Abstract
The majority of putative disease-modifying treatments in development for Alzheimer’s disease are directed against the amyloid-β (Aβ) peptide. Among the anti-Aβ therapeutic approaches, the most extensively developed is immunotherapy—specifically, passive immunization through administration of exogenous monoclonal antibodies (mAbs). Although testing of mAbs has been fraught with failure and confusing results, the experience gained from these trials has provided important clues for better treatments. This review summarizes the experience to date with anti-Aβ mAbs to enter clinical trials for Alzheimer’s disease and examines the evidence for clinical efficacy and the major problems with safety—i.e., amyloid-related imaging abnormalities. As mAbs differ considerably with regard to their epitopes and the conformations of Aβ that they recognize (monomers, oligomers, protofibrils, fibrils), the consequences of targeting different species are also considered. An often-cited explanation for the failure of anti-Aβ mAb trials is that they are set too late in the disease process. New trials are indeed evaluating treatments at prodromal and preclinical stages. We should expect to see additional studies of presymptomatic Alzheimer’s disease to join the ongoing prevention trials, for which mAbs continue to serve as the mainstay.
Keywords: Alzheimer’s disease, Amyloid-β, Amyloid-β, oligomers, Amyloid-related imaging abnormalities, Immunotherapy, Monoclonal antibodies
The amyloid hypothesis of Alzheimer’s disease (AD) holds that the accumulation of the amyloid-β (Aβ) peptide leads to synaptic dysfunction, neurodegeneration, and ultimately symptoms (1). The vast majority of potential disease-modifying treatments developed in recent years are directed against Aβ, including inhibitors of the synthetic enzymes gamma-secretase and beta-secretase, and Aβ aggregation inhibitors. However, the most elaborated anti-Aβ approach is immunotherapy, including both active vaccines to stimulate the immune system to produce its own antibodies and passive immunization through the administration of exogenous antibodies.
The advantage of active immunotherapy is long-term antibody production from short-term drug administration at limited cost. Conversely, immune response may be inconsistent or lacking, especially in older individuals, and adverse reactions— if immunologically based—may also be long-lasting. Initial experience with active vaccines was marred by an ill-fated trial of AN1792 (full-length Aβ42 with QS-21 adjuvant) that was halted following the occurrence of T cell-mediated meningoencephalitis in 6% of treated participants (2). Second-generation vaccines such as ACC-001 (3–5) and CAD106 (6,7) have sought to generate anti-Aβ antibodies restricted to the N-terminus, while avoiding T cell epitopes at the C-terminus (8,9). CAD106 is the only vaccine to advance to phase 3 trials and has been selected for the Alzheimer Prevention Initiative APOE ε4 homozygote study (https://clinicaltrials.gov; Identifier: NCT02565511) (10).
In contrast to active vaccination, passive immunization has the advantages of ensuring consistent antibody titers and allowing control of adverse events by stopping treatment. The major drawbacks of monoclonal antibodies (mAbs) are the need for repeated administrations and the associated cost of production (11). Over the past approximately 15 years several mAbs have been engineered to bind and clear Aβ (Table 1) and have advanced to human trials (Table 2). Although the testing of mAbs has been fraught with failure and confusing results, the experience gained from these trials has provided important clues to enable the development of better treatments.
Table 1.
Monoclonal Antibodies Bind Different Epitopes and Conformations of Amyloid-β
| Antibody | Manufacturer | Origin | Subclass | Epitope | Conformations Recognized | ARIA-E | ||
|---|---|---|---|---|---|---|---|---|
| Monomer | Oligomer | Fibril | ||||||
| Bapineuzumab | Pfizer Inc./Janssen Pharmaceuticals, Inc. | Humanized | IgG1 | AA 1–5 | Yes | Yes | Yes | High |
| Solanezumab | Eli Lilly and Company | Humanized | IgG1 | AA 16–26 | Yes | No | No | Low |
| Gantenerumab | Hoffman-La Roche | Human | IgG1 | AA 3–12, 18–27 | Weak | Yes | Yes | High (?) |
| Crenezumab | Genentech, Inc. | Humanized | IgG4 | AA 13–24 | Yes | Yes | Yes | Low |
| Ponezumab | Pfizer Inc. | Humanized | IgG2 | AA 30–40 | Yes | No | No | None |
| BAN2401 | BioArctic Neuroscience, AB/Eisai Co., Ltd. | Humanized | IgG1 | Protofibrils | — | — | — | — |
| Aducanumab | Biogen, Inc. | Human | IgG1 | AA 3–6 | No | Yes | Yes | High |
Epitope, Conformations Recognized, and ARIA-E are explained further in the text. Dashes indicate absence of information.
AA, amino acid; ARIA-E, amyloid-related imaging abnormalities-edema; Ig, immunoglobulin.
Table 2.
Selected Trials of Anti-Amyloid-β Monoclonal Antibodies for Alzheimer’s Disease
| Drug | Publication | Phase | Sample | Participants | Age, Years | Dose | Duration, Weeks |
Efficacy | ARIA-E | Biomarkers |
|---|---|---|---|---|---|---|---|---|---|---|
| Bapineuzumab | Salloway et al., 2009 (15) | 2 | 234 | Mild-moderate AD | 50–85 | 0.15, 0.5, 1, 2 mg/kg IV every 3 months | 78 | Failed primary end points | 17%, retrospective analysis | No effect on CSF Aβ42, tau, or p-tau |
| Bapineuzumab | Rinne et al., 2010 (19) | 2 | 28 | Mild-moderate AD | 50–80 | 0.5, 1, 2 mg/kg IV every 3 months | 78 | Retrospective analysis combined with Salloway, 2009 | ↓Cortical 11C-PiB compared with baseline and placebo | |
| Bapineuzumab | Salloway et al., 2014 (21) | 3 | 2204 | Mild-moderate AD | 50–88 | 0.5, 1, 2 mg/kg IV every 3 months | 78 | Failed primary end points | 15.3% of APOE ε4 carriers, 4.2%, 9.4%, and 14.2% of three dose groups in noncarriers | ↓Cortical 11C-PiB and ↓CSF p-tau in APOE ε4+ |
| Solanezumab | Farlow et al., 2012 (27) | 2 | 52 | Mild-moderate AD | >50 | 100, 400, 1600 mg/month IV | 52 | No cases | ↑Aβ40 and ↑Aβ42 in CSF | |
| Solanezumab | Doody et al., 2014 (28) | 3 | 2052 | Mild-moderate AD | >55 | 400 mg IV every month | 78 | Failed primary end points; ↓decline in mild AD subgroup | 0.9% solanezumab vs. 0.4% placebo | No effect on brain Aβ (PET); ↑Aβ40 and ↑Aβ42 in CSF |
| Solanezumab | Completed | 3 | 2129 | Mild AD, Aβ+ | 55–90 | 400 mg IV every month | 78 | Failed primary end point | No effect on brain Aβ or tau (PET) | |
| Gantenerumab | Ostrowitzki et al., 2012 (34) | 1 | 18 | Mild-moderate AD | 50–90 | 60, 200 mg IV every 4 weeks | 24 | 2/6 participants on 200-mg dose | ↓Cortical 11C-PiB compared with baseline | |
| Gantenerumab | Ongoing | 2/3 | 799 | Prodromal AD, Aβ+ | 50–85 | 105 or 225 mg SC every 4 weeks | 104 | Nonsignificant benefit in rapid progressors, post hoc | ||
| Crenezumab | Cummings et al., in press (38) | 2 | 431 | Mild-moderate AD | 50–80 | 300 mg SC every 2 weeks, 15 mg/kg IV every 4 weeks | 68 | Failed primary end points | 1 case, APOE ε4 homozygote | ↑CSF Aβ42 |
| Crenezumab | Completed | 2 | 91 | Mild-moderate AD | 50–80 | 300 mg SC every 2 weeks, 15 mg/kg IV every 4 weeks | 68 | Failed primary end points | No effect on brain Aβ (PET); ↑Aβ in CSF | |
| Crenezumab | Ongoing | 3 | Mild-prodromal AD, Aβ+ | 50–85 | 100 | |||||
| BAN2401 | Ongoing | 2 | Mild-prodromal AD, Aβ+ | 50–90 | 2.5, 5, 10 mg/kg IV every 2 weeks, 5, 10 mg/kg IV every 4 weeks | 78 | ||||
| Ponezumab | Landen et al., 2013 (44) | 1 | Mild-moderate AD | >50 | 10 mg/kg IV | 52 | Failed primary end points | No cases | ↓CSF Aβ42 | |
| Aducanumab | Sevigny et al., 2016 (50) | 1 | 165 | Mild-prodromal AD, Aβ+ | 50–90 | 1, 3, 6, 10 mg/kg IV every 4 weeks | 54 | Exploratory; ↓decline in CDR (10 mg/kg) and MMSE (3, 10 mg/kg) | 3%, 6%, 37%, 41% of four dose groups | ↓Cortical [18F]-florbetapir |
| Aducanumab | Ongoing | 3 | Mild-prodromal AD, Aβ+ | 50–85 | 78 |
AD, Alzheimer’s disease; Aβ+, positive for amyloid-β biomarker (PET or CSF); APOE ε4+, positive for APOE ε4; ARIA-E, amyloid-related imaging abnormalities-edema; CDR, Clinical Dementia Rating; CSF, cerebrospinal fluid; IV, intravenous; MMSE, Mini-Mental State Examination; PET, positron emission tomography; p-tau, phosphorylated tau; 11C-PiB, [11C]-Pittsburgh compound B; SC, subcutaneous.
BAPINEUZUMAB
Bapineuzumab (AAB-001; Pfizer Inc., New York, NY, and Janssen Pharmaceuticals, Inc., Raritan, NJ), a humanized immunoglobulin (Ig) G1 anti-Aβ mAb, binds the five N-terminal residues and clears both fibrillar and soluble Aβ. In 2000, Bard et al. (12) reported that in PDAPP transgenic mice, 3D6 (the murine precursor of bapineuzumab) entered the brain, decorated plaques, and induced the Fc receptor–mediated microglial phagocytosis of Aβ deposits.
Bapineuzumab was the first mAb to enter human testing after termination of the AN1792 trial. In a phase 1 single ascending dose trial, 0.5, 1.5, or 5 mg/kg of bapineuzumab was generally safe and well tolerated in 30 participants with mild to moderate AD (13). However, 3 of 10 participants in the highest dose group developed magnetic resonance imaging (MRI) abnormalities consistent with vasogenic edema, all of which later resolved. Two participants were asymptomatic, and one experienced mild, transient confusion. These events prompted the Alzheimer’s Association Research Roundtable to convene a Workgroup in July 2010, which coined the term amyloid-related imaging abnormalities (ARIA) to refer to MRI signal alterations associated with Aβ-modifying therapies— specifically, ARIA-E to denote vasogenic edema/effusions and ARIA-H to indicate microhemorrhage and hemosiderosis (14). The subsequent phase 2 trial studied intravenous bapineuzumab (0.15, 0.5, 1.0, or 2.0 mg/kg) administered every 13 weeks for 78 weeks in mild to moderate AD (15). No significant treatment differences were found for the primary efficacy end points, Alzheimer’s Disease Assessment Scale-Cognitive Subscale [ADAS-Cog11] (16,17) or Disability Assessment for Dementia (18), but prespecified exploratory analyses showed potential treatment differences for subjects who completed the study and APOE ε4 noncarriers. A parallel phase 2 study with [11C]-Pittsburgh compound B (11C-PiB) and positron emission tomography (PET) in 28 participants revealed some clearance of fibrillar Aβ (19). A retrospective review by two neuroradiologists of MRI scans from the phase 2 studies revealed that 36 participants (17%) had developed ARIA-E during bapineuzumab treatment, including 15 who were undetected during the trials. Of these participants, 28 (78%) reported no associated symptoms, whereas 8 symptomatic participants reported headache, confusion, and neuropsychiatric and gastrointestinal symptoms. Incident ARIA-H occurred in 17 (47%) of the participants with ARIA-E. Thirteen of 15 participants in whom ARIA-E was detected only retrospectively had received additional study infusions while ARIA-E was present, without any associated symptoms. ARIA-E was significantly related to higher doses of bapineuzumab and APOE s4 status (20). The results of this retrospective analysis led to the practice of using central MRI readers to assess ARIA in later AD immunotherapy programs.
The increased occurrence of ARIA-E in APOE ε4 carriers in phase 2 studies resulted in separate protocols for carriers and noncarriers in the subsequent phase 3 studies. Two 18-month trials comprising 1121 carriers and 1331 noncarriers with mild to moderate AD tested doses of bapineuzumab that varied by study administered intravenously every 13 weeks (21). Neither study revealed significant treatment differences in the primary outcomes (ADAS-Cog11 and Disability Assessment for Dementia). Evidence of mild target engagement was observed for APOE ε4 carriers only, as treatment groups differed in change in brain Aβ burden by 11C-PiB-PET (22) and cerebrospinal fluid (CSF) phosphorylated tau concentrations. Negative baseline 11C-PiB-PET scans were found in 36% of APOE ε4 noncarriers, suggesting the necessity of incorporating biomarker evidence of disease into eligibility criteria in future trials. ARIA-E occurred in 15.3% of APOE ε4 carriers who received bapineuzumab 0.5 mg/kg, including 11.4% of heterozygotes and 27.3% of homozygotes. In the noncarrier study, ARIA-E was identified in 4.2%, 9.4%, and 14.2% of participants receiving 0.5, 1.0, and 2.0 mg/kg. The 2.0-mg/kg dose was discontinued early in the trial because of a high rate of symptomatic ARIA-E. Following these results, all bapineuzumab trials were discontinued in August 2012.
SOLANEZUMAB
Solanezumab (LY2062430; Eli Lilly and Company, Indianapolis, IN), a humanized IgG1 mAb, binds the mid-domain of Aβ (residues 16–26) and increases clearance of monomers (23). Studies in transgenic PDAPP mice demonstrated that m266 (the murine precursor of solanezumab) reduced brain Aβ burden without binding Aβ deposits (24,25), opening the possibility of targeting the soluble pool of Aβ. Phase 1 and 2 studies of solanezumab revealed evidence of target engagement by dose-dependent increases in plasma and CSF total Aβ (26,27). In the phase 2 study of mild to moderate AD, 12 weeks of solanezumab treatment yielded a dose-dependent increase in CSF-free Aβ42, suggesting a shift in equilibria sufficient to mobilize Aβ42 from plaques (27).
The first phase 3 studies-EXPEDITION 1 and EXPEDITION 2—were 18-month trials of solanezumab 400 mg versus placebo (administered intravenously every 4 weeks) in 1012 and 1040 participants with mild to moderate AD (28). The original co-primary outcomes in both studies were the ADAS-Cog11 and Alzheimer’s Disease Cooperative Study-Activities of Daily Living (ADCS-ADL) (29). After analysis of data from EXPEDITION 1, the primary outcome for EXPEDITION 2 was revised to the ADAS-Cog14 in the mild AD subgroup (28). Solanezumab did not demonstrate significant benefit for the primary outcomes in either study but showed a favorable safety profile, as the incidence of ARIA-E was 0.9% with solanezumab and 0.4% with placebo. A prespecified subgroup analysis of pooled data from EXPEDITION 1 and EXPEDITION 2 showed that in participants with mild AD, there was a 34% slowing of decline for the ADAS-Cog14 and 18% for the instrumental items of the ADCS-ADL (ADCS-iADL) (23). Therefore, a third phase 3 trial, EXPEDITION 3 (NCT01900665), restricted to mild-stage AD, was launched in July 2013. Owing to the high rate of cases negative for the Aβ biomarker in EXPEDITION 1 and EXPEDITION 2, the EXPEDITION 3 trial required PET showing positive Aβ for eligibility.
In December 2016 at the Clinical Trials on Alzheimer’s Disease meeting, the negative results of EXPEDITION 3 were presented (30). In 2129 participants with mild AD (confirmed by positive Aβ on PET), solanezumab provided a nonsignificant 11% slowing of decline on the primary outcome, the ADAS-Cog14. This effect size was smaller than in the pooled subgroup analysis from EXPEDITION 1 and EXPEDITION 2. Several secondary outcomes favored solanezumab, including the Clinical Dementia Rating Sum of Boxes (CDR-SB), Mini-Mental State Examination (MMSE), and ADCS-iADL; however, these analyses were not corrected for multiplicity. Solanezumab had no effect on Aβ and tau PET biomarkers. Based on the results of EXPEDITION 3, the development of solanezumab for dementia was discontinued.
The excellent safety profile of solanezumab and encouraging trends in the exploratory analyses in mild AD led to its inclusion in two secondary prevention trials, which are continuing in the hope that earlier intervention may yield more substantial benefit. The A4 study (NCT02008357) started in February 2014 and will enroll 1150 cognitively normal individuals 65–85 years of age who have positive Aβ on PET scans (31). The Dominantly Inherited Alzheimer Network (DIAN) selected solanezumab (and gantenerumab, described below) for its phase 2/3 trial in individuals at risk for and with early-stage autosomal-dominant AD (NCT01760005) (32).
GANTENERUMAB
Gantenerumab (Hoffman-La Roche, Basel, Switzerland), the first fully human IgG1 anti-Aβ mAb, binds a conformational epitope expressed on Aβ fibrils (33). This epitope encompasses both N-terminal (3–12) and central (18–27) amino acids of Aβ and thus requires that the peptide be folded with the midregion near the N-terminus. In PS2APP transgenic mice, gantenerumab significantly reduced Aβ plaques by recruiting microglia and prevented new plaque formation without altering plasma Aβ levels (33).
In phase 1 trials in mild to moderate AD, gantenerumab treatment, including up to seven intravenous infusions (60 or 200 mg) every 4 weeks, reduced brain Aβ burden as measured by 11C-PiB PET (34). Gantenerumab was generally safe and well tolerated, but two of six participants in the 200-mg group experienced ARIA-E (34).
In 2010, a phase 2 trial of gantenerumab was launched in 360 participants with prodromal AD and CSF evidence of Aβ deposition using doses of 105 mg or 225 mg administered subcutaneously every 4 weeks for 2 years. In 2012, the trial was expanded to a phase 2/3 registration trial of 799 participants (NCT01224106). Co-primary end points included CDR-SB and change in brain Aβ levels on [18F]-florbetapir PET. However, the b trial was terminated in December 2014 following an interim futility analysis. At the Alzheimer’s Association International Conference meeting in July 2015, the study results were presented and revealed no significant treatment effects for CDR-SB or change in brain Aβ levels (35). However, post hoc subgroup analyses suggested that participants with fast progression—i.e., participants whose hippocampal volume and CDR-SB score declined most rapidly—may have benefited, especially individuals with higher serum levels of gantenerumab. The incidence of ARIA-E and ARIA-H ranged from 0.4% to 14%, increasing with gantenerumab dose and APOE ε4 status. These results were interpreted as supporting the continuation of gantenerumab trials using higher doses (35). Gantenerumab (along with solanezumab) is also being evaluated by DIAN in a phase 2/3 trial in individuals at risk for and with early-stage autosomal-dominant AD (NCT01760005) (32).
CRENEZUMAB
Crenezumab (MABT5102A; Genentech, Inc., South San Francisco, CA) was engineered on an IgG4 backbone to minimize the activation of Fc gamma receptors (36). In transgenic mice, it reduced the Fc gamma receptor-mediated activation of microglia and triggered less release of the proinflammatory cytokine tumor necrosis factor alpha, thought to contribute to neurotoxicity, as well as ARIA (36). Crenezumab prefers the mid-domain of the Aβ peptide (residues 13–24) (37) and binds multiple conformations of Aβ (monomers, oligomers, fibrils), with a 10-fold higher affinity for oligomers versus monomers (36,38). The epitope recognized by crenezumab overlaps that of solanezumab, explaining their observed cross-reactivity but not their different binding profiles for various species of Aβ (39). However, Ultsch et al. (37) reported that crenezumab and solanezumab actually target slightly different epitopes (residues 13–24 vs. 16–26, respectively). The authors suggested that solanezumab-bound Aβ possesses an alpha-helical structure between residues 21 and 26, whereas crenezumab-bound Aβ has a random coil structure between residues 21 and 24. They further proposed that the alpha-helical epitope is present in monomeric Aβ but absent from aggregated species, potentially explaining solanezumab’s preference for monomers but crenezumab’s recognition of multiple species, including oligomers (37). Phase 1 studies in mild to moderate AD produced no cases of ARIA-E following single doses (0.3–10 mg/kg intravenously) or multiple ascending doses (0.5–5 mg/kg intravenously) of crenezumab (36), allowing higher doses in phase 2.
The major phase 2 trial (NCT01343966) enrolled 431 participants with mild to moderate AD who received either low-dose SC crenezumab 300 mg or placebo biweekly (n = 184) or high-dose intravenous crenezumab 15 mg/kg or placebo every 4 weeks (n = 247) for 68 weeks (38,40). No significant treatment benefits were observed for the primary (ADAS-Cog12 and CDR-SB) or secondary outcomes at either dose. However, in a post hoc subgroup analysis of the high-dose cohort, crenezumab treatment was observed to attenuate decline on the ADAS-Cog12 in the mildest subgroup (MMSE 22–26). A parallel 91-participant biomarker study reported no treatment effects on brain fibrillar Aβ by PET, but CSF Aβ rose slightly with treatment (41,42). Adverse events were balanced between treatment groups, and only one case of ARIA-E was reported in an APOE ε4 homozygote receiving the high dose.
These data were interpreted as supporting the testing of crenezumab at even higher doses in prodromal to mild AD (confirmed by positive Aβ on PET). A phase 3 study (NCT02670083) is ongoing in participants with prodromal to mild AD (MMSE 22–30) using a higher dose of crenezumab (38). Participants are randomly assigned to receive intravenous crenezumab or placebo every 4 weeks for 100 weeks, and the primary outcome measure is the CDR-SB. Crenezumab is also being evaluated in a secondary prevention paradigm as part of an Alzheimer Prevention Initiative trial of 300 cognitively normal presenilin 1 carriers from the world’s largest early-onset AD kindred in Antioquia, Colombia (NCT01998841) (10).
PONEZUMAB
Ponezumab (PF-04360365; Pfizer Inc.), a humanized IgG2 mAb, targets the C-terminus of Aβ40 (residues 30–40) (43). Compared with IgG1, IgG2 antibodies have a lower propensity to induce immune effector function (44). A number of phase 1 trials tested safety, pharmacokinetics, and pharmacodynamics of ponezumab in mild to moderate AD (44–46). These trials pointed to a favorable safety profile without evidence of ARIA, but the antibody was poorly detectable in CSF. Two subsequent phase 2 studies revealed no clinical efficacy, and development of ponezumab for AD was discontinued (44).
BAN2401
BAN2401 (BioArctic Neuroscience AB, Stockholm, Sweden, and Eisai Co., Ltd., Tokyo, Japan), a humanized IgG1 mAb, selectively binds and clears soluble Aβ protofibrils. It was derived from the E22G Arctic mutation in the amyloid precursor protein and has been shown to reduce Aβ protofibrils in the brain and CSF of Tg-ArcSwe mice (47). In a phase 1/2a study using single and multiple ascending intravenous doses (48), BAN2401 was well tolerated with no cases of ARIA-E. A phase 2b 18-month trial testing five different intravenous doses was launched in January 2013 in prodromal or mild AD (confirmed by positive Aβ on PET) (NCT01767311) (49).
ADUCANUMAB
Aducanumab (BIIB037; Biogen, Inc., Cambridge, MA), a fully human IgG1 mAb, selectively reacts with Aβ aggregates, including soluble oligomers and insoluble fibrils (50). It binds the N-terminus (residues 3–6) and recognizes a conformational epitope present on aggregated species of Aβ but absent from monomers. Aducanumab was developed by screening libraries of memory B cells from healthy elderly individuals for reactivity against aggregated Aβ. In Tg2576 mice, an analog of aducanumab was shown to cross the blood-brain barrier, bind parenchymal Aβ, and reduce soluble and insoluble Aβ in a dose-dependent manner (50).
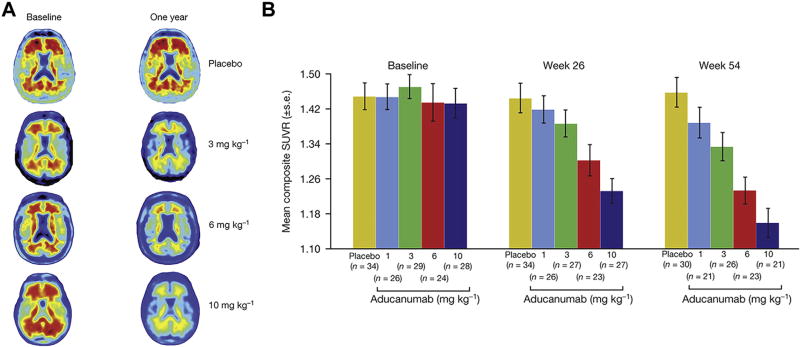
A phase 1b clinical trial has been completed in which participants with prodromal or mild AD and Aβ-positive PET scans who received 1 year of monthly intravenous infusions of aducanumab (1, 3, 6, or 10 mg/kg) evidenced reduced brain fibrillar Aβ in a dose- and time-dependent manner (Figure 1) (50). The phase 1b study was not powered for efficacy; however, exploratory analysis of clinical assessments demonstrated dose-dependent slowing of progression at 1 year. CDR-SB scores declined less with aducanumab treatment, with the greatest slowing for the 10-mg/kg dose. MMSE scores likewise declined less with aducanumab treatment, with the greatest slowing at 3 and 10 mg/kg.
Figure 1.
Amyloid plaque reduction with aducanumab. (A) Example amyloid positron emission tomography images at baseline and week 54. Individuals were chosen based on visual impression and standard uptake value ratio (SUVR) change relative to average 1-year response for each treatment group (n = 40, n = 32, n = 30, and n = 32). Axial slice shows anatomical regions in posterior brain putatively related to Alzheimer’s disease pathology. (B) SUVR values at baseline, week 26, and week 54. For the 10 mg/kg group, the SUVR composite mean value was 1.16 after 54 weeks of treatment, a value near the cut-point of 1.10 that defines a positive scan (66). Some participants at end point fell below this cutoff and would no longer have met eligibility requirement for study entry at screening. [Reprinted by permission from Sevigny et al. (50).]
The most common adverse effects were ARIA, which occurred at higher levels than in any previous anti-Aβ mAb study. ARIA-E was observed at some point during the trial in no participants in the placebo group compared with 1 (3%), 2 (6%), 11 (37%), and 13 (41%) participants receiving 1, 3, 6, and 10 mg/kg aducanumab, with increased incidence in APOE ε4 carriers. Of the 27 participants who developed ARIA-E, 15 (56%) continued treatment (50).
Based on the promising interim analysis of the phase 1b study, in August 2015, Biogen launched two identical 18-month pivotal phase 3 studies to evaluate the efficacy of monthly doses of aducanumab in slowing cognitive and functional impairment as measured by the CDR-SB. Each trial is enrolling 1350 participants with prodromal or mild AD, as confirmed by Aβ-positive PET (NCT02477800 and NCT02484547).
EFFICACY AND SAFETY OF mAbs
To date, no mAb targeting Aβ has demonstrated significant efficacy. A meta-analysis of immunotherapies by Penninkilampi et al. (51) broadly found no significant treatment differences for typical primary outcome measures, such as the ADAS-Cog, ADCS-ADL, or CDR-SB. However, as noted by the authors, the divergence of mechanisms would argue for considering these agents individually. Solanezumab and crenezumab—both targeting mid-domain Aβ epitopes—have evidenced some post hoc trends for treatment effects in mild-stage AD (23,28,38,40). Solanezumab has completed phase 3 testing in mild-stage AD without meeting its efficacy end points; however, it continues in preclinical AD trials (31,32) to see if small effects are amplified with earlier intervention. The most promising results to date have been reported for aducanumab, which demonstrated substantial reductions in brain fibrillar Aβ in an early-phase study, accompanied by slowing of clinical decline at higher doses (50). These results have also provided encouragement for gantenerumab, a similar N-terminal antibody, to continue trials using higher doses (35).
Overall, the safety and tolerability profile of mAbs targeting Aβ has been acceptable. The aforementioned meta-analysis found no difference between pooled treatment and placebo groups in the incidence of adverse events, serious adverse events, and death (51). ARIA, the most concerning safety issue, occurs with N-terminal mAbs that clear fibrillar Aβ—bapineuzumab, gantenerumab, and aducanumab. ARIA-E is strongly associated with drug dose and APOE ε4 status but is also generally (approximately 78%) asymptomatic and self-limiting (20,21) and may not require temporary suspension of treatment (20). Serious complications are rare and must be balanced against the alternative outcome of untreated AD. The same frequency of ARIA events that was dose limiting in early trials of bapineuzumab and gantenerumab has more recently— perhaps fortuitously—been tolerated with aducanumab and associated with possible clinical benefit.
TARGETS OF ANTI-Aβ mAbs
The lack of efficacy thus far with anti-Aβ mAbs may bolster the case against the amyloid hypothesis of AD (52). However, encouraging results with some antibodies make it equally difficult to dismiss this hypothesis altogether. Converging evidence over the past 2 decades has suggested that the most neurotoxic species of Aβ is the soluble oligomer (1,53), which has emerged as the central target for disease-modifying treatments, including mAbs. Moreover, transgenic mouse models have suggested that therapeutic interventions reducing fibrillar Aβ at the cost of augmenting soluble species could actually be harmful (54), although mAbs that target fibrils may also target oligomers. In this regard, the clearance of fibrillar Aβ on a PET scan is perhaps not an essential goal of treatment but may occur as an epiphenomenon to the clearance of oligomers.
As reviewed by Montoliu-Gaya and Villegas (8), mAbs directed against the N-terminus of Aβ may be most effective in clearing the toxic aggregated species of Aβ. Transgenic mouse models have demonstrated that these antibodies inhibit Aβ aggregation and disaggregate preexisting Aβ fibrils (12,55,56). However, as described by Lu et al. (57), using seeded fibril growth from brain extract and data from solid-state nuclear magnetic resonance and electron microscopy, Aβ40 monomers aggregate in oligomers and fibrils with multiples of three units, in which N-termini are exposed, whereas hydrophobic C-termini are inaccessible to antibodies (8). If a similar structure held true for Aβ42, mAbs targeting the N-terminus would likely be most efficient in clearing Aβ oligomers. The success of N-terminal antibodies in clearing aggregated Aβ may also be related to microglial activation and phagocytosis, which is hypothesized to be a common feature of bapineuzumab, gantenerumab, and aducanumab (12,34,50,58).
Thus far, a tight coupling has been observed between mAbs that target aggregated Aβ and the occurrence of ARIA. If ARIA-E is caused by increased trafficking to and clearance of fibrillar Aβ from cerebral vessels (20), mAbs could be designed with conformationally specific epitopes selective for soluble aggregated species (oligomers and protofibrils) and avoid ARIA-E. Alternatively, if ARIA-E has an inflammatory component (14), antibodies may be designed to avoid inflammation. In this regard, it is unclear whether the infrequency of ARIA-E with crenezumab is related to its IgG4 structure or its mid-domain epitope. Preclinical studies have suggested that it binds all forms of Aβ, including fibrils (36). However, more clinical testing is needed to see if it clears plaques. If, in fact, ARIA-E is more related to inflammation, single-chain variable fragments and other structures lacking the microglia-activating Fc fragment could emerge as promising therapies (59,60). They may offer an alternative, noninflammatory approach to the clearance of Aβ, potentially avoiding ARIA that occurs with complete antibodies.
MECHANISM OF Aβ CLEARANCE BY mAbs: BRAIN ENTRY VERSUS PERIPHERAL SINK
Not fully resolved is whether brain entry of anti-Aβ mAbs is necessary, although many experts have attributed the failure of these agents to poor central nervous system penetration (only approximately 0.1% cross the blood-brain barrier) (11). Novel attempts to improve antibody penetration into brain have included targeting receptors on the blood-brain barrier to induce active transport of antibodies into the central nervous system or delivering the genes encoding antibodies and inducing expression in the subject (8).
The peripheral sink hypothesis of mAbs is based on transport of Aβ across the blood-brain barrier as well as an equilibrium between Aβ in brain and periphery (61,62). By draining plasma Aβ, this equilibrium can be altered to leach Aβ from brain without any direct action of antibodies. Ponezumab exploited the peripheral sink effect—at least for plasma Aβ40— but failed to meet clinical end points. Solanezumab continues to test this hypothesis (62), which may still have hope if instituted in preclinical stages (A4 and DIAN-TU) (31,32).
IMPORTANCE OF HIGHER DOSES
The failure of anti-Aβ mAb trials has raised questions about the need for higher doses. For solanezumab, the combination of insignificant efficacy and excellent safety begs the question of whether higher doses would have yielded significant effects for the primary outcomes (30) and whether these should still be considered for ongoing studies in preclinical AD (31,32). Similarly, the encouraging results with aducanumab pose a conundrum following disappointing results with other N-terminal antibodies—bapineuzumab and gantenerumab. Both antibodies share with aducanumab similar pharmacodynamic effects of fibrillar Aβ clearance on PET scans and ARIA-E, although at lower rates than aducanumab. Would higher doses of these drugs produce similar effects (35)?
IMPORTANCE OF STAGE OF DISEASE
An often-cited explanation for the failure of anti-Aβ immunotherapy trials is that they are set too late in the disease process (9,30). Obviously, earlier intervention with a disease-modifying treatment, including anti-Aβ mAbs, is advantageous. Less clear is whether early intervention is necessary for any treatment benefit—i.e., whether an Aβ cascade is initiated such that deterioration can no longer be slowed, or whether, in the setting of advanced Aβ deposition, modest Aβ clearance is simply irrelevant. If Aβ accumulation largely precedes cognitive impairment and is nearly complete by the dementia stage (63–65), later intervention with Aβ-lowering therapies may prove ineffective. Empirical trial evidence for this viewpoint is quite sparse and perhaps limited to the post hoc analyses from the phase 3 solanezumab trial (23) and the phase 2 crenezumab trial (38,40), suggesting clinical efficacy restricted to mild AD subgroups. Unquestionably, the field is moving earlier, as a number of ongoing trials are evaluating treatment effects in prodromal (gantenerumab) or prodromal and mild (aducanumab, crenezumab, gantenerumab, BAN2401) AD. Moreover, we should expect additional studies of preclinical AD to join the ongoing secondary prevention trials: A4 (solanezumab) (31), Alzheimer Prevention Initiative (crenezumab) (10), and DIAN-TU (solanezumab, gantenerumab) (32).
In conclusion, although the development of mAbs for AD has been beset by disappointing results, these failures contain important clues as well as evidence of promise. We have learned that mAbs vary considerably in how they interact with the Aβ peptide and that these differences may bear on whether they target the neurotoxic conformations—Aβ oligomers and protofibrils. These differences also impact the clearance of fibrillar Aβ and the occurrence of important side effects (ARIA-E). Encouraging early results with high-dose aducanumab have suggested the need for higher doses of mAbs broadly. Immunotherapy trials may be started too late in disease—when too much Aβ has accumulated and the Aβ cascade is irrevocably initiated. We can expect new trials to be initiated ever earlier in the course of AD and can expect mAbs to play a central role.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by the National Institute on Aging (Grant No. P50-AG047270) and National Center for Advancing Translational Science (Grant No. UL1-TR000142).
I thank the staffs of the Yale Alzheimer’s Disease Research Unit and the Yale Center for Clinical Investigation Hospital Research Unit for their excellent work in the conduct of therapeutic trials.
CHvD reports having received consulting fees from Janssen Pharmaceuticals, Inc., Eli Lilly and Company, Genentech, Inc., and Merck and Co. and having received grant support from Genentech, Inc., Merck and Co., Eli Lilly and Company, Janssen Pharmaceuticals, Inc., Novartis International AG, Toyama, Pfizer Inc., Forum Pharmaceuticals, TauRx Therapeutics Ltd., Eisai Co., Ltd., Hoffman-La Roche, and Biogen, Inc.
References
- 1.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Suzuki H, Yoshiyama T. Vanutide cridificar and the QS-21 adjuvant in Japanese subjects with mild to moderate Alzheimer’s disease: Results from two phase 2 studies. Curr Alzheimer Res. 2015;12:242–254. doi: 10.2174/1567205012666150302154121. [DOI] [PubMed] [Google Scholar]
- 4.Pasquier F, Sadowsky C, Holstein A, Leterme Gle P, Peng Y, Jackson N, et al. Two phase 2 multiple ascending-dose studies of vanutide cridificar (ACC-001) and QS-21 adjuvant in mild-to-moderate Alzheimer’s disease. J Alzheimer Dis. 2016;51:1131–1143. doi: 10.3233/JAD-150376. [DOI] [PubMed] [Google Scholar]
- 5.van Dyck CH, Sadowsky C, Prince G, Leterme G, Booth K, Peng Y, et al. Vanutide cridificar (ACC-001) and QS-21 adjuvant in individuals with early Alzheimer’s disease: Amyloid imaging positron emission tomography and safety results from a phase 2 study. J Prev Alzheimers Dis. doi: 10.14283/jpad.2016.91. (in press) [DOI] [PubMed] [Google Scholar]
- 6.Farlow MR, Andreasen N, Riviere ME, Vostiar I, Vitaliti A, Sovago J, et al. Long-term treatment with active Abeta immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer’s disease: Randomised, double-blind, placebo-controlled, first-inhuman study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 8.Montoliu-Gaya L, Villegas S. Abeta-Immunotherapeutic strategies: a wide range of approaches for Alzheimer’s disease treatment. Expert Rev Mol Med. 2016;18:e13. doi: 10.1017/erm.2016.11. [DOI] [PubMed] [Google Scholar]
- 9.Sarazin M, Dorothee G, de Souza LC, Aucouturier P. Immunotherapy in Alzheimer’s disease: Do we have all the pieces of the puzzle? Biol Psychiatry. 2013;74:329–332. doi: 10.1016/j.biopsych.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer’s Prevention Initiative: A plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemere CA. Immunotherapy for Alzheimer’s disease: Hoops and hurdles. Mol Neurodegener. 2013;8:36. doi: 10.1186/1750-1326-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 13.Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A, et al. A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord. 2010;24:198–203. doi: 10.1097/WAD.0b013e3181c53b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367–385. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–2070. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: Additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S13–S21. [PubMed] [Google Scholar]
- 17.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier S, Gelinas I, Gauthier L. Functional disability in Alzheimer’s disease. Int Psychogeriatr. 1997;9(suppl 1):163–165. doi: 10.1017/s1041610297004857. [DOI] [PubMed] [Google Scholar]
- 19.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: A phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–372. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 20.Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: A retrospective analysis. Lancet Neurol. 2012;11:241–249. doi: 10.1016/S1474-4422(12)70015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu E, Schmidt ME, Margolin R, Sperling R, Koeppe R, Mason NS, et al. Amyloid-beta 11C-PiB-PET imaging results from 2 randomized bapineuzumab phase 3 AD trials. Neurology. 2015;85:692–700. doi: 10.1212/WNL.0000000000001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, et al. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 24.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: A measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 26.Siemers ER, Friedrich S, Dean RA, Gonzales CR, Farlow MR, Paul SM, et al. Safety and changes in plasma and cerebrospinal fluid amyloid beta after a single administration of an amyloid beta monoclonal antibody in subjects with Alzheimer disease. Clin Neuropharmacol. 2010;33:67–73. doi: 10.1097/WNF.0b013e3181cb577a. [DOI] [PubMed] [Google Scholar]
- 27.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012;8:261–271. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 28.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 29.Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11(suppl 2):S33–S39. [PubMed] [Google Scholar]
- 30.The Lancet Neurology. Solanezumab: Too late in mild Alzheimer’s disease? Lancet Neurol. 2017;16:97. doi: 10.1016/S1474-4422(16)30395-7. [DOI] [PubMed] [Google Scholar]
- 31.Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: Stopping AD before symptoms begin? Sci Transl Med. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, et al. The DIAN-TU Next Generation Alzheimer’s prevention trial: Adaptive design and disease progression model. Alzheimers Dement. 2017;13:8–19. doi: 10.1016/j.jalz.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, et al. Gantenerumab: A novel human anti-Abeta antibody demonstrates sustained cerebral amyloid-beta binding and elicits cell-mediated removal of human amyloid-beta. J Alzheimers Dis. 2012;28:49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 34.Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 35.Lasser R, Ostrowitzki S, Scheltens P, Boada M, Dubois B, Dorflinger E, et al. Efficacy and safety of gantenerumab in prodromal Alzheimer’s disease: Results from scarlet road—a global, multicenter trial. Alzheimers Dement. 2015;11(suppl):331–332. [Google Scholar]
- 36.Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, et al. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ultsch M, Li B, Maurer T, Mathieu M, Adolfsson O, Muhs A, et al. Structure of crenezumab complex with Abeta shows loss of beta-hairpin. Sci Rep. 2016;6:39374. doi: 10.1038/srep39374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Scheltens P, et al. Double-blind, placebo-controlled, randomized Phase II study of the anti-amyloid-beta antibody crenezumab (MABT5102A) in mild-to-moderate Alzheimer’s Disease (ABBY) Neurology (in press) [Google Scholar]
- 39.Crespi GA, Hermans SJ, Parker MW, Miles LA. Molecular basis for mid-region amyloid-beta capture by leading Alzheimer’s disease immunotherapies. Sci Rep. 2015;5:9649. doi: 10.1038/srep09649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings J, Cho W, Ward M, Friesenhahn M, Brunstein F, Honigberg L, et al. A randomized, double-blind, placebo-controlled phase 2 study to evaluate the efficacy and safety of crenezumab in patients with mild to moderate Alzheimer’s disease. Presented at Alzheimer’s Association International Conference (AAIC); Copenhagen, Denmark. July 16, 2014.2014. [Google Scholar]
- 41.Salloway S, Cho W, Clayton D, Honigberg L, Rabe C, Friesenhahn M, et al. Amyloid PET imaging results from a study to evaluate the impact of crenezumab on fibrillar amyloid in patients with mild-to-moderate Alzheimer’s disease. Presented at Clinical Trials on Alzheimer’s Disease (CTAD; Philadelphia, PA. November 20, 2014.2014. [Google Scholar]
- 42.Honigberg L, Clayton D, Cho W, Rabe C, Friesenhahn M, Ward M, et al. Biomarker results from the crenezumab anti-Aβ phase 2 biomarker trial. Presented at Clinical Trials on Alzheimer’s Disease (CTAD); Philadelphia, PA. November 20–22, 2014.2014. [Google Scholar]
- 43.La Porte SL, Bollini SS, Lanz TA, Abdiche YN, Rusnak AS, Ho WH, et al. Structural basis of C-terminal beta-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer’s disease. J Mol Biol. 2012;421:525–536. doi: 10.1016/j.jmb.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 44.Landen JW, Zhao Q, Cohen S, Borrie M, Woodward M, Billing CB, Jr, et al. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: A phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol. 2013;36:14–23. doi: 10.1097/WNF.0b013e31827db49b. [DOI] [PubMed] [Google Scholar]
- 45.Burstein AH, Zhao Q, Ross J, Styren S, Landen JW, Ma WW, et al. Safety and pharmacology of ponezumab (PF-04360365) after a single 10-minute intravenous infusion in subjects with mild to moderate Alzheimer disease. Clin Neuropharmacol. 2013;36:8–13. doi: 10.1097/WNF.0b013e318279bcfa. [DOI] [PubMed] [Google Scholar]
- 46.Miyoshi I, Fujimoto Y, Yamada M, Abe S, Zhao Q, Cronenberger C, et al. Safety and pharmacokinetics of PF-04360365 following a single-dose intravenous infusion in Japanese subjects with mild-to-moderate Alzheimer’s disease: A multicenter, randomized, double-blind, placebo-controlled, dose-escalation study. Int J Clin Pharmacol Ther. 2013;51:911–923. doi: 10.5414/CP201816. [DOI] [PubMed] [Google Scholar]
- 47.Tucker S, Moller C, Tegerstedt K, Lord A, Laudon H, Sjodahl J, et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-beta protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis. 2015;43:575–588. doi: 10.3233/JAD-140741. [DOI] [PubMed] [Google Scholar]
- 48.Logovinsky V, Satlin A, Lai R, Swanson C, Kaplow J, Osswald G, et al. Safety and tolerability of BAN2401 — a clinical study in Alzheimer’s disease with a protofibril selective Abeta antibody. Alzheimers Res Ther. 2016;8:14. doi: 10.1186/s13195-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lannfelt L, Moller C, Basun H, Osswald G, Sehlin D, Satlin A, et al. Perspectives on future Alzheimer therapies: amyloid-beta protofibrils—a new target for immunotherapy with BAN2401 in Alzheimer’s disease. Alzheimers Res Ther. 2014;6:16. doi: 10.1186/alzrt246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 51.Penninkilampi R, Brothers HM, Eslick GD. Safety and efficacy of anti-amyloid-beta immunotherapy in Alzheimer’s disease: A systematic review and meta-analysis. J Neuroimmune Pharmacol. 2017;12:194–203. doi: 10.1007/s11481-016-9722-5. [DOI] [PubMed] [Google Scholar]
- 52.Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18:794–799. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- 53.Walsh DM, Selkoe DJ. A beta oligomers—a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 54.Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007;282:23818–23828. doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- 55.Horikoshi Y, Mori T, Maeda M, Kinoshita N, Sato K, Yamaguchi H. Abeta N-terminal-end specific antibody reduced beta-amyloid in Alzheimer-model mice. Biochem Biophys Res Commun. 2004;325:384–387. doi: 10.1016/j.bbrc.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 56.Bussiere T, Bard F, Barbour R, Grajeda H, Guido T, Khan K, et al. Morphological characterization of Thioflavin-S-positive amyloid plaques in transgenic Alzheimer mice and effect of passive Abeta immunotherapy on their clearance. Am J Pathol. 2004;165:987–995. doi: 10.1016/s0002-9440(10)63360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular structure of beta-amyloid fibrils in Alzheimer’s disease brain tissue. Cell. 2013;154:1257–1268. doi: 10.1016/j.cell.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci. 2004;24:6144–6151. doi: 10.1523/JNEUROSCI.1090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kou J, Kim H, Pattanayak A, Song M, Lim JE, Taguchi H, et al. Anti-amyloid-beta single-chain antibody brain delivery via AAV reduces amyloid load but may increase cerebral hemorrhages in an Alzheimer’s disease mouse model. J Alzheimers Dis. 2011;27:23–38. doi: 10.3233/JAD-2011-110230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang YJ, Pollard A, Zhong JH, Dong XY, Wu XB, Zhou HD, et al. Intramuscular delivery of a single chain antibody gene reduces brain Abeta burden in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2009;30:364–376. doi: 10.1016/j.neurobiolaging.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Lee DH. Sink hypothesis and therapeutic strategies for attenuating Abeta levels. Neuroscientist. 2011;17:163–173. doi: 10.1177/1073858410381532. [DOI] [PubMed] [Google Scholar]
- 62.Liu YH, Wang YR, Xiang Y, Zhou HD, Giunta B, Manucat-Tan NB, et al. Clearance of amyloid-beta in Alzheimer’s disease: Shifting the action site from center to periphery. Mol Neurobiol. 2015;51:1–7. doi: 10.1007/s12035-014-8694-9. [DOI] [PubMed] [Google Scholar]
- 63.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jack CR, Jr, Wiste HJ, Lesnick TG, Weigand SD, Knopman DS, Vemuri P, et al. Brain beta-amyloid load approaches a plateau. Neurology. 2013;80:890–896. doi: 10.1212/WNL.0b013e3182840bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knopman DS, Jack CR, Jr, Lundt ES, Weigand SD, Vemuri P, Lowe VJ, et al. Evolution of neurodegeneration-imaging biomarkers from clinically normal to dementia in the Alzheimer disease spectrum. Neurobiol Aging. 2016;46:32–42. doi: 10.1016/j.neurobiolaging.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, et al. Amyloid-beta imaging with Pittsburgh compound B and florbetapir: Comparing radiotracers and quantification methods. J Nucl Med. 2013;54:70–77. doi: 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]