Mycobacterium mantenii is a scotochromogenic non-tuberculous mycobacterium (NTM). Van Ingen et al. [1] described this bacterium for the first time in 2009 from the cervical lymph node samples from two immunocompetent children, respiratory samples from two adults and a water sample from Zambia. Since then, only two cases of disseminated M. mantenii infection have been described in immunocompromised patients [2], [3]. This mycobacterium has also been isolated in environmental samples from Ghana [4] and the Czech Republic [5].
In this report, two new cases involving immunocompetent girls with cervicofacial lymphadenitis due to M. mantenii are presented, and the reliability of matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) for identifying M. mantenii is evaluated.
Patients and methods
Case 1
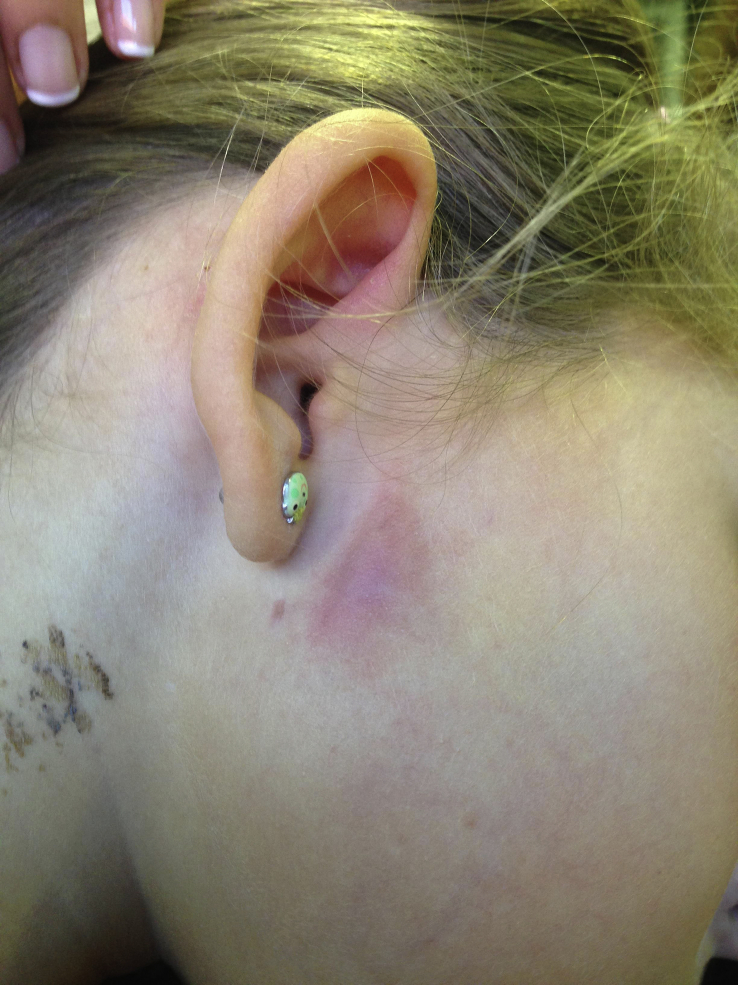
A 5-year-old girl referred to University Hospital of León (CAULE) for preauricular adenitis with 1 month of evolution had previously been treated unsatisfactorily with amoxicillin–clavulanic acid. The patient had no relevant history. On physical examination, right preauricular adenopathy was observed. A tuberculin skin test and a QuantiFERON test were both negative. Ultrasonography showed a 2-cm adenopathy with preserved fatty hilum. The patient was otherwise asymptomatic. Curettage of the adenopathy yielded abundant necrotic material for microbiological and histological examination. Anatomopathological assessment revealed necrotizing granulomatous inflammation. Empirical treatment with ciprofloxacin and clarithromycin was initiated; ciprofloxacin was discontinued when the microbiology results had been obtained. Three months of treatment with clarithromycin was completed, resulting in partial response. At 6 months follow up, the lesion had decreased slightly in size, but there remained a preauricular adenopathy with a diameter of approximately 1 cm, a gummy consistency and overlying erythematous, violaceous skin (Fig. 1).
Fig. 1.
Case 2
A 3-year-old girl was referred to CAULE for left submandibular adenitis with 1 month of evolution, associated catarrh at onset and fever on the first day. She received amoxicillin–clavulanic acid treatment for 15 days without improvement. In a physical examination, a conglomerate of mobile left submandibular adenopathies and laterocervical submandibular microadenopathies were detected. A tuberculin skin test was 13 mm and a QuantiFERON test was negative. A chest X-ray produced normal findings. In an ultrasound examination, multiple altered lymph nodes were observed in both laterocervical chains. Fine-needle aspiration yielded abundant purulent liquid that was sent for microbiological study. Based on a suspicion of NTM adenitis, empiric treatment with azithromycin and ciprofloxacin was initiated, but ciprofloxacin was suspended when the microbiology results were obtained. The evolution of the patient's condition was suggestive of spontaneous scrofula. Antibiotic treatment was continued for 4 months. Initially, the post-scrofula scar exhibited a thickened, irregular and erythematous appearance with progressive improvement at 9 months of follow up.
For both patients, basic analytic results were normal and there were negative serological findings for cytomegalovirus, Epstein–Barr virus, Toxoplasma, human immunodeficiency virus and Bartonella.
The microbiological study of both samples included PCR for Mycobacterium tuberculosis complex (Xpert1 MTB/Rif PCR, Cepheid AB, Solna, Sweden), auramine staining and culturing in mycobacteria growth indicator tube (MGIT, Becton-Dickinson and Company, Sparks, USA) medium (Becton-Dickinson, Oxford, United Kingdom) and Löwenstein–Jensen medium (Becton Dickinson). In both cases, PCR results were negative. Auramine staining was positive for the first patient and negative for the second patient. MGIT cultures for the first and second patients were positive at 12 and 30 days of incubation, respectively. Strains were subcultured in Löwenstein–Jensen medium. The strains were yellow (scotochromogenic). Identification via MALDI-TOF MS (Bruker Daltonics Inc., Bremen, Germany) indicated that these strains were M. mantenii, with scores ≥2.08. Both strains were sent to the National Centre for Mycobacteria (Instituto Carlos III, Majadahonda, Madrid) to confirm their identification using phenotypic and genotypic approaches and to perform an antibiotic sensitivity study. PCR-restriction fragment length polymorphism of the hsp65 gene with the restriction enzyme BstEII resulted in two bands (235 and 210 bp), and digestion with the enzyme HaeIII yielded three bands (130, 105 and 60 bp). Because the chromogenic strain did not match the Mycobacterium avium pattern, a fragment of the 1470 bp 16S rRNA gene was sequenced. The obtained sequences were compared with those of the GenBank database, which indicated 100% agreement with M. mantenii NLA000800224 (FJ232521) sequences. The profiles of the two strains of M. mantenii obtained by random amplified polymorphic DNA–restriction fragment length polymorphism with IS986 and enterobacterial repetitive intergenic consensus PCR showed different patterns.
Drug susceptibility on MGIT 960 was made following the manufacturer's recommendations. The strain from the first patient was sensitive to cycloserine (minimum inhibitory concentrations (MIC) <20 μg/ml) and clarithromycin (MIC <2 mg/L), and the strain from the second patient was only sensitive to clarithromycin (MIC < 2 mg/L).
Discussion
To our knowledge, the aforementioned cases are the second group of published cases involving lymphadenitis caused by M. mantenii. Both cases fit the typical description of this disease in that they involved 1- to 5-year-old immunocompetent girls with unilateral lymphadenopathy and preauricular or submandibular localization. Cervical lymphadenitis is the most common NTM infection in immunocompetent children. The incidence of this condition is low, which contributes to delays in diagnosis; diagnosis typically follows several therapeutic failures with systemic antibiotics [6], [7], [8], [9], as occurred in the cases described here. The majority of cases are caused by M. avium complex [6], [7], [8], [9], although the spectrum of disease-causing NTM species is broad [10], [11], [12], [13]. NTMs are ubiquitous, but a direct link between their environmental prevalence and their involvement in mycobacterial lymphadenitis has not been established [8].
The optimal treatment of NTM lymphadenitis is controversial. The gold standard is the complete removal of the affected nodes, although complications and recurrence can occur [14], [15], [16]. Certain authors believe that in the absence of large, randomized studies, treatment should be individualized [17]. Our patients progressed satisfactorily after curettage or spontaneous drainage combined with treatment with an antimycobacterial agent.
New genetic methods have allowed for the identification of new NTM species [10], some of which are closely related both phenotypically and phylogenetically. Mycobacterium mantenii shares 99% identity for the 16S rRNA gene with Mycobacterium scrofulaceum, Mycobacterium seoulense and Mycobacterium saskatchewanense [1]. Moreover, it is misidentified by the INNO-LiPA Mycobacteria test and the GenoType Mycobacterium CM assay as a member of the M. avium–M. intracellulare–M. scrofulaceum group and as M. intracellulare, respectively. This similarity may have contributed to the underdiagnosis of M. mantenii infections and the overestimation of M. scrofulaceum and M. avium complex in cervical lymphadenitis [18].
There was 100% agreement between MALDI-TOF MS and 16S rRNA sequencing results with respect to the identification of the two strains of M. mantenii in this study. Hence, MALDI-TOF MS is a reliable, rapid and cost-effective diagnostic tool for the identification of M. mantenii and may therefore be useful for providing guidance regarding the antibiotic treatment of choice for this NTM, which is resistant to most antibiotics.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Van Ingen J., Lindeboom J.A., Hartwig N.G., de Zwaan R., Tortoli E., Dekhuijzen R.P.P. Mycobacterium mantenii sp. nov., a pathogenic, slowly growing, scotochromogenic mycobacterium. Int J Syst Evol Microbiol. 2009;59:2772–2787. doi: 10.1099/ijs.0.010405-0. [DOI] [PubMed] [Google Scholar]
- 2.Honda Y., Tanizaki H., Otsuka A., Shirakashi M., Imura Y., Mimori T. Disseminated Mycobacterium mantenii infection with multiple purulent cutaneous lesions. Acta Derm Venereol. 2015;95:1028–1029. doi: 10.2340/00015555-2117. [DOI] [PubMed] [Google Scholar]
- 3.Hase I., Morimoto K., Sakagami T., Kazumi Y., Ishii Y., van Ingen J. Disseminated Mycobacterium gordonae and Mycobacterium mantenii infection with elevated anti-IFN-γ neutralizing autoantibodies. J Infect Chemother. 2015;21:468–472. doi: 10.1016/j.jiac.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Aboagye S.Y., Danso E., Ampah K.A., Nakobu Z., Asare P., Otchere I.D. Isolation of nontuberculous mycobacteria from the environment of Ghanian communities where buruli ulcer is endemic. Appl Environ Microbiol. 2016;82:4320–4329. doi: 10.1128/AEM.01002-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slany M., Jezek P., Fiserova V., Bodnarova M., Stork J., Havelkova M. Mycobacterium marinum infections in humans and tracing of its possible environmental sources. Can J Microbiol. 2012;58:39–44. doi: 10.1139/w11-104. [DOI] [PubMed] [Google Scholar]
- 6.Wolinsky E. Mycobacterial lymphadenitis in children: a prospective study of 105 nontuberculous cases with long-term follow-up. Clin Infect Dis. 1995;20:954–963. doi: 10.1093/clinids/20.4.954. [DOI] [PubMed] [Google Scholar]
- 7.Griffith D.E., Aksamit T., Brown-Elliot B.A., Catanzaro A., Daley C., Gordin F. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 8.Tortoli E. Epidemiology of cervico-facial pediatric lymphadenitis as a result of nontuberculous mycobacteria. Int J Mycobact. 2012;1:165–169. doi: 10.1016/j.ijmyco.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Tebruegge M., Pantazidou A., MacGregor D., Gonis G., Leslie D., Sedda L. Nontuberculous mycobacterial disease in children—epidemiology, diagnosis and management at a tertiary center. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tortoti E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev. 2014;27:727–752. doi: 10.1128/CMR.00035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriques B., Hoffner S.E., Petrini B., Juhlin I., Wåhlén P., Källenius G. Infection with Mycobacterium malmoense in Sweden: a report of 221 cases. Clin Infect Dis. 1994;18:596–600. doi: 10.1093/clinids/18.4.596. [DOI] [PubMed] [Google Scholar]
- 12.Grange J.M., Yates M.D., Pozniak A. Bacteriologically confirmed non-tuberculous mycobacterial lymphadenitis in South East England: a recent increase in the number of cases. Arch Dis Chil. 1995;72:516–517. doi: 10.1136/adc.72.6.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiménez-Montero B., Baquero-Artigao F., Saavedra-Lozano J., Tagarro-García A., Blázquez-Gamero D., Cilleruelo-Ortega M.J. Comparison of Mycobacterium lentiflavum and Mycobacterium avium-intracellulare complex lymphadenitis. Pediatr Infect Dis J. 2014;33:28–34. doi: 10.1097/INF.0000000000000007. [DOI] [PubMed] [Google Scholar]
- 14.Zimmermann P., Tebruegge M., Curtis N., Ritz N. The management of non-tubercular cervicofacial lymphadenitis in children: a systematic review and meta-analysis. J Infect. 2015;71:9–18. doi: 10.1016/j.jinf.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Loeffler A.M. Treatment options for nontuberculous mycobacterial adenitis in children. Pediatr Infect Dis J. 2004;23:957–958. doi: 10.1097/01.inf.0000142503.93438.f1. [DOI] [PubMed] [Google Scholar]
- 16.Lindeboom J.A., Kuijper E.J., Bruijnesteijn van Coppenraet E.S., Lindeboom R., Prins J.M. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: a multicenter, randomized controlled trial. Clin Infect Dis. 2007;44:1057–1064. doi: 10.1086/512675. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann P., Curtis N., Tebruegge M. Nontuberculous mycobacterial disease in childhood–update on diagnostic approaches and treatment. J Infect. 2017;74(Suppl. 1):S136–S142. doi: 10.1016/S0163-4453(17)30204-9. [DOI] [PubMed] [Google Scholar]
- 18.Tortoli E., Pecorari M., Fabio G., Messinò M., Fabio A. Commercial DNA-probes for mycobacteria incorrectly identify a number of less frequently encountered species. J Clin Microbiol. 2010;48:307–310. doi: 10.1128/JCM.01536-09. [DOI] [PMC free article] [PubMed] [Google Scholar]