Abstract
BACKGROUND
The number of pediatric hematopoietic cell transplant (HCT) patients who survive pediatric intensive care unit (PICU) admission is increasing, yet little is known about their functional morbidity after PICU discharge. We hypothesized that relative to controls, pediatric HCT patients who survive PICU admission would have greater rates of new functional morbidity at the time of PICU discharge, and only a minority of these patients would return to their functional baseline by the end of the hospitalization.
METHODS
We performed a retrospective cohort study with secondary data analysis of the Trichotomous Outcomes in Pediatric Critical Care (TOPICC) dataset. The pediatric HCT cohort was identified by querying ICD-9 diagnostic codes. A control group consisted of previously healthy patients matched 4:1 on age, sex, and illness severity, as estimated by the Pediatric Risk of Mortality (PRISM) score. We benchmarked our findings by comparing to a previously healthy group of children with lower respiratory tract infections. Functional impairment was measured by the Functional Status Scale, wherein new morbidity was defined as an increase of ≥3 points relative to the pre-hospital baseline.
RESULTS
Relative to matched controls, HCT patients had similar admission PRISM score (p=0.516) but greater PICU mortality (12.9% [11/85] vs. 6.2% [21/340], p=0.035). However, among those who survived to PICU discharge, HCT patients had similar rates of new morbidity at PICU discharge (14.9% [11/74] vs. 17.2% [55/319], p=0.622) and similar rates of resolution of new morbidity by hospital discharge (54.5% [6/11] vs. 60.0% [33/55], p=0.737). Relative to the LRTI comparison group, HCT patients had both greater admission PRISM score (p<0.001) and greater PICU mortality (12.9% [11/85] vs. 1.6% [5/308], p<0.001). However, among those who survived to PICU discharge, HCT patients again displayed similar rates of new morbidity at PICU discharge (14.9% [11/74] vs 22.1% [67/303], p=0.168) as well as resolution of new morbidity by hospital discharge (54.5% [6/11] vs. 71.6% [48/67], p=0.299).
CONCLUSIONS
For pediatric HCT patients, PICU survival with new functional morbidity is as prevalent an outcome as PICU mortality. Although pediatric HCT patients have greater PICU mortality than age-, sex-, and PRISM-matched controls, they have similar rates of new functional morbidity at PICU discharge and similar resolution of new functional morbidity at hospital discharge. Future interventions focused on improving functional status in pediatric HCT survivors of critical illness are warranted.
Keywords: hematopoietic stem cell transplantation, intensive care units, pediatric, quality of life, recovery of function, critical illness, critical care outcomes
BACKGROUND
Critically ill pediatric hematopoietic cell transplant (HCT) patients have nearly eight times greater odds for pediatric intensive care unit (PICU) mortality than do other critically ill children (1). Recent publications suggest mortality rates of up to 16% per PICU admission, with significantly higher mortality for patients requiring invasive mechanical ventilation (42.5%) or renal replacement therapy (51.9%) (1,2). Although trends suggest that mortality has decreased over time for these patients, historical comparisons are confounded by the increasingly heterogeneous cohorts, varied timing of PICU admission, and lack of illness severity standards that allow for controlled comparisons (3,4). Nonetheless, as the number of pediatric HCT PICU survivors increase, it is becoming increasingly important to assess a broad set of outcomes beyond PICU mortality.
Frameworks for assessing both functional status and health-related quality of life currently exist in pediatric critical care research and include metrics such as the Pediatric Overall Performance Category (POPC), the Pediatric Cerebral Performance Category (PCPC), and the Pediatric Quality of Life Inventory (PQOLI) (5). Recently, the Functional Status Scale (FSS) has been introduced as a more robust scale for assessing gradations of functional status over multiple domains (6). Studies such as the Trichotomous Outcomes in Pediatric Critical Care (TOPICC) have applied the FSS in the general PICU population and have found that up to 36% of children demonstrate new functional impairment at the time of PICU discharge (7). Additional studies demonstrate persistent functional impairment in 10–13% of PICU survivors when assessed at 2 years (8–14).
Among pediatric HCT patients who survive to PICU discharge, functional status decline at PICU discharge and at hospital discharge has not been assessed. However, one large study identified that pediatric HCT patients discharged from intensive care had similar 1-year survival and functional outcomes compared to pediatric HCT patients who did not require intensive care; this suggests that for many pediatric HCT patients, morbidity acquired during critical illness may be recoverable over time (15). Therefore, we aimed to extend the growing body of knowledge on functional status changes after pediatric critical illness to the particularly vulnerable population of pediatric HCT patients in order to more fully characterize the breadth of significant clinical outcomes after critical illness in pediatric HCT patients.
METHODS
Study Design
We performed a retrospective cohort study using a secondary data analysis of the Trichotomous Outcomes in Pediatric Critical Care (TOPICC) dataset. The TOPICC study enrolled 10,078 patients less than 18 years of age who were admitted between December 4, 2011 and April 7, 2013 to one of eight PICUs of the Collaborative Pediatric Critical Care Research Network (CPCCRN). Where patients had multiple PICU admissions during a single hospital stay, the first PICU admission was used. Where patients had multiple PICU admissions during multiple hospital stays, the first PICU admission within the first hospital stay was used and the rest were excluded.
Cohort
HCT recipients were identified within the TOPICC database by querying the following ICD-9 diagnosis codes: V42.81 (bone marrow replaced by transplant), V42.82 (peripheral stem cells replaced by transplant), 41.0 with all subcodes (bone marrow or hematopoietic stem cell transplant), 279.5 with all subcodes (graft-versus-host disease), and 996.85 (complications of transplanted bone marrow). For each patient, we recorded age, sex, race, and ethnicity. We used the PRISM score as a measurement of admission physiologic dysfunction (admission illness severity). The PRISM score weights vital sign and laboratory derangements within the first four hours of PICU admission according to their association with PICU mortality (16,17). We also measured critical care resource utilization according to use of invasive mechanical ventilation, vasoactive infusions, and renal replacement therapy, including intermittent or continuous hemodialysis, hemofiltration, or hemodiafiltration. Due to the challenges of retrospectively ascertaining PICU admission indication from lists of diagnosis codes, we were unable to describe each patient’s primary PICU admission indication.
Control Group
To account for varying demographics and admission illness severity, we matched each HCT patient to 4 non-HCT control patients of the same age group (<1 year, 1–4.99 years, 5–12.99 years, 13–17.99 years), same sex, PRISM score within 2 points, and who had no chronic medical conditions. In cases where an HCT patient had more than 4 possible controls, 4 controls were selected randomly using a computer-based random number generator. For three instances where one HCT patient had fewer than 4 possible controls, age- and sex-matched controls were selected according to patients with the next closest PRISM score.
Comparison Group
We anticipated that the matched control group would be heterogeneous in terms of PICU admission indication. As patients with lower respiratory tract infections (LRTI) compose a large portion of general PICU admissions (17) and 73–88% of pediatric HCT PICU admissions are indicated for LRTI and other types of respiratory failure (15,18–20), we identified a group of pediatric patients with LRTI to serve as a useful clinical benchmark against which to compare the pediatric HCT population. The LRTI comparison group was identified by querying the TOPICC database for non-HCT patients with ICD-9 diagnosis codes 480–487, including all subcodes. We then excluded patients with any chronic medical condition as indicated on the original TOPICC case report form.
Measurements
The Functional Status Scale (FSS) measures six domains of daily function (mental status, sensory, communication, motor, feeding, and respiratory), each on a five-point scale from normal function to very severe dysfunction, to produce a global assessment of no, mild, moderate, severe, or very severe impairment (total scores of 6–7, 8–9, 10–15, 16–21, and 22–30, respectively) (6). In contrast, the Pediatric Overall Performance Category (POPC) and the Pediatric Cerebral Performance Category (PCPC) are abbreviated assessments scored from one to five that estimate global functioning as normal, mild disability, moderate disability, severe disability, or coma/vegetative (5). In the TOPICC study, the FSS, POPC, and PCPC were each measured at pre-hospital baseline, at PICU discharge and at hospital discharge by review of medical records and discussion with bedside caregivers.
Outcomes
The primary outcome was the trichotomous outcome of mortality, survival with new functional morbidity, or survival without new functional morbidity, measured at PICU discharge and again at hospital discharge. New morbidity was defined as a change in FSS score of ≥3 points relative to the pre-hospital baseline. The secondary outcome was the prevalence of moderate/severe functional status impairment, defined as FSS score ≥10, measured at the time of PICU discharge and again at hospital discharge.
Statistics
Distributions of categorical variables were described with percentages and compared with Chi-squared or Fisher exact tests. Distributions of continuous variables were described with median and interquartile ranges (IQR) and compared with Wilcoxon rank sum tests. All tests are two-sided.
RESULTS
Cohort
Of the 10,014 PICU admissions in the TOPICC database that were accompanied by ICD-9 codes, we identified 85 admissions for pediatric HCT patients (0.8%), 340 admissions for previously healthy children matched 4:1 to HCT patients on age, sex, and PRISM score (3.4%), and 308 admissions for previously healthy children with LRTI (3.1%) Characteristics of the HCT patients, the matched control group, and the LRTI comparison group are depicted in Table 1.
Table 1.
Characteristics of Pediatric Hematopoietic Cell Transplant Patients
| HCT Patients (n=85) | Matched Control Group* (n=340) | LRTI Comparison Group (n=308) | |||
|---|---|---|---|---|---|
| Demographics | |||||
| Age (median, IQR) * | 7.1 (3.7–13.4) | 7.9 (2.8–13.3) | p=0.969 | 1.2 (0.4–4.4) | p<0.001 |
| Female (n, %)* | 37 (43.5) | 148 (43.5) | p=1.000 | 144 (46.8) | p=0.598 |
| Race (n, %) | p=0.430 | p=0.365 | |||
| Black | 14 (16.5) | 73 (21.5) | 68 (22.1) | ||
| White | 48 (56.5) | 167 (49.1) | 149 (48.4) | ||
| Other/Unknown | 23 (27.1) | 100 (29.4) | 91 (29.6) | ||
| Ethnicity (n, %) | p=0.145 | p=0.158 | |||
| Hispanic/Latino | 11 (12.9) | 56 (16.5) | 53 (17.2) | ||
| Non-Hispanic/Latino | 56 (65.9) | 184 (54.1) | 167 (42.5) | ||
| Unknown | 18 (21.2) | 100 (29.4) | 88 (22.4) | ||
| PICU Admission Illness Severity | |||||
| PRISM (median, IQR) * | 5 (0–10) | 4.5 (0–9) | p=0.516 | 2 (0–5) | p<0.001 |
| Baseline Functional Status Scores (median, IQR) | |||||
| Functional Status Score | 7 (6–10) | 6 (6-6) | p<0.001 | 6 (6-6) | p<0.001 |
| Pediatric Overall Performance Category | 3 (2–3) | 1 (1-1) | p<0.001 | 1 (1-1) | p<0.001 |
| Pediatric Cerebral Performance Category | 1 (1–2) | 1 (1-1) | p<0.001 | 1 (1-1) | p<0.001 |
| Baseline Functional Status Class (n, %) | p<0.001 | p<0.001 | |||
| No/mild impairment (FSS score <10) | 60 (70.6) | 338 (99.4) | 303 (98.4) | ||
| Moderate/severe impairment (FSS score ≥10) | 25 (29.4) | 2 (0.6) | 5 (1.6) | ||
| PICU Supportive Care (n, %) | |||||
| At Least One Supportive Therapy | 47 (55.3) | 140 (41.2) | p=0.019 | 161 (52.3) | p=0.621 |
| Mechanical Ventilation | 31 (36.5) | 125 (36.8) | p=0.956 | 154 (50) | p=0.027 |
| Renal Replacement Therapy | 5 (5.9) | 8 (2.4) | p=0.148 | 5 (1.6) | p=0.043 |
| Vasoactive Infusions | 30 (35.3) | 74 (21.8) | p=0.010 | 57 (18.5) | p=0.001 |
Legend: Functional status represents pre-hospital baseline.
Patients in the matched control group were matched 4:1 to HCT patients on age, sex, and PRISM score.
HCT Patients Compared to Matched Controls
Relative to the matched controls, HCT patients had similar distribution of age, sex, race, ethnicity, and PRISM score, suggesting successful patient-control matching. However, HCT patients had worse baseline functional status on the FSS, POPC, and PCPC scores (p<0.001) and more frequently used vasoactive infusions (p=0.010), but had similar rates of invasive mechanical ventilation (p=0.956) and renal replacement therapy (p=0.148).
HCT Patients Compared to LRTI Comparison Group
Relative to the LRTI comparison group, the HCT patients had similar distribution of sex, race, and ethnicity, but were older (p<0.001), had worse baseline functional status on the FSS, POPC, and PCPC scores (p<0.001), had higher PICU admission illness severity as assessed by the PRISM score (p<0.001), more frequently used renal replacement therapy and vasoactive infusions (p=0.027, p=0.001), but less frequently used invasive mechanical ventilation (p=0.027).
Change in Functional Status Between PICU Admission and PICU Discharge
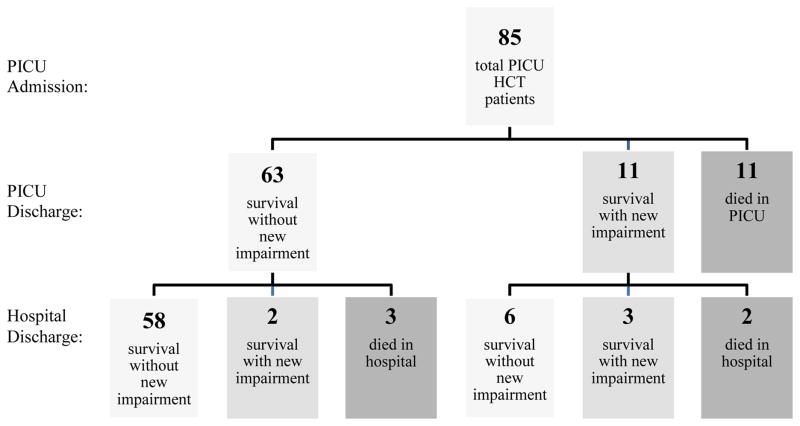
Longitudinal changes in functional status of the 85 pediatric HCT patients are illustrated in Figure 1. At PICU discharge, 63 maintained their functional baseline (ΔFSS score <3), 11 developed new impairment (ΔFSS score ≥3), and 11 died. There was no statistically significant difference in rates of PICU death among patients with moderate/severe baseline impairment vs. patients no/mild baseline impairment vs. (16.0% [4/25] vs. 11.7% [7/60], p=0.724). Similarly, among those discharged alive from the PICU, there was no statistically significant difference in the development of new functional impairment among patients with moderate/severe baseline impairment vs. no/mild baseline impairment (9.5% [2/21] vs. 17.0% [9/53], p=0.718).
Figure 1. Trichotomous Longitudinal Outcomes of Pediatric Hematopoietic Cell Transplant Patients Admitted to the Pediatric Intensive Care Unit.
New impairment defined as increase in Functional Status Scale (FSS) score of at least 3 points relative to pre-hospital baseline.
Change in Functional Status Between PICU Discharge and Hospital Discharge
Of 63 HCT patients discharged from the PICU at their functional baseline, 58 were discharged from the hospital at their functional baseline (ΔFSS score <3), two were discharged from the hospital with new morbidity (ΔFSS score ≥3), and three died in the hospital. Of the 11 HCT patients discharged from the PICU with new functional impairment, six were discharged from the hospital with recovery to their functional baseline, three were discharged from the hospital with persistent new morbidity, and two died in the hospital. Of the three patients discharged from the hospital with persistent new morbidity relative to baseline, the first had persistent deficits in communication, motor skills, and mental function, the second had persistent deficits in feeding and motor function, and the third had persistent deficits in feeding and respiratory function. There was a non-significant trend towards greater hospital death among HCT patients discharged from the PICU with new functional impairment vs. HCT patients discharged from the PICU at their baseline functional status (18.2% [2/11] vs. 4.8% [3/63], p=0.157). Post hoc power analysis indicates that a sample size of 325 HCT patients requiring PICU admission would be necessary to demonstrate statistical significance for this difference in hospital mortality between those who survive PICU admission with vs. without new functional morbidity (χ2 test, α=0.05, β=0.2). In comparison to new functional impairment, there was no difference in hospital death among HCT patients with moderate/severe impairment at PICU discharge vs. HCT patients with no/mild impairment at PICU discharge (10.7% [3/28] vs. 4.3% [2/46], p=0.360).
Comparison of HCT Patients vs. Controls at PICU Discharge
At the time of PICU discharge, HCT patients had greater mortality than both matched controls (12.9% [11/85] vs 6.2% [21/340], p=0.035) and the LRTI comparison group (12.9% [11/85] vs. 1.6% [5/308], p<0.001, Table 2). Among patients who survived to PICU discharge, HCT patients also had higher FSS scores at that time than the matched controls (median 8.5, IQR 6–11 vs. 6, IQR 6–8, p<0.001) and the LRTI comparison group (median 8.5, IQR 6–11 vs. 7, IQR 6–8, p=0.002) and had greater prevalence of moderate-to-severe functional impairment than the matched controls (37.8% [28/74] vs. 15.4% [49/319], p<0.001) and the LRTI comparison group (37.8% [28/74] vs. 13.5% [41/303], p<0.001). Among patients who survived to PICU discharge, HCT patients displayed similar rates of new morbidity at PICU discharge relative to matched controls (14.9% [11/74] vs. 17.2% [55/319], p=0.622) and the LRTI comparison group (14.9% [11/74] vs 22.1% [67/303], p=0.168).
Table 2.
Outcomes of Pediatric Hematopoietic Cell Transplant Patients
| HCT Cohort (n=85) | Matched Control Group (n=340) | LRTI Comparison Group (n=308) | ||||
|---|---|---|---|---|---|---|
| PICU Discharge | Functional Status Scores (median, IQR) | |||||
| Functional Status Score | 8.5 (6–11) | 6 (6–8) | p<0.001 | 7 (6–8) | p=0.002 | |
| Pediatric Overall Performance Category | 3 (2–4) | 2 (1–2) | p<0.001 | 2 (1–2) | p<0.001 | |
| Pediatric Cerebral Performance Category | 1 (1–3) | 1 (1-1) | p<0.001 | 1 (1-1) | p<0.001 | |
| Functional Class (n, %) | p<0.001 | p<0.001 | ||||
| No/mild impairment (FSS score <10) | 46 (54.1) | 270 (79.4) | 262 (85.1) | |||
| Moderate/severe impairment (FSS score ≥10) | 28 (32.9) | 49 (14.4) | 41 (13.3) | |||
| Dead | 11 (12.9) | 21 (6.2) | 5 (1.6) | |||
| Functional Status Change (n, %) | p=0.159 | p=0.034 | ||||
| No new impairment (ΔFSS score <3) | 63 (74.1) | 264 (77.6) | 236 (76.6) | |||
| New impairment (ΔFSS score ≥3) | 11 (12.9) | 55 (16.2) | 67 (21.8) | |||
| Dead | 11 (12.9) | 21 (6.2) | 5 (1.6) | |||
| Hospital Discharge | Functional Status Scores (median, IQR) | |||||
| Functional Status Score | 8 (6–10) | 6 (6–7) | p<0.001 | 6 (6-6) | p<0.001 | |
| Pediatric Overall Performance Category | 3 (2–4) | 2 (1–2) | p<0.001 | 1 (1–2) | p<0.001 | |
| Pediatric Cerebral Performance Category | 1 (1–4) | 1 (1-1) | p<0.001 | 1 (1-1) | p<0.001 | |
| Functional Class (n, %) | p<0.001 | p<0.001 | ||||
| No/mild impairment (FSS score <10) | 45 (52.9) | 296 (87.1) | 284 (92.2) | |||
| Moderate/severe impairment (FSS score ≥10) | 24 (28.2) | 21 (6.2) | 18 (5.8) | |||
| Dead | 16 (18.8) | 23 (6.8) | 6 (1.9) | |||
| Functional Status Change (n, %) | p=0.001 | p<0.001 | ||||
| No new impairment (ΔFSS score <3) | 64 (75.3) | 295 (86.8) | 283 (91.9) | |||
| New impairment (ΔFSS score ≥3) | 5 (5.9) | 22 (6.5) | 19 (6.2) | |||
| Dead | 16 (18.8) | 23 (6.8) | 6 (1.9) | |||
Legend: Functional status change at PICU discharge and at hospital discharge are relative to the pre-hospital baseline. Functional class and functional status change at PICU discharge and at hospital discharge were compared as ordered trichotomous categorical variables.
Comparison of HCT Patients vs. Controls at Hospital Discharge
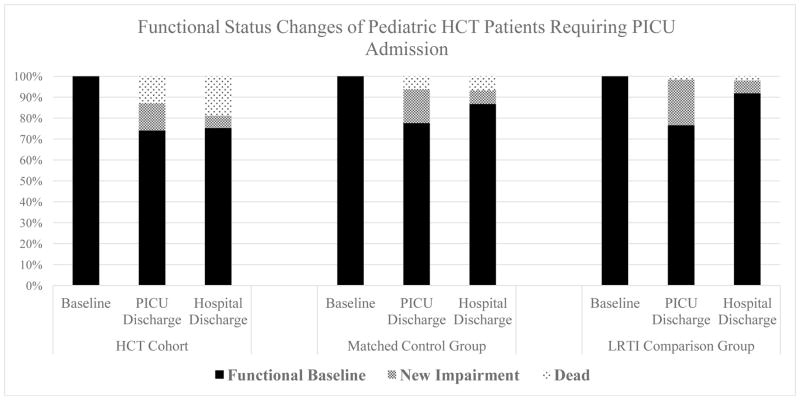
At the time of hospital discharge, the HCT cohort again had greater hospital mortality than both the matched controls (18.8% [16/85] vs. 6.8% [23/340], p<0.001) and the LRTI comparison group (18.8% [16/85] vs. 1.9% [6/308], p<0.001, Figure 2). Among patients who survived to hospital discharge, HCT patients also had higher FSS scores at that time than did the matched controls (median 8, IQR 6–10 vs. 6, IQR 6–7, p<0.001) and the LRTI comparison group (median 8, IQR 6–10 vs. 6, IQR 6-6, p<0.001); they also had greater prevalence of moderate-to-severe functional impairment than did the matched controls (34.7% [24/69] vs. 6.6% [21/317], p<0.001) and the LRTI comparison group (34.7% [24/69] vs. 6.0% [18/302], p<0.001). Among patients who survived to PICU discharge, HCT patients had similar rates of new morbidity at hospital discharge when compared to matched controls (7.2% [5/69] vs. 6.9% [22/317], p=0.928) and the LRTI comparison group (7.2% [5/69] vs. 6.3% [19/302], p=0.787). Among patients discharged from the PICU with new morbidity, pediatric HCT patients displayed similar rates of resolution of new morbidity at hospital discharge relative to matched controls (54.5% [6/11] vs. 60.0% [33/55], p=0.737) and the LRTI comparison group (54.5% [6/11] vs. 71.6% [48/67], p=0.299).
Figure 2. Functional Status Changes of Pediatric HCT Patients Requiring PICU Admission.
New impairment defined as increase in Functional Status Scale (FSS) score of at least 3 points relative to pre-hospital baseline.
DISCUSSION
This study adds to a growing body of knowledge regarding functional outcomes of pediatric HCT patients who survive critical illness. First, we identified that the group of HCT patients that are discharged from the PICU with new functional morbidity is equally as large as the group of HCT patients who do not survive PICU admission. This finding suggests a clinically relevant outcome that has to date received little formal investigation and may be an appropriate target for interventional trials. Second, we identified that while HCT patients are more likely to die in the PICU, those who survive have similar rates of new morbidity at PICU discharge and similar rates of resolution of new morbidity at hospital discharge when compared to matched controls. This suggests that a subset of pediatric HCT survivors of critical illness may have reversible functional status decline at PICU discharge and might benefit from interventions to improve functional status. Third, we identified a high-risk group of PICU survivors; HCT patients with new functional morbidity at PICU discharge appeared to have greater risk of hospital mortality than did HCT patients discharged from the PICU at their functional baseline.
Prevalence of New Functional Morbidity after PICU Discharge
This study identifies a large subgroup (12.9%) of pediatric HCT patients who survive critical illness but acquire new functional morbidity that is evident at the time of PICU discharge. This subgroup constitutes as many patients as those who die from critical illness, suggesting that the outcome of survival with new functional morbidity is not only clinically relevant, but significantly prevalent. Previous studies assessing functional status at PICU discharge have been performed in non-HCT cohorts and identify functional impairment in up to 36% of children at the time of PICU discharge (8–14). Our findings are novel, as assessment of functional status at PICU and hospital discharge in pediatric HCT patients has not been undertaken before. A growing number of studies report significant long-term morbidity in survivors of critical illnesses such as acute respiratory distress syndrome (21–24). While our study did not follow patients beyond hospital discharge, Duncan et al. found that 1-year survival and organ function tests in pediatric HCT survivors of critical illness were similar to those of pediatric HCT patients who did not require intensive care (15). This suggests that for pediatric HCT survivors of critical illness, ongoing recovery from critical-illness related decline in functional status is indeed possible. Currently, the Center for International Blood & Marrow Transplant Research (CIBMTR), the National Institutes of Health (NIH), the Pediatric Blood & Marrow Transplant Consortium (PBMTC), and the European Society for Blood & Marrow Transplantation (EBMT) have recommended following all pediatric HCT patients for long-term cardiopulmonary, renal, and multiorgan toxicity, as well as for neurodevelopmental outcomes and health-related quality of life (25–27). Based on the results of this study, we advocate for close long-term monitoring for functional deficits in pediatric HCT survivors of critical illness who do not regain their baseline functional status.
Resolution of New Functional Morbidity between PICU Discharge and Hospital Discharge
This study also suggests that while pediatric HCT patients who survive to PICU discharge have greater rates of moderate/severe impairment, they have similar rates of new functional impairment when compared to matched controls and a general group of non-chronically ill children with LRTI. Given the well-established higher risk of PICU mortality among pediatric HCT patients, one might expect that pediatric HCT patients would have a higher risk of new morbidity and perhaps lower rates of resolution of new morbidity. While survival without new morbidity, survival with new morbidity, and non-survival are a single continuum of outcomes, the ability to avert escalation along this continuum likely varies among different patient groups. We therefore speculate that due to lower functional morbidity at baseline, the matched controls and LRTI comparison group may have been better able to survive critical illness, resulting in discharge with new morbidity, whereas pediatric HCT patients may have been less able to survive critical illness, limiting the number of survivors with new functional morbidity in favor of more non-survivors.
Interestingly, although matched on PRISM score, the HCT group had greater usage of vasoactive infusions than did the matched controls. This may suggest that pediatric HCT patients have different underlying pathobiology of critical illness, they may have more rapid and severe progression of critical illness beyond the 4 hour PRISM observation window, or it may indicate different physician management strategies with respect to treating multiorgan failure in critically ill pediatric HCT patients vs. matched controls. However, the 12.9% mortality of pediatric HCT PICU patients in our study is comparable to other large studies and is consistent with an international trend towards decreasing PICU mortality of pediatric HCT patients over time (1,3,19,28). The reasons for decreasing PICU mortality in this population may include improvements in transplant conditioning regimens, infection surveillance, and treatment of post-transplant complications, as well as a bias towards more frequent admissions with lower illness severity; nonetheless, as the number of pediatric HCT survivors of critical illness grows, the need to follow survivors for short- and long-term morbidity will become increasingly important (29–33).
In addition to having similar rates of new functional impairment at PICU discharge, pediatric HCT patients discharged from the PICU with new functional impairment were as likely to be discharged from the hospital with resolution of the new functional impairment as were the matched controls and the LRTI comparison group. This suggests that a portion of pediatric HCT survivors of critical illness have the potential to make significant functional recovery after critical illness; hence, the full spectrum of rehabilitative services, including physical, occupational, and speech therapy where appropriate, should be considered in order to maximize chances of functional recovery (34,35).
Patients at High Risk for Future Death or Disability
This study identifies a particularly high-risk subgroup of pediatric HCT survivors of critical illness that merit close attention for persistent or progressive comorbidities. The long-term clinical outcomes of pediatric HCT patients who survive critical illness but develop new functional morbidity remain unknown, particularly with respect to survivors of mechanical ventilation. With our cohort of 85 pediatric HCT patients, we demonstrated a non-significant trend towards worsened hospital survival rates among pediatric HCT patients discharged from the PICU with vs. without new functional morbidity (18.2% [2/11] vs. 4.8% [3/63], p=0.157). Post hoc sample size calculations indicate that a sample size of 325 HCT patients requiring PICU admission is necessary to demonstrate statistical significance for this difference in hospital mortality between those who survive PICU admission with vs. without new functional morbidity (χ2 test, α=0.05, β=0.2). Therefore, we advocate for continued assessment of functional status in pediatric HCT patients who survive critical illness. Future interventions aimed at early and aggressive medical and rehabilitative intervention where appropriate may be particularly impactful in reducing subsequent morbidity and mortality in this high-risk cohort of PICU survivors.
Our study has several strengths. First, the proportion of HCT patients in this PICU cohort (0.8%, 85/10,014) is consistent with the proportion of HCT patients in our previous study of greater than one hundred PICUs in the Virtual Pediatric Systems (VPS) database (0.6%, 1,102/192,956) (1). This suggests external validity of this dataset with respect to the larger VPS dataset, which does not document functional status scores. Second, this study benefits from rigorous application of the FSS by trained assessors with checks of inter-rater reliability. Third, our study assesses functional status at pre-critical illness baseline, at PICU discharge, and at hospital discharge, allowing longitudinal characterization of functional status changes throughout the course of critical illness and in-hospital recovery. Our study has several weaknesses. First, owing to the complexity of multiple diagnostic codes in a retrospective analysis, we were unable to assign each patient a primary reason for PICU admission. Second, our study lacked granular data on hematopoietic stem cell transplant characteristics commonly associated with adverse clinical outcomes, such as underlying disease, donor type and HLA match, conditioning regimen, and post-transplant toxicities including graft versus host disease. Importantly, PRISM score alone may not be adequately suitable to approximate illness severity in pediatric HCT patients; incorporation of transplant-specific risk-factors such as those described above may allow more precise prognostication of mortality risk (36,37). Third, our study included only the first PICU admission for each patient during the study interval and therefore does not directly address functional outcomes after iterative episodes of critical illness. Future studies that combine transplant- and critical care-specific data are needed to better delineate subgroups of pediatric HCT patients at high-risk for both mortality as well as new or persistent functional morbidity after critical illness.
CONCLUSIONS
For pediatric HCT patients, PICU survival with new functional morbidity is as prevalent an outcome as PICU mortality. Relative to previously healthy age-, sex-, and PRISM-matched controls and previously healthy patients with LRTI, pediatric HCT patients continue to have elevated rates of PICU mortality. However, pediatric HCT patients who survive PICU admission have similar rates of new and recoverable functional impairment and thus should receive aggressive rehabilitation services aimed at maximizing recovery. Future interventions focused on improving functional status in pediatric HCT survivors of critical illness are warranted.
Highlights.
Survival with new functional morbidity is as prevalent an outcome as mortality.
New functional morbidity is recoverable in many patients.
Patients without functional recovery may be at-risk for in-hospital mortality.
Acknowledgments
Funding: This work was supported by the NIH NICHD K12HD000850 (Zinter), the Pediatric Blood and Marrow Transplant Foundation (Zinter), the National Marrow Donor Program Amy Strelzer Manasevit Grant (Zinter), and by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services: UG1HD050096, UG1HD049981, UG1HD049983, UG1HD063108, UG1HD083171, UG1HD083166, UG1HD083170 and U01HD049934.
We acknowledge the investigators of the Collaborative Pediatric Critical Care Research Network who partook in data acquisition that made this study possible. We also acknowledge our patients and families who participated in this study.
Footnotes
Disclosures: None
Contributions:
Study concept and design: Zinter, McQuillen
Acquisition of data: Zinter, Holubkov, Pollack
Analysis and interpretation of data: Zinter, Holubkov, Steurer, Dvorak, Duncan, Sapru, Tamburro, McQuillen, Pollack
Drafting of the manuscript: Zinter, McQuillen
Critical revision of the manuscript for important intellectual content: Zinter, Holubkov, Steurer, Dvorak, Duncan, Sapru, Tamburro, McQuillen, Pollack
Statistical analysis: Zinter, Holubkov, Steurer, McQuillen
Administrative, technical, or material support: Holubkov
Study supervision: Zinter, McQuillen
Approval of final manuscript: Zinter, Holubkov, Steurer, Dvorak, Duncan, Sapru, Tamburro, McQuillen, Pollack
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New Insights Into Multicenter PICU Mortality Among Pediatric Hematopoietic Stem Cell Transplant Patients. Crit Care Med. 2015;43:1986–1994. doi: 10.1097/CCM.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinter MS, DuBois SG, Spicer A, Matthay K, Sapru A. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. 2014;40:1536–1544. doi: 10.1007/s00134-014-3389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamburro RF, Barfield RC, Shaffer ML, Rajasekaran S, Woodard P, Morrison RR, et al. Changes in outcomes (1996–2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9:270–277. doi: 10.1097/PCC.0b013e31816c7260. [DOI] [PubMed] [Google Scholar]
- 4.van Gestel JP, Bollen CW, van der Tweel I, Boelens JJ, van Vught AJ. Intensive care unit mortality trends in children after hematopoietic stem cell transplantation: a meta-regression analysis. Crit Care Med. 2008;36:2898–2904. doi: 10.1097/CCM.0b013e318186a34a. [DOI] [PubMed] [Google Scholar]
- 5.Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015;41:1235–1246. doi: 10.1007/s00134-015-3780-7. [DOI] [PubMed] [Google Scholar]
- 6.Pollack MM, Holubkov R, Glass P, Dean JM, Meert KL, Zimmerman J, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009;124:e18–28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollack MM, Holubkov R, Funai T, Berger JT, Clark AE, Meert K, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015;43:1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polic B, Mestrovic J, Markic J, Mestrovic M, Capkun V, Utrobicic I, et al. Long-term quality of life of patients treated in paediatric intensive care unit. Eur J Pediatr. 2013;172:85–90. doi: 10.1007/s00431-012-1843-0. [DOI] [PubMed] [Google Scholar]
- 9.Jayshree M, Singhi SC, Malhi P. Follow up of survival and quality of life in children after intensive care. Indian Pediatr. 2003;40:303–309. [PubMed] [Google Scholar]
- 10.Butt W, Shann F, Tibballs J, Williams J, Cuddihy L, Blewett L, et al. Long-term outcome of children after intensive care. Crit Care Med. 1990;18:961–965. doi: 10.1097/00003246-199009000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Taylor A, Butt W, Ciardulli M. The functional outcome and quality of life of children after admission to an intensive care unit. Intensive Care Med. 2003;29:795–800. doi: 10.1007/s00134-003-1690-6. [DOI] [PubMed] [Google Scholar]
- 12.Ong C, Lee JH, Leow MK, Puthucheary ZA. Functional Outcomes and Physical Impairments in Pediatric Critical Care Survivors: A Scoping Review. Pediatr Crit Care Med. 2016;17:e247–59. doi: 10.1097/PCC.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 13.Pollack MM, Holubkov R, Funai T, Clark A, Berger JT, Meert K, et al. Pediatric intensive care outcomes: development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15:821–827. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack MM, Holubkov R, Funai T, Clark A, Moler F, Shanley T, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014;168:671–676. doi: 10.1001/jamapediatrics.2013.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan CN, Lehmann LE, Cheifetz IM, Greathouse K, Haight AE, Hall MW, et al. Clinical outcomes of children receiving intensive cardiopulmonary support during hematopoietic stem cell transplant. Pediatr Crit Care Med. 2013;14:261–267. doi: 10.1097/PCC.0b013e3182720601. [DOI] [PubMed] [Google Scholar]
- 16.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Pollack MM, Holubkov R, Funai T, Dean JM, Berger JT, Wessel DL, et al. The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med. 2016;17:2–9. doi: 10.1097/PCC.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamas A, Otheo E, Ros P, Vazquez JL, Maldonado MS, Munoz A, et al. Prognosis of child recipients of hematopoietic stem cell transplantation requiring intensive care. Intensive Care Med. 2003;29:91–96. doi: 10.1007/s00134-002-1549-2. [DOI] [PubMed] [Google Scholar]
- 19.Chima RS, Daniels RC, Kim MO, Li D, Wheeler DS, Davies SM, et al. Improved outcomes for stem cell transplant recipients requiring pediatric intensive care. Pediatr Crit Care Med. 2012;13:e336–42. doi: 10.1097/PCC.0b013e318253c945. [DOI] [PubMed] [Google Scholar]
- 20.Diaz MA, Vicent MG, Prudencio M, Rodriguez F, Marin C, Serrano A, et al. Predicting factors for admission to an intensive care unit and clinical outcome in pediatric patients receiving hematopoietic stem cell transplantation. Haematologica. 2002;87:292–298. [PubMed] [Google Scholar]
- 21.Ruhl AP, Huang M, Colantuoni E, Karmarkar T, Dinglas VD, Hopkins RO, et al. Healthcare utilization and costs in ARDS survivors: a 1-year longitudinal national US multicenter study. Intensive Care Med. 2017;43:980–991. doi: 10.1007/s00134-017-4827-8. [DOI] [PubMed] [Google Scholar]
- 22.Pfoh ER, Wozniak AW, Colantuoni E, Dinglas VD, Mendez-Tellez PA, Shanholtz C, et al. Physical declines occurring after hospital discharge in ARDS survivors: a 5-year longitudinal study. Intensive Care Med. 2016;42:1557–1566. doi: 10.1007/s00134-016-4530-1. [DOI] [PubMed] [Google Scholar]
- 23.Ward SL, Turpin A, Spicer AC, Treadwell MJ, Church GD, Flori HR. Long-Term Pulmonary Function and Quality of Life in Children After Acute Respiratory Distress Syndrome: A Feasibility Investigation. Pediatr Crit Care Med. 2017;18:e48–e55. doi: 10.1097/PCC.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM. Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatr Crit Care Med. 2017;18:e122–e130. doi: 10.1097/PCC.0000000000001070. [DOI] [PubMed] [Google Scholar]
- 25.Lawitschka A, Guclu ED, Varni JW, Putz M, Wolff D, Pavletic S, et al. Health-related quality of life in pediatric patients after allogeneic SCT: development of the PedsQL Stem Cell Transplant module and results of a pilot study. Bone Marrow Transplant. 2014;49:1093–1097. doi: 10.1038/bmt.2014.96. [DOI] [PubMed] [Google Scholar]
- 26.DeFilipp Z, Duarte RF, Snowden JA, Majhail NS, Greenfield DM, Miranda JL, et al. Metabolic Syndrome and Cardiovascular Disease after Hematopoietic Cell Transplantation: Screening and Preventive Practice Recommendations from the CIBMTR and EBMT. Biol Blood Marrow Transplant. 2016;22:1493–1503. doi: 10.1016/j.bbmt.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/PBMTC First International Conference on Late Effects after Pediatric Hematopoietic Cell Transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant. 2012;18:162–171. doi: 10.1016/j.bbmt.2011.12.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Gestel JP, Bierings MB, Dauger S, Dalle JH, Pavlicek P, Sedlacek P, et al. Outcome of invasive mechanical ventilation after pediatric allogeneic hematopoietic SCT: results from a prospective, multicenter registry. Bone Marrow Transplant. 2014 doi: 10.1038/bmt.2014.147. [DOI] [PubMed] [Google Scholar]
- 29.Heimall J, Buckley RH, Puck J, Fleisher TA, Gennery AR, Haddad E, et al. Recommendations for Screening and Management of Late Effects in Patients with Severe Combined Immunodeficiency after Allogenic Hematopoietic Cell Transplantation: A Consensus Statement from the Second Pediatric Blood and Marrow Transplant Consortium International Conference on Late Effects after Pediatric HCT. Biol Blood Marrow Transplant. 2017;23:1229–1240. doi: 10.1016/j.bbmt.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh AL, Patel P, Sweiss K, Chowdhery R, Dudek S, Rondelli D. Decreased pulmonary function in asymptomatic long-term survivors after allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant. 2016;51:283–285. doi: 10.1038/bmt.2015.216. [DOI] [PubMed] [Google Scholar]
- 31.Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15:817–826. doi: 10.1016/j.bbmt.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Bratton SL, Van Duker H, Statler KD, Pulsipher MA, McArthur J, Keenan HT. Lower hospital mortality and complications after pediatric hematopoietic stem cell transplantation. Crit Care Med. 2008;36:923–927. doi: 10.1097/01.CCM.0B013E318161FAC1. [DOI] [PubMed] [Google Scholar]
- 33.Naeem N, Reed MD, Creger RJ, Youngner SJ, Lazarus HM. Transfer of the hematopoietic stem cell transplant patient to the intensive care unit: does it really matter? Bone Marrow Transplant. 2006;37:119–133. doi: 10.1038/sj.bmt.1705222. [DOI] [PubMed] [Google Scholar]
- 34.Baumann FT, Kraut L, Schule K, Bloch W, Fauser AA. A controlled randomized study examining the effects of exercise therapy on patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:355–362. doi: 10.1038/bmt.2009.163. [DOI] [PubMed] [Google Scholar]
- 35.Wieczorek B, Ascenzi J, Kim Y, Lenker H, Potter C, Shata NJ, et al. PICU Up!: Impact of a Quality Improvement Intervention to Promote Early Mobilization in Critically Ill Children. Pediatr Crit Care Med. 2016;17:e559–e566. doi: 10.1097/PCC.0000000000000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider DT, Lemburg P, Sprock I, Heying R, Gobel U, Nurnberger W. Introduction of the oncological pediatric risk of mortality score (O-PRISM) for ICU support following stem cell transplantation in children. Bone Marrow Transplant. 2000;25:1079–1086. doi: 10.1038/sj.bmt.1702403. [DOI] [PubMed] [Google Scholar]
- 37.Demaret P, Pettersen G, Hubert P, Teira P, Emeriaud G. The critically-ill pediatric hemato-oncology patient: epidemiology, management, and strategy of transfer to the pediatric intensive care unit. Ann Intensive Care. 2012;2 doi: 10.1186/2110-5820-2-14. 14-5820-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]