Abstract
Lymphangioleiomyomatosis (LAM) is a rare and progressive neoplastic disease of young woman, characterized by the proliferation of abnormal smooth muscle-like cells (LAM cells) in the lungs and axial lymphatics. A 44-year-old woman was referred to our hospital because pleural effusion was detected during a health checkup. She had chylothorax, chylous ascites, and chyluria, and her computed tomography scan showed a solid tumor in the pelvis. Surgical biopsy was performed; she was diagnosed as having LAM. We could not control the fluid collection and chyluria using standard medical treatments. Therefore, we chose to administer sirolimus, and her symptoms dramatically improved. The mechanism of chyluria presumably involved LAM cell infiltrates in the ureter via the lymphatic vessel flow, which causes LAM to develop because of ureter wall exposure.
Keywords: Lymphangioleiomyomatosis, Chylothorax, Chylous ascites, Chyluria, Sirolimus, Ezetimibe
1. Introduction
Lymphangioleiomyomatosis (LAM) is a rare disease that affects 3.4–7.8 persons per 1 million [1], and it occurs exclusively in women of reproductive age [2]. Pathologically, it is characterized by the proliferation of abnormal smooth muscle-like cells (LAM cells) in the lungs and axial lymphatics, including the mediastinum and retroperitoneum, and lymphangiogenesis in the lesion [3]. Patients with LAM often develop respiratory symptoms and signs, such as exertional dyspnea, pneumothorax, and hemosputum [4]. Approximately 15% of patients with this disease have been diagnosed based on the presence of chylothorax, chylous ascites, renal angiomyolipomas, and lymphangioleiomyomatosis of the retroperitoneum and pelvis [4].
We herein describe our experience with one patient with LAM who had chylous ascites and chyluria due to LAM cells infiltrating the ureter. Her condition remarkably improved after administering sirolimus.
2. Case report
A 44-year-old woman was found to have pleural effusion on a chest radiograph during an annual health checkup in July 2016. Her computed tomography (CT) scan showed pleural effusion, ascites fluid, and a nodular lesion of the pelvis; therefore, we suspected peritoneal cancer. There was no familial history of tuberous sclerosis complex. She had a history of tonsillectomy at 28 years of age and lumbar disc herniation at 36 years of age.
The physical examination at the initial visit revealed that her height was 156 cm, body weight was 48.6 kg, body temperature was 36.1 °C, blood pressure was 104/62 mmHg, radial pulse rate was 80/min, and respiratory rate was 18/min. No superficial lymphadenopathy was identified on palpation. She had neither anemia nor jaundice. Auscultations of her heart and lung sound were normal. The abdomen was slightly distended, which indicated the presence of ascites.
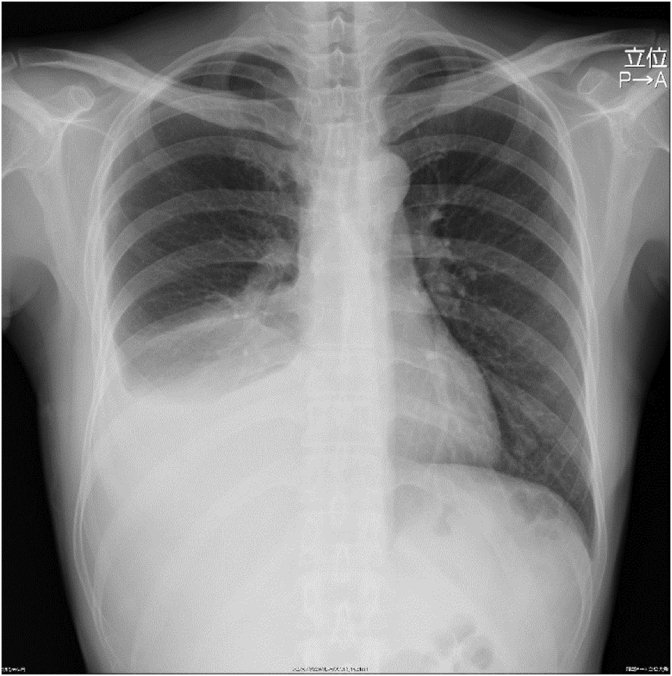
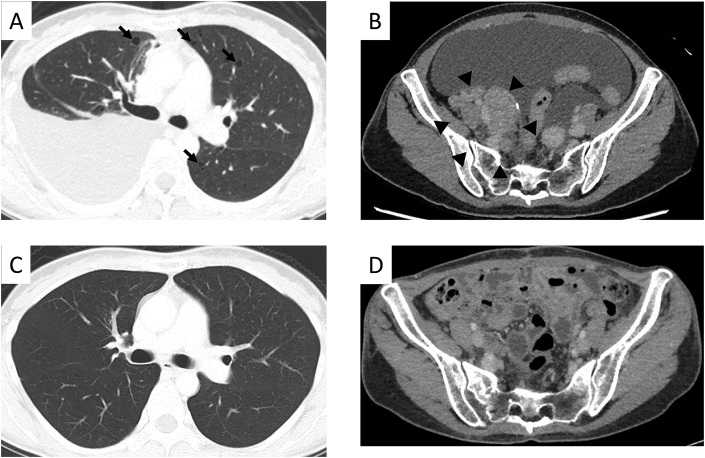
Results of the biochemical examination of blood at the initial visit were normal, except for the hemoglobin level of 10.5 g/dL (reference range 11.6–14.8 g/dL). Regarding tumor markers, the carbohydrate antigen 125 level was 628 U/mL (reference range 0.0–35.0 U/mL). As for results of the pulmonary function test, percentage vital capacity (%VC) and 1% forced expiratory volume (FEV1.0%) were within normal range (%VC: 83.8%; FEV1.0%: 98.4%), but the percentage diffusing capacity of the lung carbon monoxide (%DLco) and percentage diffusing capacity of the lung carbon monoxide/alveolar ventilation (%DLco/VA) were slightly decreased (%DLco: 76.0%; %DLco/VA: 72.0%). The chest radiograph revealed right pleural effusion with mild to moderate retention (Fig. 1), and the chest CT scan showed moderate accumulation of the right pleural effusion with multiple thin-walled cysts in both lung fields (Fig. 2A). The enhanced abdominal CT scan demonstrated ascites and slightly heterogeneous tumorous lesions of the right pelvis (Fig. 2B). However, there was no evidence of any renal angiomyolipomas. Even the magnetic resonance imaging scan of the pelvic area showed similar findings.
Fig. 1.
Radiological findings on admission. Chest radiograph revealing right pleural effusion.
Fig. 2.
Computed tomography (CT) scans (1.25-mm slice) on admission (A, B) and after 2 months of sirolimus treatment (C, D). A: Chest CT scan revealing moderate accumulation of the right pleural effusion and multiple thin-walled cysts (arrows) in both lung fields. B: Enhanced abdominal CT scan demonstrating ascites and a solid tumor (arrowheads) in the right pelvis. C: Chest CT scan revealing that the multiple small cysts in both lungs are stable and pleural effusion retention is decreased. D: Enhanced abdominal CT scan showing that the quantity of ascites fluid and size of the tumor lesion are smaller.
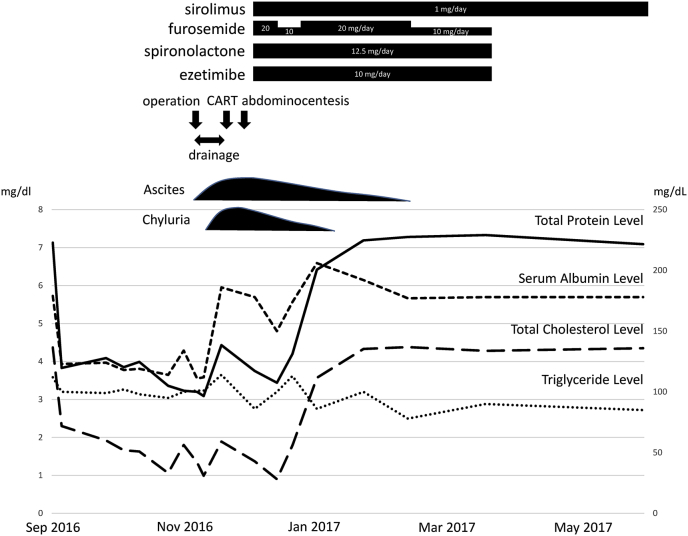
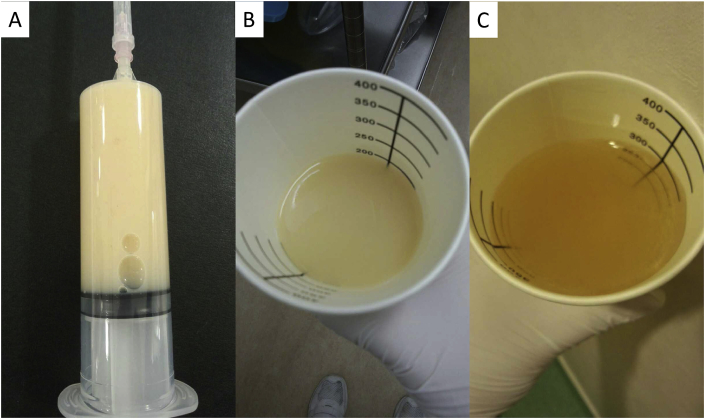
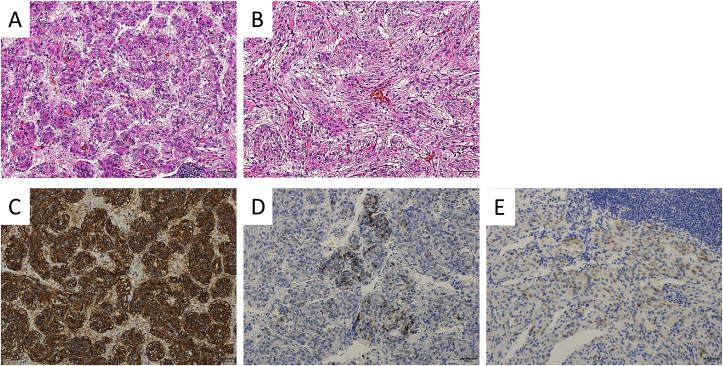
The patient's clinical course is presented in Fig. 3. She did not have an appetite because of the presence of ascites fluid with severe retention at the initial visit. The ascites fluid was chylous ascites (Fig. 4A). Despite the use of diuretics (furosemide and spironolactone), albumin therapy, abdominocentesis, and cell-free and concentrated ascites reinfusion therapy, we could not control the ascites fluid, and the patient developed significant hypoproteinemia and hypoalbuminemia. In November, enucleation of the right retroperitoneal tumor was performed. According to the histopathological examination, the cell that had a kind of circular nucleus from a uniform circle in acidophilic cytoplasm stained by hematoxylin-eosin formed a strand from a vacuole nest and multiplied (Fig. 5A). Additionally, a part of the same type of cell became spindle-shaped and was complex (Fig. 5B). The immunohistochemical stain was positive for smooth muscle actin (Fig. 5C), HMB45 (Fig. 5D), and MelanA (Fig. 5E), leading to the diagnosis of LAM. Postoperatively, we noticed that chyluria (Fig. 4B) had worsened, and a diagnosis of nephrotic syndrome was made (daily urinary protein level: 11,258 mg/day; daily urinary albumin level: 4675.45 mg/day). There was no oval fat body or fatty cast with urinary sediment, so the cause of nephrotic syndrome was regarded as chyluria due to lymphatic vessel obstruction with LAM, not a glomerular abnormality. Although lymphangiography was performed to confirm the position of the fistula in the thoracic duct, we could not detect it. We hypothesized that stenosis or occlusion of the lymphatic vessel by tumor invasion within the pelvis would exist.
Fig. 3.
Patient's clinical course.
Fig. 4.
Photographs before and after treatment. A: Photograph of the chylous ascites. B: Photograph of chyluria. C: Photograph of the transparent urine.
Fig. 5.
Results of the histopathological examination. A: The cell that had a kind of circular nucleus from a uniform circle in stained acidophilic cytoplasm forms a strand from a vacuole nest and multiplies (hematoxylin-eosin stain [HE], ×200). B: A part of the same type of cell became spindle-shaped and complex (HE, ×200). C–E: The immunohistochemical stain is positive for smooth muscle actin (C), HMB45 (D), and MelanA (E).
Next, the oral administration of 1 mg/day each of sirolimus and ezetimibe, which is a small intestine cholesterol transporter inhibitor, was started. After 10 days of treatment with sirolimus, her urine became transparent (Fig. 4C), and hypoalbuminemia improved dramatically. Twenty days later, ascites fluid retention decreased, and pitting edema of the extremities improved; thus, she was discharged.
Two months later, the serum albumin level became within normal range, and the enhanced abdominal CT scan revealed that the quantity of ascites fluid and size of the tumor lesion were smaller (Fig. 2D). Additionally, the multiple small cysts in both lungs were stable on the chest CT scan (Fig. 2C). Ten months later, %VC and FEV1.0% were unchanged (%VC: 92.4%; FEV1.0%: 108.6%), but %DLco and %DLco/VA were improved (%DLco: 92.0%; %DLco/VA: 74.8%). Currently, she has experienced no adverse events with sirolimus.
3. Discussion
The lymphatic vessels from both lower limbs, genital organs, and organs in the pelvis merge at the first and second lumbar heights after the aorta, and form the cisterna chyli. Then, the lymphatic vessels shift in the thoracic cavity from the aortic hiatus and vein corner to vein circulation. Fifty to 90% of the lymph flow of the cisterna chyli comes from the intestinal tract and liver [5]. Furthermore, the lymph flow increases by 200 times with the intake of fatty food [6]. It seems that chyle lymph is cloudy macroscopically, because emulsified fat is abundant and the principal ingredient is protein and fat, particularly glyceride (chylomicron) [7].
The cause of chyluria is impaired circulation of lymph flow due to various causes, including filariasis, a congenital anomaly, traumatic injury, surgical procedure, and the presence of a tumor and aneurysm [8], [9]. Regarding the mechanism of chyluria, it is thought that some of the chyle lymph flows backward in the kidney lymph system near the cisterna chyli and becomes mixed with the urine due to a failed part of the renal calyx or renal pelvis [10]. For example, McCormick and colleagues reported that chyluria occurred because of abnormal lymphatic flow and reflux due to a congenital anomaly [11]. In contrast, since LAM cells may infiltrate fatty tissue around the retroperitoneum from the retroperitoneal lymph node, it is considered that LAM cells infiltrate a ureter, which is a retroperitoneal organ, through the lymphatic vessel flow, and chyluria occurs by lymphatic vessel flow, which causes LAM to develop due to ureter wall exposure [12]. In the present patient, we thought that LAM cells of the tumor in the right pelvis infiltrated the right ureter wall, and this infiltration induced chyluria. Additionally, chyluria may occur naturally and can develop or be exacerbated by surgical stress after the abdominal cavity lymph system is damaged [13]. The mechanism of chyluria in our patient was considered the same as that in a previous case report [14]; chyluria developed postoperatively, the lymph system was damaged by surgical stress, and lymph flow was occluded.
Pseudomenopause therapy, such as GnRH therapy and bronchodilatation therapy, for chronic obstructive pulmonary disease has been used previously with the treatment for LAM [15]. However, with recent clarification of the pathophysiology, it was recognized that the mammalian target of rapamycin plays an important role in the increase of LAM cells [16], [17]. Moreover, it was shown that sirolimus, which was the repressor, could become the molecular target drug [18]. The effectiveness and safety of sirolimus for LAM have been reported by large-scale clinical trials, such as the CAST trial [18] of 2009 and Multicenter International LAM Efficacy of Sirolimus Trial [19] of 2011, and it was assumed that sirolimus had an effect on the index of breathing function. Furthermore, Taveira-DaSilva and colleagues reported that sirolimus is effective for chyluria with LAM, and its effect occurs early [20]. Additionally, Ando et al. reported that after giving low-dose sirolimus to three patients with chylothorax and chylous ascites complicated with LAM, chylothorax in two patients completely disappeared and chylous ascites decreased [21]. There is no definitive treatment for chyluria with LAM. The treatment of chyluria generally includes a low-fat meal and medium-chain triglyceride diet as noninvasive treatment, and instillation of sclerosing solutions (such as silver nitrate) into the renal pelvis and surgical or retroperitoneoscopic renal pedicle lymphatic disconnection as invasive treatment [8], [9]. Additionally, Contini and colleagues reported that estrogen-suppressive therapy with triptorelin, a synthetic analogue of Gn-RH, is effective for chyluria with LAM [22].
In the current patient, chylothorax, chylous ascites, and chyluria improved after oral low-dose sirolimus (1 mg/day) was taken immediately, and reaccumulation was not shown during the 4-month follow-up. Moreover, we thought that we reduced fat absorption in the intestinal tract by administering ezetimibe, which inhibits a cholesterol transporter (Niemann-Pick C1 like 1) that selectively emerges on the surface of epithelium cells in the proximal small intestine, and it controls cholesterol absorption with the consumption of a low-fat meal [23]. There is no report about the effectiveness of ezetimibe for chyluria with LAM, and it is unknown how ezetimibe contributed to this patient's clinical course. However, it is thought that the administration of ezetimibe reduces intralymphatic pressure; Tanaka et al. reported [23] that ezetimibe partially contributed to the improvement of chyluria. In our patient, although the internal use of ezetimibe was discontinued because sirolimus was started 2 months later, there has been no relapse of chyluria.
Chyluria is a rare symptom of LAM, which we consider the mechanism of chyluria. In the future, it is very important to accumulate information on patients similar to ours and to discuss the mechanism of chyluria to improve patients' clinical course.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Acknowledgements
The authors thank Dr. Kuniaki Seyama (Division of Respiratory Medicine, Juntendo University Faculty of Medicine and Graduate School of Medicine) for providing useful advice about the patient’ treatment.
References
- 1.Harknett E.C., Chang W.Y., Byrnes S., Johnson J., Lazor R., Cohen M.M., Gray B., Geiling S., Telford H., Tattersfield A.E., Hubbard R.B., Johnson S.R. Use of variability in national and regional data to estimate the prevalence of lymphangioleiomyomatosis. QJM. 2011;104:971–979. doi: 10.1093/qjmed/hcr116. [DOI] [PubMed] [Google Scholar]
- 2.Taylor J.R., Ryu J., Colby T.V., Raffin T.A. Lymphangioleiomyomatosis. Clinical course in 32 patients. N. Engl. J. Med. 1990;323:1254–1260. doi: 10.1056/NEJM199011013231807. [DOI] [PubMed] [Google Scholar]
- 3.Seyama K., Kumasaka T., Kurihara M., Mitani K., Sato T. Lymphangioleiomyomatosis: a disease involving the lymphatic system. Lymphatic Res. Biol. 2010;8:21–31. doi: 10.1089/lrb.2009.0018. [DOI] [PubMed] [Google Scholar]
- 4.Seyama K., Ando K., Hoshika Y., Suzuki Y., Takekawa H. Current understanding and perspectives of lymphangioleiomyomatosis. Nippon Rinsho. 2013;71:1103–1108. [PubMed] [Google Scholar]
- 5.Kuroda S., Aoki H., Shiozaki S., Harano M., Onoda T., Ohno S., Higaki K., Ninomiya M., Takakura N. Clinical study on chylous acites following hepato-pancreatic surgery. Jpn. J. Gastroenterol. Surg. 2006;39:631–636. [Google Scholar]
- 6.Leibovitch I., Mor Y., Golomb J., Ramon J. The diagnosis and management of postoperative chylous ascites. J. Urol. 2002;167:449–457. doi: 10.1016/S0022-5347(01)69064-5. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki R., Morita H., Sugenoya Y., Mizobuchi M., Yamamoto W., Ideura T., Yoshimura A. A case report of chronic chyluria probably due to Bancroftian filariasis, which showed hypoproteinemia. Nihon Jinzo Gakkai Shi. 2001;43:63–68. [PubMed] [Google Scholar]
- 8.Cheng J.T., Mohan S., Nasr S.H., D'Agati V.D. Chyluria presenting as milky urine and nephrotic-range proteinuria. Kidney Int. 2006;70:1518–1522. doi: 10.1038/sj.ki.5001703. [DOI] [PubMed] [Google Scholar]
- 9.Graziani G., Cucchiari D., Verdesca S., Balzarini L., Montanelli A., Ponticelli C. Chyluria associated with nephrotic-range proteinuria. pathophysiology, clinical picture and therapeutic options. Nephron Clin. Pract. 2011;119:c248–c254. doi: 10.1159/000329154. [DOI] [PubMed] [Google Scholar]
- 10.Koga S., Arakaki Y., Matsuoka M., Ohyama C. Clinical research on chyluria. Jpn. J. Limnol. 1985;8:51–53. [Google Scholar]
- 11.McCormick A., Rosenberg S., Tier K., Balest A. A case of a central conducting lymphatic anomaly responsive to sirolimus. Pediatrics. 2016;137 doi: 10.1542/peds.2015-2694. [DOI] [PubMed] [Google Scholar]
- 12.Kumasaka T. Pathology and pathophysiology of lymphangioleiomyomatosis. Kokyuu. 2009;28:679–688. [Google Scholar]
- 13.Cardenas A., Chopra S. Chylous ascites. Am. J. Gastroenterol. 2002;97:1896–1900. doi: 10.1111/j.1572-0241.2002.05911.x. [DOI] [PubMed] [Google Scholar]
- 14.Makino Y., Shimanuki Y., Fujiwara N., Morio Y., Sato K., Yoshimoto J., Gunji Y., Suzuki T., Sasaki S., Iwase A., Kawasaki S., Takahashi K., Seyama K. Peritoneovenous shunting for intractable chylous ascites complicated with lymphangioleiomyomatosis. Intern. Med. 2008;47:281–285. doi: 10.2169/internalmedicine.47.0475. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi H., Bando M., Yoshizumi N., Kogawara H., Yamasawa H., Sugiyama Y. A case of lymphangioleiomyomatosis with refractory chylous pleural effusions and ascites that was treated with sirolimus. Annal. Jpn Respir. Soc. 2016;5:101–105. [Google Scholar]
- 16.Taveira-DaSilva A.M., Moss J. Management of lymphangioleiomyomatosis. F1000Prime Rep. 2014;6:116. doi: 10.12703/P6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henske E.P., McCormack F.X. Lymphangioleiomyomatosis - a wolf in sheep's clothing. J. Clin. Invest. 2012;122:3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissler J.J., McCormack F.X., Young L.R., Elwing J.M., Chuck G., Leonard J.M., Schmithorst V.J., Laor T., Brody A.S., Bean J., Salisbury S., Franz D.N. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N. Engl. J. Med. 2008;358:140–151. doi: 10.1056/NEJMoa063564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormack F.X., Inoue Y., Moss J., Singer L.G., Strange C., Nakata K., Barker A.F., Chapman J.T., Brantly M.L., Stocks J.M., Brown K.K., Lynch J.P., 3rd, Goldberg H.J., Young L.R., Kinder B.W., Downey G.P., Sullivan E.J., Colby T.V., McKay R.T., Cohen M.M., Korbee L., Taveira-DaSilva A.M., Lee H.S., Krischer J.P., Trapnell B.C., National Institutes of Health Rare Lung Diseases Consortium. MILES Trial Group Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N. Engl. J. Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taveira-DaSilva A.M., Hathaway O., Stylianou M., Moss J. Changes in lung function and chylous effusions in patients with lymphangioleiomyomatosis treated with sirolimus. Ann. Intern. Med. 2011;l54:797–805. doi: 10.1059/0003-4819-154-12-201106210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ando K., Kurihara M., Kataoka H., Ueyama M., Togo S., Sato T., Doi T., Iwakami S., Takahashi K., Seyama K., Mikami M. Efficacy and safety of low-dose sirolimus for treatment of lymphangioleiomyomatosis. Respir. Invest. 2013;51:175–183. doi: 10.1016/j.resinv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Contini P., Schiavina M., Schiavina R., Tavalazzi F., Fabiani A., Di Scioscio V., Spagnolo P., Richeldi L. Efficacy of hormonal suppression in a patient with chyluria due to lymphangioleiomyomatosis. Multidiscip. Respir. Med. 2011;6:313–317. doi: 10.1186/2049-6958-6-5-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka S., Tsuruya K., Tsuchimoto A., Eriguchi M., Kitazono T. Successful treatment of massive proteinuria and severe chyluria by inhibition of cholesterol absorption with ezetimibe in a patient with filariasis. Clin. Kinesiol. J. 2012;5:449–452. doi: 10.1093/ckj/sfs110. [DOI] [PMC free article] [PubMed] [Google Scholar]