Abstract
Amyloid β (Aβ) plaques are a key histopathological hallmark of Alzheimer’s disease (AD) and soluble Aβ species are believed to play an important role in the clinical development of this disease. Emerging biomarker data demonstrates that Aβ plaque deposition begins decades before the onset of clinical symptoms, suggesting that understanding the biological determinants of the earliest steps in the development of AD pathology may provide key opportunities for AD treatment and prevention. Although a clinical association between sleep disruption and AD has long been appreciated, emerging clinical studies and insights from the basic neurosciences has shed important new light on how sleep and Aβ homeostasis may be connected in the setting of AD. Aβ, like many interstitial solutes, is cleared in part through the exchange of brain interstitial fluid and cerebrospinal fluid (CSF) along a brain-wide network of perivascular pathways recently termed the ‘glymphatic’ system. Glymphatic function is primarily a feature of the sleeping, rather than the waking brain, and is slowed in the aging and post-traumatic brain. These changes may underlie the diurnal fluctuations in interstitial and CSF Aβ levels observed in both the rodent and human. These and other emerging studies suggest that age-related sleep disruption may be one key factor rendering the aging brain vulnerable to Aβ deposition and the development of AD. If true, sleep may represent a key modifiable risk factor or therapeutic target in the pre-clinical phases of AD.
Keywords: Alzheimer’s, glymphatic, astrocytes, sleep, aquaporin-4, cerebrospinal fluid, CSF, interstitial fluid, perivascular
Alzheimer’s disease (AD) is characterized by extracellular deposition of amyloid β (Aβ)-containing plaques, intracellular neurofibrillary tangles comprised of hyper-phosphorylated tau, and associated progressive cognitive impairment. The determinants of progressive pathology in AD remain incompletely understood, and the failure of recent clinical trials aimed at reducing production or aggregation of Aβ (see(1) for review) has raised doubts about the sufficiency of the “amyloid cascade hypothesis”, which postulates that Aβ deposition is the key event initiating the pathogenic processes in AD. Notwithstanding, Aβ deposition measured via the cerebrospinal fluid (CSF) or by Aβ positron-emission tomography (PET) is among the earliest events in the neuropathological cascade characterizing sporadic AD and is considered a key driver of the disease.
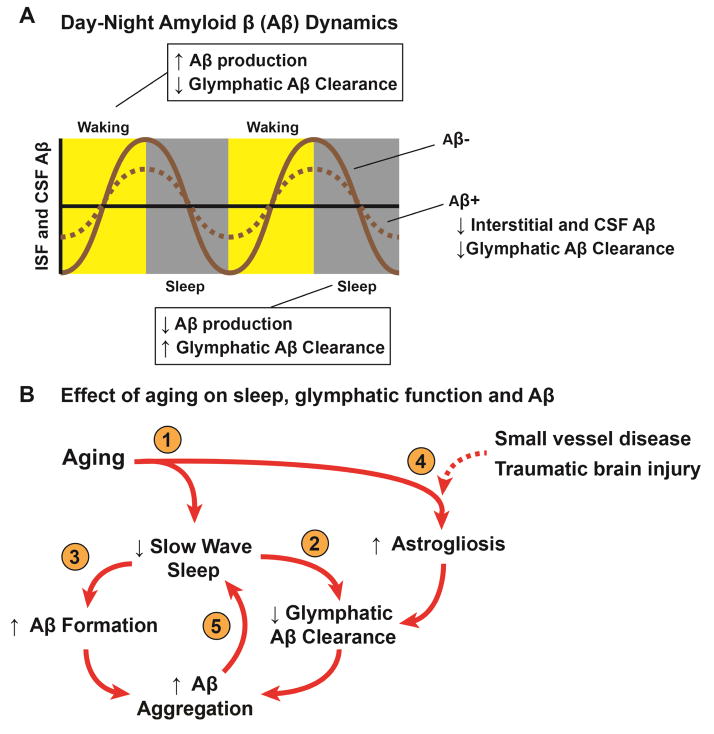
Alzheimer’s-related changes, including regional hypometabolism, cortical and hippocampal atrophy, neuroinflammation, and Aβ and tau aggregation develop over decades prior to the onset of clinical dementia. Accordingly, a “preclinical” phase of AD has been proposed characterized by the presence of Aβ and tau aggregates detectible by CSF- or PET-based measurement prior to onset of clinical symptoms(2, 3), offering a potential window of opportunity for therapeutic intervention. Increasing sleep disruption, slowing clearance of Aβ, and increasing Aβ deposition are each present in the preclinical stage. While each is strongly linked to cognitive decline and AD diagnosis, emerging research suggests that they may be linked biologically (Figure 1A–B). The glymphatic system is a brain-wide perivascular network that supports the exchange interstitial fluid (ISF) and CSF, facilitating the clearance of interstitial solutes, including Aβ and tau from the brain parenchyma (4–6). Experimental studies demonstrate that glymphatic function is primarily active during sleep(7), and is impaired in the aging and post-traumatic brain(8–10). In this review we provide a framework for understanding the relationship between Aβ dynamics, sleep, and ISF flow in the context of AD and discuss the potential role that age-related impairment of glymphatic function may play in linking these features in the preclinical phase of AD.
Figure 1. Relationship between sleep, aging and Aβ in the setting of Alzheimer’s disease.
(A) Schematic depicting diurnal fluctuations in interstitial and CSF Aβ levels measured in mice(55, 98) and human subjects(55, 106), respectively. During waking, Aβ levels increase while during sleep Aβ levels decline. These changes are thought to be attributable to increased metabolic demand and slowed glymphatic Aβ clearance during waking and more rapid glymphatic Aβ clearance during sleep and reduced metabolic burden during slow wave sleep. In the setting of Aβ plaques, the amplitude of the diurnal Aβ fluctuation declines as interstitial Aβ is sequestered into insoluble plaques and is unavailable for glymphatic clearance(98, 106). (B) Aging, sleep disruption, and glymphatic pathway impairment may constitute a feed-forward cycle promoting Aβ plaque deposition in the aging brain. (1) Sleep disruption, particularly of slow wave sleep, and astrogliosis are frequent features of the aging brain. (2) Reduced slow wave activity impairs glymphatic Aβ clearance, which is greatest in the sleeping brain(67). (3) Metabolic demand increases with loss of slow wave sleep, increasing Aβ formation(120). (4) Astrogliosis associated with aging, small vessel disease, traumatic brain injury or amyloid plaques is associated with impaired glymphatic pathway function, perhaps via impairment of perivascular AQP4 localization(9, 10, 76, 121). (5) The presence of Aβ aggregates specifically inhibits slow wave activity(122).
Interstitial Aβ dynamics
Aβ is a soluble, normally secreted peptide resulting from the proteolytic cleavage of amyloid precursor protein (APP), a large membrane-spanning glycoprotein(11, 12), in neuronal and glial cell types(13, 14). Among the many Aβ subspecies, Aβ1-40 is produced most abundantly, exchanges most readily with the CSF and is associated with the cerebral vasculature(12). The Aβ1-42 species is the primary component of amyloid plaques due to its greater propensity to aggregate into oligomeric and fibrillary forms(15, 16). Aβ is released into the ISF where it can remain soluble or aggregate into insoluble plaques. While the presence of insoluble Aβ plaques is necessary for diagnosis of AD, their presence alone does not predict dementia severity. Rather, soluble Aβ oligomeric species isolated from human brain tissue are most strongly associated with neurocognitive decline(17, 18) and are directly synaptotoxic when evaluated within in situ and in vivo experimental models of learning and behavior (19).
Brain amyloid β clearance
While familial AD, caused by mutations in Aβ-related genes, results from the increased production of the pro-fibrillary Aβ1-42, there is compelling evidence that impairment in clearance Aβ clearance promotes the mis-aggregation of Aβ into plaques in the more common setting of sporadic AD. Declining CSF Aβ1-42 levels sensitively discriminate AD status(20). In AD patients with measurement of both CSF Aβ1-42 and Aβ PET binding, an inverse relationship between these markers has been identified(21, 22), supporting the notion that increased brain Aβ plaque burden is accompanied by a reduction in the amount of soluble Aβ exchanging between the brain ISF and the CSF. These changes in Aβ exchange are evident even at the earliest stages of AD, as levels of soluble amyloid (Aβ1-42) in the CSF are reduced in preclinical stages of the disease(2, 23). In pulse-chase radio-labeling studies carried out in human subjects, the rate of Aβ1-40 or Aβ1-42 production did not differ between AD subjects and cognitively-intact controls, while the rate of Aβ clearance was significantly slowed in AD subjects(24). Similar results were observed in cognitively-intact aging subjects, which showed no significant change in the rate of Aβ production with advancing age, but did report a decline in Aβ clearance with advancing age(25). These findings suggest that the failure of the brain to eliminate Aβ is a key step in AD pathogenesis.
The clearance of Aβ from the brain has been attributed to several different processes, including local proteolytic degradation(26, 27), phagocytosis by microglial cells(30), receptor- or carrier-mediated efflux across the blood-brain barrier (BBB)(28, 29), and clearance along perivascular spaces surrounding cerebral arteries(30, 31). Recently, the ‘glymphatic’ system, a brain-wide perivascular network supporting the clearance of interstitial solutes to the CSF, has been described and its role in the clearance of interstitial Aβ has been established(4–6). Once in the CSF, Aβ may be cleared to the periphery along CSF efflux pathways including arachnoid villi of the dural sinuses, across the cribriform plate(32), across the blood-CSF barrier at the choroid plexus(33, 34), or taken up by meningeal lymphatic vessels associated with dural sinuses(35–37). While experimental and clinical evidence supports the role of each of these respective processes in the clearance of Aβ from the brain, the relative contribution of each remains unclear, and may vary based upon such factors as age, brain region, physiological state, or the presence of pathology. The access of Aβ to each of these clearance pathways is dictated by the dynamics of ISF and solute exchange.
Dynamics of interstitial solute exchange
The brain extracellular space comprises approximately 20% of the overall brain volume(38), and is a narrow (typically 30–70 nm in width(39)), tortuous, matrix-filled space that separates neighboring cells and processes. The ISF is derived from several sources, including water produced from cellular metabolism, water crossing the BBB, and water from the CSF-containing compartments including the ventricles and subarachnoid spaces(38).
The movement of interstitial solutes within the extracellular compartment are governed by process of diffusion and bulk flow, or convection(38, 40). With diffusion, random thermal motion causes solutes to move passively down their concentration gradients. Diffusion is influenced by solute size, with larger molecules diffusing more slowly than small molecules; by the size and nature of the extracellular compartment, with a larger extracellular space facilitating diffusion; and by the chemical interactions between the solute and the extracellular matrix and cell surfaces that it encounters(38). Bulk flow involves the pressure-driven movement of a fluid and solutes in much the same manner as a river’s current entrains the movement of objects suspended beneath and floating upon its surface. The rate of solute movement driven by bulk flow depends upon the hydrostatic or osmotic forces driving movement of the bulk fluid and is independent of the size of the solute, so long as its size does not approach the dimensions of the extracellular space to constrain its movement.
Aβ is taken up and degraded locally by several different cell types, including microglia, astrocytes, neurons, and vascular smooth muscle cells(32, 41, 42). In addition, Aβ is cleared across the BBB through the activity of both low density lipoprotein receptor-related protein 1 (LRP1) and P-glycoprotein (PGP) and (28, 43, 44). In radio-tracer clearance assays, inhibition of LRP1-mediated clearance slows the efflux of 125I-Aβ1-40 by >70%(45). A decline in LRP1-mediated clearance of Aβ is observed in the aged brain(28, 44), while expression of PGP declines in AD subjects(46). While these data suggest that LRP1-mediated trans-endothelial efflux is a major route for Aβ clearance under the reported experimental conditions in rodents, how physiological regulation, regional variability and pathology-associated changes in BBB efflux transporter expression and function influences interstitial Aβ clearance has not been exhaustively defined either in experimental animal studies or in human subjects.
Interstitial solutes not degraded locally or cleared across the BBB exchange with CSF within the ventricular or the subarachnoid compartments(47, 48). In brain regions closely associated with ventricular walls or the pial surface, diffusion may primarily account for this exchange. However, over the larger distances separating much of the neuropil from these CSF compartments, diffusion alone cannot account for the exchange of interstitial solutes with the CSF. Instead this exchange is supported by bulk flow. Whether bulk flow occurs throughout the wider interstitial compartment, or is restricted to perivascular spaces and white matter tracks connecting the interstitium to distant CSF compartments remains controversial(40, 48–51). Recent modeling studies have suggested that solute exchange between the interstitium and perivascular spaces is mediated by diffusion while studies employing dynamic contrast-enhanced (DCE)-MRI in rodents(5, 52), non-human primates(53) and human subjects(54) suggest that CSF-ISF exchange is occurring macroscopically along perivascular pathways throughout brain tissue. The presence of this ISF-CSF exchange is reflected in the close temporal relationship between interstitial and CSF Aβ dynamics both in experimental animals and human subjects in which both interstitial and CSF Aβ increase during waking and decline during sleep(55). Many factors, both physiological and pathological, may influence the kinetics of this exchange. For example, MRI- and PET-based approaches to imaging the exchange of water from the BBB to the ventricular compartment have demonstrated that experimental animals with Aβ plaque burden and subjects with AD exhibit slowed ISF-CSF exchange kinetics(56, 57). To begin to makes sense of the specific changes in the aging and AD brain underlying these alterations in ISF-CSF exchange, the molecular, cellular and anatomical basis of these exchange pathways must be considered.
Cellular and anatomical basis for ISF-CSF exchange
Different anatomical elements of neural tissue exhibit distinct properties of ISF exchange and flow. For example, water movement occurs differently within white matter tracts than in gray matter, with more rapid diffusion along the axis of axon bundles than along axes orthogonal to the axon tract or within the gray matter(58). Similar differences are observed in the rate and extent of bulk flow observed between white and gray matter, with more rapid bulk flow taking place through the extracellular spaces along white matter tracks than within gray matter. In the same way that axon fibers organize bulk flow, perivascular spaces within the white matter and gray matter serve as permissive anatomical pathways for the bulk movement of ISF and solutes, with more rapid movement of solutes along the spaces surrounding the brain vasculature than through the wider extracellular compartment. By providing permissive routes for rapid movement of ISF(40, 59–61), white matter tracts and perivascular spaces serve as critical scaffolds organizing solute exchange throughout brain tissue.
Perivascular pathways also provide an anatomical link between the CSF of the subarachnoid space and the brain interstitium. Tracers injected into the subarachnoid CSF move rapidly over the brain surface along pathways surrounding cerebral surface arteries, entering the brain parenchyma along perivascular spaces surrounding penetrating cerebral arteries(5, 6). Tracers with a large molecular weight (2000 kD) follow these routes from the subarachnoid space to the basal lamina surrounding terminal capillary beds within the brain parenchyma, indicating continuity between these compartments that extends the length of the cerebrovascular tree. Smaller molecular weight tracers (<70 kD) exchange relatively freely between perivascular spaces and the surrounding interstitium, suggesting that the pathway between perivascular compartments and the wider brain extracellular space is restricted by solute size(6), likely resulting from perivascular astrocytic endfoot ensheathment of the cerebral vasculature(62).
While exchange of brain ISF with extra-ventricular CSF, particularly along spaces immediately surrounding cerebral blood vessels has long been observed(40, 63), the extent and significance of this exchange has recently begun to be characterized. Dynamic imaging approaches have shown CSF movement into and through the brain interstitium and clearance of interstitial solutes to the CSF to be more rapid and extensive than previously appreciated(5, 6, 52). The anatomical routes for CSF and ISF exchange remain the controversial. Studies in rodents report that CSF tracers enter the brain along perivascular spaces surrounding penetrating arteries while ISF tracers are cleared from the brain along perivascular spaces surrounding deep draining veins(6, 10, 52, 64–67), yet other studies carried out in rodents report that interstitial solutes are cleared from the brain along perivascular spaces surrounding arteries(68, 69) or drain towards the ventricular CSF compartment(70, 71). DCE-MRI studies carried out in nonhuman primates and human subjects confirm that gadolinium-based contrast agents injected intrathecally exchange through the brain parenchyma along peri-arterial routes(53, 54, 72).
The perivascular exchange of CSF and ISF has important implications for understanding cerebral amyloid angiopathy (CAA), the deposition principally of Aβ1-40 in the walls of penetrating and leptomeningeal arteries commonly observed among subjects with AD and associated clinically with lobar hemorrhages(73). The deposition of Aβ in the walls of penetrating arteries has been cited as evidence that brain ISF and Aβ are cleared from the brain along perivascular spaces surrounding arteries and in the opposite direction of blood flow(31). This model is supported by experimental studies demonstrating that tracers and labeled Aβ injected intraparenchymally into rodents is rapidly detected in perivascular spaces surrounding intracortical arteries(74, 75). The observation, including in mice(6, 67), rats(5), nonhuman primates(53), and human subjects(54), that CSF tracers enter the brain along perivascular spaces surrounding cerebral arteries suggest a different potential role in the pathogenesis of CAA. The movement of subarachnoid CSF containing Aβ inward along peri-arterial spaces may promote deposition of Aβ in the vascular wall when the downstream pathways of CSF-ISF exchange are impaired(10, 76), particularly in the setting of aging and vascular injury when vascular smooth muscle uptake of Aβ may be impaired(42).
Astrocytes facilitate and organize fluid and solute movement throughout brain tissue, including supporting exchange between the ISF and CSF compartments. Astrocytes comprise the glia limitans externa, a laminar external boundary of the brain parenchyma that faces the subpial compartment and the perivascular Virchow-Robin spaces that surround penetrating blood vessels. Within the brain tissue, astrocytes extend perivascular endfeet that completely ensheathe the brain vasculature(77). The exchange of ISF and CSF along perivascular spaces appears dependent upon the astroglial water channel aquaporin-4 (AQP4), a transmembrane water channel that is localized specifically to the perivascular astrocytic endfeet that surround the cerebral vasculature(6). In the initial description of the glymphatic system in 2012, deletion of the Aqp4 gene in mice was observed to nearly abolish the perivascular exchange of CSF and ISF, and dramatically slow the clearance of solutes, including Aβ, from the brain interstitium. Although a recent experimental study in mice failed to observe a similar effect of Aqp4 gene deletion on CSF tracer influx into brain tissue(50), work from four different research labs using four different transgenic mouse lines has confirmed the dependence of perivascular CSF-ISF exchange upon AQP4(78). In related work, deletion of the Aqp4 gene in mice In a transgenic mouse line that spontaneously develops Aβ plaques, deletion of the Aqp4 gene markedly accelerates the development of Aβ plaques and neurocognitive deficits(79). Based on results from a recent modeling study, AQP4 is believed to support the diffusion of water from perivascular spaces, through the brain parenchyma via the gap-junction coupled astroglial syncytium, providing a rapid cellular pathway to bridge perivascular CSF influx and ISF efflux routes(80).
Reactive astrogliosis is a key cellular feature in the aging and AD brain. Associated both with Aβ plaques and neurofibrillary pathology, reactive astrocytes exhibit alterations in the homeostatic functions that they serve, including impaired metabolic support for neurons through the astrocyte-neuron lactate shuttle, dysregulation of neurovascular coupling, and slowed astrocytic uptake of Aβ, which likely contributes to the pathogenesis of AD(81). In addition to changes that impact the local production and degradation of Aβ, reactive astrogliosis also appears to impact mechanisms of perivascular Aβ clearance. Loss of perivascular AQP4 localization is a common feature of reactive astrocytes, including in the setting of aging(10), ischemic injury(82), and traumatic brain injury(83). In each case, loss of perivascular AQP4 localization was associated with impaired perivascular CSF-ISF exchange and slowed clearance of interstitial solutes, including Aβ(9, 10, 76). Studies in human autopsy tissue have similarly demonstrated that changes in AQP4 expression, including the loss of perivascular localization, are associated with the development of AD pathology, including Aβ plaques(8, 84–86).
Sleep
A number of robust longitudinal studies(87) have confirmed early observations of sleep quality as a significant predictor of dementia status(88). Sleep disruptions are evident in early stages of clinically detectible cognitive decline relative to cognitively normal adults(89, 90), observations that were particularly pronounced in individuals at increased genetic risk of developing AD(90).
Sleep states and Alzheimer’s disease
Adult humans cycle through different sleep states about every 90 minutes(91). This cycle typically consists initially of non-rapid eye movement (NREM). NREM is classified into early (Stage 1), characterized as transitioning from wake-like alpha waves (8–13 Hz) to theta waves (4–7 Hz). Stage 2 NREM features the appearance of K-complexes (briefly negative sharp wave followed immediately by a positive inflection) and sleep spindles (brief bursts of very high frequency waves (11–16 Hz)). Stage 3 NREM, more commonly termed slow wave sleep (SWS), is marked by EEG frequencies of 0.5–2 Hz, or delta waves. From SWS, humans transition into brief episodes of rapid eye movement (REM), which has an EEG frequency of 30–80 Hz, a pattern that is similar to waking EEG patterns. Throughout the course of the night, human subjects will pass through 3–4 cycles of NREM SWS and REM sleep, with early cycles being dominated by longer bouts of SWS and later cycles dominated by longer periods of REM sleep(92).
In adults, advancing age is marked by significant reductions in sleep efficiency, SWS and REM sleep time, and by increased Stage 1 and 2 sleep time and sleep fragmentation(93). Reduction in deep (stage 3) sleep and disruption of the diurnal (sleep/wake) patterns are more prominent among AD subjects(94), among whom disturbed sleep is linked to increased severity of symptoms(95).
Sleep and amyloid β
Beyond the association between sleep disruption and AD risk, there is strong evidence of a direct relationship between sleep and brain Aβ. Qualitative measures, including shorter sleep duration and poor quality of sleep, are associated with higher levels of Aβ burden measured by Aβ PET in cognitively intact older adults(96), and poor objective sleep quality is associated with low CSF Aβ(97) in healthy older adults. In a mouse model of amyloidosis that develops spontaneous Aβ plaques, increased time awake was positively correlated with ISF Aβ levels(98) while sleep deprivation accelerated Aβ plaque formation(99). These results support a possible causal relationship between sleep and AD pathology(100). If validated, then this would suggest that sleep disruption may serve as a potentially modifiable clinical target for intervention for insipient AD(101).
Mechanistic considerations
A number of mechanisms have been proposed to explain link between Aβ, AD and sleep(102, 103). Neuronal activity, which drives Aβ production in waking states, is slowed during SWS, suggesting that sleep-wake differences in neuronal activity may underlie these relationships(103). In support of this interpretation are human serial CSF sampling studies reporting highest Aβ levels at the end of awake periods(99) and lowest levels following sleep(104). CSF Aβ levels decline over one night of sleep in healthy adults, an effect that was completely eliminated with a single night of sleep deprivation(104). When accounting for the estimated 6 hour delay in cleared Aβ arriving and lumbar sampling site(105), these lowest levels are coincident the predicted reduced neuronal activity of SWS(103). However, the concomitant impact of high metabolic demand and presumably increased Aβ production during light sleep and REM sleep that are interspersed with SWS on interstitial and CSF Aβ levels remains to be established. In addition to sleep stage-dependent changes in Aβ production, it is also not yet known whether differences in local degradation or BBB efflux of Aβ contribute to sleep-wake differences in interstitial and CSF Aβ levels.
In mice, diurnal fluctuations in ISF Aβ are lost in the presence of Aβ plaques(98). In human subjects, diurnal CSF Aβ fluctuations are similarly reduced among subjects positive for Aβ aggregation measured by PET(106). These findings suggest that by pulling soluble Aβ out of solution and into aggregates, Aβ aggregates reduced the availability of soluble Aβ to clearance mechanisms such as BBB efflux pathways or perivascular clearance routes.
A second explanation for the diurnal fluctuations in ISF and CSF Aβ implicates sleep-state dependent changes in glymphatic clearance of Aβ. Perivascular CSF-ISF exchange is markedly increased in the sleeping compared to the waking brain(67). In experimental studies carried out in mice, under conditions of both natural sleep and anesthesia with ketamine and xylazine, CSF tracer influx into the cortex was dramatically increased compared to the rates in the same animals in the waking state. These patterns of CSF influx were paralleled by differences in solute (including Aβ) clearance, clearance being markedly more rapid from the sleeping compared to the waking brain. Interestingly, these sleep-wake differences in glymphatic pathway function appeared to attributable in part to changes in extracellular volume fraction, with expansion of the extracellular space observed in the sleeping compared to the waking state. These effects appear to be mediated through changes in cortical noradrenergic tone, as blockade of cortical noradrenergic signaling restored glymphatic pathway function in the waking brain to levels observed in the sleeping brain(67).
Different anesthetics also appear to exert disparate effects on perivascular CSF-ISF exchange. In a pharmacokinetic study evaluating rates of interstitial solute efflux, interstitial tracer was cleared 1.5-times faster from ketamine/xylazine-anesthetized animals than awake behaving animals(47). In contrast, anesthesia with sodium pentobarbital slowed insterstitial solute efflux by more than 10-fold compared to ketamine/xylazine. In one recent study, glymphatic function appeared to be reduced under anesthesia with isofluorane or ketamine alone (without the alpha 1 adrenergic agonist xylazine) compared to the waking brain(107). In a second recent study, glymphatic function was markedly greater in rats treated with low-dose isofluorane plus the alpha 1 adrenergic agonist dexmeditomidine compared to isofluorane alone(108). These studies demonstrate that interstitial and glymphatic dynamics in the anesthetized or sedated brain are not necessarily the same as those observed in the sleeping brain.
To date, these studies have only been conducted in rodents using electrophysiological recordings through a craniotomy, and haven’t been confirmed in human subjects. If validated in human subjects, then sleep-wake changes in the dimensions of the brain extracellular space will have important implications not only for the clearance of interstitial solutes such as Aβ, but also for the diffusion and distribution of neurotrophic factors and neuromodulators. Indeed, the potential role of cortical noradrenergic tone in the regulation of sleep-wake changes in glymphatic pathway function are particularly interesting in light of observed changes in the central noradrenergic signaling axis, including increases in CSF norepinephrine levels with AD progression(109–111).
Enlarged perivascular spaces
MRI-visible perivascular spaces (ePVS) have traditionally been considered benign radiographic features that are increasingly associated with different neurological conditions, including clinical and diagnostic features of amyloid pathology. Post mortem evaluation has linked ePVS with CAA(112, 113) and increased Aβ plaque burden(113). Further, in vivo work employing MRI has identified a strong correlation between radiologically visible ePVS and AD status (114, 115), cerebral small vessel disease(116), and CAA(112). Sleep efficiency was negatively correlated with total perivascular space volume, and duration of N3 negatively correlated with PVS volume, but not apnea, hypopnea or duration of N1, N2, or REM(117). These studies suggest that enlarged perivascular space burden may reflect impaired perivascular glymphatic function. In support of this notion, a recent case series evaluating perivascular CSF influx and clearance in human subjects reported that perivascular influx and interstitial clearance were both slowed in the setting of normal pressure hydrocephalus (NPH)(54). Importantly, in subjects diagnosed with NPH, ePVS are often observed on in vivo MRI(118) while loss of perivascular AQP4 localization is observed post mortem upon histopathological evaluation(119).
Summary
We have outlined the basis of an established relationship between clinical symptoms of AD, a canonical pathological hallmark of the disease (Aβ), and sleep. We have discussed the ways that sleep may interact with the systems responsible for maintaining brain homeostasis in the context of AD. As both reductions in sleep efficiency and Aβ accumulation occur in the decades preceding clinical AD, it is difficult to identify the causative relationship, if any between these two observations. This has resulted in the notion that sleep disruption and amyloidopathy occur as a cycle of inter-related events (Figure 1A–B); amyloid pathology alters cellular and molecularly governed sleep patterns, while the inability of the brain to engage in successful sleep renders it vulnerable to Aβ pathology(100).
Footnotes
Disclosures
Research in the Iliff lab is funded by grants from the NIA (AG054456), NINDS (NS089709), the Paul G. Allen Family Foundation; a Sponsored Collaborative Agreement with GlaxoSmithKline, and a Sponsored Research Agreement with Gilead Pharmaceuticals. Dr. Iliff reports receiving lecture fees from GlaxoSmithKline, Shire, Genentech, and Neurim Pharmaceuticals. Dr. Boespflug reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Golde TE, Schneider LS, Koo EH. Anti-Aβ Therapeutics in Alzheimer’s Disease: The Need for a Paradigm Shift. Neuron. 2011;69:203–213. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon MJ, Iliff JJ. Regulation of cerebrospinal fluid (CSF) flow in neurodegenerative, neurovascular and neuroinflammatory disease. Biochim Biophys Acta. 2016;1862:442–451. doi: 10.1016/j.bbadis.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, et al. Association of Perivascular Localization of Aquaporin-4 With Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017;74:91–99. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 9.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 12.Golde TE, Eckman CB, Younkin SG. Biochemical detection of Aβ isoforms: implications for pathogenesis, diagnosis, and treatment of Alzheimer’s disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 13.Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proceedings of the National Academy of Sciences. 1993;90:2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann T, Bieger SC, Bruhl B, Tienari PJ, Ida N, Allsop D, et al. Distinct sites of intracellular production for Alzheimer’s disease A[beta]40/42 amyloid peptides. Nat Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- 15.Jarrett JT, Berger EP, Lansbury PT. The carboxy terminus of the beta. amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- 16.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 17.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Konrad V, et al. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Annals of Neurology. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 18.Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–358. doi: 10.1016/j.nbd.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosa MI, Perucchi J, Medeiros LR, Fernandes B, Fernandes Dos Reis ME, Silva BR. Accuracy of cerebrospinal fluid Abeta(1-42) for Alzheimer’s disease diagnosis: a systematic review and meta-analysis. J Alzheimers Dis. 2014;40:443–454. doi: 10.3233/JAD-132264. [DOI] [PubMed] [Google Scholar]
- 21.Fagan AM, Mintun MA, Mach RH, Lee S-Y, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Annals of Neurology. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 22.Grimmer T, Riemenschneider M, Förstl H, Henriksen G, Klunk WE, Mathis CA, et al. Beta Amyloid in Alzheimer’s Disease: Increased Deposition in Brain Is Reflected in Reduced Concentration in Cerebrospinal Fluid. Biological Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo J-A, Lim J-H, Sul A-R, Lee M, Youn YC, Kim H-J. Cerebrospinal Fluid β-Amyloid(1–42) Levels in the Differential Diagnosis of Alzheimer’s Disease—Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0116802. doi: 10.1371/journal.pone.0116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson BW, Elbert DL, Mawuenyega KG, Kasten T, Ovod V, Ma S, et al. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015;78:439–453. doi: 10.1002/ana.24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saido T, Leissring MA. Proteolytic Degradation of Amyloid β-Protein. Cold Spring Harbor Perspectives in Medicine. 2012;2:a006379. doi: 10.1101/cshperspect.a006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saido TC, Iwata N. Metabolism of amyloid β peptide and pathogenesis of Alzheimer’s disease: Towards presymptomatic diagnosis, prevention and therapy. Neuroscience Research. 2006;54:235–253. doi: 10.1016/j.neures.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-[beta] peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 30.Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO. Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol. 2003;29:106–117. doi: 10.1046/j.1365-2990.2003.00424.x. [DOI] [PubMed] [Google Scholar]
- 31.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masseguin C, LePanse S, Corman B, Verbavatz JM, Gabrion J. Aging affects choroidal proteins involved in CSF production in Sprague-Dawley rats. Neurobiol Aging. 2005;26:917–927. doi: 10.1016/j.neurobiolaging.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, et al. Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS. 2011;8:21. doi: 10.1186/2045-8118-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14:977–979. doi: 10.1016/S1474-4422(15)00221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Kanekiyo T, Cirrito JR, Liu CC, Shinohara M, Li J, Schuler DR, et al. Neuronal clearance of amyloid-beta by endocytic receptor LRP1. J Neurosci. 2013;33:19276–19283. doi: 10.1523/JNEUROSCI.3487-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanekiyo T, Liu CC, Shinohara M, Li J, Bu G. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci. 2012;32:16458–16465. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci. 2015;7:136. doi: 10.3389/fnagi.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bell RD, Sagare AP, Friedman AE, Bedi GS, Holtzman DM, Deane R, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–918. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wijesuriya HC, Bullock JY, Faull RL, Hladky SB, Barrand MA. ABC efflux transporters in brain vasculature of Alzheimer’s subjects. Brain Res. 2010;1358:228–238. doi: 10.1016/j.brainres.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 47.Groothuis DR, Vavra MW, Schlageter KE, Kang EW, Itskovich AC, Hertzler S, et al. Efflux of drugs and solutes from brain: the interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J Cereb Blood Flow Metab. 2007;27:43–56. doi: 10.1038/sj.jcbfm.9600315. [DOI] [PubMed] [Google Scholar]
- 48.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319–328. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 49.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS. Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife. 2017:6. doi: 10.7554/eLife.27679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holter KE, Kehlet B, Devor A, Sejnowski TJ, Dale AM, Omholt SW, et al. Interstitial solute transport in 3D reconstructed neuropil occurs by diffusion rather than bulk flow. Proc Natl Acad Sci U S A. 2017;114:9894–9899. doi: 10.1073/pnas.1706942114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratner V, Gao Y, Lee H, Elkin R, Nedergaard M, Benveniste H, et al. Cerebrospinal and interstitial fluid transport via the glymphatic pathway modeled by optimal mass transport. Neuroimage. 2017;152:530–537. doi: 10.1016/j.neuroimage.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goulay R, Flament J, Gauberti M, Naveau M, Pasquet N, Gakuba C, et al. Subarachnoid Hemorrhage Severely Impairs Brain Parenchymal Cerebrospinal Fluid Circulation in Nonhuman Primate. Stroke. 2017;48:2301–2305. doi: 10.1161/STROKEAHA.117.017014. [DOI] [PubMed] [Google Scholar]
- 54.Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. 2017 doi: 10.1093/brain/awx191. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki Y, Nakamura Y, Yamada K, Igarashi H, Kasuga K, Yokoyama Y, et al. Reduced CSF Water Influx in Alzheimer’s Disease Supporting the beta-Amyloid Clearance Hypothesis. PLoS One. 2015;10:e0123708. doi: 10.1371/journal.pone.0123708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Igarashi H, Suzuki Y, Kwee IL, Nakada T. Water influx into cerebrospinal fluid is significantly reduced in senile plaque bearing transgenic mice, supporting beta-amyloid clearance hypothesis of Alzheimer’s disease. Neurol Res. 2014;36:1094–1098. doi: 10.1179/1743132814Y.0000000434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. Journal of magnetic resonance imaging: JMRI. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 59.Wang P, Olbricht WL. Fluid mechanics in the perivascular space. J Theor Biol. 2011;274:52–57. doi: 10.1016/j.jtbi.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14:69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42–49. doi: 10.1152/ajprenal.1980.238.1.F42. [DOI] [PubMed] [Google Scholar]
- 62.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 63.Weed LH. Studies on Cerebro-Spinal Fluid. No. III: The pathways of escape from the Subarachnoid Spaces with particular reference to the Arachnoid Villi. The Journal of medical research. 1914;31:51–91. [PMC free article] [PubMed] [Google Scholar]
- 64.Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol. 1990;52:431–439. [PubMed] [Google Scholar]
- 65.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 66.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246:F835–844. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- 69.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991;545:103–113. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 70.Bedussi B, van der Wel NN, de Vos J, van Veen H, Siebes M, VanBavel E, et al. Paravascular channels, cisterns, and the subarachnoid space in the rat brain: A single compartment with preferential pathways. J Cereb Blood Flow Metab. 2017;37:1374–1385. doi: 10.1177/0271678X16655550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bedussi B, van Lier MG, Bartstra JW, de Vos J, Siebes M, VanBavel E, et al. Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS. 2015;12:23. doi: 10.1186/s12987-015-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol Open. 2015;4 doi: 10.1177/2058460115609635. 2058460115609635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 74.Hawkes CA, Sullivan PM, Hands S, Weller RO, Nicoll JA, Carare RO. Disruption of arterial perivascular drainage of amyloid-beta from the brains of mice expressing the human APOE epsilon4 allele. PLoS One. 2012;7:e41636. doi: 10.1371/journal.pone.0041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- 76.Wang M, Ding F, Deng S, Guo X, Wang W, Iliff JJ, et al. Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J Neurosci. 2017;37:2870–2877. doi: 10.1523/JNEUROSCI.2112-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the CNS castle. Fluids and barriers of the CNS. 2011;8:4. doi: 10.1186/2045-8118-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mestre H, Kress BT, Zou W, Pu T, Murlidharan G, Castellanos Rivera RM, et al. Aquaporin-4 dependent glymphatic solute transport in rodent brain. bioRxiv. 2017 doi: 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener. 2015;10:58. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Asgari M, de Zelicourt D, Kurtcuoglu V. How astrocyte networks may contribute to cerebral metabolite clearance. Sci Rep. 2015;5:15024. doi: 10.1038/srep15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osborn LM, Kamphuis W, Wadman WJ, Hol EM. Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol. 2016;144:121–141. doi: 10.1016/j.pneurobio.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 82.Wang M, Iliff JJ, Liao Y, Chen MJ, Shinseki MS, Venkataraman A, et al. Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci. 2012;32:17948–17960. doi: 10.1523/JNEUROSCI.1860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, et al. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33:834–845. doi: 10.1038/jcbfm.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoshi A, Yamamoto T, Shimizu K, Ugawa Y, Nishizawa M, Takahashi H, et al. Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J Neuropathol Exp Neurol. 2012;71:750–759. doi: 10.1097/NEN.0b013e3182632566. [DOI] [PubMed] [Google Scholar]
- 85.Moftakhar P, Lynch MD, Pomakian JL, Vinters HV. Aquaporin expression in the brains of patients with or without cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2010;69:1201–1209. doi: 10.1097/NEN.0b013e3181fd252c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perez E, Barrachina M, Rodriguez A, Torrejon-Escribano B, Boada M, Hernandez I, et al. Aquaporin expression in the cerebral cortex is increased at early stages of Alzheimer disease. Brain Res. 2007;1128:164–174. doi: 10.1016/j.brainres.2006.09.109. [DOI] [PubMed] [Google Scholar]
- 87.Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:361–370. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 89.Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, et al. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J Int Neuropsychol Soc. 2012;18:490–500. doi: 10.1017/S135561771200001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hita-Yanez E, Atienza M, Gil-Neciga E, Cantero JL. Disturbed sleep patterns in elders with mild cognitive impairment: the role of memory decline and ApoE epsilon4 genotype. Curr Alzheimer Res. 2012;9:290–297. doi: 10.2174/156720512800107609. [DOI] [PubMed] [Google Scholar]
- 91.Hartmann E. The 90-Minute Sleep-Dream Cycle. Archives of General Psychiatry. 1968;18:280–286. doi: 10.1001/archpsyc.1968.01740030024004. [DOI] [PubMed] [Google Scholar]
- 92.AASM. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL, USA: American Academy of Sleep Medicine; [Google Scholar]
- 93.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 94.Prinz PN, Peskind ER, Vitaliano PP, Raskind MA, Eisdorfer C, Zemcuznikov N, et al. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 95.Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6:347–352. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-Reported Sleep and β-Amyloid Deposition in Community-Dwelling Older Adults. JAMA neurology. 2013;70:1537–1543. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ju YS, McLeland JS, Toedebusch CD, et al. Sleep quality and preclinical alzheimer disease. JAMA Neurology. 2013;70:587–593. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4:150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang J-E, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat Rev Neurol. 2014;10:115–119. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends in Neurosciences. 39:552–566. doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lim MM, Gerstner JR, Holtzman DM. The sleep–wake cycle and Alzheimer’s disease: what do we know? Neurodegenerative disease management. 2014;4:351–362. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev. 2017;31:102–111. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ooms S, Overeem S, Besse K, Rikkert M, Verbeek M, Claassen JR. Effect of 1 night of total sleep deprivation on cerebrospinal fluid β-amyloid 42 in healthy middle-aged men: A randomized clinical trial. JAMA Neurology. 2014;71:971–977. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- 105.Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–861. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lucey BP, Mawuenyega KG, Patterson BW, Elbert DL, Ovod V, Kasten T, et al. Associations Between beta-Amyloid Kinetics and the beta-Amyloid Diurnal Pattern in the Central Nervous System. JAMA Neurol. 2017;74:207–215. doi: 10.1001/jamaneurol.2016.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gakuba C, Gaberel T, Goursaud S, di Palma C, Quenault I, Martinez De Lizarrondo S, et al. General anesthesia impairs the glymphatic system. Anesthesiology. 2016 doi: 10.7150/thno.19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R, et al. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology. 2017;127:976–988. doi: 10.1097/ALN.0000000000001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer’s disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Szot P, White SS, Greenup JL, Leverenz JB, Peskind ER, Raskind MA. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: evidence of compensatory changes. Neuroscience. 2007;146:471–480. doi: 10.1016/j.neuroscience.2007.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elrod R, Peskind ER, DiGiacomo L, Brodkin KI, Veith RC, Raskind MA. Effects of Alzheimer’s disease severity on cerebrospinal fluid norepinephrine concentration. Am J Psychiatry. 1997;154:25–30. doi: 10.1176/ajp.154.1.25. [DOI] [PubMed] [Google Scholar]
- 112.Charidimou A, Jaunmuktane Z, Baron JC, Burnell M, Varlet P, Peeters A, et al. White matter perivascular spaces: an MRI marker in pathology-proven cerebral amyloid angiopathy? Neurology. 2014;82:57–62. doi: 10.1212/01.wnl.0000438225.02729.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med. 2003;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- 114.Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE. Visible Virchow-Robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook Dementia Study. J Alzheimers Dis. 2015;43:415–424. doi: 10.3233/JAD-132528. [DOI] [PubMed] [Google Scholar]
- 115.Chen W, Song X, Zhang Y Alzheimer’s Disease Neuroimaging I. Assessment of the Virchow-Robin Spaces in Alzheimer disease, mild cognitive impairment, and normal aging, using high-field MR imaging. AJNR Am J Neuroradiol. 2011;32:1490–1495. doi: 10.3174/ajnr.A2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10:376–381. doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Berezuk C, Ramirez J, Gao F, Scott CJ, Huroy M, Swartz RH, et al. Virchow-Robin Spaces: Correlations with Polysomnography-Derived Sleep Parameters. Sleep. 2015;38:853–858. doi: 10.5665/sleep.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tarnaris A, Tamangani J, Fayeye O, Kombogiorgas D, Murphy H, Gan YC, et al. Virchow-Robin spaces in idiopathic normal pressure hydrocephalus: a surrogate imaging marker for coexisting microvascular disease? Acta Neurochir Suppl. 2012;113:33–37. doi: 10.1007/978-3-7091-0923-6_7. [DOI] [PubMed] [Google Scholar]
- 119.Eide PK, Hansson HA. Astrogliosis and impaired aquaporin-4 and dystrophin systems in idiopathic normal pressure hydrocephalus. Neuropathol Appl Neurobiol. 2017 doi: 10.1111/nan.12420. [DOI] [PubMed] [Google Scholar]
- 120.Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag. 2014;4:351–362. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peng W, Achariyar TM, Li B, Liao Y, Mestre H, Hitomi E, et al. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2016;93:215–225. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, et al. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18:1051–1057. doi: 10.1038/nn.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]