Dual modulation of endocannabinoid transport and fatty acid amide hydrolase protects against excitotoxicity. J Neurosci. 2005;25:7813–7820. doi: 10.1523/JNEUROSCI.2347-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharlamov EA, Lepsveridze E, Meparishvili M, Solomonia RO, Lu B, Miller ER, Kelly KM, Mtchedlishvili Z. Alterations of GABAA and glutamate receptor subunits and heat shock protein in rat hippocampus following traumatic brain injury and in posttraumatic epilepsy. Epilepsy Res. 2011;95:20–34. doi: 10.1016/j.eplepsyres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Real E, Kessels HW, Malinow R. GluR1 links structural and functional plasticity at excitatory synapses. J Neurosci. 2007;27:13706–13718. doi: 10.1523/JNEUROSCI.3503-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. The invisible wounds of war. Healing the brain, healing the mind. Science. 2011;333:514–517. doi: 10.1126/science.333.6042.514. [DOI] [PubMed] [Google Scholar]
- Moore DF, Jaffee MS. Military traumatic brain injury and blast. Neuro Rehabilitation. 2010;26:179–181. doi: 10.3233/NRE-2010-0553. [DOI] [PubMed] [Google Scholar]
- Muller D, Buchs PA, Stoppini L. Time course of synaptic development in hippo-campal organotypic cultures. Dev Brain Res. 1993;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Naidoo V, Karanian DA, Vadivel SK, Locklear JR, Wood JT, Nasr M, Quizon PMP, Graves EE, Shukla V, Makriyannis A, Bahr BA. Equipotent inhibition of fatty acid amide hydrolase and monoacylglycerol lipase – dual targets of the endocannabinoid system to protect against seizure pathology. Neurotherapeutics. 2012;9:801–813. doi: 10.1007/s13311-011-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Orlovska S, Pedersen MS, Benros ME, Mortensen PB, Agerbo E, Nordentoft M. Head injury as risk factor for psychiatric disorders: a nationwide register-based follow-up study of 113,906 persons with head injury. Am J Psychiatr. 2014;171:463–469. doi: 10.1176/appi.ajp.2013.13020190. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Lacaille JC. Long-term synaptic plasticity in hippocampal feedback inhibitory networks. Prog Brain Res. 2008;169:241–250. doi: 10.1016/S0079-6123(07)00014-3. [DOI] [PubMed] [Google Scholar]
- Perkinton MS, Sihra TS, Williams RJ. Ca2+-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons. J Neurosci. 1999;19:5861–5874. doi: 10.1523/JNEUROSCI.19-14-05861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piehler T, Zander N, Benjamin R. Primary explosive blast-induced traumatic brain injury model in PC12 cell culture. Bio-protocol. 2016;6:e1907. [Google Scholar]
- Rao MV, McBrayer MK, Campbell J, Kumar A, Hashim A, Sershen H, Stavrides PH, Ohno M, Hutton M, Nixon RA. Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J Neurosci. 2014;34:9222–9234. doi: 10.1523/JNEUROSCI.1132-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Gavin CF, Rubio MD, Kramar EA, Chen LY, Jia Y, Huganir RL, Muzyczka N, Gall CM, Miller CA, Lynch G, Rumbaugh G. Myosin IIb regulates actin dynamics during synaptic plasticity and memory formation. Neuron. 2010;67:603–617. doi: 10.1016/j.neuron.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WB, Arianpour R, Deacon RM, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM. The role of hippocampal glutamate receptor-A-dependent synaptic plasticity in conditional learning: the importance of spatiotemporal discontiguity. J Neurosci. 2004;24:7277–7282. doi: 10.1523/JNEUROSCI.1093-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober ME, Requena DF, Davis LJ, Metzger RR, Bennett KS, Morita D, Niedzwecki C, Yang Z, Wang KK. Alpha II Spectrin breakdown products in immature Sprague Dawley rat hippocampus and cortex after traumatic brain injury. Brain Res. 2014;1574:105–112. doi: 10.1016/j.brainres.2014.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Hall BJ, Patrick GN. Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J Neurosci. 2010;30:16718–16729. doi: 10.1523/JNEUROSCI.3686-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbold M, Diaz A, Martins ET, Rufino A, Amante LN, Thais ME, Quevedo J, Hohl A, Linhares MN, Walz R. Psychiatric disorders and traumatic brain injury. Neuropsychiatr Dis Treat. 2008;4:797–816. doi: 10.2147/ndt.s2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G, Ramlackhansingh AF, Edison P, Hellyer P, Cole J, Veronese M, Leech R, Greenwood RJ, Turkheimer FE, Gentleman SM, Heckemann RA, Matthews PM, Brooks DJ, Sharp DJ. Amyloid pathology and axonal injury after brain trauma. Neurology. 2016;86:821–828. doi: 10.1212/WNL.0000000000002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlov SI, Larner SF, Kirk DR, Atkinson J, Hayes RL, Wang KW. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. J Neurotrauma. 2009;26:913–921. doi: 10.1089/neu.2008.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szinyei C, Behnisch T, Reiser G, Reymann KG. Inositol 1,3,4,5-tetrakisphosphate enhances long-term potentiation by regulating Ca2+ entry in rat hippocampus. J Physiol. 1999;516:855–868. doi: 10.1111/j.1469-7793.1999.0855u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SN, Gibson TR, Thompson BM, Deng Y, Hall ED. Relationship of calpain-mediated proteolysis to the expression of axonal and synaptic plasticity markers following traumatic brain injury in mice. Exp Neurol. 2006;201:253–265. doi: 10.1016/j.expneurol.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Trinchese F, Fa' M, Liu S, Zhang H, Hidalgo A, Schmidt SD, Yamaguchi H, Yoshii N, Mathews PM, Nixon RA, Arancio O. Inhibition of calpains improves memory and synaptic transmission in a mouse model of Alzheimer disease. J Clin Invest. 2008;118:2796–2807. doi: 10.1172/JCI34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderklish PW, Bahr BA. The pathogenic activation of calpain: a marker and mediator of cellular toxicity and disease states. Int J Exp Pathol. 2000;81:323–339. doi: 10.1111/j.1365-2613.2000.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderklish P, Neve RL, Bahr BA, Arai A, Hennegriff M, Lynch G. Translational suppression of a glutamate receptor subunit impairs long-term potentiation. Synapse. 1992;12:333–337. doi: 10.1002/syn.890120410. [DOI] [PubMed] [Google Scholar]
- Vornov JJ, Tasker RC, Coyle JT. Delayed protection by MK-801 and tetrodotoxin in a rat organotypic hippocampal culture model of ischemia. Stroke. 1994;25:457–464. doi: 10.1161/01.str.25.2.457. [DOI] [PubMed] [Google Scholar]
- Wei J, Xiong Z, Lee JB, Cheng J, Duffney LJ, Matas E, Yan Z. Histone modification of Nedd4 ubiquitin ligase controls the loss of AMPA receptors and cognitive impairment induced by repeated stress. J Neurosci. 2016;36:2119–2130. doi: 10.1523/JNEUROSCI.3056-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V, Mulhall E. Iraq and Afghanistan Veterans of America, New York. Washington DC: Iraq and afghanistan veterans of america; 2009. Invisible Wounds: Psychological and Neurological Injuries Confront a New Generation of Veterans; pp. 1–24. [Google Scholar]
- Wisniewski ML, Hwang J, Bahr BA. Submicromolar Aβ42 reduces hippocampal glutamate receptors and presynaptic markers in an aggregation-dependent manner. Biochim Biophys Acta. 2011;1812:1664–1674. doi: 10.1016/j.bbadis.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander NE, Piehler T, Boggs ME, Banton R, Benjamin R. In vitro studies of primary explosive blast loading on neurons. J Neurosci Res. 2015;93:1353–1363. doi: 10.1002/jnr.23594. [DOI] [PubMed] [Google Scholar]
- Zander NE, Piehler T, Banton R, Boggs M. The effect of explosive blast loading on human neuroblastoma cells. Anal Biochem. 2016;504:4–6. doi: 10.1016/j.ab.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Zhou H, Chen L, Gao X, Luo B, Chen J. Moderate traumatic brain injury triggers rapid necrotic death of immature neurons in the hippocampus. J Neuropathol Exp Neurol. 2012;71:348–359. doi: 10.1097/NEN.0b013e31824ea078. [DOI] [PMC free article] [PubMed] [Google Scholar]


