Abstract
Oxidative stress is one hypothesized mechanism linking anthropometric, behavioral, and medical risk factors with cardiovascular disease (CVD). We evaluated cross-sectional associations between CVD risk factors and biomarkers of oxidative stress, and investigated these biomarkers as predictors of incident diabetes and hypertension among premenopausal women. F2-isoprostane (F2-IsoP) and metabolite (15-F2t-IsoP-M), reliable biomarkers of oxidative stress, were measured in urine samples collected at enrollment from 897 premenopausal women (ages 35-54) enrolled in the Sister Study cohort without a CVD history. Blood pressure, waist circumference, and body mass index (BMI) were measured at enrollment by trained study personnel. Diabetes and cigarette smoking were self-reported via enrollment questionnaires. Over a maximum follow-up of 11.5 years, participants self-reported incident diabetes and hypertension diagnoses on mailed questionnaires. In cross-sectional analyses, both F2-IsoP and 15-F2t-IsoP-M were positively associated with BMI, waist circumference, diastolic blood pressure, and current smoking. F2-IsoP was elevated among those with diabetes, and 15-F2t-IsoP-M increased with higher systolic blood pressure. Prospective analyses suggested an increased hypertension risk among those with elevated 15-F2t-IsoP-M (highest vs. lowest quartile: hazard ratio=2.34; 95% CI: 1.20-4.56). Our results suggest that urinary F2-IsoP and 15-F2t-IsoP-M are positively associated with adiposity measures, blood pressure, and cigarette smoking. Further investigation is warranted to evaluate 15-F2t-IsoP-M as a predictor of hypertension.
Keywords: oxidative stress, F2-isoprostanes, hypertension, cardiovascular disease, body mass index
Graphical abstract
Introduction
An overabundance of reactive oxygen species (ROS) relative to antioxidant defense, termed oxidative stress, may play a critical role in the pathogenesis of several chronic diseases, including cardiovascular diseases (CVD). ROS are hypothesized to drive the development and progression of atherosclerosis through the peroxidation of polyunsaturated fatty acids (PUFAs) in lipoproteins, producing a chronic inflammatory state that leads to plaque formation and rupture.[1] Thus markers of lipid peroxidation may be useful indicators of vascular disease risk.
Classical risk factors for atherosclerosis and subsequent occlusive events, such as obesity and cigarette smoking, have been associated with increased lipid peroxidation, a potential pathway through which these factors may influence vascular disease development.[1] F2-isoprostanes (F2-IsoPs), stable products of the peroxidation of arachidonic acid, are widely considered the current ‘gold standard’ measure of oxidative stress in vivo,[2] and elevations in both urinary and plasma concentrations of these biomarkers have been observed among current smokers and individuals with diabetes, hypertension, and obesity.[3–5] However, most previous investigations of relationships between F2-IsoPs and these key CVD risk factors have been conducted in small, clinical cohorts, often comprised of patients with advanced disease. Furthermore, the few studies to date in healthy populations have relied exclusively on the measurement of plasma F2-IsoPs or unmetabolized F2-IsoPs in urine.[3–5] F2-IsoPs measured in plasma may be subject to autoxidation during sample collection and storage,[6] while unmetabolized F2-IsoPs in urine may reflect local F2-IsoP production in the kidneys, rather than systemic oxidative stress.[7] Thus the predominant urinary F2-isoprostane metabolite, 2,3-dinor-5,6-dihydro-15-F2t-isoprostane (15-F2t-IsoP-M), which is independent of local renal production, may be a better marker of systemic oxidative stress in vivo. To our knowledge, associations between 15-F2t-IsoP-M and major CVD risk factors have not been comprehensively evaluated in a young, CVD-free population.
The objective of this study was to examine cross-sectional associations between risk factors for CVD and oxidative stress, as measured by urinary F2-IsoP and 15-F2t-IsoP-M, in a cohort of premenopausal women without a history of cardiovascular conditions. Additionally, we evaluated whether urinary F2-IsoP and 15-F2t-IsoP-M concentrations were associated with incident diabetes and hypertension over a maximum follow-up of 11.5 years.
Materials and methods
Study population
Participants in these analyses were controls in a case-control study of oxidative stress and breast cancer risk nested within the prospective Sister Study cohort.[8] Between 2003 and 2009, over 50,000 women from the U.S. and Puerto Rico were recruited into the Sister Study through a national multi-media campaign and a network of breast cancer professionals and volunteers. Women were eligible to participate in the Sister Study if they were ages 35 to 74 years and free of breast cancer themselves at enrollment, but had a sister with a breast cancer diagnosis. All participants provided written informed consent. The study was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences, the National Institutes of Health, and the Copernicus Group.
Sister Study participants eligible for inclusion in the control sample were ages 35 to 54 years, premenopausal, had at least one intact ovary, and had a urine sample collected at enrollment. Women were considered premenopausal if they self-reported at least one menstrual cycle within the 12 months prior to enrollment, or were aged 54 years and younger and their only reason for not experiencing menses was hysterectomy (without bilateral oophorectomy). A total of 922 women remained breast cancer free as of December 31, 2012 and were selected as control participants. For these analyses, we excluded women who reported a history of heart attack, angina, stroke, transient ischemic attack, or congestive heart failure (N=23) on enrollment questionnaires. We also excluded those classified as having type 1 diabetes, defined as a self-reported diabetes diagnosis before age 30, due to their small number (N=2). Thus final analyses include 897 women.
CVD risk factor assessment
During a home visit at Sister Study enrollment, trained study personnel used standardized protocols to measure blood pressure, height, weight, and waist circumference. Three sitting measurements, approximately 1–2 minutes apart, were taken for systolic and diastolic pressure. If both arms could be used, measurements were taken from alternating arms, starting with the left arm (Left→Right→Left). Otherwise, three readings were taken from the available arm. The average of the three readings was used in all analyses. Height and weight were measured without shoes, and waist circumference was measured using a cloth tape measure over skin or lightweight clothing. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Sociodemographic information and current cigarette smoking, physical activity, and use of medications for high blood pressure were assessed via questionnaire at Sister Study enrollment. Participants also self-reported any previous diagnosis of diabetes, age at diabetes diagnosis, and use of diabetes medication on enrollment questionnaires. We classified women as having type 2 diabetes if they reported a diabetes diagnosis at age 30 years or older and/or were currently taking diabetes medication (self-reported diagnosis only: N=11; diabetes medication only: N=5; self-reported diagnosis and diabetes medication: N=15). According to the methods described by D’Agostino et al,[9, 10] we also calculated an absolute measure of 10-year general CVD risk. This risk score was developed in the Framingham Heart Study and incorporates information on non-laboratory-based risk factors, including gender, age, systolic blood pressure, hypertension treatment, smoking, diabetes, and BMI.
Incident diabetes and hypertension events were ascertained via detailed follow-up questionnaires, completed by participants every 2–3 years, and brief health update questionnaires completed annually. Women were asked to report a diagnosis of diabetes or hypertension, as well as the date of diagnosis.
Oxidative stress measurement
Sister Study participants provided first morning urine samples during the enrollment home visit. Urine samples were shipped frozen to the Eicosanoid Core Laboratory at Vanderbilt University Medical Center, where gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI MS) was used to measure F2-IsoP and 15-F2t-IsoP-M. Detailed protocols for these methods have been published. [11–13] To account for urine diluteness, all values of F2-IsoP and 15-F2t-IsoP-M were adjusted for creatinine concentrations and are reported as ng/mg of creatinine (ng/mg Cr).
Statistical analysis
Values of F2-IsoP and 15-F2t-IsoP-M were log-transformed to approximate a normal distribution. Unadjusted and multivariable linear regression models, with log-transformed F2-IsoP and 15-F2t-IsoP-M as the dependent variable, were used to evaluate cross-sectional associations with continuous (age, systolic blood pressure, diastolic blood pressure, BMI, waist circumference, 10-year CVD risk score) and dichotomous (current smoking, prevalent diabetes,) variables. Due to evidence of collinearity between BMI and waist circumference, and between systolic and diastolic blood pressure, these variables were not entered together in the same linear regression models. We further considered adjustment for education level (less than Bachelor’s degree, Bachelor’s degree, higher than Bachelor’s degree), race (white, non-white), and physical activity (total MET [metabolic equivalent] hours/week). Estimates were largely unchanged; therefore, we present results without adjustment for these additional variables. For associations between F2-IsoP and 15-F2t-IsoP-M and systolic and diastolic blood pressure measures, we performed sensitivity analyses excluding those who reported taking high blood pressure medication at enrollment (N=114).
Using general linear models, we calculated multivariable-adjusted geometric means of F2-IsoP and 15-F2t-IsoP-M within categories of all risk factor variables. BMI and waist circumference were categorized according to established guidelines,[14, 15] and systolic blood pressure, diastolic blood pressure, and 10-year CVD risk score were categorized using study-specific quartiles.
Cox proportional hazards regression models were used to estimate associations between F2-IsoP and 15-F2t-IsoP-M and incident diabetes and hypertension. Person-time at risk was defined independently for each outcome, as the time between Sister Study enrollment and date of self-reported diagnosis or date of last contact, whichever came first. Participants who reported a diagnosis prior to Sister Study enrollment were excluded from analyses specific to that outcome (diabetes: N=46; hypertension: N=144). For incident hypertension analyses, we further excluded any others who reported high blood pressure medication use at enrollment (N=3), or had a systolic blood pressure ≥140 or a diastolic blood pressure ≥90, as measured at the enrollment home visit (N=2). F2-IsoP and 15-F2t-IsoP-M were categorized into quartiles for analyses of hypertension. Multivariable models for hypertension were adjusted for age, education, race, physical activity, BMI, and current smoking at enrollment. Tests for linear trend were conducted by including F2-IsoP or 15-F2t-IsoP-M in the model as a continuous variable. Due to the small number of incident diabetes events, we dichotomized F2-IsoP and 15-F2t-IsoP-M at the median for these analyses. In sensitivity analyses, we varied the cutpoint used for dichotomization (e.g. 25th or 75th percentile). Multivariable models for diabetes were adjusted for age, education, race, physical activity, and BMI at enrollment. The proportional hazards assumption was checked by visual assessment of log-log plots. All analyses were performed with Sister Study Data Release 5.0.1 using SAS 9.4 (SAS Institute, Cary, NC).
Results
Participant characteristics are shown in Table 1. The average age at enrollment was 47 years (SD= 4). The majority of participants were non-Hispanic white (87%) and non-smokers (91%). The mean BMI was 27 kg/m2 (SD=7). Few participants had type 2 diabetes (3%), a diastolic blood pressure ≥90 (3%), or a systolic blood pressure ≥140 (2%) at enrollment.
Table 1.
Participant characteristics at enrollment (N=897)
| N | % | |
|---|---|---|
|
Age
|
|
|
| Mean, SD
|
47
|
4
|
| <45
|
243
|
27
|
| 45-49
|
308
|
34
|
| 50+
|
346
|
39
|
|
Race/ethnicity
|
|
|
| Non-Hispanic white
|
784
|
87
|
| Non-Hispanic black
|
57
|
6
|
| Hispanic
|
34
|
4
|
| Other
|
22
|
2
|
|
Education
|
|
|
| Less than Bachelor’s degree
|
370
|
41
|
| Bachelor’s degree
|
284
|
32
|
| Higher than Bachelor’s degree
|
243
|
27
|
|
BMI (kg/m2)
|
|
|
| Mean, SD
|
27
|
7
|
| <25
|
418
|
47
|
| 25-29
|
244
|
27
|
| 30+
|
234
|
26
|
|
Waist
|
|
|
| Mean, SD
|
83
|
15
|
| <=80
|
442
|
49
|
| 80.1-88
|
163
|
18
|
| 88+
|
290
|
32
|
|
Current smoking
|
|
|
| No
|
820
|
91
|
| Yes
|
77
|
9
|
| Total physical activity (MET-hrs/week), median (IQR)
|
44
|
(28, 66)
|
|
Type 2 Diabetes*
|
|
|
| No
|
866
|
97
|
| Yes
|
31
|
3
|
|
Diastolic blood pressure (mmHg)†
|
|
|
| <64
|
207
|
23
|
| 64-70
|
255
|
28
|
| 71-77
|
198
|
22
|
| 78-89
|
212
|
24
|
| ≥90
|
23
|
3
|
|
Systolic blood pressure (mmHg)‡
|
|
|
| <101
|
208
|
23
|
| 101-109
|
232
|
26
|
| 110-118
|
240
|
27
|
| 119-139
|
194
|
22
|
| ≥140
|
21
|
2
|
|
Currently taking high blood pressure medication
|
|
|
| No
|
783
|
87
|
| Yes
|
114
|
13
|
|
10-year CVD risk (quartiles)
|
|
|
| <2.0%
|
201
|
22
|
| 2.0-2.8%
|
238
|
27
|
| 2.9-4.1%
|
225
|
25
|
| ≥4.2% | 230 | 26 |
Defined as a self-reported diabetes diagnosis at age 30 years or older and/or use of oral diabetes medication
Categories defined by the 25th (64), 50th (71), and 75th (78) percentiles, and 90 mmHg, the value of diastolic blood pressure used to define hypertension
Categories defined by the 25th (101), 50th (110), and 75th (119) percentiles, and 140 mmHg, the value of systolic blood pressure used to define hypertension
Abbreviations: CVD, cardiovascular disease; BMI, body mass index; MET, metabolic equivalent; SD, standard deviation; IQR, interquartile range
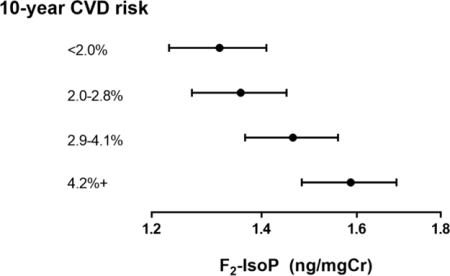
In multivariable linear regression models, urinary F2-IsoP and 15-F2t-IsoP-M were positively associated with BMI (both p<0.001) and waist circumference (both p<0.001) (Table 2). Both F2-IsoP and 15-F2t-IsoP-M increased with increasing diastolic blood pressure (F2-IsoP: p=0.031; 15-F2t-IsoP-M: p<0.001), while only 15-F2t-IsoP-M increased significantly with systolic blood pressure (p=0.004). Associations with blood pressure measures remained similar when 114 women taking high blood pressure medication at enrollment were excluded, for both F2-IsoP (systolic: β=0.046, p=0.180; diastolic: β=0.063, p =0.007) and 15-F2t-IsoP-M (systolic: β=0.087, p=0.002; diastolic: β=0.086, p<0.001). F2-IsoP was elevated among current smokers (p=0.046) and those with type 2 diabetes (p=0.009). Patterns were similar for 15-F2t-IsoP-M, though the association with type 2 diabetes appeared somewhat weaker (p=0.128). Age was inversely associated with 15-F2t-IsoP-M (p=0.016), but was not strongly associated with F2-IsoP. Both F2-IsoP and 15-F2t-IsoP-M increased with increasing 10-year CVD risk score (both p<0.001). Geometric means of F2-IsoP and 15-F2t-IsoP-M according to categorical variables are shown in Figure 1.
Table 2.
Regression coefficients for CVD risk factors in relation to change in log F2-IsoP or 15-F2t-IsoP-M (ng/mg Cr)
|
|
F2-IsoP
|
15-F2t-IsoP-M
|
||||||
|---|---|---|---|---|---|---|---|---|
|
|
Unadjusted
|
Adjusteda
|
Unadjusted
|
Adjusted*
|
||||
| β | P | β | p | B | p | β | p | |
| Systolic BP, per 20 mmHg
|
0.109
|
<0.001
|
0.019
|
0.524
|
0.169
|
<0.001
|
0.070
|
0.004
|
| Diastolic BP, per 10 mmHg†
|
0.100
|
<0.001
|
0.046
|
0.031
|
0.138
|
<0.001
|
0.072
|
<0.001
|
| BMI, per 5 kg/m2
|
0.092
|
<0.001
|
0.079
|
<0.001
|
0.121
|
<0.001
|
0.101
|
<0.001
|
| Waist circumference, per 5 cm‡
|
0.099
|
<0.001
|
0.080
|
<0.001
|
0.135
|
<0.001
|
0.109
|
<0.001
|
| Current smoking
|
0.169
|
0.006
|
0.122
|
0.046
|
0.216
|
<0.001
|
0.165
|
<0.001
|
| Type 2 diabetes
|
0.390
|
<0.001
|
0.249
|
0.009
|
0.326
|
<0.001
|
0.118
|
0.128
|
| Age, per 5 years
|
0.015
|
0.439
|
0.026
|
0.182
|
0.020
|
0.234
|
0.038
|
0.016
|
| 10-year CVD risk | 3.081 | <0.001 | 4.205 | <0.001 | ||||
Adjusted for systolic blood pressure, BMI, current smoking, prevalent diabetes, age
Adjusted for BMI, current smoking, prevalent diabetes, age
Adjusted for systolic blood pressure, current smoking, prevalent diabetes, age Abbreviations: CVD, cardiovascular disease; F2-IsoP, F2-isoprostane; 15-F2t-IsoP-M, F2-isoprostane metabolite; BMI, body mass index; Cr, creatinine
Figure 1.
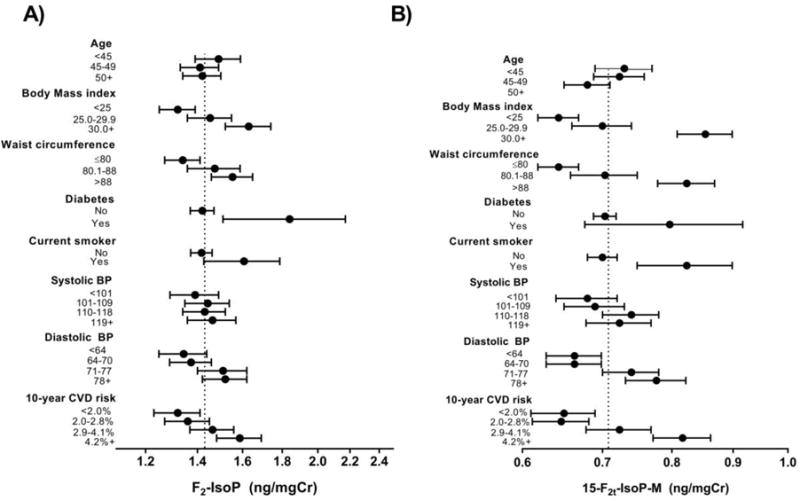
Geometric mean (95% CI) values of A) F2-IsoP and B) 15-F2t-IsoP-M according to CVD risk factors among 897 premenopausal women. Means according to 10-year CVD risk are unadjusted. All other means adjusted for current smoking, prevalent diabetes and age. All means except for those according to waist circumference are additionally adjusted for body mass index. All means except for those according to diastolic blood pressure are additionally adjusted for systolic blood pressure.
Units: Body mass index, kg/m2; waist circumference, cm; systolic blood pressure, mmHg; diastolic blood pressure, mmHg
With adjustment for age and BMI at enrollment, neither F2-IsoP nor 15-F2t-IsoP-M concentrations above the median were strongly associated with incident diabetes (Table 3). Patterns remained similar when alternative cutpoints (e.g. 25th or 75th percentile) were used to dichotomize F2-IsoP and 15-F2t-IsoP-M (data not shown). F2-IsoP was also not associated with risk of hypertension in the adjusted model. However, hypertension risk appeared to increase with 15-F2t-IsoP-M (HR for highest vs lowest quartile: 2.34; 95% CI: 1.20, 4.56; ptrend=0.060), though adjusted estimates were noticeably attenuated compared to unadjusted estimates.
Table 3.
Hazard ratios (HR) and 95% confidence intervals (CI) for incident diabetes and hypertension
| N events | N person-years | Unadjusted HR (95% CI) | Adjusted HR (95% CI)* | |
|---|---|---|---|---|
|
Diabetes
|
|
|
|
|
| F2-IsoP (ng/mg Cr)
|
|
|
|
|
| <1.38
|
14
|
3500.02
|
1
|
1
|
| 1.38+
|
23
|
3484.71
|
1.66 (0.85, 3.22)
|
1.14 (0.57, 2.29)
|
| Continuous (per ng/mg Cr)
|
|
|
1.22 (0.95, 1.58)
|
0.98 (0.73, 1.30)
|
| 15-F2t-IsoP-M (ng/mg Cr)
|
|
|
|
|
| <0.69
|
13
|
3550.93
|
1
|
1
|
| 0.69+
|
24
|
3433.81
|
1.91 (0.97, 3.75)
|
0.95 (0.45, 2.02)
|
| Continuous (per ng/mg Cr)
|
|
|
2.21 (1.24, 3.94)
|
1.01 (0.48, 2.10)
|
|
Hypertension
|
|
|
|
|
| F2-IsoP (ng/mg Cr)
|
|
|
|
|
| <1.00
|
20
|
1390.29
|
1
|
1
|
| 1.00-1.37
|
28
|
1443.45
|
1.37 (0.77, 2.44)
|
1.41 (0.79, 2.53)
|
| 1.38-1.94
|
29
|
1521.51
|
1.34 (0.76, 2.37)
|
1.19 (0.66, 2.14)
|
| 1.94+
|
37
|
1387.80
|
1.80 (1.04, 3.13)
|
1.31 (0.73, 2.35)
|
| Continuous (per ng/mg Cr)
|
|
|
1.23 (1.06, 1.43)
|
1.06 (0.90, 1.24)
|
| Ptrend
|
|
|
0.006
|
0.482
|
| 15-F2t-IsoP-M (ng/mg Cr)
|
|
|
|
|
| <0.53
|
14
|
1555.09
|
1
|
1
|
| 0.53-0.68
|
28
|
1510.49
|
2.07 (1.09, 3.93)
|
1.98 (1.04, 3.77)
|
| 0.69-0.93
|
33
|
1461.65
|
2.53 (1.36, 4.74)
|
2.34 (1.24, 4.43)
|
| 0.94+
|
39
|
1215.83
|
3.41 (1.85, 6.31)
|
2.34 (1.20, 4.56)
|
| Continuous (per ng/mg Cr)
|
|
|
2.33 (1.59, 3.40)
|
1.51 (0.98, 2.33)
|
| Ptrend | <0.001 | 0.060 |
Diabetes: adjusted for age, BMI, education, race, and physical activity; Hypertension: adjusted for age, BMI, education, race, physical activity, and current smoking
Abbreviations: F2-IsoP, F2-isoprostane; 15-F2t-IsoP-M, F2-isoprostane metabolite; Cr, creatinine
Discussion
A number of behavioral, anthropometric, and medical factors may be associated with increases in lipid peroxidation, a potential mechanism through which these factors may contribute to CVD development and progression. In this study of premenopausal women without a CVD history, cross-sectional analyses suggested a positive association between F2-IsoP and 15-F2t-IsoP-M and BMI, waist circumference, blood pressure, diabetes, and smoking. These findings were reflected in the strong linear increase in F2-IsoP and 15-F2t-IsoP-M with increasing 10-year CVD risk score. In contrast, after adjustment for other risk factors, 15-F2t-IsoP-M appeared to decrease with age. Additionally, our analyses suggested that elevated 15-F2t-IsoP-M may be associated with an increased risk of developing hypertension.
Our findings for smoking and BMI are consistent with most previous investigations. The association between smoking and oxidative stress is well-established in the literature,[3, 16] reflecting the large number of oxidants present in cigarette smoke.[17] Several studies in diverse populations have also reported elevated concentrations of urinary or plasma F2-isoprostanes among overweight (BMI 25.0-29.9 kg/m2) individuals and those with obesity (BMI ≥ 30.0 kg/m2).[3, 5, 18–22] Though fewer have evaluated associations with measures of central adiposity, such as waist circumference, our findings and those of others[4, 5, 18] suggest a strong linear relationship, similar to that for BMI. A number of mechanisms have been proposed to explain associations between adiposity and oxidative stress, including alterations in inflammatory markers and adipokines.[5]
In our cross-sectional analyses, both F2-IsoP and 15-F2t-IsoP-M were elevated among participants with diabetes, though the association with the metabolite was noticeably attenuated with adjustment for BMI and other risk factors. Other cross-sectional studies have also suggested higher F2-IsoP concentrations among Type 2 diabetics.[3, 23] In a report from the Framingham Heart Study, which included men and women with and without a history of CVD, urinary F2-IsoPs were positively associated with blood glucose and diabetes in age- and sex-adjusted models. The association with glucose remained significant in models adjusted for other CVD risk factors.[3] It remains unknown, however, whether diabetes is a cause or a consequence of oxidative stress,[24] and prospective studies to date have produced conflicting results. In the Insulin Resistance Atherosclerosis Study (IRAS), an inverse association was observed between several urinary F2-IsoPs and diabetes risk,[25, 26] while a non-significant positive association was reported among Framingham Heart Study participants.[27] Given the small number of incident diabetes events in our sample, we were limited in our ability to evaluate F2-IsoP and 15-F2t-IsoP-M as predictors of diabetes risk. However, our results were not suggestive of an increased risk among those with elevated F2-IsoP or 15-F2t-IsoP-M.
The vasoconstricting and inflammatory properties of F2-isoprostanes suggest their potential involvement in the development of hypertension. However, findings from previous studies of relationships between oxidative stress and blood pressure in humans have been inconsistent.[3, 4, 28–35] Some have demonstrated positive associations between systolic blood pressure and F2-isoprostanes[3, 4, 32] or higher F2-isoprostane concentrations among hypertensives than among normotensives,[28, 33] though these associations are often attenuated by adjustment for BMI and other lifestyle and clinical factors.[3, 28, 32] Results of other cross-sectional and case-controls studies have suggested little association between F2-isoprostanes and blood pressure,[30, 31, 34, 35] while a recent study among elderly Swedish men observed an inverse association between F2-isoprostanes and a 24-hour measure of both systolic and diastolic blood pressure.[29]
In the current study, blood pressure measures were positively associated with F2-isoprostanes in cross-sectional analyses, with particularly strong associations for 15-F2t-IsoP-M. These associations were apparent even at a relatively low distribution of blood pressure; the median values of systolic and diastolic blood pressure in our sample were only 110 and 71 mmHg, respectively, well below the clinical cutpoints for hypertension. Interestingly, our results also suggested that elevated 15-F2t-IsoP-M may be associated with incident hypertension. To our knowledge, this relationship has not been previously reported. In our sample, hypertension risk appeared to plateau between the 3rd and 4th quartiles of 15-F2t-IsoP-M. This may suggest a protective effect at the lowest 15-F2t-IsoP-M concentrations, but the absence of a linear dose-response relationship. Further research in larger and more diverse samples is warranted to prospectively characterize dose-response relationships between oxidative stress biomarkers and hypertension risk.
Strengths of the current study include the standardized assessment of anthropometric measures and blood pressure. The evaluation of 15-F2t-IsoP-M, in addition to F2-IsoP, is also a notable strength. Our study also has limitations. For assessment of smoking status, diabetes, and medication use, we relied on self-reported information, which may be subject to inaccuracies. However, we were still able to detect associations between F2-IsoP and 15-F2t-IsoP-M and several CVD risk factors, suggesting that these relationships are apparent even in young populations without a CVD history. Our definition of diabetes at enrollment included a small number of women who used diabetes medication but did not report a diabetes diagnosis (N=5). The definition of incident diabetes over follow-up did not include medication use. However, exclusion of women with medication only-defined diabetes at enrollment did not meaningfully alter the observed positive association with prevalent disease, providing confidence that this difference in definition is unlikely to have influenced our results for incident diabetes. We also relied on self-report of a physician diagnosis for our analyses of incident hypertension and diabetes, and were unable to account for potential differences in physician visits (or opportunities for hypertension/diabetes detection) according to enrollment characteristics. Finally, our sample was comprised entirely of premenopausal women, most of whom were non-Hispanic white. Thus our findings may not generalize to postmenopausal women, males, or individuals of other race/ethnicities.
Conclusions
Oxidative stress is one hypothesized mechanism linking several key risk factors with cardiovascular disease. Results of the current study suggest that urinary F2-IsoP and 15-F2t-IsoP-M, reliable biomarkers or oxidative stress, are positively associated with adiposity measures, blood pressure, and cigarette smoking. Our findings also suggest that elevated 15-F2t-IsoP-M may be a predictor of incident hypertension. Further studies in larger, more diverse samples are warranted to confirm this association.
Highlights.
Oxidative stress may be a mechanistic link between key risk factors and CVD.
F2-isoprostanes (F2-IsoP) were positively associated with blood pressure measures.
Higher F2-IsoP was also associated with adiposity, diabetes, and current smoking.
Elevated F2-isoprostane metabolite in urine may predict incident hypertension.
Acknowledgments
Funding
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01-ES044005) and the Avon Foundation (02-2012-085).
Abbreviations
- CVD
cardiovascular disease
- F2-IsoP
F2-isoprostane
- 15-F2t-IsoP-M
F2-isoprostane metabolite
- BMI
body mass index
- Cr
creatinine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
None
References
- 1.Davies SS, Roberts LJ., 2nd F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free radical biology & medicine. 2011;50(5):559–66. doi: 10.1016/j.freeradbiomed.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milne GL, Yin H, Brooks JD, Sanchez S, JacksonRoberts L, 2nd, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods in enzymology. 2007;433:113–26. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 3.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arteriosclerosis, thrombosis, and vascular biology. 2003;23(3):434–9. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 4.Alkazemi D, Egeland GM, Roberts LJ, 2nd, Kubow S. Isoprostanes and isofurans as non-traditional risk factors for cardiovascular disease among Canadian Inuit. Free radical research. 2012;46(10):1258–66. doi: 10.3109/10715762.2012.702900. [DOI] [PubMed] [Google Scholar]
- 5.Kanaya AM, Wassel CL, Stoddard PJ, Harris TB, Cummings SR, Kritchevsky SB, Goodpaster BH, Green C, Satterfield S, Gross MD. F2-isoprostanes and adiposity in older adults. Obesity (Silver Spring, Md) 2011;19(4):861–7. doi: 10.1038/oby.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Il’yasova D, Scarbrough P, Spasojevic I. Urinary biomarkers of oxidative status. Clinica chimica acta; international journal of clinical chemistry. 2012;413(19-20):1446–53. doi: 10.1016/j.cca.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow JD, Zackert WE, Yang JP, Kurhts EH, Callewaert D, Dworski R, Kanai K, Taber D, Moore K, Oates JA, Roberts LJ. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Analytical biochemistry. 1999;269(2):326–31. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]
- 8.Kent EE, Forsythe LP, Yabroff KR, Weaver KE, de Moor JS, Rodriguez JL, Rowland JH. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–7. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 10.Cardiovascular Disease (10-year risk) Framingham Heart Study. Available from: https://www.framinghamheartstudy.org/risk-functions/cardiovascular-disease/10-year-risk.php.
- 11.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nature protocols. 2007;2(1):221–6. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 12.Morrow JD, Roberts LJ., 2nd Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods in enzymology. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 13.Morales CR, Terry ES, Zackert WE, Montine TJ, Morrow JD. Improved assay for the quantification of the major urinary metabolite of the isoprostane 15-F(2t)-Isoprostane (8-iso-PGF(2alpha)) by a stable isotope dilution mass spectrometric assay. Clinica chimica acta; international journal of clinical chemistry. 2001;314(1-2):93–9. doi: 10.1016/s0009-8981(01)00637-4. [DOI] [PubMed] [Google Scholar]
- 14.Obesity: preventing and managing the global epidemic. Geneva, Switzerland: 2000. (WHO Technical Report Series). [PubMed] [Google Scholar]
- 15.Ardern CI, Janssen I, Ross R, Katzmarzyk PT. Development of health-related waist circumference thresholds within BMI categories. Obesity research. 2004;12(7):1094–103. doi: 10.1038/oby.2004.137. [DOI] [PubMed] [Google Scholar]
- 16.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ., 2nd Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. The New England journal of medicine. 1995;332(18):1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 17.Frei B, Forte TM, Ames BN, Cross CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Protective effects of ascorbic acid. The Biochemical journal. 1991;277(Pt 1):133–8. doi: 10.1042/bj2770133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB, Jr, Wagenknecht LE. Urinary F2-isoprostanes, obesity, and weight gain in the IRAS cohort. Obesity (Silver Spring, Md) 2012;20(9):1915–21. doi: 10.1038/oby.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. The Journal of clinical endocrinology and metabolism. 2003;88(10):4673–6. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 20.Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. The Journal of clinical endocrinology and metabolism. 2004;89(10):4963–71. doi: 10.1210/jc.2004-0305. [DOI] [PubMed] [Google Scholar]
- 21.Araki S, Dobashi K, Yamamoto Y, Asayama K, Kusuhara K. Increased plasma isoprostane is associated with visceral fat, high molecular weight adiponectin, and metabolic complications in obese children. European journal of pediatrics. 2010;169(8):965–70. doi: 10.1007/s00431-010-1157-z. [DOI] [PubMed] [Google Scholar]
- 22.Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors associated with oxidative stress in human populations. American journal of epidemiology. 2002;156(3):274–85. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- 23.Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS letters. 1995;368(2):225–9. doi: 10.1016/0014-5793(95)00649-t. [DOI] [PubMed] [Google Scholar]
- 24.Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2009;202(2):321–9. doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Il’yasova D, Morrow JD, Wagenknecht LE. Urinary F2-isoprostanes are not associated with increased risk of type 2 diabetes. Obesity research. 2005;13(9):1638–44. doi: 10.1038/oby.2005.201. [DOI] [PubMed] [Google Scholar]
- 26.Il’yasova D, Spasojevic I, Base K, Zhang H, Wang F, Young SP, Millington DS, D’Agostino RB, Jr, Wagenknecht LE. Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes care. 2012;35(1):173–4. doi: 10.2337/dc11-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallmeier D, Larson MG, Wang N, Fontes JD, Benjamin EJ, Fox CS. Addition of inflammatory biomarkers did not improve diabetes prediction in the community: the framingham heart study. Journal of the American Heart Association. 2012;1(4):e000869. doi: 10.1161/JAHA.112.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Annor FB, Goodman M, Okosun IS, Wilmot DW, Il’yasova D, Ndirangu M, Lakkur S. Oxidative stress, oxidative balance score, and hypertension among a racially diverse population. Journal of the American Society of Hypertension: JASH. 2015;9(8):592–9. doi: 10.1016/j.jash.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helmersson-Karlqvist J, Bjorklund-Bodegard K, Larsson A, Basu S. 24-Hour ambulatory blood pressure associates inversely with prostaglandin F(2alpha), interleukin-6 and F(2)-isoprostane formation in a Swedish population of older men. International journal of clinical and experimental medicine. 2012;5(2):145–53. [PMC free article] [PubMed] [Google Scholar]
- 30.Ward NC, Hodgson JM, Puddey IB, Mori TA, Beilin LJ, Croft KD. Oxidative stress in human hypertension: association with antihypertensive treatment, gender, nutrition, and lifestyle. Free radical biology & medicine. 2004;36(2):226–32. doi: 10.1016/j.freeradbiomed.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 31.Woodward M, Croft KD, Mori TA, Headlam H, Wang XS, Suarna C, Raftery MJ, MacMahon SW, Stocker R. Association between both lipid and protein oxidation and the risk of fatal or non-fatal coronary heart disease in a human population. Clinical science (London, England: 1979) 2009;116(1):53–60. doi: 10.1042/CS20070404. [DOI] [PubMed] [Google Scholar]
- 32.Gross M, Steffes M, Jacobs DR, Jr, Yu X, Lewis L, Lewis CE, Loria CM. Plasma F2-isoprostanes and coronary artery calcification: the CARDIA Study. Clinical chemistry. 2005;51(1):125–31. doi: 10.1373/clinchem.2004.037630. [DOI] [PubMed] [Google Scholar]
- 33.Hozawa A, Ebihara S, Ohmori K, Kuriyama S, Ugajin T, Koizumi Y, Suzuki Y, Matsui T, Arai H, Tsubono Y, Sasaki H, Tsuji I. Increased plasma 8-isoprostane levels in hypertensive subjects: the Tsurugaya Project. Hypertension research: official journal of the Japanese Society of Hypertension. 2004;27(8):557–61. doi: 10.1291/hypres.27.557. [DOI] [PubMed] [Google Scholar]
- 34.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brummer J, Gutzki FM, Berger J, Frolich JC, Boger RH. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109(7):843–8. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 35.Cracowski JL, Baguet JP, Ormezzano O, Bessard J, Stanke-Labesque F, Bessard G, Mallion JM. Lipid peroxidation is not increased in patients with untreated mild-to-moderate hypertension. Hypertension (Dallas, Tex: 1979) 2003;41(2):286–8. doi: 10.1161/01.hyp.0000050963.16405.e6. [DOI] [PubMed] [Google Scholar]


