Abstract
Elastin-like polypeptides (ELPs) constitute a genetically engineered class of ‘protein polymers’ derived from human tropoelastin. They exhibit a reversible phase separation whereby samples remain soluble below a transition temperature (Tt) but form amorphous coacervates above Tt. Their phase behavior has many possible applications in purification, sensing, activation, and nanoassembly. As humanized polypeptides, they are non-immunogenic, substrates for proteolytic biodegradation, and can be decorated with pharmacologically active peptides, proteins, and small molecules. Recombinant synthesis additionally allows precise control over ELP architecture and molecular weight, resulting in protein polymers with uniform physicochemical properties suited to the design of multifunctional biologics. As such, ELPs have been employed for various uses including as anti-cancer agents, ocular drug delivery vehicles, and protein trafficking modulators. This review aims to offer the reader a catalogue of ELPs, their various applications, and potential for commercialization across a broad spectrum of fields.
Keywords: Nanomedicine, Elastin-like Polypeptides, Drug Delivery, Biomedical Engineering
Graphical Abstract
Introduction
While ambitious visions for nanomedicine have outpaced their practical applications, nanomedicine has had a significant impact on drug delivery systems. First envisioned in 1906 by Paul Ehrlich [1], these novel drug delivery systems are aimed at improving clinical outcomes through innovations in particle size, shape, multifunctionality, site-directed delivery, and the reduction of toxicity [2]. The therapeutic index, defined as a ratio between toxic and effective doses, is one measure by which nanomedicines may be optimized. Drug toxicity depends on duration, concentration, and total exposure to drug. In particular, many chemotherapeutics have low therapeutic indices caused by dose-limiting side-effects in normal tissues. While raising the dose of an approved free drug can improve efficacy, off-target effects render this unsafe. Thus, a major rationale for exploring nanomedicines is that they can enhance the therapeutic index compared to their free drug counterpart.
One potential advantage of nanomedicine includes the capacity to construct materials that ferry diverse drugs to sites of disease for controlled release and/or site-directed delivery. By tuning the size and architecture of nanoparticles, for instance, it may be possible to enhance drug residence times and augment the therapeutic index over what could be achieved with free drug alone. Breakthroughs have been most prominent in the oncology space with the approval of nanoformulations, such as liposomal doxorubicin and albumin-bound paclitaxel, aimed at exploiting the enhanced permeability and retention (EPR) effect sometimes seen in tumors [3, 4]. Alternatively, with over 50% of all anti-cancer drugs doubling as substrates of the P-glycoprotein efflux pump, nanoparticles are a compelling solution to overcome multidrug resistance [5].
Extensive efforts to expand the utility of nanoformulations have relied on grafting polymers to their surface. Liposomes are among the best-characterized nanomedicine platforms [6]. For early liposomal formulations, rapid opsonization and detection by the mononuclear phagocyte system, which removes foreign bodies from the blood, hampered the mean residence time and therapeutic efficacy [7]. To prevent opsonization, protective polymers were later developed to sterically shield nanoparticles, which propelled liposomes to clinical approval twenty years ago [8]. Based on data from liposomes and other similarly sized particles, the surface properties for new carriers should be optimized to minimize opsonization, prevent complement activation, and eliminate clearance by the mononuclear phagocyte system. All of these factors can be strongly influenced by composition and architecture of polymers at the nanoparticle surface. Thus, advances in nanomedicine have been closely tied to the development of polymers for biological applications.
As nanomaterials, high molecular weight polymers can solubilize hydrophobic drugs [9, 10]. Polymer-drug conjugation involves the appending of a water-soluble polymer onto the drug of choice [11]. Polymers with large hydrodynamic radii can prevent renal filtration and extend the drug mean residence time. If these polymers are amphiphilic, they can also directly mediate nanoparticle assembly [12, 13]. As such, vast arrays of polymeric species, ranging from natural to synthetic, have been studied as drug carriers. Chemical polymerization produces mixtures of polymers with differing chain length, which necessitates statistical definitions of their polydispersity. The polydispersity index (PDI) is defined as the ratio of the mass-average molecular weight (Mw) to the number-average molecular weight (Mn) and characterizes the heterogeneity in a system. A monodisperse polymer possesses a PDI approaching 1 while polydisperse polymers have PDI greater than 1. The PDI is highly dependent on the mechanism of the polymerization reaction [14]. As polymers of differing molecular weights have various fates in the body, it is evident that the PDI should be controlled to the greatest extent possible [15]. Additional aspects of polymer nanomaterials to be considered include immunogenicity, biodegradability, efficiency of encapsulation and drug loading, as well as stability on the shelf and in the body.
Synthetic polymers, e.g. polyethylene glycol (PEG) and polycaprolactone (PCL), have seen widespread use as biomaterials owing to their low immunogenicity and high biocompatibility. Drawbacks related to polydispersity, linkage stability, and limited carrying capacity[16, 17] unfortunately limit the delivery applications of these platforms. More recent advances have since focused on the fabrication of hybrid nanocarrier systems. Block copolymers, for instance, can spontaneously self-assemble into nanostructures in the form of micelles, electrostatic complexes, and polymersomes due to their amphiphilic properties[2].
Compared to conventional polymers, biomaterials derived from recombinant proteins may serve as viable alternatives. The molecular techniques underpinning these ‘protein polymer’ technologies were first detailed in 1986[18, 19]. Archetypal peptides include leucine zippers[20], collagen-like polymers[21], extended recombinant polypeptide (XTEN) polymers[22], silk-like polypeptides (SLPs)[23], and elastin-like polypeptides (ELPs)[24]. These protein polymers, comprised of repeating motifs sourced from either natural or de novo engineered amino acid sequences, are generated via genetic engineering and recombinant biosynthesis. This offers an exquisite level of precision and tunability with regards to the length, molecular weight, sequence, and monodispersity of the resulting material. Owing to their origin as natural polypeptide chains, protein polymers can be biocompatible and biodegradable, leaving only small peptides and amino acids as their metabolic byproducts[25].
Genetic encoding, meanwhile, grants structural and functional control over molecular features such as secondary structures, targeting motifs, and drug conjugation sites. This provides a compelling rationale for the systematic construction of libraries varying by specific amino acid residues. The addition of natural peptides and protein domains to these polymers creates fusion proteins which often maintain the function and activity of the parent macromolecule[26]. Finally, the low costs of large-scale production in biological systems render recombinant protein polymers amenable to process scale-up[27].
Elastin-Like Polypeptides
Elastin is a polymeric extracellular matrix (ECM) protein, found in tissues as varied as the skin, lungs, blood vessels, and cartilage, which underpins the protractible nature of vertebrate tissues[28-30]. Though only one gene encodes the ~60 kDa soluble precursor, tropoelastin, it exists as polymorphs containing repetitive hydrophobic motifs, largely valine and alanine, denoting elastomeric domains. Other amino acids present at significant levels include glycine and proline, which disrupts alpha helix and beta-sheet formation. These elastomeric domains occur between distinct peptides that are involved in crosslinking other tropoelastin monomers through the action of lysyl oxidase on lysine residues [31]. As biosynthesis and extrusion into the ECM proceed, the final products generated are insoluble elastin fibrils. Pioneering studies first revealed the temperature-sensitive nature of hydrolyzed α-elastin[32]. The protein remained soluble below 25°C, but when heated to 37°C, elastin phase-separated into a secondary amorphous phase known as a coacervate. Interestingly, the investigators further noted that this process was completely reversible. Those findings eventually facilitated the first chemical synthesis of an elastin-like polypeptide prior to the emergence of molecular biology as a discipline[33].
Elastin-like polypeptides (ELPs) are an artificial, biomimetic class of protein polymers inspired by the recurring hydrophobic motifs of tropoelastin[34]. Due to their broad range of applications, including in drug delivery and tissue engineering, ELPs have attracted attention throughout the scientific community[35-37]. The canonical ELP unit consists of a hydrophobic, five amino acid motif (Val-Pro-Gly-Xaa-Gly)n where the guest residue, Xaa, specifies any amino acid and n determines the number of pentapeptide repeats. Proline is usually avoided at the fourth residue since its presence can interfere with coacervation. It should be noted, however, that inclusion of amino acid side chains capable of enhancing functionality does not necessarily interfere with ELP phase behavior. The addition of tyrosine to facilitate spectrophotometric analysis[38] or lysine for crosslinking[39, 40] are two examples. Furthermore, ELPs with other repeat motifs beyond the one described have similar properties. Examples may range from other pentapeptides (e.g. IPGVG) to heptapeptide (e.g. LGAGGAG) and nonapeptide (e.g. LGAGGAGVL) repeat sequences[41].
A primary aspect of ELP biomaterials involves their ability to reversibly form coacervates following temperature changes (Figure 1a). This feature is known as the critical transition temperature (Tt) and can be explained thermodynamically in terms of the Gibbs free energy (ΔG = ΔH – TΔS). If ΔG during a temperature transition (ΔGt) is zero, then ΔHt = TtΔSt which can be rearranged to Tt = ΔHt/ΔSt. The increase in order of ELPs at Tt might appear to contradict the second law of thermodynamics— namely that the order of a system has an inverse relationship with temperature— but the complete system consisting of protein polymer and water must be considered. In the absence of a stimulus, ELPs remain soluble in aqueous solutions as random coils and have been described as intrinsically disordered proteins[42]. The hydrophobic side chains of VPGXG remain surrounded by ordered water molecules existing in a low entropy state. Once the temperature rises above Tt, water molecules clustered around the hydrophobic amino acids are expelled into the bulk water phase. This favors a gain in solvent entropy and allows non-polar side chains to form intra- and intermolecular interactions with neighboring ELP molecules. Hydrophobic interactions, meanwhile, facilitate folding and dynamic assembly into more ordered secondary structures as type II β-turn spirals [24].
Figure 1. Depiction of reversible phase separation by Elastin-like polypeptides (ELPs).
A. ELPs are soluble below a transition temperature, Tt, but undergo coacervation at temperatures above Tt. B. The linear relationship between Tt and concentration can be studied by measuring optical density as a function of temperature. Three different ELPs of varying length and hydrophobicity phase separate above the indicated lines.
Employing the model protein sequence (GVGVP)n, Urry demonstrated that these hydrophobic associations are responsible for a shift from complete solubility in pure water at 20°C to a state of phase separation at 40°C with a dynamic structured state of 63% water/37% polypepeptide by weight at physiological temperatures. The self-assembled form is subsequently denatured at 70°C to a disordered state consisting of 32% water/68% polypeptide [43]. This phase separation phenomena produces a distinct relationship between the hydrophobicity of an ELP and its transition temperature. In fact, Urry maintains that every interaction and/or modification of which a polymer is capable can be characterized as a function of its effect on Tt [24]. The higher the molecular weight or hydrophobicity of an ELP, such as the amino acid-based hydrophobicity scale, the lower the energy required for surrounding water molecules to enter the bulk water and thus the lower the heat required to induce hydrophobic assembly[44]. In short, ELPs with high molecular weights and/or hydrophobic guest residues exhibit lower transition temperatures than ELPs with low molecular weights and/or hydrophilic guest residues. (Figure 1b).
This phase separation property is completely reversible like an on/off switch at temperatures below the Tt, making ELPs an attractive scaffold as a smart biomaterial that can respond to temperature[45]. ELPs can be clinically designed to exploit their thermal responsiveness. One example is an ELP that remains soluble at room temperature for ease of injection but phase separates at 37°C within the target tissue to form an insoluble depot[46]. The sharp (2-3°C range) phase separation observed can be tuned to occur between 0-100°C and exists primarily as a function of guest residue hydrophobicity, molecular weight, and concentration[47].
One study suggests that the presence of mild detergents in solution does not curtail phase separation[48]. Phase separation for certain ELPs may also be triggered by external factors such as alterations in ionic strength. Increasing concentrations of a kosmotropic salt, for instance, reduces the ELP Tt[49]. Modeling approaches that involve the prediction of transition temperatures further allow investigators to tune ELP design[50]. In addition, certain ELP derivatives have also been described that can induce phase separation in response to pH changes resulting from ionizable guest residues[51], electrical current[52], redox triggers[53], magnetism[54], and light[55].
Molecular Cloning
Concatemerization was the original technique employed to genetically engineer libraries of ELP[56]. This method relies on the self-ligation of repetitive genetic sequences thus creating oligomers of various lengths through single-step synthesis. Although offering a rapid means of ELP gene construction, the primary drawback remains the fact that absolute control over length is forfeited. In other words, genes constructed through concatemerization exist as a distribution of DNA oligomers with varying chain lengths, rather than allowing a distinct length to be specified. While each bacterial colony arises from a single length of ELP gene, in practice it is difficult to target a gene of a specific molecular weight. Another disadvantage is that concatemerization of genes encoding large ELPs (>100 kDa) is challenging.
Another method, known as recursive directional ligation by plasmid reconstruction (PRe-RDL), improves on the shortfalls associated with concatemerization[27]. The mechanism for construction of these plasmids involves the use of Type IIs restriction enzymes that cut to the 3’ direction of their recognition site. By exact placement of recognition sites before the start codon and after the stop codon, these enzymes can produce complementary sticky ends inside the ELP gene. Two batches of such plasmids can be doubly digested with the unique type IIs enzyme and a second enzyme found elsewhere on the plasmid. By gel purification, the two halves of the plasmid, each containing the entire ELP gene, can be ligated together. This reconstitutes the entire plasmid (origin of replication, antibiotic resistance, ribosome binding site, etc.), while retaining only the restriction sites proximal to the start and stop codon for the ELP. More importantly, the resulting gene now encodes an in-frame fusion of both plasmids. This can be used to double the length of an ELP gene, or to graft different genes that encode for block copolymers[57]. PRe-RDL can be repeated in multiple iterations to generate larger plasmids and lends itself to the eventual expression of entire ELP libraries with specific molecular weights. By use of the Type IIs restriction enzymes, no restriction sites encode amino acids that are expressed in the resulting protein polymer. As such, PRe-RDL is not limited to the construction of ELP genes and can generate any protein polymer composed from repetitive DNA sequences. Beyond PRe-RDL, other methods employed in the genesis of functional ELPs include polymerase chain reaction (PCR)[58], seamless cloning[59], and overlap extension rolling circle amplification (OERCA)[60, 61].
Protein expression and physicochemical characterization
ELPs have been commonly expressed in E. coli, owing to cost effectiveness and ease of scale up, but expression from other organisms as varied as yeast[62], fungus[63], and plants[64] has also been detailed. In addition, ELPs exhibiting more elaborate architectures at the genetic level have been designed such as the diblock copolymers pioneered by the laboratories of Conticello[65] and Chilkoti[36]. These protein polymers exhibit self-assembly arising from the amphiphilic properties imparted by two distinct blocks of differing polarities. This mediates partitioning of diblock copolymers under aqueous conditions into spherical micelles that consist of a hydrophobic ELP core and a hydrophilic ELP corona. The resulting nanoparticle is stable over a 5–10°C range between the critical micelle temperature (CMT) of the hydrophobic block and the bulk phase transition temperature mediated by the hydrophilic ELP corona. The hydrophobic core itself may be employed in the encapsulation of hydrophobic drugs to increase their solubility[66] while the hydrophilic corona can be imbued with functional groups to enhance targeting[67, 68]. Further increases in temperature mediate formation of micron-sized aggregates more characteristic of the bulk ELP phase separation.
In general, micelle assembly may improve a protein's pharmacokinetics and biodistribution since the usual micelle hydrodynamic radius (5 < Rh <50 nm) permits evasion of the mononuclear phagocyte system and limits glomerular filtration[6]. Similar to monomer ELPs, altering the amino acid sequence, molecular weight, and hydrophilic-to-hydrophobic ratio of the block copolymer will dictate CMT, determine hydrodynamic radius, and yield control over micelle stability. As an example, an ELP's particular properties may be tuned to allow formation of a micelle under all physiological temperatures for drug delivery purposes. It should further be noted that triblock copolymers[65], dendrimers[69], vesicles[70], and chemically crosslinked hydrogels[71] have also been described.
ELP purification from E. coli lysate relies on the exploitation of an ELP's previously mentioned thermal responsiveness. This method is called inverse transition cycling (ITC) and serves as a facile means of separating protein polymers from cellular debris without the need for chromatography. In brief, after cell membrane disruption, the ELP phase transition is triggered by heating above the Tt, allowing ELP coacervates to be collected via centrifugation. Once the supernatant is removed, the ELP pellet is resolubilized in cold buffer and centrifuged below the ELP Tt. This provides a means of eliminating insoluble cellular debris and other contaminants captured in the pellet, completing one cycle of ITC. Multiple ITC rounds effectively raise ELP purity to levels above 95%, rendering the material suitable for study. Following purification, ELPs can also be lyophilized for long-term storage. Useful workflows detailing this entire purification process can be found in many general reviews of the ELP literature[27, 72].
The versatility of these protein polymers is further underscored by the fact that ELP sequences can be appended to other proteins as demonstrated in 1999 by Chilkoti et al[34], forming fusions at either the N-terminus or C-terminus capable of imparting unique biological activities. Interestingly, one study elucidated that orientation of the protein relative to the ELP played a large role in determining yields obtained of the resulting fusion protein[73]. Localizing the ELP segment at the C-terminal end of the target protein (protein-ELP) proved superior to N-terminal ELP constructs (ELP-protein). At the most basic level, protein-ELP fusions can serve as purification tags. These fusion proteins may also function as macromolecule carriers aimed at increasing molecular weights to retard filtration by the kidneys. Diblock copolymers have subsequently been manipulated to exploit the presence of peptide or protein moieties on an ELP micelle corona and allow high-avidity uptake[66, 74].
Small molecule drugs conjugated with ELPs, meanwhile, gain properties of thermally-induced phase separation while retaining their bioactivity. For instance, one system involved the chemical conjugation of an ELP to the chemotherapeutic, doxorubicin[12, 75]. In another study, micelle formation was exploited to present multivalent targeting motifs[68], which enhanced cellular uptake. ELPs have been utilized in tandem with recombinant oligopeptide fusions involving cell-penetrating peptides[76], a c-myc oncogene inhibitor[77], and recombinant protein fusions with interleukin-1 receptor antagonist[78]. Surfaces coated with an ELP fused to the RGD or fibronectin CS5 cell binding sequence retained the ability to support in vitro endothelial cell adhesion and spreading[79]. Additional uses of ELPs include purification of holoenzymes such as D-Amino acid oxidase [80], isolation of multisubunit enzymes like RNA polymerase [81], entrapment of small molecules[66], and the generation of ELPs hybridized with other polymers [71].
The genetically-encoded nature of ELPs permits the generation of monodisperse biopolymers[25]. Considering that polymer molecular weight plays a large role with regard to in vivo drug carrier disposition, ELP monodispersity lends itself to the control of pharmacokinetic parameters such as biodistribution and clearance rate. Other studies were integral in establishing the foundations for using microPET imaging as a non-invasive means of tracking ELP biodistribution and image-driven pharmacokinetics[82]. This was accomplished by conjugating a chelating agent, AmBaSar, onto the ELP prior to radiolabeling with 64Cu. In contrast to the classical method of obtaining blood samples to deduce pharmacokinetics, this imaging modality allows for the application of image-driven pharmacokinetic modeling[83]. These studies confirmed that a low molecular weight soluble ELP (<40 kDa) was cleared rapidly by the kidney, and that high molecular weight soluble ELPs (>70 kDa) were retained in the blood for long enough to generate an EPR-based enhancement in a breast cancer xenograft model. Interestingly, a nanoparticle composed of an ELP diblock copolymer demonstrated slightly enhanced hepatic clearance. An advantage afforded by microPET imaging coupled with image-driven pharmacokinetics involves data acquisition that neither compromises the physiological system under investigation nor suffers limitations encountered when removing blood and radiolabeled agent from the subject.
As a biotherapeutic, ELPs have agreeable pharmacokinetics with terminal circulation half-lives ranging from 8 to 12 hours in mice[12, 82, 84]. Based on prior work in small animal models, ELPs also exhibit biocompatibility and low immunogenicity[85-87]. One study by Cho et al[88] involved the application of immune-tolerant elastin-like polypeptides (iTEPs) as cytotoxic T-lymphocyte vaccine carriers. iTEP design places an emphasis on humoral tolerance through the absence of epitopes that can bind T and B cells. The motif selected to generate the repeats of iTEPs A to D were sourced from the homologous peptide sequences of mouse and human elastins. In addition, this 18 residue motif is longer than either MHC class II-restricted TCR epitopes or linear BCR epitopes to further avoid immunogenicity. Despite lacking the canonical pentapeptide sequences, iTEPs still retain phase transition properties. Each of the four iTEPs elicited negligible iTEP-specific antibody titers relative to an ovalbumin (OVA) control. Another interesting finding was the fact that in vivo aggregation of hydrophobic constructs (iTEPs A, C, and D) did not influence immunogenicity in any manner differing from soluble forms (iTEP B). At the biophysical level, this negligible immunogenicity may originate from the high energy levels required for host antibodies to overcome when binding to the predominantly random coiled structure of solvated ELPs at potential epitopes[44, 89, 90]. In combination with their lack of positive or negative charge, limited number of primary epitopes, and the similarity of their pentameric repeat to short fragments of human tropoelastin, the possibility remains that they have a low likelihood of producing an adaptive immune response. This particular interpretation has been borne out by data from PhaseBio Inc, which suggest that ELPs are safe in human clinical trials[91].
It should be expected that the biological composition of these protein polymers mediates their biodegradation into peptides and amino acids readily removed from the body. One in vitro study by our laboratory[92] demonstrated that transformed PTEN-deficient mouse hepatocytes enzymatically degrade ELP nanoparticles (Figure 2). The kinetics of biodegradation have also been analyzed in vivo using a 59.4 kDa 14C-labeled ELP. In that study, intravenous administration led to a relatively modest degradation rate of ~2.5 wt % per day, pointing to the fact that ELPs can achieve in vivo stability for therapeutic applications[84].
Figure 2. Uptake and degradation of ELP nanoparticle.
Rhodamine-conjugated S48I48 (red) was incubated with transformed murine hepatocytes for 0, 2, 4, 24 h at 37°C and imaged using live-cell confocal fluorescence microscopy. Cells were counterstained with lysotracker green to show low pH compartments associated with lysosomal protease activity. Differential interference contrast (DIC) imaging illustrated cell morphology. ELP nanoparticles were visible within and on the cell surface after 2 h; intracellular staining significantly decreased after 24-h incubation. The arrow indicates internalized nanoparticles with the lysosomes. Scale bar: 10 μm. Reprinted from [92] with permission of John Wiley and Sons.
There have been reports that ELPs do not significantly affect whole blood clotting time in dogs or induce red blood cell hemolysis in rabbits[37]. Though all ELPs are eventually subject to clearance, ELP solubility ultimately affects these degradation kinetics. Ex vivo experiments deduced that soluble ELPs below Tt experience enzymatic degradation by elastases as well as collagenases. The formation of coacervates above an ELP transition temperature partially blocked proteolysis by collagenase. In contrast, an elastase degrades both soluble and coacervate ELP[92]. While there may be conditions where ELP coacervation is protective against a proteolytic environment, this appears to vary case-by-case.
In the design of next generation, clinically-relevant nanomedicines, ELPs have demonstrated evidence supporting the following advantages: i) Genetically-engineered precision—as the amino acid sequences of ELPs can be tailored at the genetic level, their biosynthesis affords excellent control over architecture, rendering monodisperse polymers from which to build multifunctional therapeutics. ii) Safety—ELPs appear to be biocompatible, biodegradable, and non-immunogenic. iii) Environmentally-responsiveness—the phase behavior of the ELP can be tailored to assemble multivalent nanoparticles in aqueous solution without solvents or crosslinkers, while maintaining the activity of fusion proteins.
Applications
The versatility of ELPs as nanomedicines is driving interest in their adoption across a broad spectrum of disciplines as depicted in Figure 3. Anti-cancer strategies rank chief among their applications, with oncology capturing about 35% of total market revenue in the nanomedicine sector[93, 94]. Beyond oncology, however, there exists an exciting landscape of pioneering methods involving ELPs in fields as diverse as gene delivery, ophthalmology, and maternal-fetal medicine. What follows is a catalogue of these various applications as they pertain to translational approaches and the potential for eventual commercialization.
Figure 3.
The various applications of ELPs as nanomedicines
Cancer
According to the American Cancer Society, over 1.6 million new cases of cancer will be diagnosed in 2015[95]. Though currently the second leading cause of death in the United States, it is expected to surpass heart diseases in coming years[96]. This has prompted researchers to accelerate innovations in anti-cancer nanomedicine, and ELPs are one emerging tool in the field. Since the breadth of ELP cancer research extends beyond the purview of this article, only a few instructive examples will be detailed.
One of the first cancer-based applications was performed by Dr. Chilkoti and colleagues, who demonstrated that hyperthermia could enhance tumor localization of ELPs in SKOV-3 ovarian carcinoma and D-54MG glioma cell lines[89]. Liu et al further solidified this link between mild hyperthermia and tumor penetration by studying the sequestering of 14C-radiolabeled ELPs using FaDu tumor grafts in nude mice models[84]. For a soluble ELP (Tt = 40°C), a terminal plasma half-life approaching 8.7 hours was achieved. Once the tumors were heated to 42°C, the thermally responsive [14C]ELP1 exhibited a 1.5 fold increase in accumulation rates over a thermally unresponsive [14C]ELP2 control.
Conjugation of small molecule drugs to ELPs represented another early advance aimed at decreasing toxicities and enhancing therapeutic efficacy. In one system, an N-terminal lysine present on the ~60 kDa ELP1-150 was conjugated to a pH-sensitive hydrazone bond[75]. This bond mediated attachment of a maleimide linker ferrying doxorubicin, an anticancer agent which acts through inhibition of topoisomerase. Following endosomal uptake in FaDu cells, the pH responsive ELP-hydrazone portion is severed in the acidic lysosome, allowing free doxorubicin to reach the nucleus and effect its cytotoxicity. Interestingly, in vivo studies revealed similar cytotoxicity following administration of both free doxorubicin and doxorubicin-ELP despite the differing intracellular distribution rates. Modifications to the length and structure of the doxorubicin-ELP maleimide linker offers an additional means of tuning aspects of the release profile without dramatically altering ELP Tt. Most prominently, it was deduced that shorter linkers between doxorubicin-ELP conjugates enhanced drug release[97]. This approach was further refined to deliver up to 8 doxorubicin molecules covalently linked to one end of the ELP, which promoted the assembly of ELP-coated nanoparticles [12]. These chimeric polypeptides were the first ELPs reported to block tumor growth in vivo; furthermore, they appeared to be effective after only a single dose.
Building on those studies, a research team based at the Samsung Advanced Institute of Technology led by Kim and colleagues took a hybrid approach by merging ELPs with other drug delivery technologies[98]. Temperature-sensitive liposomes were generated with DPPC/Chol/DSPE-PEG serving as the primary lipids, which incorporated a stearyl group appended to the amino terminus of an ELP via an amide bond called SA-V3. Doxorubicin was encapsulated inside the resulting ELP liposomal formulation at a ratio of 1:0.2 (w/w, phospholipid:doxorubicin). Most notably, over 80% of the encapsulated drug exhibited release within 5 minutes following a temperature increase to 42°C. Liposomes lacking SA-V3, by contrast, experienced doxorubicin release rates of less than 10% after administration of mild hyperthermia. Six-week old male BALB/c nude mice were later used to implant tumors deriving from EMT-6, a murine mammary cell line. Overall stable blood circulation times were observed with the in vivo half-life of encapsulated doxorubicin persisting for about 2.5 hours. Finally, a study was conducted to determine drug accumulation after preheating a tumor for 30 minutes at 42°C. ELP liposomes displayed significantly better tumor localization at 6 and 12 hours after preheating; furthermore, these levels exceeded the concentration achieved for free drug by 31-fold. Additional hybridized systems, including PEG-functionalized ELPs[99], are also currently under investigation by several other groups.
Alternative strategies have focused on designing protein polymers capable of overcoming transport barriers, such as the P-glycoprotein efflux pump [76, 100, 101]. Bidwell and Raucher validated this approach using a cell-penetrating peptide (CPP) appended to an ELP in MCF-7 breast carcinoma cells[77]. In this study, penetratin (AntP), a 16 amino acid sequence capable of endocytic uptake, was designed in tandem with a peptide (H1) inhibitor of the c-Myc proto-oncogene's transcriptional activities. In vitro aggregation at 42°C, mediated by the temperature-sensitive AntP-ELP-H1, enhanced cell death 2-fold versus controls containing non-heated cells or thermally unresponsive ELP. Further advances have led to many derivations and in vivo applications of the original CPP-ELP concept in combination with peptides as varied as Bac[102], p21[103, 104], and Tat[105] against neoplasms. Moreover, research into the uptake mechanisms of CPP-ELPs deduced via flow cytometry that endocytosis of these polymers occurs in a caveolae-independent manner[101].
Other tumor-targeting modalities have also been described in ELP-related literature. One topic explored in the MacKay laboratory targets angiogenesis. Unregulated blood vessel growth remains a stable hallmark of many solid tumors which require access to nutrients in the bloodstream. Such neovasculature differentially overexpresses integrin αV heterodimers and can thus be targeted by RGD-containing peptides[106, 107]. Certain antagonists, most prominently disintegrins derived from viper venoms, offer a natural source of high affinity ligands binding these proteins. While contortrostatin (CN) is a prototypal member of the disintegrin family obtained directly from venom[108], recombinant approaches have since been employed by Markland and colleagues to generate vicrostatin (VCN) by bacterial fermentation, which have been evaluated in murine models of breast and prostate cancer[109].
Fusion of VCN to a ~73 kDa ELP sequence G(VPGAG)192Y, also known as A192, formed spherical multimers while retaining VCN's integrin-binding and receptor-mediated uptake capacities as demonstrated on HUVECs[110]. When compared to a control ELP fused to a linear RGD segment, the VCN-A192 construct outperformed with a 30-fold lower IC50 in vitro. The higher molecular weight of VCN-A192 also solves a pressing challenge inherent in the use of free VCN— namely, the rapid clearance of this small peptide therapeutic via renal filtration. An orthotopic murine model of breast carcinoma demonstrated that VCN-A192 not only slowed clearance but also promoted tumor accumulation relative to either A192 or VCN alone.
Parallel in scope to those efforts, the Chilkoti laboratory established a means for controllable tumor targeting using micelle-forming ELP diblock copolymers. This rationale arises from the fact that, while high affinity nanoparticles dictate enhanced accumulation at target sites, high affinity may introduce toxicities involving healthy tissues expressing the same receptor at reduced levels. Solving this issue necessitates development of a construct with a low affinity for receptors in healthy tissue that shift to a high avidity form in response to a trigger—such as the application of heat. To that end, the GRGDS peptide targeting the αVβ3 integrin was fused to an ELP diblock copolymer[74]. While slight binding was demonstrated in the monomeric form, above the critical micelle temperature these peptide-decorated nanoparticles drove high-avidity uptake by endothelial cells. More recent studies have focused on a RGD-TRAIL-ELP and its apoptotic activity in both human colorectal carcinoma (COLO-205) and human breast cancer (MDA-MB-231) cell lines[111]
Subsequent studies have expanded the use of ELP diblock copolymers with data indicating that larger proteins, consisting of >100 residues, fused to diblock ELP segments similarly preserve biological moieties and their functions without abolishing ELP micelle formation. A significant set of experiments conducted by Shi et al[66] validated this ELP drug delivery strategy using a hydrophobic, immunosuppressive macrolide derived from Streptomyces hygroscopicus known as rapamycin. In contrast to direct conjugation of small molecules onto a polymer platform, this novel departure utilizes high affinity binding between rapamycin and its cognate human receptor, FKBP12 (KD=0.2 nM). FKBP was fused to an ELP diblock copolymer, which presented FKBP on the corona of a resulting nanoparticle stable at physiological temperatures. Following passive uptake, biodegradation, and drug release, freed rapamycin binds to native FKBP and inhibits mTOR-mediated cell cycling (Figure 4A). mTOR is a serine/threonine kinase bridging upstream signal inputs (e.g. PI3K/AKT pathway) with downstream effectors through signaling cascades and is thus commonly exploited during tumorigenesis[112, 113]. While the anti-proliferative properties of rapamycin have been found in neoplasms of the breast, prostate, and colon, the free drug's utility is tempered by low solubility, low bioavailability, and dose-limiting side effects in the lungs, kidneys, and liver. ELP diblock copolymers exhibiting high avidity for rapamycin were designed to prevent filtration/accumulation in the kidney and permeability into normal tissues, raise its poor solubility, and increase the tolerated dose.
Figure 4. Design of Elastin-like polypeptide (ELP) nanoparticles that carry an anti-proliferative drug at both their core and corona.
(A) High-avidity interaction between a small molecule drug (rapamycin) and its cognate target protein (FKBP) decorated at surface of an ELP nanoparticle. The nanoparticles assemble nanoparticles above a critical micelle temperature (CMT). (B) Dynamic light scattering of FKBP-decorated FSI and plain SI nanoparticles shows that protein modification minimally affects CMT or hydrodynamic radius. (C) Tumor growth inhibition by FSI-rapamycin versus free rapamycin (0.75 mg/kg BW). Free rapamycin mice were sacrificed at Day 24 due to toxicity. Reprinted from [66] with permission of Elsevier.
Fusion between FKBP and a 39 kDa diblock copolymer ELP (SI) self-assembles into a stable nanoparticle (FSI) with a 24 nm hydrodynamic radius and a CMT of 24.5°C. Relative to plain SI, the hydrodynamic radius and stability of FSI was nearly identical (Figure 4B). A novel two-phase solvent evaporation method had to be adapted to facilitate encapsulation of rapamycin into FSI. Both TEM and DLS confirmed that the radii of FSI nanoparticles following drug encapsulation were only slightly larger than the preceding values.
The FSI release half-life was explored via dialysis under sink conditions, which revealed a biexponential release profile, with a fast phase (1.9 hr half-life) followed by a slow (57.8 hr half-life) terminal phase. This biphasic behavior is illustrative of the fact that FSI not only encapsulates a reservoir of rapamycin within its hydrophobic core (i.e. rapid release) but also retains approximately 30% of the drug on FKBP decorating the micelle corona. To confirm this mechanism, rapamycin was solubilized within the unmodified diblock copolymer SI alone, which released all of its contents with a 2.2 hr half-life, similar to FSI's initial release phase. In another manuscript, it was demonstrated that free FKBP-ELP released drug under sink conditions with a monoexponential decay and a half-life of just 13 hours[114]. Together, these data suggest that by coassembly of multiple copies of FKBP at the surface of a nanoparticle, it may be possible to cooperatively drive avidity for target small molecules. This observation could have many potential uses; however, in the case of FSI, it appears to promote long duration release of rapamycin from the carrier.
In vitro cell proliferation assays confirmed rapamycin-bound FSI (IC50=0.28 nM) was just as effective as free drug (IC50=0.27 nM) in reducing the viability of MDA-MB-468 breast cancer cells sensitive to the drug. In contrast, neither FSI-bound nor free drug affected the rapamycin-insensitive MDA-MB-231 cell line. This FSI formulation also extended drug solubility at least 10-fold above that of the free drug. Tumor regression studies conducted in orthotopic breast cancer xenografts led to the premature withdrawal of mice committed to dosing regimens with free rapamycin due to cumulative toxicities. FSI, on the other hand, surpassed its free counterpart by mitigating drug toxicities without sacrificing cytostatic efficacy (Figure 4C).
Another notable anti-cancer strategy revolves around merging ELP technology and antibody therapeutics. Antibodies have figured prominently in the world of biotechnology with the first therapeutic mAb (muromonab-CD3 for prevention of acute transplant rejection) approved by the FDA in 1986[115]. Rituximab, a chimeric mAb capable of binding CD20 to induce apoptosis in B-cell malignancies, constitutes one example which has since been deemed an essential medicine by the World Health Organization[116]. While single chain variable fragments (scFv) have been proposed as a versatile class of antibody therapeutics[117], their small size leads to poor tumor retention and rapid clearance (~4 hrs). To address these shortcomings, many investigators have turned to bioconjugation with high molecular weight polymers as a means of prolonging scFv half-lives[118]. Applying those findings to ELPs, anti-CD20 scFv was generated through fusion to the ~73 kDa A192 monomer by Aluri and coworkers[119].
Dynamic light scattering, phase diagrams, and transmission electron microscopy revealed that anti-CD20 scFv-ELPs unexpectedly formed nanoworm structures with a hydrodynamic radius of 85.7 ± 16.5 nm. In contrast, the unmodified A192 ELP exhibited a more typical value of 6.7 ± 0.2 nm. As the A192 ELP is not a diblock copolymer, its simple addition provides no mechanism for particle assembly. Instead, it appears that particle assembly is mediated by the scFv domain. scFv are frequently challenging to produce and stabilize; furthermore, a likely explanation for the nanoassembly of the scFv-ELP is that the scFv domains partially aggregate to form the core of a particle held together by noncovalent intermolecular associations. Instead of flocculating, the high molecular weight A192 protein polymer sterically stabilizes these colloids. More surprising, in vitro studies confirmed binding of CD20 receptor and efficient induction of apoptosis by the nanoworms. Viability assays revealed an IC50=32 μM in Raji cells and IC50=41 μM for SU-DHL-7 B-cell lines. Tumor regression was examined using Raji xenografs in athymic nude mice. Median survival times for the ELP nanoworms were significantly longer than for monoclonal Rituximab or PBS. Notably, upon cessation of therapy, these tumors continued to proliferate. Nevertheless, the surprising stabilization of these nanostructures and the finding that they maintain efficacy suggests this class of nanoparticles may be suitable for additional study.
Ocular diseases
With the National Eye Institute reporting an economic burden for eye diseases surpassing 139 billion USD in 2014, ocular therapeutics represent another urgent growth opportunity[120]. In particular, novel ELP-based solutions have been devised to combat corneal wounds[121] and Sjögren's syndrome. Each year, about 2.4 million eye injuries occur in the US[122], some of which are exacerbated into sight-threatening conditions.
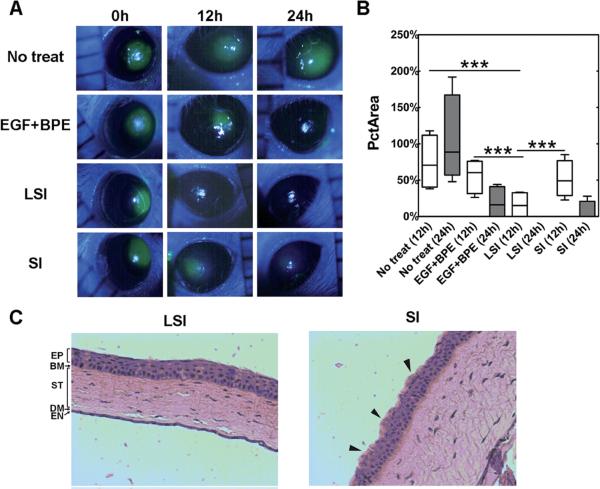
The discovery of lacritin[123], a mitogen found in tears promoting survival of corneal epithelial cells, provided a compelling rationale for the design of a lacritin-ELP fusion (LSI) exhibiting multivalent presentation to treat ocular abrasion wounds by Wang et al[124]. This fusion was composed of the lacritin domain appended to the ELP diblock copolymer discussed above (SI), which by itself assembles nanoparticles (Rh ~ 25 nm) above 25°C. A shift in particle diameter at a temperature of 18.4°C was observed for LSI, but interestingly, the construct preassembled into 30-40 nm nanoparticles even below this transition temperature. Larger LSI nanoparticles approaching 140 nm were subsequently isolated at physiological temperatures. To assay LSI's mitogenic activity in vitro, a scratch was applied to confluent human corneal epithelial cells and the healing process was observed over 24 hrs. At concentrations as low as 10 nM, LSI accelerated scratch wound healing in a manner comparable to a positive control containing epidermal growth factor (EGF) and bovine pituitary extract (BPE). In vivo efficacy studies were then investigated via induction of circular defect in the corneal epithelium in NOD mice (Figure 5A). Mice were segregated into four groups— KSFM media supplement with BPE (50 μg ml−1) + EGF 5 ng ml−1, LSI (100 μM), plain SI (100 μM), or no treatment— then allowed to heal for 24 hrs following two rounds of topical administration. Fluorescein staining and percentage of initial wound area values both confirmed that treatment with LSI outperformed SI, BPE + EGF co-treatment, and no treatment controls (Figure 5B).
Figure 5. Lacritin-ELP nanoparticles heal corneal wounds in mice.
A 2 mm defect in the corneal epithelium of female non-obese diabetic (NOD) mice was monitored using fluorescein staining at 0, 12 and 24 h with or without treatment by LSI, SI, and a positive control EGF + BPE. (A) Representative images showing the time-lapse healing of the corneal wound. (B) LSI at both 12 and 24 h significantly (***p = 0.001, n = 4) decreased the percentage of initial wound area (PctArea) compared to SI, EGF + BPE, and no treatment groups. (C) After 24 h, corneas were fixed, sectioned across the defect, and stained by hematoxylin and eosin. The corneal epithelium of the LSI treatment group revealed normal pathology. Although reduced fluorescein staining was observed at late times in the SI group, the epithelium did not recover fully, as evidenced by its irregular surface (black arrows). Reprinted from [124] with permission of The Royal Society of Chemistry.
These findings were corroborated through histological analyses showing improved morphology of the corneal epithelia after LSI-mediated healing (Figure 5C). Follow-up LSI studies further emphasized the critical contribution of multivalency to this efficacy. In comparison to a thermally-insensitive ELP control (LS96), LSI induced more profound corneal wound healing over a 12 hr timeframe. More recent data indicates that Lacritin-ELPs are also capable of phase separating in the lacrimal gland, where they mimic the functional effects of free lacritin by promoting tear protein exocytosis [125].
The autoimmune disease known as Sjögren's syndrome (SjS) affects approximately 4 million patients with 9 out of 10 being women[126]. The primary symptoms include debilitating dry eye and dry mouth. The MacKay group, in collaboration with the Hamm-Alvarez laboratory, therefore sought to further adapt the FSI rapamycin nanoformulation discussed earlier to encompass ocular indications[114]. This rationale arises from the fact that, while this small molecule drug acts as an immunosuppressant, it has never been investigated as a means of treating the severe autoimmune inflammation of the lacrimal gland, called dacryoadenitis, using the NOD model that replicates some of the pathology found in SjS. The physicochemical characterizations and release profiles of FSI were consistent with the earlier breast cancer study.
The non-obese diabetic (NOD) mouse model was selected for in vivo work owing to its recapitulation of symptoms, including autoimmune-mediated lymphocytic infiltration of lacrimal gland, characteristic changes in the spectrum of tear proteins secreted, and development of serum autoantibodies, regularly seen in human SjS. FSI, free drug, PBS, and untreated control groups were assessed for their efficacy in reducing lymphocytic infiltration of the lacrimal gland using image analysis of the percent area infiltrated by lymphocytes. FSI exhibited a ~50% reduction in infiltration area following only 3 injections in one week. Free rapamycin likewise displayed a similar decrease when examined relative to both PBS and untreated mice; however, this efficacy was accompanied by histopathology consistent with renal toxicity. Nephrotoxicity was observed for free rapamycin, which exhibited a greater degree of kidney tubule vacuolization relative to FSI-encapsulated drug. Toxicological studies involving FSI-bound versus free drug showed that the former was far better tolerated. Only 4% of FSI-treated mice, for instance, had bruised tails post-injection. Injections with the free drug conversely led to a 70% increase in edematous tails with 26% becoming bruised and 4% ending up necrotic. When lacrimal gland gene expression was assessed by qPCR, the FSI-encapsulated drug shifted transcriptional patterns for a panel of genes associated with the mTOR pathway in comparison to a similar dose of the free drug. Beyond simply adjusting the toxicity profile for rapamycin, these data suggest that carriers like FSI may also play a large role in the delivery of the drug to diseased target tissue, modulating a change in disease pathology. ELP constructs, such as FSI and others, may have broader utility in other autoimmune and inflammatory disease conditions.
Cardiovascular diseases
Cardiovascular diseases (CVDs) mark the primary cause of death worldwide with 600,000 fatalities reported each year in the US alone. Similarly sobering is the fact that CVD accounts for 313 billion USD in costs due to health expenditures and lost productivity[127]. Among the risk factors for CVD, hypertension remains most amenable to pharmacological modulation as indicated by the broad range of antihypertensive medications on the market[128]. PhaseBio Inc. has pioneered an ELP-based treatment for essential hypertension using a VIP-ELP fusion protein known as Vasomera™. Vasoactive intestinal peptide (VIP) is a neuropeptide, consisting of 28 amino acid residues, which acts as a ligand of the G-protein coupled receptors VPAC1 and VPAC2[129]. As VIP promotes heart contractility and induces coronary vasodilation, therapeutic applications against hypertension provide a compelling rationale for its exploitation.
Fusion of ELP to an analogue of VIP specific for VPAC2 effectively produced a long-acting biotherapeutic, exceeding the brief half-life (<2 min) of the parent peptide, due to the greater molecular weight imparted by the ELP segment[130]. To deduce the VIP-ELP's effect on blood pressure, SHR rats were induced to develop pulmonary arterial hypertension and then received either VIP-ELP (3-6 mg/kg) or placebo (0.9% NaCl) delivered via single bolus doses intravenously or intratracheally. This led to rapid reductions in pulmonary artery pressure (−24 ± 3% from 41 ± 1 to 31 ± 1 mmHg) and sustained (>5 min) vasorelaxation independent of administrate route (IV: −17 ± 4% versus IT: −28 ± 4%). Long-term hemodynamic studies were conducted in conscious rats where VIP-ELPs mediated dose-dependent blood pressure decreases that were sustainable for about 12 hours post-dosing.
VIP-ELP was subsequently investigated for its potential in mitigating heart failure using rat models of doxorubicin-induced cardiomyopathy[131]. Daily intravenous administration of VIP-ELPs combated myocardial dysfunction and prevented muscle wasting. These protein polymers also reduced the energetic demands of diseased cardiac tissue while simultaneously improving left ventricular systolic/diastolic functioning. When canines were given the VIP-ELPs, both groups with healthy and failing hearts exhibited a dose-dependent increases in cardiac function within the 0.1 to 1 μg/kg/min range[132].
Those precedents established the foundation for Phase I single ascending dose trials by PhaseBio in patients with pulmonary arterial hypertension[133]. Introduced through once weekly dosing regimens, VIP-ELPs were found to be safe and well-tolerated by subcutaneous and intravenous routes. They also demonstrated a prolonged, dose-dependent reduction in blood pressure. Recent work now focuses on treating the cardiac dysfunction associated with both Duchenne and Becker muscular dystrophy. This novel biotherapeutic has since been awarded Orphan Designation status by the FDA.
Maternal-fetal medicine
Pregnant women have largely been overlooked as a patient population by the pharmaceutical industry owing to the fear of formulating an agent that, while beneficial to the mother, may pose risks to the fetus[134]. Drug development for pregnancy-related disorders has therefore been a slow process. Preeclampsia, also known as pregnancy-induced hypertension, is one such disorder characterized by elevated blood pressure, swelling, and proteinuria. Left undetected, this condition can escalate to eclampsia which threatens both mother and child; one estimate indicates this causes 40 to 60% of all maternal deaths in developing countries[135]. The only effective treatment involves induction of delivery.
Encouraging breakthroughs by Bidwell and colleagues, however, suggest a novel solution to the problems of maternal drug delivery from his earlier work using SynB1-ELP, a cell penetrating peptide fusion[136]. Pregnant Sprague Dawley rats were given a bolus dose (100 mg/kg) intravenously to acquire pharmacokinetics data between an ELP control and SynB1-ELP. While plain ELPs possessed a higher initial plasma concentration (3,168 μg/mL) relative to SynB1-ELP (2,376 μg/mL), the latter exhibited faster extravasation; this indicates that the SynB1 moiety mediated quicker sequestering into tissue. After this rapid distribution in the first 60 minutes, a slower terminal half-life prevailed with SynB1-ELP terminal half-life lasting 190.2 min in contrast to 77.5 min for ELP alone.
Four hours after the bolus dose, tissues were removed and examined ex vivo using whole organ fluorescence imaging to deduce factors such as biodistribution, fetal uptake, and placental deposition. It was observed that ELP and SynB1-ELP constructs accumulated at significant amounts in placental tissue, although only minute levels were detected in the fetuses. Microscopic examination of placental tissues using a cytokeratin counterstain, meanwhile, confirmed that ELP existed within trophoblast cell cytoplasm yet remained excluded from the fetal side of the chorionic villi as intended. The effects of a 5 day continuous infusion of the two ELP groups was subsequently explored. Chronic infusion had a moderating effect on the biodistribution of SynB1-ELP when compared to ELP. In other words, the tissue levels of the two achieved similar values. Beyond this difference, results were comparable to those identified following a bolus dose. Fetal tissue invasion by either of the ELP groups was negligible.
Diabetes
Diabetes describes a cluster of chronic metabolic diseases, involving high blood glucose levels and insulin insufficiency, which affects more than 300 million people worldwide; the CDC further predicts that a staggering 1 in 3 Americans could be stricken by 2050[137]. Within this patient population, type 2 diabetes essentially amounts to 90 to 95 percent of all cases diagnosed in adults. One striking study pursued by Schellenberger et al focused on fusion of exenatide, a therapeutic analogue of glucagon-like peptide 1 improving glycemic control, to the recombinant polypeptide known as XTEN[22]. Based on allometric scaling in various animal models, this fusion protein was extrapolated to enhance the peptide's plasma half-life in humans from 2.4 hrs to 139 hrs, which could potentially enable once monthly administration.
These results provided suitable grounds for ELP experts to expand into new lines of investigation. More specifically, the 30 amino acid glucagon-like peptide 1 (GLP-1), which possesses a short half-life (<2 min), was chosen as an ELP fusion candidate due to its role in promoting insulin release from pancreatic β-cells[138]. Two 50 kDa (GLP-1)-ELPs were designed with the first property of room temperature solubility and the ability to form a stable, coacervate-based drug depot following subcutaneous injection into C57BL/6J mice; the second fusion served as a control which remained soluble at body temperature. Both constructs were examined in vitro for stability against neutral endopeptidase, a protease known to degrade GLP-1. Surprisingly, neither the soluble or depot-forming ELPs experienced degradation even after 18 hr incubation with the enzyme at 20 or 37°C. In contrast, the native GLP-1 was almost completely degraded. This underpins the fact that the increased molecular weight heightened GLP-1 stability and could be considered in the formulation of longer-acting therapies. GLP-1, however, still proved more potent (EC50=0.113 nM) in binding GLP-1 receptor versus depot-capable (EC50= 4.75 nM) and soluble (EC50= 5.59 nM) configurations.
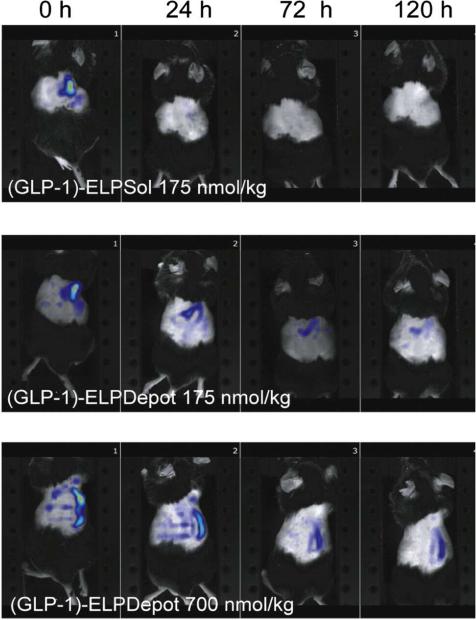
According to biodistribution studies determined by NIR fluorescence tomography (Fig. 6), the GLP-1 depot was retained at its injection site for over 120 hours, while the soluble GLP-1 formulation disappeared 24 hours post-injection. In vivo efficacy data later demonstrated that (GLP-1)-ELP formulation steadily reduced blood glucose levels up to ~30% in a dose-dependent manner (e.g. 175, 350, and 700 nmol/kg over 24, 72, and 144 hrs respectively). Alternatively, the soluble (GLP-1)-ELP rapidly precipitated a 60% glucose level decline at a dose of 175 nmol/kg, causing a peak and valley response indicative of more rapid onset and loss of activity. These results affirm that GLP-1 ELP fusions can reduce blood glucose levels across a range of doses at intervals that depend on the phase behavior of the ELP. Based on these exciting findings, (GLP-1)-ELP underwent Phase IIB trials as listed in Table 1 under the trade name Glymera™ for once weekly treatment of hyperglycemia associated with type 2 diabetes[91]. Additional work has been conducted with a proinsulin-ELP formulation, capable of being converted into mature insulin following subcutaneous administration, alongside novel coformulations involving (GLP-1)-ELP.
Figure 6.
NIR tomography images of 175 nmol/kg (GLP-1)-ELPSol, 175 nmol/kg (GLP-1)-ELPDepot, and 700 nmol/kg (GLP-1)-ELPDepot at 0, 24, 72 and 120 hr after subcutaneous injection. Reprinted from [138] with permission of Elsevier.
Table 1.
Elastin-like polypeptide (ELP) nanomedicines under commercial investigation.
| Product/Agent | Indications | Status | Company | References |
|---|---|---|---|---|
| siEVI1-ELP | Various cancers (breast, ovarian, pancreatic, and lung) | Preclinical | PeptiMed | [139] |
| Liposome-ELP conjugates | Breast cancer | Preclinical | Samsung | [98] |
| VIP-ELP (Vasomera™) | Pulmonary arterial hypertension, cardiomyopathies, cystic fibrosis | Phase I | PhaseBio | [130-133, 140, 141] |
| GLP1-ELP (Glymera™) | Type II diabetes | Phase IIB | PhaseBio | [61, 91, 138] |
Another prominent area in diabetes management involves treating chronic wounds such as pressure sores and foot ulcers. In the most extreme cases, amputations are performed to stem tissue damage. With the advent of biotechnology, however, unique solutions have emerged in the form of recombinant growth factors aimed at facilitating wound re-epithelialization. Exogenous keratinocyte growth factor (KGF) is a monomeric peptide shown to augment healing within wounds of complete and partial thickness in animal models[142]. Since KGF is administered topically, its activity and bioavailability are both attenuated, necessitating large quantities to evoke the desired clinical outcomes.
A KGF-ELP fusion was generated as a means of improving KGF efficacy by Yarmush and colleagues[143]. Chief among their findings was the fact that KGF-ELP formed into 500 nm nanoparticles despite the ELP segment having been designed linearly rather than as a diblock copolymer. These results are not unexpected, however, considering that several ELP fusions discussed previously have displayed similar behaviors[119, 124, 144]. Seeking to determine whether KGF-ELP could act on its receptor (KGFR), proliferation assays in A431 epithelial cells were performed. Despite the apparent involvement in the KGF domain in mediating particle assembly, proliferation rates were comparable for KGF-ELP and exogenous KGF (2.31-fold versus 2.0-fold respectively). A control ELP alone did not produce this growth response. Another encouraging observation was the fact that KGF-ELPs phosphorylated downstream effectors (e.g. ERK) of the receptor although at diminished levels relative to KGF. Finally, in vivo studies were conducted employing genetically diabetic male mice due to retarded wound healing. Treatments between KGF-ELP and free KGF revealed that the former induced slightly more prominent re-epithelialization (36% coverage versus 31% coverage respectively).
Regenerative medicine
Regenerative medicine seeks to restore lost functionality and promote self-healing of damaged tissues. Regarding applications of the ELP platform, the Heilshorn group pioneered a single-step process for rapid yet reproducible peptide conjugation coupled with crosslinking of ELP hydrogels[145]. In this case, a 15 amino acid peptide (QK) capable of augmented binding and stability relative to vascular endothelial growth factor (VEGF) was selected for ELP fusion. 2D cell proliferation studies using HUVECs determined that QK-ELPs recapitulated proliferation at both 10 nM and 1 μM relative to soluble QK in unmodified ELP hydrogels. Further work revealed a 10-fold increase in the normalized protrusion area of HUVEC spheroids, defined as a ratio of the cross-sectional area of protruding cells in the xy plane to the cross-sectional area of the spheroid itself, within a 3D hydrogel system.
ELP depot formulations yield another means of combating certain ailments including osteoarthritis due to the avascular nature of diseased sites. Intra-articular injections of 14C-labeled ELP depots in rat models led to sustained release with an 85 hr half-life. In dramatic contrast, the soluble ELP control only exhibited a 4 hr half-life[146]. A similar osteoarthritis-based study utilized ELPs fused to the interleukin-1 receptor antagonist (IL-1Ra) to recapitulate bioactivities of the parent molecule[78].
In the field of neural inflammation, one strategy employed to treat sciatica involved covalent conjugation of curcumin, a small molecule drug that binds tumor necrosis factor (TNFα). The appended ELP possessed a degradable carbamate linker to mediate drug release. Studies in vitro confirmed protection against cytotoxicity induced by TNFα at rates comparable to free drug. Intramuscular injections of curcumin-ELP at the sciatic nerve, meanwhile, demonstrated 5-fold higher levels of the drug at 96 hrs over its free counterpart[147].
Muscle proliferation and differentiation have likewise been investigated in tandem with ELP protein polymers. According to Ciofani et al, H9c2 rat myoblasts were affixed to polystyrene substrates with two different ELPs either containing or lacking cross-linking domains, and quantitative fluorescence confirmed greater adhesion and proliferation by these groups relative to a bare polystyrene control. Measuring the sizes of myotubes during growth further demonstrated that ELPs (length=980 μm, width=200 μm) outclassed their counterparts (length=280 μm, width=60 μm) as potential substrates for muscle tissue regeneration[148].
Infectious diseases
Anti-infective drugs, such as antibiotics and antivirals, are anticipated to comprise a market surpassing 80 billion USD by 2017[149]. Since current treatments for systemic bacterial infections have largely been confined to intravenous antibiotic delivery, the sustained release potential of injectable, in situ forming ELP depots was explored by Adams et al[150]. In this study, ELPs with periodic lysine residues were covalently cross-linked to β-[Tris(hydroxymethyl) phosphine] proprionic acid (THPP) and loaded with either vancomycin or cefazolin. Therapeutic concentrations of the two drugs were released at all times. Vancomycin (drug release time constant (τ) =1170 ± 90 hrs) proved more effective than cefazolin (τ =32 ± 2 hrs) in prolonging release rates at ELP concentrations of 225 mg/mL. Additionally, both vancomycin and cefazolin ELP depots exhibited inhibition of B. subtilis. Another small molecule antibiotic, doxycycline, was explored by Amruthwar and Janorkar, which focused on delivery from a hydrogel scaffold[151].
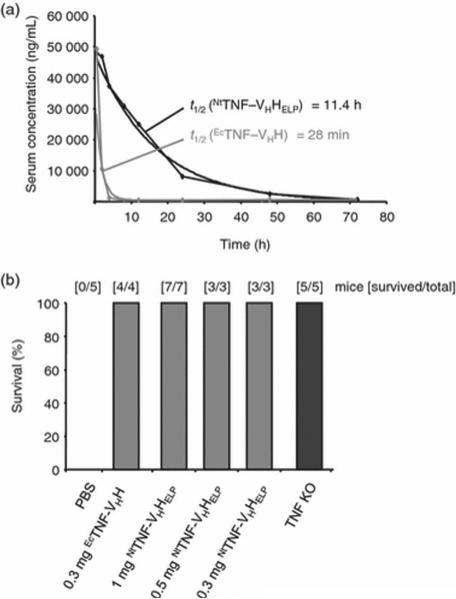
Interestingly, while E. coli remains the most common organism employed for ELP biosynthesis, plant-based expression systems are also gaining traction[26, 64]. A study by Scheller and colleagues[152] used transgenic tobacco plants (Nicotiana tabacum) to produce ELP fusions of camel-derived heavy chain antibodies targeting tumor necrosis factor (TNF). Previous data from this group established the viability of this approach since ELP fusions yielded a 40-fold increase in scFv levels over conventional methods[153]. In addition, the scFv ELPs remained stable within mature seeds for long periods at room temperature and demonstrated comparable binding affinities to their cognate antigen as compared to controls lacking an ELP. In this set of studies, the dissociation constants of anti-TNF ELP (Kd=1.3±0.098 nM) versus its non-ELP, E.coli derived counterpart (Kd=0.59±0.032 nM) against TNF were comparable. Both constructs likewise prevented TNF-mediated cell death in vitro in a dose-dependent manner. Strikingly, anti-TNF ELPs provided an 11.4 hr serum half-life in C57/BL6 mice whereas the non-ELP bound antibody only exhibited a 28 min half-life (Figure 7A). Subsequent experiments revealed that the ELP fusion was just as effective as the non-ELP antibody in septic shock models (Figure 7B).
Figure 7. Serum half-life of NtTNF-VHHELP and neutralization of LPS/D-Gal toxicity by NtTNF-VHHELP.
(A) Three mice were intravenously injected with 100 μg plant-derived NtTNF-VHHELP and for control with 100 μg EcTNF-VHH. Serum samples were prepared over a time range post-injection. (B) LPS/D-gal-induced septic shock was blocked by NtTNF-VHHELP and EcTNF-VHH. Survival was monitored 24-h post-injection. Reprinted from [152] with permission of John Wiley and Sons.
With regard to antiviral strategies, plant-derived scFv ELPs have been similarly instructive. As a proof of concept, the 2F5 mAb directed against HIV-1 glycoprotein 41 was adapted into an ELP fusion protein[154]. The binding kinetics of the 2F5-derived antibodies were similar to CHO-derived controls, but ELPs strangely yielded absolute antigen binding activities surpassing 100% (152.3% 2F5-ELP versus 94.1% CHO2F5), though confounding factors were not elucidated. In follow-up studies, another anti-HIV mAb (2G12) with neutralizing abilities was also investigated[155]. Fusion of ELP to various antibody domains led to increasing declines in 2G12 neutralization activity (IC50= 5.24 to 29.44 μg/mL) relative to the CHO-derived Ab (IC50=3.71 μg/mL).
Certain studies have also employed the ELP platform in the development of novel vaccine candidates. Seeking a vaccine against the parasitic flatworm Schistosoma japonicum, Solomon and colleagues[156] designed a cleavable ELP-thioredoxin linker fused to the 67 kDa Sj67 surface membrane protein. Although the ELP fusion enhanced solubility of recombinant Sj67, the protein was not amenable to transition cycling and other methods (e.g. size-exclusion chromatography) were employed for purification. The preferential IgG binding to rSj67 (4.7-fold greater median fluorescence) in the sera of 13 schistosomiasis japonica infected volunteers relative to five uninfected controls was confirmed using bead-based ELISA. Additional diseases for which potential ELP-based vaccines are being explored include tuberculosis [157, 158], influenza[159, 160], and Psittacine beak and feather disease[161].
The development of cytotoxic T-lymphocyte vaccine carriers using the previously mentioned iTEPs has also been described[88]. In this manuscript, a diblock copolymer consisting of hydrophobic iTEPA and hydrophilic iTEPB was fused to pOVA containing the SIINFEKL epitope. The resulting construct formed a micelle with mean diameters of 81.2 ± 14.2 nm at 5 μM and 71.9 ± 20.8 nm at 25 μM respectively. After incubation with dendritic cells, surface presentation of SIINFEKL was more pronounced for the iTEP nanoparticles compared to soluble iTEPB–pOVA or OVA. Follow-up studies deduced that CD8+ T-cells co-cultured with iTEP-pretreated dendritic cells exhibited more activity than DC-treated T-cells (4.38-fold), OVA/DC-treated T-cells (3.81-fold), and iTEPB–pOVA/DC-treated T-cells (2.9-fold). Subcutaneous immunization of C57BL/6 mice likewise revealed that iTEP NPs were capable of activating greater numbers of cytotoxic T-lymphocytes in vivo than either OVA or free SIINFEKL peptide.
Gene delivery
Since its first exploration in the 1990s, gene therapy—the paradigm of treating diseases through delivery of corrective genes to the patient—has presented researchers both with a compelling tool to revolutionize medicine and the unfortunate reality that more must be done to perfect the technology. Viruses are considered the standard vehicles for gene delivery where many strategies focus on encapsulating functional genes into a viral vector capable of transducing target cells. However, this method alone possesses various pitfalls; gene incorporation via adenoviral vectors is transient due to episomal regulation[162], while retrovirally-introduced genes are prone to random integration which leads to insertional mutagenesis[163].
Adeno-associated virus (AAV), by contrast, is a single-stranded DNA virus that has been demonstrated to be a safe vector for constitutive, therapeutic expression of various diseases; another beneficial attribute of AAV lies in its capacity to transduce both dividing and non-dividing cells for gene delivery[164, 165]. Seeking to enhance transduction into human neural stem cells and fibroblasts, an AAV r3.45 vector generated through directed evolution was adsorbed nonspecifically onto tissue culture plates containing hydrophobic monoblock ELPs with 128 repeats of VPGVG[166]. 24 hr incubation with ELP substrate provided an AAV immobilization rate greater than 50%. Dissociation kinetics for adsorbed AAV was favorable with <14% of the immobilized vector being released after 4 days to allow for sustained delivery. As such, the ELP-functionalized AAV system lessened the amount of vector required to induce cellular transduction when compared against a bolus delivery control.
Beyond viral gene therapy, ELPs have also been reported to mediate plasmid DNA delivery[167]. One study focused on the treatment of critical limb ischemia [168] using an injectable system comprised of ELP hollow spheres within an in situ-forming ELP gel scaffold to deliver plasmid eNOS and IL-10. H&E stains of C57BL/6 mouse sections were performed following an in vivo subcutaneous study to determine blood vessel density (e.g. angiogenesis) and inflammation post-administration. Two treatment groups, eNOS (20 μg) and IL-10 (10 μg)/eNOS (20 μg), exhibited higher blood vessel density by day 14 than controls or IL-10 treatment groups. Inflammation was measured using the volume fraction of infiltrated inflammatory cells. By day 7, this volume fraction decreased 30% for IL-10 and IL-10/eNOS treatment groups compared to ELP and eNOS, although no statistically significant difference could be observed by day 14. Unilateral hind limb ischemia was then induced in the mice to deduce how treatment groups influence angiogenesis. Assessing clinical severity, the saline group demonstrated minimal functional recovery with 3/7 animals in the group exhibiting severely necrotic limbs; the ELP, IL-10, and eNOS groups fared slightly better than saline. The eNOS/IL-10 group, by contrast, outperformed with 5/7 ischemic mice experiencing functional recovery. Follow-up studies involving skeletal muscle samples stained with H&E to further investigate angiogenesis revealed that eNOS and IL-10/eNOS groups were superior to saline, ELP, and IL-10 groups by day 21. In terms of inflammation, the volume fraction of inflammatory cells was reduced by 50% for IL-10 (10 μg), IL-10 (10 μg)/eNOS (20 μg), and eNOS on day 21 relative to saline.
The rise of gene silencing via RNA interference further equips researchers with another tool needed to circumvent ailments at the genetic level. First discovered in plants, RNA interference relies on the cleavage of either endogenous or exogenous dsRNA (e.g. viral infection, laboratory transfection) by the endoribonuclease Dicer[169]. This results in the emergence of 21-30 long nucleotides, known as small interfering RNA (siRNA), which are partitioned into RNA-induced silencing complexes. After activation, siRNAs acquire the ability to seek and destroy mRNA transcripts in order to halt protein expression. PeptiMed Inc, for example, has formulated ELPs as part of their anti-cancer strategy aimed at enhancing uptake and delivery of siRNA targeting the proto-oncogene EVI1[139].
Respiratory diseases
Cystic fibrosis, an autosomal recessive disorder arising from defects in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR), is the second most common inherited disease after sickle cell anemia in the US[170]. This illness disproportionately affects Caucasians (1 in 2,500 birth incidence) with at least 30,000 Americans suffering from the disease[171]. Cystic fibrosis is primarily characterized by the presence of thick, sticky mucus responsible for blocking the airways, causing lung damage, and promoting chronic lung infections. Recent studies based at PhaseBio Inc have demonstrated that VIP-ELPs can be repurposed towards treatment of Cystic fibrosis[141]. Similar to recent drugs on the market acting as channel potentiators (e.g. ivacaftor), VIP-ELPs were capable of regulating Cl− ions. Most excitingly, VIP-ELP effectively recapitulated both membrane insertion and channel functioning of misfolded ΔF508 (the most prevalent CFTR mutation) in human nasal epithelial cells.
Conclusions
The purpose of this review has been to describe state-of-the-art developments involving elastin-like polypeptides as a therapy-potentiating platform technology. With a myriad of applications already disclosed, one can reason that these protein polymers might soon provide a succinct yet versatile means of solving real clinical challenges. If their safety and efficacy can be demonstrated in one of these many settings, this advance may open the door to using these dynamic materials to treat a wide range of conditions. In closing, elastin-like polypeptides are poised to render more tangible the next era of healthcare and pharmaceutical science—nanomedicine.
Acknowledgements
This manuscript was made possible by the National Institute of Health R01EB020053 to J.A.M., NIH EY011386 to S.F.H-A UL1RR031986 to the SC CTSI and P30 CA014089 to the Norris Comprehensive Cancer Center, the USAMRMC/TATRC grant W81XWH1210538, the Stop Cancer Foundation, the USC Ming Hsieh Institute, the USC Wright Foundation, and the USC Whittier Foundation.
J.A.M., S.F.H., and J.P.D. are inventors on patents related to uses for elastin-like polypeptides.
List of Abbreviations
- AAV
Adeno-associated virus
- AntP
Penetratin
- BPE
Bovine pituitary extract
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- Chol
Cholesterol
- CMT
Critical micelle temperature
- CN
Contortrostatin
- CPP
Cell-penetrating peptide
- CVD
Cardiovascular disease
- DIC
Differential interference contrast
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DSPE-PEG
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000]
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- ELP
Elastin-like polypeptide
- eNOS
Endothelial nitric oxide synthase
- EPR
Enhanced permeability and retention effect
- FKBP
FK506 binding protein 12
- FSI
FKBP-S48I48
- ΔG
Change in Gibbs free energy
- GLP-1
Glucagon-like peptide 1
- ΔH
Change in enthalpy
- H&E
Hematoxylin and eosin
- IL-1Ra
Interleukin 1 receptor antagonist
- IL-10
Interleukin 10
- ITC
Inverse transition cycling
- iTEP
Immune-tolerant elastin-like polypeptides
- KGF
Keratinocyte growth factor
- KGFR
Keratinocyte growth factor receptor
- LSI
Lacritin-S48I48
- mAb
Monoclonal antibody
- Mn
Number-average molecular weight
- mTOR
Mammalian target of rapamycin
- Mw
Mass-average molecular weight
- NOD
Non-obese diabetic
- OERCA
Overlap extension rolling circle amplification
- OVA
Ovalbumin
- PCL
Polycaprolactone
- PCR
Polymerase chain reaction
- PDI
Polydispersity index
- PEG
Polyethylene glycol
- PRe-RDL
Recursive directional ligation by plasmid reconstruction
- qPCR
Quantitative polymerase chain reaction
- ΔS
Change in entropy
- scFv
Single chain variable fragment
- siRNA
Small interfering RNA
- SI
S48I48
- SjS
Sjögren's syndrome
- SLP
Silk-like polypeptide
- THPP
β-[Tris(hydroxymethyl) phosphine] proprionic acid
- TNFα
Tumor necrosis factor alpha
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- T
Temperature
- Tt
Transition temperature
- VCN
Vicrostatin
- VEGF
Vascular endothelial growth factor
- VIP
Vasoactive intestinal peptide
- XTEN
Extended recombinant polypeptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
J.D. is co-founder of a start-up company, S-aima Biopharmaceutics, commercializing elastin-like polypeptides.
References
- 1.Strebhardt K, Ullrich A. Paul Ehrlich's magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8:473–480. doi: 10.1038/nrc2394. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman AS. The origins and evolution of “controlled” drug delivery systems. Journal of Controlled Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Cho K, Wang X, Nie S, Chen Z, Shin DM. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clinical Cancer Research. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 4.Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: A royal gate for targeted anticancer nanomedicines. Journal of Drug Targeting. 2007;15:457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- 5.Sharom FJ. The P-glycoprotein efflux pump: how does it transport drugs? The Journal of membrane biology. 1997;160:161–175. doi: 10.1007/s002329900305. [DOI] [PubMed] [Google Scholar]
- 6.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends in Pharmacological Sciences. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Owens DE, 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International journal of pharmaceutics. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Barenholz Y. Doxil(R)--the first FDA-approved nano-drug: lessons learned. Journal of controlled release : official journal of the Controlled Release Society;2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 10.Mitragotri S, Anderson DG, Chen X, Chow EK, Ho D, Kabanov AV, Karp JM, Kataoka K, Mirkin CA, Petrosko SH, Shi J, Stevens MM, Sun S, Teoh S, Venkatraman SS, Xia Y, Wang S, Gu Z, Xu C. Accelerating the Translation of Nanomaterials in Biomedicine. ACS nano. 2015;9:6644–6654. doi: 10.1021/acsnano.5b03569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vicent MJ, Duncan R. Polymer conjugates: nanosized medicines for treating cancer. Trends Biotechnol. 2006;24:39–47. doi: 10.1016/j.tibtech.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.MacKay JA, Chen M, McDaniel JR, Liu W, Simnick AJ, Chilkoti A. Self-assembling chimeric polypeptide-doxorubicin conjugate nanoparticles that abolish tumours after a single injection. Nat Mater. 2009;8:993–999. doi: 10.1038/nmat2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai-Kato K, Nishiyama N, Kozaki M, Nakanishi T, Matsuda Y, Hirano M, Hanada H, Hisada S, Onodera H, Harashima H, Matsumura Y, Kataoka K, Goda Y, Okuda H, Kawanishi T. General considerations regarding the in vitro and in vivo properties of block copolymer micelle products and their evaluation. Journal of Controlled Release. 2015;210:76–83. doi: 10.1016/j.jconrel.2015.05.259. [DOI] [PubMed] [Google Scholar]
- 14.Rogošić M, Mencer HJ, Gomzi Z. Polydispersity index and molecular weight distributions of polymers. European Polymer Journal. 1996;32:1337–1344. [Google Scholar]
- 15.Yamaoka T, Tabata Y, Ikada Y. Fate of water-soluble polymers administered via different routes. Journal of pharmaceutical sciences. 1995;84:349–354. doi: 10.1002/jps.2600840316. [DOI] [PubMed] [Google Scholar]
- 16.Veronese FM. Peptide and protein PEGylation: a review of problems and solutions. Biomaterials. 2001;22:405–417. doi: 10.1016/s0142-9612(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 17.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer. 2006;6:688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan N, Kumar S. Ordered and disordered proteins as nanomaterial building blocks, Wiley Interdisciplinary Reviews. Nanomedicine and Nanobiotechnology. 2012;4:204–218. doi: 10.1002/wnan.1160. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari FA, Richardson C, Chambers J, Causey SC, Pollock TJ, Capello J, Crissman JW. Construction of synthetic DNA and its use in large polypeptide synthesis. Google Patents. 1993 [Google Scholar]
- 20.Hakoshima T. Leucine Zippers. eLS, John Wiley & Sons, Ltd; 2001. [Google Scholar]
- 21.Luo T, Kiick KL. Collagen-like peptides and peptide–polymer conjugates in the design of assembled materials. European Polymer Journal. 2013;49:2998–3009. doi: 10.1016/j.eurpolymj.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schellenberger V, Wang C.-w., Geething NC, Spink BJ, Campbell A, To W, Scholle MD, Yin Y, Yao Y, Bogin O, Cleland JL, Silverman J, Stemmer WPC. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nat Biotech. 2009;27:1186–1190. doi: 10.1038/nbt.1588. [DOI] [PubMed] [Google Scholar]
- 23.Valluzzi R, Winkler S, Wilson D, Kaplan DL. Silk: molecular organization and control of assembly. 2002 doi: 10.1098/rstb.2001.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urry DW. Physical Chemistry of Biological Free Energy Transduction As Demonstrated by Elastic Protein-Based Polymers. The Journal of Physical Chemistry B. 1997;101:11007–11028. [Google Scholar]
- 25.Frandsen JL, Ghandehari H. Recombinant protein-based polymers for advanced drug delivery. Chemical Society Reviews. 2012;41:2696–2706. doi: 10.1039/c2cs15303c. [DOI] [PubMed] [Google Scholar]
- 26.Floss DM, Schallau K, Rose-John S, Conrad U, Scheller J. Elastin-like polypeptides revolutionize recombinant protein expression and their biomedical application. Trends in Biotechnology. 2010;28:37–45. doi: 10.1016/j.tibtech.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Hassouneh W, MacEwan SR, Chilkoti A. Fusions of elastin-like polypeptides to pharmaceutical proteins. Methods in enzymology. 2012;502:215–237. doi: 10.1016/B978-0-12-416039-2.00024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldock C, Oberhauser AF, Ma L, Lammie D, Siegler V, Mithieux SM, Tu Y, Chow JYH, Suleman F, Malfois M, Rogers S, Guo L, Irving TC, Wess TJ, Weiss AS. Shape of tropoelastin, the highly extensible protein that controls human tissue elasticity. Proceedings of the National Academy of Sciences. 2011;108:4322–4327. doi: 10.1073/pnas.1014280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urry DW, Luan CH, Peng SQ. Molecular biophysics of elastin structure, function and pathology. Ciba Foundation symposium. 1995;192:4–22. doi: 10.1002/9780470514771.ch2. discussion 22-30. [DOI] [PubMed] [Google Scholar]
- 30.Partridge SM, Davis HF, Adair GS. The chemistry of connective tissues. 2. Soluble proteins derived from partial hydrolysis of elastin. The Biochemical journal. 1955;61:11–21. doi: 10.1042/bj0610011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciofani G, Genchi GG, Mattoli V, Mazzolai B, Bandiera A. The potential of recombinant human elastin-like polypeptides for drug delivery. Expert Opinion on Drug Delivery. 2014;11:1507–1512. doi: 10.1517/17425247.2014.926885. [DOI] [PubMed] [Google Scholar]
- 32.Urry DW, Starcher B, Partridge SM. Coacervation of Solubilized Elastin effects a Notable Conformational Change. Nature. 1969;222:795–796. doi: 10.1038/222795a0. [DOI] [PubMed] [Google Scholar]
- 33.Cox BA, Starcher BC, Urry DW. Communication: Coacervation of tropoelastin results in fiber formation. J Biol Chem. 1974;249:997–998. [PubMed] [Google Scholar]
- 34.Meyer DE, Chilkoti A. Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotech. 1999;17:1112–1115. doi: 10.1038/15100. [DOI] [PubMed] [Google Scholar]
- 35.Meyer DE, Chilkoti A. Genetically Encoded Synthesis of Protein-Based Polymers with Precisely Specified Molecular Weight and Sequence by Recursive Directional Ligation: Examples from the Elastin-like Polypeptide System. Biomacromolecules. 2002;3:357–367. doi: 10.1021/bm015630n. [DOI] [PubMed] [Google Scholar]
- 36.Chilkoti A, Dreher MR, Meyer DE. Design of thermally responsive, recombinant polypeptide carriers for targeted drug delivery. Advanced Drug Delivery Reviews. 2002;54:1093–1111. doi: 10.1016/s0169-409x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 37.Mackay JA, Chilkoti A. Temperature sensitive peptides: Engineering hyperthermia-directed therapeutics. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2008;24:483. doi: 10.1080/02656730802149570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastuszka MK, Janib SM, Weitzhandler I, Okamoto CT, Hamm-Alvarez S, MacKay JA. A Tunable and Reversible Platform for the Intracellular Formation of Genetically Engineered Protein Microdomains. Biomacromolecules. 2012;13:3439–3444. doi: 10.1021/bm301090x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim DW, Nettles DL, Setton LA, Chilkoti A. Rapid Cross-Linking of Elastin-like Polypeptides with (Hydroxymethyl)phosphines in Aqueous Solution. Biomacromolecules. 2007;8:1463–1470. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reichheld SE, Muiznieks LD, Stahl R, Simonetti K, Sharpe S, Keeley FW. Conformational Transitions of the Cross-linking Domains of Elastin during Self-assembly. Journal of Biological Chemistry. 2014;289:10057–10068. doi: 10.1074/jbc.M113.533893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowalczyk T, Hnatuszko-Konka K, Gerszberg A, Kononowicz AK. Elastin-like polypeptides as a promising family of genetically-engineered protein based polymers. World Journal of Microbiology & Biotechnology. 2014;30:2141–2152. doi: 10.1007/s11274-014-1649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts S, Dzuricky M, Chilkoti A. Elastin-like polypeptides as models of intrinsically disordered proteins. FEBS letters. 2015;589:2477–2486. doi: 10.1016/j.febslet.2015.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urry DW, Trapane TL, Prasad KU. Phase-structure transitions of the elastin polypentapeptide–water system within the framework of composition–temperature studies. Biopolymers. 1985;24:2345–2356. doi: 10.1002/bip.360241212. [DOI] [PubMed] [Google Scholar]
- 44.Urry DW, Urry KD, Szaflarski W, Nowicki M. Elastic-contractile model proteins: Physical chemistry, protein function and drug design and delivery. Advanced Drug Delivery Reviews. 2010;62:1404–1455. doi: 10.1016/j.addr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Pastuszka MK, Okamoto CT, Hamm-Alvarez SF, MacKay JA. Flipping the Switch on Clathrin-Mediated Endocytosis using Thermally Responsive Protein Microdomains. Advanced functional materials. 2014;24:5340–5347. doi: 10.1002/adfm.201400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDaniel JR, Callahan DJ, Chilkoti A. Drug delivery to solid tumors by elastin-like polypeptides. Advanced drug delivery reviews. 2010;62:1456–1467. doi: 10.1016/j.addr.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valiaev A, Lim DW, Schmidler S, Clark RL, Chilkoti A, Zauscher S. Hydration and Conformational Mechanics of Single, End-Tethered Elastin-like Polypeptides. Journal of the American Chemical Society. 2008;130:10939–10946. doi: 10.1021/ja800502h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thapa A, Han W, Simons RH, Chilkoti A, Chi EY, López GP. Effect of Detergents on the Thermal Behavior of Elastin-like Polypeptides. Biopolymers. 2013;99:55–62. doi: 10.1002/bip.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho Y, Zhang Y, Christensen T, Sagle LB, Chilkoti A, Cremer PS. Effects of Hofmeister Anions on the Phase Transition Temperature of Elastin-like Polypeptides. The Journal of Physical Chemistry B. 2008;112:13765–13771. doi: 10.1021/jp8062977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen T, Hassouneh W, Trabbic-Carlson K, Chilkoti A. Predicting Transition Temperatures of Elastin-Like Polypeptide Fusion Proteins. Biomacromolecules. 2013;14:1514–1519. doi: 10.1021/bm400167h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Urry DW. Molecular Machines: How Motion and Other Functions of Living Organisms Can Result from Reversible Chemical Changes. Angewandte Chemie International Edition in English. 1993;32:819–841. [Google Scholar]
- 52.Jung Y, Bayley H, Movileanu L. Temperature-Responsive Protein Pores. Journal of the American Chemical Society. 2006;128:15332–15340. doi: 10.1021/ja065827t. [DOI] [PubMed] [Google Scholar]
- 53.Urry DW, Hayes LC, Gowda DC, Harris CM, Harris RD. Reduction-driven polypeptide folding by the Tt mechanism. Biochemical and Biophysical Research Communications. 1992;188:611–617. doi: 10.1016/0006-291x(92)91100-5. [DOI] [PubMed] [Google Scholar]
- 54.Ciofani G, Genchi GG, Guardia P, Mazzolai B, Mattoli V, Bandiera A. Recombinant human elastin-like magnetic microparticles for drug delivery and targeting. Macromolecular bioscience. 2014;14:632–642. doi: 10.1002/mabi.201300361. [DOI] [PubMed] [Google Scholar]
- 55.Shimoboji T, Ding ZL, Stayton PS, Hoffman AS. Photoswitching of Ligand Association with a Photoresponsive Polymer–Protein Conjugate. Bioconjugate Chemistry. 2002;13:915–919. doi: 10.1021/bc010057q. [DOI] [PubMed] [Google Scholar]
- 56.Haider M, Leung V, Ferrari F, Crissman J, Powell J, Cappello J, Ghandehari H. Molecular Engineering of Silk-Elastinlike Polymers for Matrix-Mediated Gene Delivery: Biosynthesis and Characterization. Molecular Pharmaceutics. 2005;2:139–150. doi: 10.1021/mp049906s. [DOI] [PubMed] [Google Scholar]
- 57.Janib SM, Pastuszka M, Aluri S, Folchman-Wagner Z, Hsueh PY, Shi P, Yi A, Cui H, Mackay JA. A quantitative recipe for engineering protein polymer nanoparticles. Polymer chemistry. 2014;5:1614–1625. doi: 10.1039/C3PY00537B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurihara H, Morita T, Shinkai M, Nagamune T. Recombinant extracellular matrix-like proteins with repetitive elastin or collagen-like functional motifs. Biotechnology letters. 2005;27:665–670. doi: 10.1007/s10529-005-4477-8. [DOI] [PubMed] [Google Scholar]
- 59.McMillan RA, Lee TAT, Conticello VP. Rapid Assembly of Synthetic Genes Encoding Protein Polymers. Macromolecules. 1999;32:3643–3648. [Google Scholar]
- 60.Amiram M, Quiroz FG, Callahan DJ, Chilkoti A. A highly parallel method for synthesizing DNA repeats enables the discovery of 'smart' protein polymers. Nature materials. 2011;10:141–148. doi: 10.1038/nmat2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. Injectable protease-operated depots of glucagon-like peptide-1 provide extended and tunable glucose control. Proceedings of the National Academy of Sciences. 2013;110:2792–2797. doi: 10.1073/pnas.1214518110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sallach RE, Conticello VP, Chaikof EL. Expression of a recombinant elastin-like protein in pichia pastoris. Biotechnology Progress. 2009;25:1810–1818. doi: 10.1002/btpr.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herzog RW, Singh NK, Urry DW, Daniell H. Expression of a synthetic protein-based polymer (elastomer) gene in Aspergillus nidulans. Applied microbiology and biotechnology. 1997;47:368–372. doi: 10.1007/s002530050942. [DOI] [PubMed] [Google Scholar]
- 64.Conley AJ, Joensuu JJ, Jevnikar AM, Menassa R, Brandle JE. Optimization of elastin-like polypeptide fusions for expression and purification of recombinant proteins in plants. Biotechnology and Bioengineering. 2009;103:562–573. doi: 10.1002/bit.22278. [DOI] [PubMed] [Google Scholar]
- 65.Wright ER, Conticello VP. Self-assembly of block copolymers derived from elastin mimetic polypeptide sequences. Advanced Drug Delivery Reviews. 2002;54:1057–1073. doi: 10.1016/s0169-409x(02)00059-5. [DOI] [PubMed] [Google Scholar]
- 66.Shi P, Aluri S, Lin YA, Shah M, Edman M, Dhandhukia J, Cui H, MacKay JA. Elastin-based protein polymer nanoparticles carrying drug at both corona and core suppress tumor growth in vivo. Journal of controlled release : official journal of the Controlled Release Society. 2013;171:330–338. doi: 10.1016/j.jconrel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun G, Hsueh PY, Janib SM, Hamm-Alvarez S, MacKay JA. Design and cellular internalization of genetically engineered polypeptide nanoparticles displaying adenovirus knob domain. J Control Release. 2011;155:218–226. doi: 10.1016/j.jconrel.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dreher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoti A. Temperature Triggered Self-Assembly of Polypeptides into Multivalent Spherical Micelles. Journal of the American Chemical Society. 2008;130:687–694. doi: 10.1021/ja0764862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kojima C, Irie K. Synthesis of temperature-dependent elastin-like peptide-modified dendrimer for drug delivery. Peptide Science. 2013;100:714–721. doi: 10.1002/bip.22276. [DOI] [PubMed] [Google Scholar]
- 70.Park WM, Champion JA. Thermally Triggered Self-Assembly of Folded Proteins into Vesicles. Journal of the American Chemical Society. 2014;136:17906–17909. doi: 10.1021/ja5090157. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Cai L, Paul A, Enejder A, Heilshorn SC. Hybrid Elastin-like Polypeptide– Polyethylene Glycol (ELP-PEG) Hydrogels with Improved Transparency and Independent Control of Matrix Mechanics and Cell Ligand Density. Biomacromolecules. 2014;15:3421–3428. doi: 10.1021/bm500969d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacEwan SR, Hassouneh W, Chilkoti A. Non-chromatographic Purification of Recombinant Elastin-like Polypeptides and their Fusions with Peptides and Proteins from Escherichia coli. 2014:e51583. doi: 10.3791/51583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christensen T, Amiram M, Dagher S, Trabbic-Carlson K, Shamji MF, Setton LA, Chilkoti A. Fusion order controls expression level and activity of elastin-like polypeptide fusion proteins. Protein science : a publication of the Protein Society. 2009;18:1377–1387. doi: 10.1002/pro.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simnick AJ, Valencia CA, Liu R, Chilkoti A. Morphing low-affinity ligands into high-avidity nanoparticles by thermally triggered self-assembly of a genetically encoded polymer. ACS Nano. 2010;4:2217–2227. doi: 10.1021/nn901732h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dreher MR, Raucher D, Balu N, Michael Colvin O, Ludeman SM, Chilkoti A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. Journal of controlled release : official journal of the Controlled Release Society. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 76.Massodi I, Bidwell GL, 3rd, Raucher D. Evaluation of cell penetrating peptides fused to elastin-like polypeptide for drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2005;108:396–408. doi: 10.1016/j.jconrel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 77.Bidwell GL, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Molecular Cancer Therapeutics. 2005;4:1076–1085. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 78.Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: Sustained release of a local antiinflammatory therapeutic. Arthritis & Rheumatism. 2007;56:3650–3661. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 79.Liu JC, Heilshorn SC, Tirrell DA. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules. 2004;5:497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 80.Du K, Sun J, Song X, Song C, Feng W. Enhancement of the solubility and stability of d-amino acid oxidase by fusion to an elastin like polypeptide. Journal of Biotechnology. 2015;212:50–55. doi: 10.1016/j.jbiotec.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 81.Fong BA, Gillies AR, Ghazi I, LeRoy G, Lee KC, Westblade LF, Wood DW. Purification of Escherichia coli RNA polymerase using a self-cleaving elastin-like polypeptide tag. Protein Science. 2010;19:1243–1252. doi: 10.1002/pro.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Janib SM, Liu S, Park R, Pastuszka MK, Shi P, Moses AS, Orosco MM, Lin YA, Cui H, Conti PS, Li Z, MacKay JA. Kinetic quantification of protein polymer nanoparticles using non-invasive imaging. Integrative Biology. 2013;5:183–194. doi: 10.1039/c2ib20169k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin S, Seo JW, Zhang H, Qi J, Curry F-RE, Ferrara KW. An imaging-driven model for liposomal stability and circulation. Molecular pharmaceutics. 2009;7:12–21. doi: 10.1021/mp900122j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu W, Dreher MR, Furgeson DY, Peixoto KV, Yuan H, Zalutsky MR, Chilkoti A. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. Journal of controlled release : official journal of the Controlled Release Society. 2006;116:170–178. doi: 10.1016/j.jconrel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 85.Megeed Z, Cappello J, Ghandehari H. Genetically engineered silk-elastinlike protein polymers for controlled drug delivery. Adv Drug Deliv Rev. 2002;54:1075–1091. doi: 10.1016/s0169-409x(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 86.Cappello J, Crissman JW, Crissman M, Ferrari FA, Textor G, Wallis O, Whitledge JR, Zhou X, Burman D, Aukerman L, Stedronsky ER. In-situ self-assembling protein polymer gel systems for administration, delivery, and release of drugs. Journal of controlled release : official journal of the Controlled Release Society. 1998;53:105–117. doi: 10.1016/s0168-3659(97)00243-5. [DOI] [PubMed] [Google Scholar]
- 87.Nouri FS, Wang X, Chen X, Hatefi A. Reducing the Visibility of the Vector/DNA Nanocomplexes to the Immune System by Elastin-Like Peptides. Pharm Res. 2015 doi: 10.1007/s11095-015-1683-5. [DOI] [PubMed] [Google Scholar]
- 88.Cho S, Dong S, Parent KN, Chen M. Immune-tolerant elastin-like polypeptides (iTEPs) and their application as CTL vaccine carriers. Journal of Drug Targeting. 2015:1–12. doi: 10.3109/1061186X.2015.1077847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer DE, Kong GA, Dewhirst MW, Zalutsky MR, Chilkoti A. Targeting a genetically engineered elastin-like polypeptide to solid tumors by local hyperthermia. Cancer research. 2001;61:1548–1554. [PubMed] [Google Scholar]
- 90.Urry DW, Parker TM. Mechanics of elastin: molecular mechanism of biological elasticity and its relationship to contraction. Journal of muscle research and cell motility. 2002;23:543–559. [PubMed] [Google Scholar]
- 91.Pharmaceutics P. Phase 2b Multicenter, Randomized, Double-Blind, Placebo- and Active-Controlled. Parallel-Group Study to Assess the PD Response and Safety of Three Dose Levels of Glymera Injection Following 20 Weeks of Weekly SC Dosing in Adults With T2DM. 2012 [Google Scholar]
- 92.Shah M, Hsueh P-Y, Sun G, Chang HY, Janib SM, MacKay JA. Biodegradation of elastin-like polypeptide nanoparticles. Protein Science. 2012;21:743–750. doi: 10.1002/pro.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pillai G. Nanomedicines for Cancer Therapy: An Update of FDA Approved and Those under Various Stages of Development. SOJ Pharmacy & Pharmaceutical Sciences. 2014;1:13. [Google Scholar]
- 94.Wang R, Billone PS, Mullett WM. Nanomedicine in Action: An Overview of Cancer Nanomedicine on the Market and in Clinical Trials. Journal of Nanomaterials. 2013;2013:12. [Google Scholar]
- 95.Society AC. Cancer Facts and Figures. 2015 [Google Scholar]
- 96.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 97.Furgeson DY, Dreher MR, Chilkoti A. Structural optimization of a “smart” doxorubicin– polypeptide conjugate for thermally targeted delivery to solid tumors. Journal of Controlled Release. 2006;110:362–369. doi: 10.1016/j.jconrel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Park SM, Cha JM, Nam J, Kim MS, Park S-J, Park ES, Lee H, Kim HR. Formulation Optimization and In Vivo Proof-of-Concept Study of Thermosensitive Liposomes Balanced by Phospholipid, Elastin-Like Polypeptide, and Cholesterol. PLoS ONE. 2014;9:e103116. doi: 10.1371/journal.pone.0103116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Eldijk MB, Smits FCM, Vermue N, Debets MF, Schoffelen S, van Hest JCM. Synthesis and Self-Assembly of Well-Defined Elastin-Like Polypeptide–Poly(ethylene glycol) Conjugates. Biomacromolecules. 2014;15:2751–2759. doi: 10.1021/bm5006195. [DOI] [PubMed] [Google Scholar]
- 100.Ryu JS, Raucher D. Elastin-like polypeptide for improved drug delivery for anticancer therapy: preclinical studies and future applications. Expert Opinion on Drug Delivery. 2015;12:653–667. doi: 10.1517/17425247.2015.974546. [DOI] [PubMed] [Google Scholar]
- 101.Bidwell GL, Raucher D. Cell Penetrating Elastin-like Polypeptides for Therapeutic Peptide Delivery. Advanced drug delivery reviews. 2010;62:1486–1496. doi: 10.1016/j.addr.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryu JS, Raucher D. Elastin-like polypeptides: the influence of its molecular weight on local hyperthermia-induced tumor accumulation. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V. 2014;(88):382–389. doi: 10.1016/j.ejpb.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 103.Walker LR, Ryu JS, Perkins E, McNally LR, Raucher D. Fusion of cell-penetrating peptides to thermally responsive biopolymer improves tumor accumulation of p21 peptide in a mouse model of pancreatic cancer. Drug Design, Development and Therapy. 2014;8:1649–1658. doi: 10.2147/DDDT.S60451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ryu JS, Raucher D. Anti-tumor efficacy of a therapeutic peptide based on thermo-responsive elastin-like polypeptide in combination with gemcitabine. Cancer Letters. 2014;348:177–184. doi: 10.1016/j.canlet.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 105.Massodi I, Bidwell GL, 3rd, Davis A, Tausend A, Credit K, Flessner M, Raucher D. Inhibition of ovarian cancer cell metastasis by a fusion polypeptide Tat-ELP. Clinical & experimental metastasis. 2009;26:251–260. doi: 10.1007/s10585-009-9237-z. [DOI] [PubMed] [Google Scholar]
- 106.Boudreau NJ, Varner JA. The Homeobox Transcription Factor Hox D3 Promotes Integrin α5β1 Expression and Function during Angiogenesis. Journal of Biological Chemistry. 2004;279:4862–4868. doi: 10.1074/jbc.M305190200. [DOI] [PubMed] [Google Scholar]
- 107.Reiss S, Sieber M, Reiss S, Sieber M, Oberle V, Wentzel A, Spangenberg P, Claus R, Kolmar H, Lösche W. Inhibition of platelet aggregation by grafting RGD and KGD sequences on the structural scaffold of small disulfide-rich proteins. Platelets. 2006;17:153–157. doi: 10.1080/09537100500436663. [DOI] [PubMed] [Google Scholar]
- 108.Minea R, Swenson S, Costa F, Chen TC, Markland FS. Development of a Novel Recombinant Disintegrin, Contortrostatin, as an Effective Anti-Tumor and Anti-Angiogenic Agent. Pathophysiology of Haemostasis and Thrombosis. 2005;34:177–183. doi: 10.1159/000092419. [DOI] [PubMed] [Google Scholar]
- 109.Minea RO, Helchowski CM, Zidovetzki SJ, Costa FK, Swenson SD, Markland FS., Jr. Vicrostatin – An Anti-Invasive Multi-Integrin Targeting Chimeric Disintegrin with Tumor Anti-Angiogenic and Pro-Apoptotic Activities. PLoS ONE. 2010;5:e10929. doi: 10.1371/journal.pone.0010929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Janib SM, Gustafson JA, Minea RO, Swenson SD, Liu S, Pastuszka MK, Lock LL, Cui H, Markland FS, Conti PS, Li Z, MacKay JA. Multimeric Disintegrin Protein Polymer Fusions That Target Tumor Vasculature. Biomacromolecules. 2014;15:2347–2358. doi: 10.1021/bm401622y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang K, Duan N, Zou W, Zhang C, Lai Y, Shen P, Hua Z. Fused hydrophobic elastin-like-peptides (ELP) enhance biological activity of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) Protein and peptide letters. 2015;22:1000–1006. doi: 10.2174/0929866522666150824162015. [DOI] [PubMed] [Google Scholar]
- 112.Banaszynski LA, Liu CW, Wandless TJ. Characterization of the FKBP·Rapamycin·FRB Ternary Complex. Journal of the American Chemical Society. 2005;127:4715–4721. doi: 10.1021/ja043277y. [DOI] [PubMed] [Google Scholar]
- 113.Yuan R, Kay A, Berg W, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. Journal of Hematology & Oncology. 2009;2:45. doi: 10.1186/1756-8722-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shah M, Edman MC, Janga SR, Shi P, Dhandhukia J, Liu S, Louie SG, Rodgers K, Mackay JA, Hamm-Alvarez SF. A rapamycin-binding protein polymer nanoparticle shows potent therapeutic activity in suppressing autoimmune dacryoadenitis in a mouse model of Sjogren's syndrome. J Control Release. 2013;171:269–279. doi: 10.1016/j.jconrel.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. mAbs. 2015;7:9–14. doi: 10.4161/19420862.2015.989042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sousou T, Friedberg J. Rituximab In Indolent Lymphomas. Seminars in hematology. 2010;47:133–142. doi: 10.1053/j.seminhematol.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotech. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 118.Johnson RN, Kopeckova P, Kopecek J. Synthesis and evaluation of multivalent branched HPMA copolymer-Fab' conjugates targeted to the B-cell antigen CD20. Bioconjug Chem. 2009;20:129–137. doi: 10.1021/bc800351m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aluri SR, Shi P, Gustafson JA, Wang W, Lin Y-A, Cui H, Liu S, Conti PS, Li Z, Hu P, Epstein AL, MacKay JA. A Hybrid Protein–Polymer Nanoworm Potentiates Apoptosis Better than a Monoclonal Antibody. ACS nano. 2014;8:2064–2076. doi: 10.1021/nn403973g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Institute NE. Eye Disease Statistics. 2014 [Google Scholar]
- 121.Haddadin RI, Vora GK, Chodosh J. Corneal trauma following keratoplasty. International ophthalmology clinics. 2013;53:23–32. doi: 10.1097/IIO.0b013e3182a12b3b. [DOI] [PubMed] [Google Scholar]
- 122.A.A.o. Ophthalmology Eye Health Statistics [Google Scholar]
- 123.Karnati R, Laurie DE, Laurie GW. Lacritin and the tear proteome as natural replacement therapy for dry eye. Experimental eye research. 2013;117:39–52. doi: 10.1016/j.exer.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang W, Despanie J, Shi P, Edman MC, Lin Y-A, Cui H, Heur M, Fini ME, Hamm-Alvarez SF, MacKay JA. Lacritin-mediated regeneration of the corneal epithelia by protein polymer nanoparticles. Journal of Materials Chemistry B. 2014;2:8131–8141. doi: 10.1039/C4TB00979G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang W, Jashnani A, Aluri SR, Gustafson JA, Hsueh PY, Yarber F, McKown RL, Laurie GW, Hamm-Alvarez SF, MacKay JA. A thermo-responsive protein treatment for dry eyes. J Control Release. 2015;199:156–167. doi: 10.1016/j.jconrel.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patel R, Shahane A. The epidemiology of Sjögren’s syndrome. Clinical Epidemiology. 2014;6:247–255. doi: 10.2147/CLEP.S47399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Healthline Heart Disease by the Numbers: Facts, Statistics, and You [Google Scholar]
- 128.Oparil S, Schmieder RE. New Approaches in the Treatment of Hypertension. Circulation Research. 2015;116:1074–1095. doi: 10.1161/CIRCRESAHA.116.303603. [DOI] [PubMed] [Google Scholar]
- 129.Dvorakova MC, Kruzliak P, Rabkin SW. Role of neuropeptides in cardiomyopathies. Peptides. 2014;61:1–6. doi: 10.1016/j.peptides.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 130.Yeh ST. Novel Vasoactive Intestinal Peptide-ELP Fusion Protein VPAC-Agonists Trigger Sustained Pulmonary Artery Vaso-Relaxation in Rats with Acute Hypoxia-Induced Pulmonary Hypertension. Hypertension. 2012;60:A184. [Google Scholar]
- 131.del Rio CL, Youngblood B, Yeh ST, Georgopoulos L, Arnold S, Wallery J, Hamlin RL. Evaluation of Vasomera™, A Novel VPAC2-selective Vasoactive Intestinal Peptide Agonist, in Rats with Doxorubicin-Induced Cardiomyopathy: Evidence for Chronic Cardio-Protection. Circulation. 2012;126:A18796. [Google Scholar]
- 132.del Rio CL, George R, Kloepfer P, Ueyama Y, Youngblood B, Georgopoulos L, Arnold S, Hamlin RL. Vasomera™, a novel VPAC2-selective Vasoactive intestinal peptide agonist, enhance contractility and decreases myocardial demand in dogs with both normal hearts and with pacing-induced dilated cardiomyopathy. Journal of the American College of Cardiology. 2013;61 [Google Scholar]
- 133.Pharmaceutics P. Phase 1, Randomized, Double-Blind, Placebo-Controlled Exploratory Study That Will Assess the Safety, Tolerability. Pharmacokinetics and Hemodynamic Response to a Single 30 Minute Intravenous Infusion of Vasomera™ (PB1046) in Adult Subjects With Stage 1 or 2 Essential Hypertension. 2013 [Google Scholar]
- 134.Bidwell GL, George EM. Maternally sequestered therapeutic polypeptides – a new approach for the management of preeclampsia. Frontiers in Pharmacology. 2014;5:201. doi: 10.3389/fphar.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.N.I.o.C.H.a.H. Development How many women are affected by or at risk of preeclampsia? [Google Scholar]
- 136.George EM, Liu H, Robinson GG, Bidwell GL. A polypeptide drug carrier for maternal delivery and prevention of fetal exposure. Journal of Drug Targeting. 2014;22:935–947. doi: 10.3109/1061186X.2014.950666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.C.f.D.C.a. Prevention. Number of Americans with Diabetes Projected to Double or Triple by 2050 [Google Scholar]
- 138.Amiram M, Luginbuhl KM, Li X, Feinglos MN, Chilkoti A. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. Journal of Controlled Release. 2013;172:144–151. doi: 10.1016/j.jconrel.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Primiano T. Specific delivery of immunostimulatory RNA via nanoparticles blocks growth of primary and disseminated ovarian tumors. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. 2015;15:A287. [Google Scholar]
- 140.Youngblood BL YS, Georgopoulos L, Arnold S, Wallery J, Hamlin RL, del Rio CL. Vasomera™, A Novel VPAC2-Selective Vasoactive Intestinal Peptide Agonist Evidence for Chronic Cardio-Protection in Rats with Doxorubicin-Induced Cardiomyopathy. Heart Failure Society of America. 2012 [Google Scholar]
- 141.Pharmaceutics P. VIP-ELP fusion molecules PB1120 and PB1046 correct F508del-CFTR dysfunction. 2015 [Google Scholar]
- 142.Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine & growth factor reviews. 1998;9:153–165. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 143.Koria P, Yagi H, Kitagawa Y, Megeed Z, Nahmias Y, Sheridan R, Yarmush ML. Self-assembling elastin-like peptides growth factor chimeric nanoparticles for the treatment of chronic wounds. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1034–1039. doi: 10.1073/pnas.1009881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang W, Sreekumar PG, Valluripalli V, Shi P, Wang J, Lin YA, Cui H, Kannan R, Hinton DR, Mackay JA. Protein polymer nanoparticles engineered as chaperones protect against apoptosis in human retinal pigment epithelial cells. J Control Release. 2014 doi: 10.1016/j.jconrel.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cai L, Dinh CB, Heilshorn SC. One-pot Synthesis of Elastin-like Polypeptide Hydrogels with Grafted VEGF-Mimetic Peptides. Biomaterials science. 2014;2:757–765. doi: 10.1039/C3BM60293A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. Journal of Controlled Release. 2006;115:175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 147.Sinclair SM, Bhattacharyya J, McDaniel JR, Gooden DM, Gopalaswamy R, Chilkoti A, Setton LA. A genetically engineered thermally responsive sustained release curcumin depot to treat neuroinflammation. Journal of controlled release : official journal of the Controlled Release Society. 2013;171:38–47. doi: 10.1016/j.jconrel.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ciofani G, Genchi GG, Liakos I, Athanassiou A, Mattoli V, Bandiera A. Human recombinant elastin-like protein coatings for muscle cell proliferation and differentiation. Acta biomaterialia. 2013;9:5111–5121. doi: 10.1016/j.actbio.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 149.I.I.f.H. Informatics. The Global Use of Medicines: Outlook through. 2017 [Google Scholar]
- 150.Adams SB, Shamji MF, Nettles DL, Hwang P, Setton LA. Sustained Release of Antibiotics from Injectable and Thermally Responsive Polypeptide Depots, Journal of biomedical materials research. Part B. Applied biomaterials. 2009;90:67–74. doi: 10.1002/jbm.b.31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Amruthwar SS, Janorkar AV. Preparation and characterization of elastin-like polypeptide scaffolds for local delivery of antibiotics and proteins. Journal of materials science. Materials in medicine. 2012;23:2903–2912. doi: 10.1007/s10856-012-4749-5. [DOI] [PubMed] [Google Scholar]
- 152.Conrad U, Plagmann I, Malchow S, Sack M, Floss DM, Kruglov AA, Nedospasov SA, Rose-John S, Scheller J. ELPylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock. Plant Biotechnology Journal. 2011;9:22–31. doi: 10.1111/j.1467-7652.2010.00523.x. [DOI] [PubMed] [Google Scholar]
- 153.Scheller J, Leps M, Conrad U. Forcing single-chain variable fragment production in tobacco seeds by fusion to elastin-like polypeptides. Plant Biotechnology Journal. 2006;4:243–249. doi: 10.1111/j.1467-7652.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 154.Floss DM, Sack M, Stadlmann J, Rademacher T, Scheller J, Stöger E, Fischer R, Conrad U. Biochemical and functional characterization of anti-HIV antibody–ELP fusion proteins from transgenic plants. Plant Biotechnology Journal. 2008;6:379–391. doi: 10.1111/j.1467-7652.2008.00326.x. [DOI] [PubMed] [Google Scholar]
- 155.Floss DM, Sack M, Arcalis E, Stadlmann J, Quendler H, Rademacher T, Stoger E, Scheller J, Fischer R, Conrad U. Influence of elastin-like peptide fusions on the quantity and quality of a tobacco-derived human immunodeficiency virus-neutralizing antibody. Plant Biotechnology Journal. 2009;7:899–913. doi: 10.1111/j.1467-7652.2009.00452.x. [DOI] [PubMed] [Google Scholar]
- 156.Solomon JS, Nixon CP, McGarvey ST, Acosta LP, Manalo D, Kurtis JD. Expression, purification, and human antibody response to a 67 kDa vaccine candidate for schistosomiasis japonica. Protein Expression and Purification. 2004;36:226–231. doi: 10.1016/j.pep.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 157.García-Arévalo C, Bermejo-Martín JF, Rico L, Iglesias V, Martín L, Rodríguez-Cabello JC, Arias FJ. Immunomodulatory Nanoparticles from Elastin-Like Recombinamers: Single-Molecules for Tuberculosis Vaccine Development. Molecular Pharmaceutics. 2013;10:586–597. doi: 10.1021/mp300325v. [DOI] [PubMed] [Google Scholar]
- 158.Floss DM, Mockey M, Zanello G, Brosson D, Diogon M, Frutos R, Bruel T, Rodrigues V, Garzon E, Chevaleyre C, Berri M, Salmon H, Conrad U, Dedieu L. Expression and Immunogenicity of the Mycobacterial Ag85B/ESAT-6 Antigens Produced in Transgenic Plants by Elastin-Like Peptide Fusion Strategy. Journal of Biomedicine and Biotechnology. 2010 doi: 10.1155/2010/274346. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Phan HT, Hause B, Hause G, Arcalis E, Stoger E, Maresch D, Altmann F, Joensuu J, Conrad U. Influence of Elastin-Like Polypeptide and Hydrophobin on Recombinant Hemagglutinin Accumulations in Transgenic Tobacco Plants. PLoS ONE. 2014;9:e99347. doi: 10.1371/journal.pone.0099347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ingrole RS, Tao W, Tripathy JN, Gill HS. Synthesis and Immunogenicity Assessment of Elastin-Like Polypeptide-M2e Construct as an Influenza Antigen. Nano LIFE. 2014;04:1450004. doi: 10.1142/s1793984414500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duvenage L, Hitzeroth II, Meyers AE, Rybicki EP. Expression in tobacco and purification of beak and feather disease virus capsid protein fused to elastin-like polypeptides. Journal of Virological Methods. 2013;191:55–62. doi: 10.1016/j.jviromet.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 162.Järås M, Brun ACM, Karlsson S, Fan X. Adenoviral vectors for transient gene expression in human primitive hematopoietic cells: Applications and prospects. Experimental Hematology. 2007;35:343–349. doi: 10.1016/j.exphem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 163.Uren AG, Kool J, Berns A, van Lohuizen M. Retroviral insertional mutagenesis: past, present and future. Oncogene. 2005;24:7656–7672. doi: 10.1038/sj.onc.1209043. [DOI] [PubMed] [Google Scholar]
- 164.Xie Q, Bu W, Bhatia S, Hare J, Somasundaram T, Azzi A, Chapman M. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci USA. 2002;99:10405–10410. doi: 10.1073/pnas.162250899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Goncalves M, Pau M, de Vries A, Valerio D. Generation of a high-capacity hybrid vector: packaging of recombinant adenoassociated virus replicative intermediates in adenovirus capsids overcomes the limited cloning capacity of adenoassociated virus vectors. Virology. 2001;288:236–246. doi: 10.1006/viro.2001.1073. [DOI] [PubMed] [Google Scholar]
- 166.Kim JS, Chu HS, Park KI, Won JI, Jang JH. Elastin-like polypeptide matrices for enhancing adeno-associated virus-mediated gene delivery to human neural stem cells. Gene Ther. 2012;19:329–337. doi: 10.1038/gt.2011.84. [DOI] [PubMed] [Google Scholar]
- 167.Dash BC, Mahor S, Carroll O, Mathew A, Wang W, Woodhouse KA, Pandit A. Tunable elastin-like polypeptide hollow sphere as a high payload and controlled delivery gene depot. Journal of Controlled Release. 2011;152:382–392. doi: 10.1016/j.jconrel.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 168.Dash BC, Thomas D, Monaghan M, Carroll O, Chen X, Woodhouse K, O'Brien T, Pandit A. An injectable elastin-based gene delivery platform for dose-dependent modulation of angiogenesis and inflammation for critical limb ischemia. Biomaterials. 2015;65:126–139. doi: 10.1016/j.biomaterials.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 169.Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS letters. 2005;579:5822–5829. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 170.O'Sullivan BP, Freedman SD. Cystic fibrosis. The Lancet. 1891-1904;373 doi: 10.1016/S0140-6736(09)60327-5. [DOI] [PubMed] [Google Scholar]
- 171.Zeitlin PL. Emerging drug treatments for cystic fibrosis. Expert Opinion on Emerging Drugs. 2007;12:329–336. doi: 10.1517/14728214.12.2.329. [DOI] [PubMed] [Google Scholar]