Abstract
Artemisinin resistance in Plasmodium falciparum threatens the remarkable efficacy of artemisinin-based combination therapies worldwide. Thus, greater insight into the resistance mechanism using monitoring tools is essential. The ring-stage survival assay is used for phenotyping artemisinin-resistance or decreased artemisinin sensitivity. Here, we review the progress of this measurement assay and explore its limitations and potential applications.
Keywords: Malaria, Artemisinin, Resistant phenotype, Ring-stage survival assay, Improvement, Application
INTRODUCTION
Malaria, which is mainly caused by Plasmodium falciparum, is a long-term worldwide public health problem. An estimated 216 million new cases occurred globally in 2016, resulting in 445 000 deaths (WHO, 2017). Despite significant progress in reducing morbidity and mortality rates in many areas of endemicity, drug resistance has become a challenging issue. Since P. falciparum developed resistance to chloroquine and sulfadoxine-pyrimethamine, malaria has spread rampantly throughout Asia and Africa over the last several decades (Snow et al., 2001; Trape et al., 1998), posing serious difficulties for its control and elimination.
Originally discovered in China, artemisinin (ART) and its derivatives, including dihydroartemisinin (DHA), artemether, and artesunate, demonstrate high performance, low toxicity, and limited cross-resistance with other antimalarial drugs (Li et al., 1979; Miller & Su, 2011). ART is at the frontline for the treatment and possible cure of malaria (Fairhurst, 2015); however, along with its global application, resistance to ART has developed and increased in many regions. Since its first detection in 2008 (Noedl et al., 2008) and 2009 (Dondorp et al., 2009) in western Cambodia, ART resistance has appeared successively in other countries of the Greater Mekong Subregion, manifesting with a reduced parasite clearance rate or prolonged in vivo parasite clearance time following treatment with ART-based combination therapies (ACTs) (Amaratunga et al., 2012; Ashley et al., 2014; Hien et al., 2012; Huang et al., 2015; Kyaw et al., 2013; Phyo et al., 2012). For many decades, Southeast Asia (SEA) has been an epicenter for the evolution of drug-resistant falciparum malaria, and the emergence of ART resistance in SEA is of great concern for the global control of falciparum malaria (Fairhurst, 2015).
RING-STAGE SURVIVAL ASSAY
Hidden within ART-resistant parasites is the ability to remain dormant in the ring stage after exposure to ART, as well as recovery at a rapid rate, resulting in numerous parasites enduring DHA-exposed dormancy (Codd et al., 2011; Teuscher et al., 2010). Due to these special characteristics, despite substantial reductions in the clinical response to ART observed in falciparum malaria, in vitro concentrations resulting in 50% growth inhibition in a conventional 48 h exposure assay were relatively low and did not contribute to slow parasite clearance or ACT failure (Dondorp et al., 2009; Saralamba et al., 2011; Woodrow & White, 2017). It is, therefore, necessary to implement rapid and exact monitoring to halt the further spread of ART-resistance.
Hence, the ring-stage survival assay (RSA) was recently established as a new protocol in the surveillance of ART resistance, and can distinguish culture-adapted isolates with fast clearance or slow-clearing rates that can survive pharmacologically relevant doses of ART (Dondorp et al., 2009; Witkowski et al., 2013a). Previous therapeutic efficacy studies have demonstrated a clear correlation between RSA in vitro and day 3 parasitemia positivity as well as mutations in the Kelch domain gene (K13) associated with resistance (Woodrow & White, 2017; Ariey et al., 2014; Wang et al., 2015; Zhang et al., 2016). Current data have shown RSA to be an important assay for ART resistance in vitro.
In RSA, young ring-stage parasite cultures (0-3 h), tightly synchronized by 5% sorbitol, are exposed to 700 nmol/L DHA or 0.1% dimethyl sulfoxide (DMSO) as controls for 6 h, then cultivated for 66 h after twice or thrice drug washing. At the end of the assay, survival rates of these isolates are calculated as the ratio under the microscope of viable parasites surviving DHA-induced incubation relative to initial conditions (<a href="http://www.wwarn.org/tools-resources/procedures/ring-stage-survival-assays-rsa-evaluate-vitro-and-ex-vivo-susceptibility" target="_blank">http://www.wwarn.org/tools-resources/procedures/ring-stage-survival-assays-rsa-evaluate-vitro-and-ex-vivo-susceptibility</a>). In general, a ≥1% survival rate is defined as an ART-resistant strain (<xref ref-type="fig" rid="F1-ZoolRes-38-6-317">Figure 1</xref>).
Figure 1.
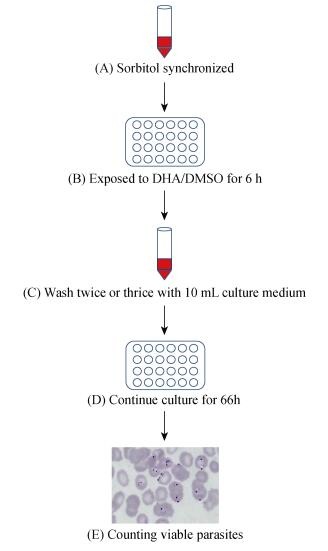
Schematic representation of the ring-stage survival assay in vitro
This method can decrease interference caused by the internal variables of the host, such as organism immunity level, allosteric effects of hemoglobin, and capability to metabolize drugs (Amaratunga et al., 2012; Witkowski et al., 2013a). RSA in vitro is proposed to give phenotypic information, thus enabling screening for reduced susceptibility to ART in prolonged clearance parasites (Witkowski et al., 2013b).
IMPROVED METHODS FOR RSA
While feasible and efficient for the surveillance of ART resistance, the RSA tool has several limitations, including sophisticated Percoll gradient centrifugation, biased assessment of the degree of sorbitol synchronization treatment, and exacting requirements for counting viable parasites (Witkowski et al., 2013a). In Whitney A. Kite's laboratory, two alternative RSA methods have been developed; that is, filtration ring-stage survival assay and sorbitol-only ring-stage survival assay. The first is essentially a filtration process in which the 0-3 h fresh post-invasion rings are obtained after filtering out the merozoites from mature forms. The latter assay performs a repeated step of high synchronization prior to measurement, with the remaining late-stage schizonts typically removed, except for the early rings. Compared with the standard RSA protocol, these modifications have shown a marked increase in phase-specificity as well as less time in culture, fewer lab resources, and lower volume of isolates (Kite et al., 2016). In addition, to limit the inherent variability of microscopic examination, Amaratunga et al. (2014) developed a quick and simple bi-color flow cytometric assay -RSA-2FACS and MitoTracker deep red FM (MTDR) -to accurately quantify observations of viable parasites applied to the RSA. In their study, mitochondrial DNA is readily dyed using the Mito Tracker deep red FM method, allowing for the selection of viable parasites from pyknotic strains (Amaratunga et al., 2014). In addition, Dogovski et al. (2015) suggested direct assessment of the drug-induced growth effects in western Cambodian parasites. For this, the RNA-binding dye SYTO-61, which can distinguish isolates in different stages, was used as a fluorescent marker to determine whether parasites that survived DHA exposure exhibited growth retardation. By comparison with no-drug controls, the decreased SYTO-61 signal in the drug-treated samples exhibited an absolute increase in the number of viable parasites (Dogovski et al., 2015).
PROSPECT AND APPLICATION OF RSA
The traditional RSA approach was first carried out by Witkowski and colleagues based on sensitivity to DHA exposure at different stages. Their results demonstrated that median ring-stage survival of laboratory lines collected in western Cambodia with slow-clearing infection was 47-fold higher than those with normal ART sensitivity in RSA0-3h (0.23 and 10.88%, respectively), whereas no significant differences were observed in RSA9-12h or trophozoite-stage survival assay (TSA18-21h) rates (Witkowski et al., 2013a). In contrast, Cui et al. (2012) demonstrated that the ART resistance phenotype was associated with the dormancy mechanism not only at the development of the ring stage, but also in trophozoites and schizonts (Cui et al., 2012). Thus, the different consequences involved in the characteristics of this phenotype require additional empirical evidence. Cooper et al. (2015) documented that DHA susceptibility using the standard RSA based on the IC50 value for ART failed to clarify resistance in P. falciparum parasites from Kampala, Uganda, with the parasitemia of almost all isolates dropping to a much lower level (≤0.025%) after the 72-h assay, revealing no sign of ART resistance. Susceptibility to ART in Cameroonian isolates was also identified using ex-vivo RSA, with the DHA-treated cultures showing almost no healthy-appearing parasites (median survival rate=0.49%, IQR=0% to 1.3%) (Menard et al., 2016). However, reduced ART drug in-vitro sensitivity of parasites from the China-Myanmar border was reported after assessment of early ring-stage survival by comparing 34 clinical isolates with the 3D7 reference standard strain (Zhang et al., 2016).
The RSA0-3h assay was recently developed to test ART resistance for P. falciparum isolates. This assay has been subsequently improved in terms of simplicity and practicality. Thus, the growing availability of RSA will increase the convenience and ease of investigating ART responses in laboratory testing.
Funding Statement
This study was funded by the National Natural Science Foundation of China (31260508, U1202226) and grants of Doctor Newcomer Scholarship from Yunnan province
REFERENCES
- 1. Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, Zhou CJ, Mao S, Anderson JM, Lindegardh N, Jiang HY, Song JP, Su XZ, White NJ, Dondorp AM, Anderson TJC, Fay MP, Mu JB, Duong S, Fairhurst RM. 2012. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. The Lancet Infectious Diseases, 12 (11): 851- 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amaratunga C, Neal AT, Fairhurst RM. 2014. Flow cytometry-based analysis of artemisinin-resistant Plasmodium falciparum in the ring-stage survival assay. Antimicrobial Agents and Chemotherapy, 58 (8): 4938- 4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, Lim P, Leang R, Duong S, Sreng S, Suon S, Chuor CM, Bout DM, Ménard S, Rogers WO, Genton B, Fandeur T, Miotto O, Ringwald P, Le Bras J, Berry A, Barale JC, Fairhurst RM, Benoit-Vical F, Mercereau-Puijalon O, Ménard D. 2014. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature, 505 (7481): 50- 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Mao S, Sam B, Sopha C, Chuor CM, Nguon C, Sovannaroth S, Pukrittayakamee S, Jittamala P, Chotivanich K, Chutasmit K, Suchatsoonthorn C, Runcharoen R, Hien TT, Thuy-Nhien NT, Thanh NV, Phu NH, Htut Y, Han KT, Aye KH, Mokuolu OA, Olaosebikan RR, Folaranmi OO, Mayxay M, Khanthavong M, Hongvanthong B, Newton PN, Onyamboko MA, Fanello CI, Tshefu AK, Mishra N, Valecha N, Phyo AP, Nosten F, Yi P, Tripura R, Borrmann S, Bashraheil M, Peshu J, Faiz MA, Ghose A, Hossain MA, Samad R, Rahman MR, Hasan MM, Islam A, Miotto O, Amato R, MacInnis B, Stalker J, Kwiatkowski DP, Bozdech Z, Jeeyapant A, Cheah PY, Sakulthaew T, Chalk J, Intharabut B, Silamut K, Lee SJ, Vihokhern B, Kunasol C, Imwong M, Tarning J, Taylor WJ, Yeung S, Woodrow CJ, Flegg JA, Das D, Smith J, Venkatesan M, Plowe CV, Stepniewska K, Guerin PJ, Dondorp AM, Day NP, White NJ. 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. The New England Journal of Medicine, 371 (5): 411- 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Codd A, Teuscher F, Kyle DE, Cheng Q, Gatton ML. 2011. Artemisinin-induced parasite dormancy: a plausible mechanism for treatment failure. Malaria Journal, 10 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper RA, Conrad MD, Watson QD, Huezo SJ, Ninsiima H, Tumwebaze P, Nsobya SL, Rosenthal PJ. 2015. Lack of artemisinin resistance in Plasmodium falciparum in Uganda based on parasitological and molecular Assays. Antimicrobial Agents and Chemotherapy, 59 (8): 5061- 5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui L, Wang ZL, Miao J, Miao M, Chandra R, Jiang HY, Su XZ, Cui LW. 2012. Mechanisms of in vitro resistance to dihydroartemisinin in Plasmodium falciparum. Molecular Microbiology, 86 (1): 111- 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dogovski C, Xie SC, Burgio G, Bridgford J, Mok S, McCaw JM, Chotivanich K, Kenny S, Gnädig N, Straimer J, Bozdech Z, Fidock DA, Simpson JA, Dondorp AM, Foote S, Klonis N, Tilley L. 2015. Targeting the cell stress response of Plasmodium falciparum to overcome artemisinin resistance. PLoS Biology, 13 (4): e1002132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NPJ, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. The New England Journal of Medicine, 361 (5): 455- 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fairhurst RM. 2015. Understanding artemisinin-resistant malaria: what a difference a year makes. Current Opinion in Infectious Diseases, 28 (5): 417- 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hien TT, Thuy-Nhien NT, Phu NH, Boni MF, Thanh NV, Nha-Ca NT, Thai LH, Thai CQ, Toi PV, Thuan PD, Long LT, Dong LT, Merson L, Dolecek C, Stepniewska K, Ringwald P, White NJ, Farrar J, Wolbers M. 2012. In vivo susceptibility of Plasmodium falciparum to artesunate in Binh Phuoc Province, Vietnam. Malaria Journal, 11 355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang F, Takala-Harrison S, Jacob CG, Liu H, Sun XD, Yang HL, Nyunt MM, Adams M, Zhou SS, Xia ZG, Ringwald P, Bustos MD, Tang LH, Plowe CV. 2015. A single mutation in K13 predominates in southern china and is associated with delayed clearance of Plasmodium falciparum following artemisinin treatment. Journal of Infectious Diseases, 212 (10): 1629- 1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kite WA, Melendez-Muniz VA, Moraes Barros RR, Wellems TE, Sá JM. 2016. Alternative methods for the Plasmodium falciparum artemisinin ring-stage survival assay with increased simplicity and parasite stage-specificity. Malaria Journal, 15 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kyaw MP, Nyunt MH, Chit K, Aye MM, Aye KH, Aye MM, Lindegardh N, Tarning J, Imwong M, Jacob CG, Rasmussen C, Perin J, Ringwald P, Nyunt MM. 2013. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS One, 8 (3): e57689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Yu PL, Chen YX, Li LQ, Gai YZ, Wang DS, Zheng YP. 1979. Synthesis of some derivatives of artemisinine. Chinese Science Bulletin, 24 (14): 667- 669. (in Chinese) [Google Scholar]
- 16. Menard S, Tchoufack JN, Maffo CN, Nsango SE, Iriart X, Abate L, Tsapi MT, Awono-Ambéné PH, AbegaMekongo FA, Morlais I, Berry A. 2016. Insight into k13-propeller gene polymorphism and ex vivo DHA-response profiles from Cameroonian isolates. Malaria Journal, 15 (1): 572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller LH, Su XZ. 2011. Artemisinin: discovery from the Chinese herbal garden. Cell, 146 (6): 855- 858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. The New England Journal of Medicine, 359 (24): 2619- 2620. [DOI] [PubMed] [Google Scholar]
- 19. Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, ler Moo C, Al-Saai S, Dondorp AM, Lwin KM, Singhasivanon P, Day NPJ, White NJ, Anderson TJC, Nosten F. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. The Lancet, 379 (9830): 1960- 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saralamba S, Pan-Ngum W, Maude RJ, Lee SJ, Tarning J, Lindegårdh N, Chotivanich K, Nosten F, Day NPJ, Socheat D, White NJ, Dondorp AM, White LJ. 2011. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proceedings of the National Academy of Sciences of the United States of America, 108 (1): 397- 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Snow RW, Trape JF, Marsh K. 2001. The past, present and future of childhood malaria mortality in Africa. Trends in Parasitology, 17 (12): 593- 597. [DOI] [PubMed] [Google Scholar]
- 22. Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. 2010. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. The Journal of Infectious Diseases, 2002 (9): 1362- 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trape JF, Pison G, Preziosi MP, Enel C, du Loû AD, Delaunay V, Samb B, Lagarde E, Molez JF, Simondon F. 1998. Impact of chloroquine resistance on malaria mortality. Comptes Rendusde l'Académie Des Sciences-Series Ⅲ-Sciences de la Vie, 321 (8): 689- 697. [DOI] [PubMed] [Google Scholar]
- 24. Wang ZL, Wang YN, Cabrera M, Zhang YM, Gupta B, Wu YR, Kemirembe K, Hu Y, Liang XY, Brashear A, Shrestha S, Li XL, Miao J, Sun XD, Yang ZQ, Cui LW. 2015. Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrobial Agents and Chemotherapy, 59 (11): 6952- 6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. WHO. 2017. World Malaria Report 2017. Geneva, Switzerland: World Health Organization, [Google Scholar]
- 26. Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, Lim P, Mao S, Sopha C, Sam B, Anderson JM, Duong S, Chuor CM, Taylor WRJ, Suon S, Mercereau-Puijalon O, Fairhurst RM, Menard D. 2013a. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. The Lancet. Infectious Diseases, 13 (12): 1043- 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, Chy S, Duong S, Leang R, Ringwald P, Dondorp AM, Tripura R, Benoit-Vical F, Berry A, Gorgette O, Ariey F, Barale JC, Mercereau-Puijalon O, Menard D. 2013b. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrobial Agents and Chemotherapy, 57 (2): 914- 923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woodrow CJ, White NJ. 2017. The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiology Reviews, 41 (1): 34- 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang YM, Wu YR, Hu Y, Wang LQ, Ruan YH, Ma N, Li SM, Wang YN, Jia DD, Xiang Z, Yang ZQ. 2016. In vitro susceptibility of Plasmodium falciparum to artemisinin drugs and K13-Propeller polymorphisms at the China-Myanmar border. Chinese Journal of Zoonoses, 32 (3): 219- 223. (in Chinese) [Google Scholar]


