Abstract
In a multicenter prospective Phase II study, we evaluated the safety and efficacy of pentostatin followed by donor lymphocyte infusion (DLI) in patients with low donor T-cell chimerism after allogeneic hematopoietic cell transplantation (HCT). Thirty-six patients with low donor blood CD3 chimerism were enrolled in this study. Thirty-five patients received a total of 41 DLIs following a dose of pentostatin, and one patient received pentostatin only. Median donor CD3 chimerism prompting the initiation of pentostatin/DLI was 28 (5 to 47)%. Responses (defined by increases in donor CD3 chimerism ≥10% maintained to day 56 post-DLI) were seen in 16 patients (44.4%) with a median rise in CD3 donor chimerism to 64 (48 to 100)%. There was a trend for better responses among the 21 patients who received first treatment within 100 days after transplant (57% response rate) compared to the 15 patients who received first treatment more than 100 days after HCT (27% response rate) (p=0.07). Fourteen patients (39%) developed grade II-IV acute GVHD, at a median of 10 (0 to 83) days after DLI. Ten patients (28%) developed extensive chronic GVHD. Seventeen patients (47%) developed new grade 4 cytopenias after DLI. There was no difference in relapse between non-responders and responders. Twenty-eight patients (78%) died, most (n= 21) due to relapse. Five of the 16 responders (31%) are alive, all disease-free, at a median 60 (range 21 to 132) months after DLI. Six of the 20 non-responders (30%) are alive at median of 47 (range 16 to 100) months after DLI, 3 in complete remission. Pentostatin/DLI had acceptable toxicity and appeared to increase low donor CD3 chimerism after HCT but had no impact on mortality.
Keywords: donor lymphocyte infusion (DLI), hematopoietic cell transplantation (HCT), low donor T-cell chimerism, pentostatin
INTRODUCTION
Allogeneic hematopoietic cell transplantation (HCT) has the potential to provide long-term survival and cure in patients with hematological malignancies [1,2]. Nonetheless, low donor chimerism and graft rejection, both associated with an increased risk of relapse, remain major causes of transplant failure [3–8].
Donor lymphocyte infusion (DLI) is an approach frequently used to treat patients with relapsed hematologic malignancies after allogeneic HCT and has been also used preemptively in patients with documented low donor chimerism [9–13]. However, DLI alone has not been sufficient to improve donor chimerism [11,14]. The hypothesis that led to this prospective study was that host immunosuppression prior to DLI might improve efficacy of DLI in reversing low donor T-cell chimerism. We chose pentostatin as the immunosuppressive drug for this study as it is a purine analog and a potent inhibitor of adenosine deaminase (ADA). Administered to humans, pentostatin is lymphocytotoxic and preferentially reduces T-cells and, to a lesser extent, B-cells and NK-cells. Recovery of immune function occurs slowly over a period of several months but does not seem to be associated with an increased incidence of secondary malignancies or opportunistic infections [15]. Pentostatin at a dose of 4 mg/m2 administered in a single infusion was chosen, as this dose had been shown to be safe without significant toxicity but sufficient to induce in vivo immunosuppressive effects when used following HCT for treatment of graft-versus-host disease (GVHD) [16].
This prospective study assessed the safety and efficacy of the combined use of pentostatin and DLI in HCT patients with low or falling donor T-cell chimerism with the primary objective being the prevention of graft rejection. GVHD, infections, and disease response were evaluated as secondary objectives.
PATIENTS AND METHODS
Patients with donor CD3 chimerism of 5 to 50% after HCT at the Fred Hutchinson Cancer Research Center, VA Puget Sound Health Care System, and the University of Utah Health Sciences Center were enrolled. The study was approved by the institutional review boards in all institutions, and all patients provided written informed consent. The protocol was registered with Clinicaltrials.gov (NCT00096161).
Donor Chimerism Monitoring after HCT
Peripheral blood chimerism was checked routinely at 1 month, 3 months, and 12 months after nonmyeloablative HCT, or more frequently if clinically indicated. Chimerism was checked after ablative transplant if clinically indicated.
Disease Assessment prior to study enrollment
Disease assessment was performed prior to study enrollment as follows:
Leukemia, MDS: bone marrow biopsy and aspirate for morphology, flow cytometry and cytogenetics/FISH/molecular testing (as clinically indicated).
Lymphoma: Bone marrow biopsy and aspirate and CT scan
Multiple myeloma: Bone marrow biopsy and aspirate, SPEP with immunofixation, UPEP with immunofixation, and imaging (X-ray, CT, MRI) as clinically indicated
Inclusion Criteria
Recipient of an allogeneic HCT from an HLA-matched or one HLA-allele mismatched, related or unrelated donor, and donor CD3 peripheral blood chimerism <50% on two evaluations (at least 14 days apart) but ≥5%. Chimerism inclusion criteria were: <50% donor CD3 peripheral blood chimerism on two separate, consecutive evaluations at least 14 days apart; or absolute decreases of donor CD3 peripheral blood chimerism of ≥20% if the second test showed <50% donor CD3 cells.
Patients with evidence of disease were eligible if the disease was stable in comparison to disease status at time of transplantation.
Exclusion Criteria
Active grade II to IV acute GVHD or extensive chronic GVHD, Karnofsky performance score <50%, evidence of relapse or disease progression after transplantation, prednisone dose >0.25mg/kg/day, or other immunosuppressive medications (study groups 1A, 1B).
Immunosuppressive Therapy
Patients who enrolled on study group 1 had to be off immunosuppressive therapy (IST) besides corticosteroids at the time of study treatment. Patients who were receiving immunosuppression therapy other than steroids had immunosuppression therapy discontinued one day prior to infusion of pentostatin (without a preceding taper). Patients who were on corticosteroids had them tapered to a dosage level less than or equal to 0.25 mg/kg/day 1to 2 weeks prior to DLI. Patients could not have active GVHD upon enrollment to the study. Patients on study group 2 were allowed to be on IST at time of study treatment. Based on the study protocol patients were transitioned to the following IST regimen: cyclosporine at a dose of 5 mg/kg q12hrs PO based on adjusted body weight from day (−3) of DLI, and MMF at 30 mg/kg/d PO based on adjusted body weight from the day of DLI. If there was no evidence of GVHD after DLI, MMF was discontinued on day 28 after DLI and cyclosporine were discontinued with or without taper at day 56 after DLI.
Treatment Plan
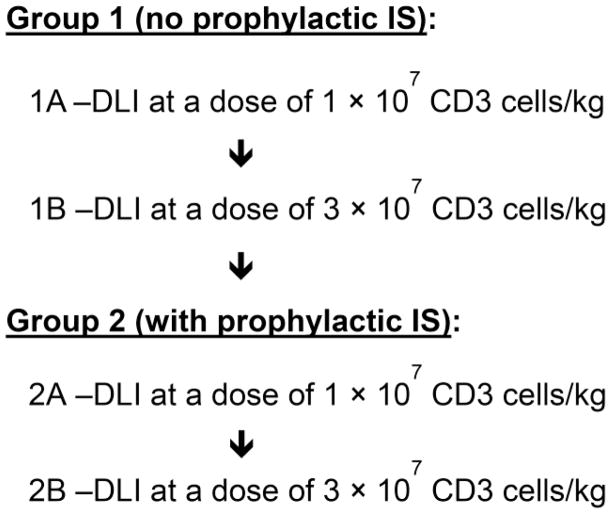
A single dose of pentostatin (4 mg/m2, adjusted for renal function) was given two days before DLI. There were two cohorts that evaluated DLI with (cohort 1) or without (cohort 2) prophylactic immunosuppression (IS). The DLI was given at one of two dose levels, A; 1 × 107 CD3 cells/kg and B; 3 × 107 CD3 cells/kg (Figure 1). For cohort 2, prophylactic immunosuppression (IS) with cyclosporine and MMF was given after DLI. Up to 20 patients could be enrolled into each study group. The enrollment to the study groups was done sequentially based on stopping rules: (i) grade IV acute GVHD >15%, and (ii) <5/10 responders. Each patient was treated on one study group. Patients could not be treated on more than one study group. An optional second pentostatin/DLI (3 × 107 CD3 cells/kg) treatment could be given at least 4 weeks after the first treatment. A second Pentostatin/DLI treatment was allowed on all study groups. DLI consisted of either fresh or cryopreserved unmodified leukapheresis product or cryopreserved G-CSF-mobilized PBSC from the original donor.
Figure 1. Study design.
Up to 20 patients could be enrolled into each study group. The enrollment to the study groups was done sequentially based on stopping rules: (i) >15% grade IV aGVHD; (ii) <5/10 responders.
Definitions
DLI-related GVHD was defined as acute [17] or chronic GVHD [18] requiring treatment. Extensive chronic GVHD was defined as generalized skin involvement, or localized skin involvement and/or hepatic dysfunction due to chronic GVHD, plus at least one of the following: (i) liver histology showing chronic aggressive hepatitis, bridging necrosis, or cirrhosis; (ii) eye involvement with Schirmer’s test with less than 5 mm wetting; (iii) positive lip biopsy; or (iv) involvement of any other target organ.
Safety was defined as <15% grade IV acute GVHD.
Response was defined as an increase of at least 10% in donor CD3 cell chimerism, maintained to day 56 after DLI.
Study Endpoints
Primary endpoint
Safety and effectiveness of DLI and pentostatin was observed in achieving sustained engraftment after graft rejection or low chimerism.
Secondary endpoints
Grades II-IV acute GVHD, chronic GVHD, relapse/progression, and survival were observed.
Statistical Methods
Overall survival and progression free survival (PFS) after DLI were estimated by the Kaplan-Meier method. Cumulative incidence of relapse after DLI was estimating using standard competing risk methods. Analyses of time-to-event endpoints was by Cox regression. Response to DLI was analyzed by chi-squared test. The power of these analyses was limited by small sample size and was generally <50% even for hypothetical differences in response rate as large as 30%; thus, negative differences should be interpreted with caution.
RESULTS
Patient Characteristics and Treatment Summary
Table 1 summarizes the patient characteristics. Thirty-six patients were treated. Diagnoses included acute myeloid leukemia (n=13), non-Hodgkin lymphoma (n=4), chronic lymphocytic leukemia (n=7), chronic myeloid leukemia (n=2), myelodysplastic syndrome (n=6), myeloproliferative disorder (n=2), multiple myeloma (n=1), and non-Hodgkin lymphoma + myelodysplastic syndrome (n=1). Twenty-three patients had evidence of underlying disease at time of study enrollment by lymphadenopathy (n=8), bone marrow morphology (CLL (n=2); myelofibrosis (n=6)) or minimal residual disease detected by flow cytometry/cytogenetics/FISH (n=7). Patients received nonmyeloablative (n=34) or ablative (n=2) conditioning, followed by PBSCs from HLA-matched related (n=17) or unrelated (n=19) donors, or one HLA-allele mismatched unrelated donor (n=2). Median age at HCT was 56 (35 to 72) years. Thirty-five patients received a total of 41 DLIs. One patient received pentostatin without DLI due to development of extensive rash following pentostatin administration, with skin biopsy consistent with GVHD.
Table 1.
Patient Characteristics
| Characteristic | N (%) /Median (range) |
|---|---|
| Patient number | 36 |
| Gender (male/female) | 31/5 |
| Patient age at HCT, years, median (range) | 56 (35–72) |
| Diagnosis, No. | |
| Acute myeloid leukemia | 13 |
| Myelodysplastic syndrome | 6 |
| Chronic lymphocytic leukemia | 7 |
| Non Hodgkin lymphoma | 4 |
| Chronic myeloid leukemia | 2 |
| Myeloproliferative disorder | 2 |
| Multiple myeloma | 1 |
| Non Hodgkin lymphoma and myelodysplastic syndrome | 1 |
| Disease status at time of study intervention | |
| CR | 13 |
| Not in CR* | 23 |
| Donor source | |
| HLA-matched related | 17 |
| HLA-matched unrelated | 18 |
| 1-allele HLA-mismatched unrelated | 1 |
| Transplant conditioning intensity | |
| Nonmyeloablative (fludarabine/TBI) | 34 |
| Myeloablative | |
| Busulfan/Cyclophosphamide | 1 |
| Fludarabine/TBI/anti-CD45 Ab | 1 |
| GVHD prophylaxis after transplant | |
| MMF/cyclosporine | 25 |
| MMF/cyclosporine/sirolimus | 2 |
| MMF/tacrolimus | 6 |
| MF/tacrolimus/sirolimus | 2 |
| Cyclosporine/methotrexate | 1 |
| Acute GVHD before DLI | |
| None | 30 |
| Grade II-III | 6 |
| Time from HCT to DLI | |
| ≤100 days | 21 |
| >100 days | 15 |
| % of donor CD3 chimerism at time of DLI, median (range) | 28 (5–47) |
Evidence of disease: residual lymphadenopathy (n=8), bone marrow morphology (CLL (n=2); myelofybrosis (n=6)), minimal residual disease detected by marrow flow cytometry/cytogenetics/FISH (n=7).
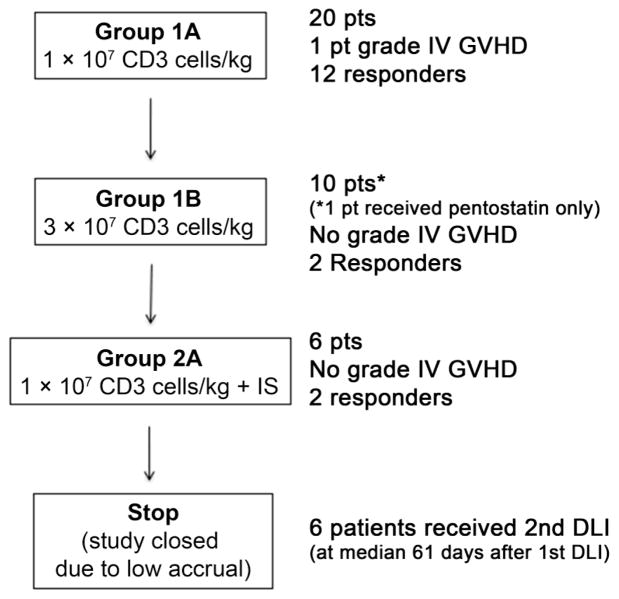
Figure 2 summarizes the study treatment. Twenty patients were treated in group 1A, ten patients were treated in group 1B, and six patients were treated in group 2A. The transition from group 1B to group 2A was done after 10 patients per the study stopping rules, as less than 5 of 10 patients on group 1B responded. Due to low accrual, the study was closed while accruing patients into group 2A. Thus, no patients were enrolled in group 2B. DLI was given at a median of 96 (54 to 339) days after HCT. Six patients received a second pentostatin/DLI treatment at a median of 61 (39 to 155) days after first DLI.
Figure 2.
Study treatment.
Donor Chimerism after DLI
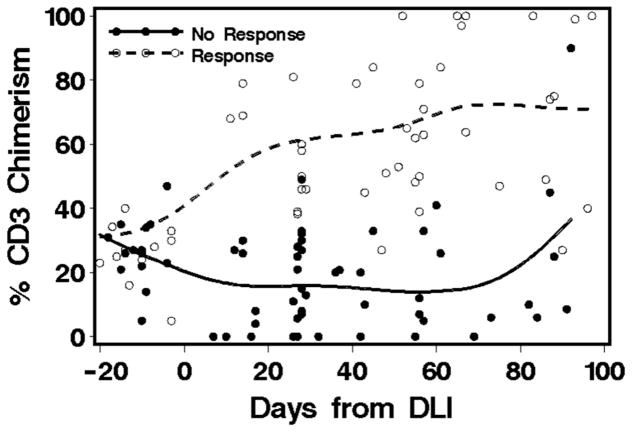
Table 2 summarizes the patients’ responses to study intervention. Median donor CD3 chimerism before pentostatin/DLI was 28% (range 5 to 47%), as measured at a median of 13 (range 3 to 21) days before DLI. Response was seen in 16 patients (44.4%) with a median increase in CD3 donor chimerism to 64% (range 48 to 100%) (Figure 3). Per study protocol, efficacy was defined as an increase of at least 10 percentage points in donor T-cell chimerism from the time of enrollment, maintained to day 56 after the last DLI. Among the 16 responders, the percentage increase in median chimerism by day 56 after DLI was 35% (range 11 to 95%). Fifteen of the 16 responders continued to have improved chimerism beyond day 56 after DLI, with a median maximum chimerism improvement of 61% (range 16to 95%). Best response was achieved among patients treated in group 1A with 12 (60%) responders. Two of 10 patients (20%) treated in group 1B responded, and two of six patients (33%) treated in group 2A responded. There was a trend for better efficacy among the 21 patients who received their first treatment within 100 days after transplant compared to the 15 patients who received their first treatment more than 100 days after HCT (57% vs. 27% responders) (p=0.07). Eleven patients (31%) rejected their graft. Diagnosis, disease status at time of DLI, donor CD3 chimerism at time of DLI, and prophylactic IS after DLI did not seem to affect the response to the study treatment (Table 3). One of six patients responded to a second pentostatin/DLI treatment.
Table 2.
Response According to Study Group and Time of Study Intervention
| Study Intervention ≤ Day 100 after HCT | Study Intervention > Day 100 after HCT | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Study Group | No. of Patients | Overall Response | No. of Patients | Responders | No. of Patients | Responders |
| 1A | 20 | 12 (60%) | 13 | 10 (77%) | 7 | 2 (29%) |
| 1B | 10 | 2 (20%) | 6 | 2 (33%) | 4 | 0 |
| 2A | 6 | 2 (33%) | 2 | 0 | 4 | 2 (50%) |
Figure 3.
Chimerism before and after DLI
Table 3.
Factors Associated with Response
| N | % Response | P | |
|---|---|---|---|
| Diagnosis | |||
| Lymphoid malignancy | 13 | 38% | |
| Myeloid malignancy | 23 | 48% | 0.59 |
| Disease status at DLI | |||
| No evidence of disease | 13 | 38% | |
| Evidence of disease | 23 | 48% | 0.59 |
| CD3 donor chimerism at DLI1 | |||
| ≤27% | 18 | 37% | |
| >27% | 17 | 56% | 0.25 |
| Time from HCT to study intervention | |||
| <100 days | 21 | 57% | |
| >100 days | 15 | 27% | 0.07 |
| DLI dose | |||
| 1×107 CD3/kg | 26 | 54% | |
| 3×107 CD3/kg | 10 | 20% | 0.07 |
| Donor | |||
| Related | 17 | 47% | |
| Unrelated | 19 | 42% | 0.77 |
| Patient age at treatment intervention* | |||
| ≤56 years | 18 | 61% | |
| >56 years | 18 | 28% | 0.04 |
| Donor age at HCT* | |||
| ≤45 years | 17 | 41% | |
| >45 years | 16 | 44% | 0.88 |
| ANC count at DLI (×103/μL)* | |||
| ≤ 2.15 | 17 | 29% | |
| >2.15 | 17 | 59% | 0.08 |
| Lymphocyte count at DLI (×103/μL)* | |||
| ≤0.46 | 16 | 25% | |
| >0.46 | 16 | 63% | 0.03 |
| Immunosuppression after DLI | |||
| No | 30 | 47% | |
| Yes | 6 | 33% | 0.55 |
Divided at median
DLI-Related Complications
GVHD
Table 4 summarizes GVHD development in the different study groups. Fourteen patients (39%) (12 responders) developed acute GVHD after DLI [grade II (n=10), III (n=3), IV (n=1)] at a median of 9 (−1 to 83) days after DLI. One patient developed grade II skin GVHD after pentostatin, and DLI was aborted. Of the 16 patients who developed acute GVHD after pentostatin/DLI, three had a prior history of GVHD. Thirteen patients (36%) developed chronic GVHD, of whom 10 (28%) developed extensive chronic GVHD at a median of 112 (13 to 347) days after DLI. One patient developed de novo extensive chronic GVHD. Among the six patients who received prophylactic IS after DLI one patient developed acute GVHD (grade II), and none developed chronic GVHD. There was no statistically significant difference in acute GVHD after DLI among patients who received or not prophylactic IS (HR=0.30 (0 to 2.4), p=0.18).
Table 4.
GVHD after Study Intervention According to Study Group
| Study Group | No. of Patients | Acute GVHD* | Extensive Chronic GVHD† |
|---|---|---|---|
| 1A | 20 | 10 (50%) | 8 (6 also developed acute |
| Grades II (n=6), III (n=3), IV (n=1) | GVHD) | ||
| 1B | 10 | 3 (30%) grade II | 2 (also developed acute GVHD) |
| 2A | 6 | 1 (17%) grade II | 0 |
Median time for acute GVHD after DLI: 9 (-1 - 83) days (one patient developed GVHD after pentostatin administration)
Median time for chronic GVHD after DLI: 112 (13 – 347) days
Cytopenia
Seventeen patients (47%) (nine non-responders; eight responders) developed new grade 4 cytopenias after DLI; nine patients developed both grade 4 neutropenia (ANC < 500/mcl) and grade 4 thrombocytopenia (<25,000/mcl); four patients developed isolated neutropenia; and four patients developed isolated thrombocytopenia. Median time between DLI and neutrophil nadir was 22 (2 to 100) days, and neutropenia lasted for median of 7 (1 to 52) days. Twelve patients received platelet transfusions started at median of 33 (1 to 98) days after DLI. Ten of the 17 patients who developed grade 4 cytopenias after DLI relapsed, six of whom were non-responders.
Infections
Twenty-six patients developed infections within 100 days after DLI. The median number of infections per patient was 1 (range 0 to 9). Eleven patients developed bacteremia, three patients developed bacterial pneumonia, three patients developed fungal pneumonia, and eight patients had CMV reactivation. Infection was the primary cause of death in three cases.
Relapse after DLI
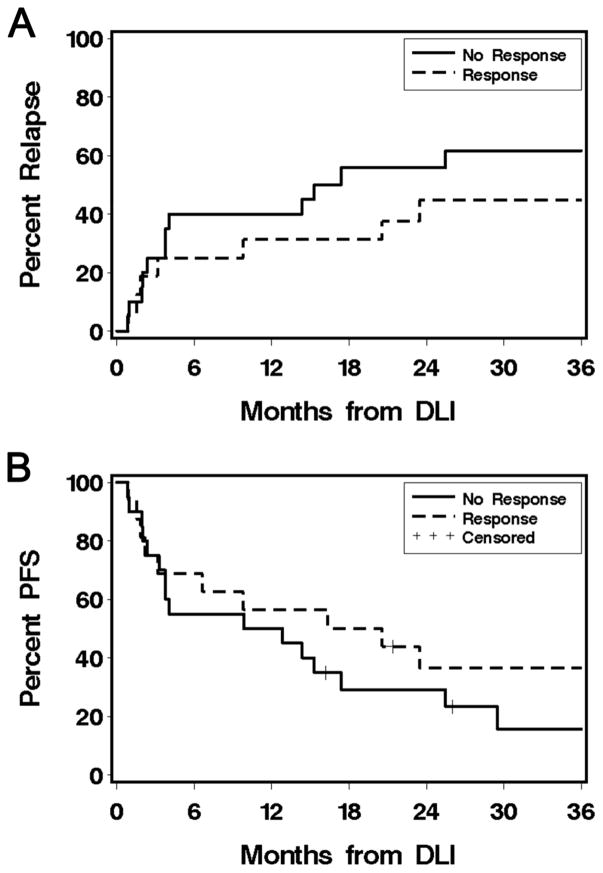
Nineteen patients (53%) experienced disease progression or relapse after DLI. Among the 20 non-responders 12 relapsed (60%), compared to seven of 16 responders (44%) (HR 0.64 (0.3 to 1.6), p=0.35) (Figure 4A). Ten of the 17 patients (59%) who developed grade 4 cytopenias after DLI relapsed, 6 of whom (60%) were non-responders. Thirteen of 24 (54%) patients who had evidence of disease at time of study intervention and six of 12 (50%) patients without evidence of disease at time of treatment experienced disease progression.
Figure 4. Outcome.
A) Relapse/progression. B) Progression-free survival.
Survival after DLI
Twenty-eight patients died at a median of 522 (67 to 2028) days after DLI. Causes of death were relapse (n=21), respiratory failure/infections (n=4), grade IV GVHD (n=2), or other causes (n=1). Five of the 16 responders (31%) are alive at median of 60 (range 21 to 132) months after DLI, all in CR. Six of the 20 non-responders (30%) are alive at median of 47 (range 16 to 100) months after DLI, 3 (50%) in complete remission (Figure 4B). Three of the six patients (50%) who received prophylactic IS after DLI died, two of them due to disease progression. Twenty two of the 30 patients (73%) who did not receive prophylactic IS died, 13 due to disease progression.
DISCUSSION
Low donor CD3 cell chimerism after transplant is associated with increased risk of graft rejection and relapse [3–8]. Although DLI has been used in patients with documented low donor chimerism [9–13], DLI alone has not been sufficient to improve donor chimerism [11,14]. Therefore, in this study we have examined the effect of adding pentostatin before DLI on donor CD3 chimerism.
The results of our prospective study demonstrated that treatment with pentostatin and DLI in patients with low donor T cell chimerism after allogeneic HCT had acceptable toxicities and the potential to improve donor T cell chimerism, with a trend for better response if DLI was given within 100 days after transplant. There was a trend of lower relapse rate among patients who responded to the study intervention compared to non-responders, but the difference was not statistically significant.
In the pediatric population it has been demonstrated that chimerism-based pre-emptive immunotherapy with fast withdrawal of immunosuppression and DLI allows patients with early post-transplant mixed chimerism to achieve similar survival rates as patients who achieved full donor chimerism spontaneously [13]. It was also demonstrated in the pediatric population that delayed intervention resulted in increased risk of relapse [18].
In the adult population, Dey et al. administered prophylactic DLI after discontinuation of immunosuppression to 16 patients with hematological malignancies 35 to 44 days after nonmyeloablative bone marrow transplant. Eleven patients, all with pre-DLI donor T-cell chimerism ≥40%, converted to full donor chimerism at median of 32 days after DLI, one patient had stable chimerism, and 4 patients, all with initial donor T-cell chimerism ≤20%, lost their graft [11]. Similar to the findings by Dey el al., our group has previously shown that DLI was ineffective in reversing donor T cell chimerism when the baseline level was <40% [14]. Our current study included only patients with ≤50% donor T-cell chimerism, and 12 of the 16 responders had baseline donor T-cell chimerism of <40%. Thus, our results suggest that adding pentostatin to DLI may improve the DLI efficacy in reversing of donor T-cell chimerism.
Recently, Solomon et al. used preemptive DLI without withdrawal of IS to improve donor T-cell chimerism in older recipients of alemtuzumab-containing reduced-intensity unrelated donor HCT [12]. Twenty-five of 36 patients on that study received DLI for <50% donor T-cell chimerism at a median of 62 days after HCT. Sixteen of the 25 patients who received preemptive DLI improved their donor chimerism. Solomon et al. reported estimated 2 years OS of 57% and DFS of 38%. They reported no cases of grade III/IV acute GVHD after DLI, but reported 20 cases (56%) of chronic GVHD. In our current study, three responders developed grade III GVHD, and one responder developed grade IV GVHD. Prophylactic IS did not seem to decrease the risk of DLI-related acute GVHD, but might have decreased the risk of chronic GVHD as none of the six patients who received prophylactic IS developed extensive chronic GVHD after DLI. Two of the six (33%) patients who received prophylactic IS responded, compared to 14 of 30 (47%) patients who did not receive prophylactic immunosuppression, but the difference was not statistically significant. There was no difference in relapse and survival between the two groups. Our group has previously demonstrated that DLI-related GVHD is DLI dose related, and seems to be lower than transplant-related-GVHD [19]. Due to the lower toxicity from GVHD after DLI compared to GVHD after HCT, DLI is usually administered without prophylactic immunosuppression. The lower incidence and severity of DLI-related GVHD compared with GVHD after transplant may be due to a number of factors, including: (i) the presence of mixed chimerism at the time of DLI, which may imply an active state of immune tolerance that decreased the risk of GVHD; and (ii) the distance between DLI and prior exposure to cytotoxic drugs, which may result in less tissue damage and cytokine release before DLI compared to time of transplant, and thus result in lower risk of GVHD after DLI. Based on our findings, we recommend considering not to administer prophylactic IS if pentostatin-DLI is given for treatment of low donor T cell chimerism after DLI.
The small study population limits our ability to draw definitive conclusions from this study. However, the acceptable toxicity and the potential of improving donor T-cell chimerism, especially if pentostatin/DLI is given within 100 days after transplant, support the consideration of early treatment with pentostatin/DLI in patients with low donor T-cell chimerism after HCT.
HIGHLIGHTS.
Pentostatin/DLI was well tolerated by patients with low donor chimerism.
Increased donor chimerism was observed in 44% of patients.
Grade II-VI acute GVHD was developed by 39% of patients (grade III/VI 11%)
28% of patients developed extensive chronic GVHD
Pentostatin/DLI did not have impact on mortality
Acknowledgments
We thank the patients who participated in the study. We thank members of the research and clinical staff at the FHCRC and referring physicians for their contribution to the care of our patients after hematopoietic cell transplantation.
This study was supported by grants CA078902, CA018029, CA118953 and CA015704 from the National Cancer Institute, National Institutes of Health, Bethesda, MD.
Footnotes
Financial disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
as of 09-01-2017
- 1.Appelbaum FR. The current status of hematopoietic cell transplantation. Annu Rev Med. 2003;54:491–512. doi: 10.1146/annurev.med.54.101601.152456. [DOI] [PubMed] [Google Scholar]
- 2.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader P, Beck J, Frey A, et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant. 1998;21:487–495. doi: 10.1038/sj.bmt.1701119. [DOI] [PubMed] [Google Scholar]
- 4.Barrios M, Jimenez-Velasco A, Roman-Gomez J, et al. Chimerism status is a useful predictor of relapse after allogeneic stem cell transplantation for acute leukemia. Haematologica. 2003;88:801–810. [PubMed] [Google Scholar]
- 5.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22:1696–1705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Simon JA, Caballero D, Diez-Campelo M, et al. Chimerism and minimal residual disease monitoring after reduced intensity conditioning (RIC) allogeneic transplantation. Leukemia. 2002;16:1423–1431. doi: 10.1038/sj.leu.2402550. [DOI] [PubMed] [Google Scholar]
- 7.Baron F, Petersdorf EW, Gooley T, et al. What is the role for donor natural killer cells after nonmyeloablative conditioning? Biol Blood Marrow Transplant. 2009;15:580–588. doi: 10.1016/j.bbmt.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storb R, Gyurkocza B, Storer BE, et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2013;31:1530–1538. doi: 10.1200/JCO.2012.45.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism defines a high-risk group of childhood acute myelogenous leukemia patients after allogeneic stem cell transplantation where pre-emptive immunotherapy may be effective. Bone Marrow Transplant. 2004;33:815–821. doi: 10.1038/sj.bmt.1704444. [DOI] [PubMed] [Google Scholar]
- 10.Rettinger E, Willasch AM, Kreyenberg H, et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood. 2011;118:5681–5688. doi: 10.1182/blood-2011-04-348805. [DOI] [PubMed] [Google Scholar]
- 11.Dey BR, McAfee S, Colby C, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–329. doi: 10.1016/s1083-8791(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SR, Sizemore CA, Zhang X, et al. Preemptive DLI without withdrawal of immunosuppression to promote complete donor T-cell chimerism results in favorable outcomes for high-risk older recipients of alemtuzumab-containing reduced-intensity unrelated donor allogeneic transplant: a prospective phase II trial. Bone Marrow Transplant. 2014;49:616–621. doi: 10.1038/bmt.2014.2. [DOI] [PubMed] [Google Scholar]
- 13.Horn B, Petrovic A, Wahlstrom J, et al. Chimerism-based pre-emptive immunotherapy with fast withdrawal of immunosuppression and donor lymphocyte infusions after allogeneic stem cell transplantation for pediatric hematologic malignancies. Biol Blood Marrow Transplant. 2015;21:729–737. doi: 10.1016/j.bbmt.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Bethge WA, Hegenbart U, Stuart MJ, et al. Adoptive immunotherapy with donor lymphocyte infusions after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Blood. 2004;103:790–795. doi: 10.1182/blood-2003-07-2344. [DOI] [PubMed] [Google Scholar]
- 15.Brogden RN, Sorkin EM. Pentostatin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in lymphoproliferative disorders. Drugs. 1993;46:652–677. doi: 10.2165/00003495-199346040-00006. [DOI] [PubMed] [Google Scholar]
- 16.Bolanos-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23:2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 18.Horn B, Soni S, Khan S, et al. Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies. Bone Marrow Transplant. 2009;43:469–476. doi: 10.1038/bmt.2008.339. [DOI] [PubMed] [Google Scholar]
- 19.Bar M, Sandmaier BM, Inamoto Y, et al. Donor lymphocyte infusion for relapsed hematological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biology of Blood & Marrow Transplantation. 2013;19:949–957. doi: 10.1016/j.bbmt.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]