Abstract
Objectives:
To identify predisposing factors, species distribution, antifungal susceptibility, and outcome.
Methods:
This study is a retrospective chart review that was conducted at a children’s hospital at King Fahad Medical City, Riyadh, Kingdom of Saudi Arabia. One hundred twenty-nine children with invasive candidiasis who were admitted between January 2010 and January 2015.
Results:
The statistical analysis results have revealed a group of risk factors; prematurity in 37 (28.7%) of patients, low birth weight in 42 (32.6%), central venous catheter in 59 (45.7%), malignancy in 21 (16.3%), immunotherapy in 20 (15.5%), and ventilator support in 60 (46.5%). More than 2-fold mortality rate in patients who had heart vegetation (odds ratio [OR]: 2.9) and patients who had Candida isolated from their blood were more than twice as likely to die as patients with Candida isolated from other sites (OR: 2.2). A total of 48.3% of patients on ventilator died versus 26.1% who were not on ventilator (p=0.009); and 43.8% of patients in the ICU died versus only 24.5% of patients who were not in the ICU (p=0.03). Candida parapsilosis exhibited the highest mortality rate (56.2%).
Conclusion:
Candida albicans is the most common isolate among all Candida species. Gender, low birth weight, prolonged ICU stay, presence of vegetation, positive blood culture, and mechanical ventilation as a strong predictive risk factors for death in children with invasive candidiasis, a finding that could be applied as prophylactic indicator in critically ill children especially neonates.
Candida species are the most common cause of invasive fungal infections in humans; Candida is considered the fourth most common isolate and is associated with the second highest case fatality rate in children with bloodstream infections. The manifestations of infection can range from non-life-threatening mucocutaneous disorders to an invasive disease that can involve any organ.1 The most frequently affected organs in children with disseminated candidiasis are the lungs, liver, kidney, and brain. The frequency of invasive candidiasis is greater in children than in adults and is particularly high in neonates.2 The most frequently implicated risk factors include the use of broad-spectrum antibacterial agents, the use of central venous catheters, the receipt of parenteral nutrition, the receipt of renal replacement therapy, neutropenia, malignancy, the use of implantable prosthetic devices, and the receipt of immunosuppressive agents.3 Candida albicans (C. albicans) is the most common invasive Candida spp. in pediatric patients, accounting for 53% of cases. Candida parapsilosis (21%) and Candida tropicalis (10%) are the most prevalent non-C. albicans spp., whereas Candida glabrata (C. glabrata) and Candida krusei (C. krusei) are infrequent.4 The Prospective Antifungal Therapy Alliance (PATH Alliance) registry describes the epidemiology and outcomes of invasive candidiasis caused by non-albicans species from 2004 to 2008, which accounted for more than 50% of all cases of invasive candidiasis.5 Currently, most Candida treatment guidelines recommend early empiric antifungal therapy for patients suspected or known to have an invasive Candida infection, pending confirmation of the species and its antimicrobial susceptibilities.6-11 The late introduction of appropriate antifungal therapy is an important contributor to the high morbidity and mortality associated with invasive Candida infections that has been reported in several studies.12-16 The mortality rate that is attributable to invasive candidiasis is approximately 86% among intensive care unit (ICU) patients and 42% among non-ICU patients.5 The assessment of these risk factors and the development of a valid risk score are important for early interventions with appropriate antifungal therapy and can reduce the burden of infection.
The objectives of our study are to acquire baseline demographic data, to identify the risk factors for the development of invasive Candida infection, and to review the antifungal susceptibility testing for different Candida species as well as to assess the clinical outcome of invasive candidiasis in pediatric patients at King Fahad Medical City (KFMC), Riyadh, Kingdom of Saudi Arabia to provide important comparative data. Prospective multi-center studies regarding pediatric invasive candidiasis have been conducted in specific regions of the world,17-19 but additional research is needed to fill important knowledge gaps in pediatric candidiasis.
Methods
This study is a retrospective chart review that was conducted at a children’s hospital at KFMC. King Fahad Medical City is a referral hospital with 250 beds of all subspecialties allocated for children. The proposal for this study was approved by the institutional review board of the KFMC, Riyadh, KSA. All pediatric patients from birth until 14 years of age based on the study proposal inclusion criteria, who were diagnosed with invasive candidiasis were recruited. Invasive candidiasis was defined as the isolation of Candida from blood, cerebrospinal fluid (CSF), or other sterile body fluids such as synovial fluid, peritoneal fluid and pleural fluid. All patients were treated in our hospital from January 2010 to January 2015. We excluded patients in whom Candida had been isolated from tissues and urine. A sample of 129 was identified: 114 from blood, 7 from cerebral spinal fluid, and 8 from other sterile body fluids. A data collection form composed of 7 sections and 51 question items was designed to gather information for this study. All necessary variables were collected and statistically analyzed. Obtained demographic and clinical data from the charts includes; age, gender, nationality, and underlying disease. Risk factors included prematurity, low birth weight, trauma, neutropenia, total parenteral nutrition, steroids, and presence of a central venous catheter, underlying gastrointestinal disease, recent surgery, recent broad-spectrum antibiotic treatment, antifungal therapy, and admission to an ICU. Each data sheet had a code.
Microbiology. Specimen processing
A minimum sample of 3 milliliters of blood was collected in pediatric blood vials and inoculated in a BACTEC instrument (Becton Dickinson) until it was determined to be positive by the machine. Gram stains were performed for all positive cultures, and samples were subcultured on blood, MacConkey, chocolate, and Sabouraud agar. Colonies on Sabouraud agar were identified as germ tube test positive for Candida albicans; other Candida species were identified using an API 20C AUX (bioMérieux, Paris, France). Antifungal susceptibility testing was performed on Mueller-Hinton agar with 2% glucose. Minimal inhibitory concentrations were determined with an E-test using a breakpoint reference from the Clinical and Laboratory Standards Institute (CLSI M27-S4) to determine the antifungal susceptibility of yeasts.20
Data management
A well-designed, consensually validated data collection form was applied to gather data for this study. It was composed of 7 sections that included 51 items. Study members collected the data. Data were then initially entered into Excel and cross-checked by the data management team members to identify missing values and anomalies before finally transferring the data to the statistical software.
Statistical analysis
Data were analyzed with the Statistical Analysis Software SAS V9.4. (SAS institute, NC, USA), and a p-value of ≤0.05 was accepted as the significance level for all statistical tests. All collected variables were analyzed using descriptive and inferential statistics. Categorical variables were described and reported as counts and percentages. Continuous variables were described and reported as means±standard deviation. Patient survival status was the main study outcome variable and was analyzed versus all demographic and clinical characteristics of patients via univariate and multivariate analyses.
Results
The majority of patients (n=123 [95.4%]) were Saudi, and almost 67 (51.9%) were males, with a mean age of 2.4±1.41 years. The reported risk factors were found to be: prematurity in 37 (28.7%) patients, low birth weight in 42 (32.6%) patients, the presence of a central venous catheter in 59 (45.7%) patients, malignancy in 21 (16.3%) patients, immunotherapy in 20 (15.5%) patients, and the required use of mechanical ventilators in 60 (46.5%) patients (Table 1). Among patients with Candida infections, C. albicans was isolated from 59 (45.7%) patients, C. tropicalis isolated from 28 (21.7%) patients, C. parapsilosis isolated from 16 (12.4%) patients, Candida famata from 7 (5.4%) patients, Candida lusitaniae from 5 (3.9%) patients, and other species were isolated from 14 (10.9%) patients (Table 2). The univariate analysis identified gender, ventilator use, low birth weight, presence of echocardiography vegetation, and intensive care as significant risk factors for poor prognosis and death in children with invasive candidiasis. The results revealed that 46.8% of females and 26.9% of males died (p=0.02). The mortality rate of patients on ventilator support was 48.3%, versus 26.1% on patients not on ventilator (p=0.009). The mortality rate of patients who stayed in the ICU was 43.8% versus 24.5% in patients who did not stay in the ICU (p=0.03) (Table 3). Multivariate analysis revealed that the mortality rate in patients with cardiac vegetation was increased almost 3 times (OR: 2.9) (Table 4). In the same time, death was noticed increased by more than 2-fold in patients with Candida isolated from their blood, those with low birth weight, those on ventilator, and those with prolonged ICU stay compared to others (Table 4). Candida parapsilosis was found to have the highest mortality rate among all Candida species (56.2%), predominantly in neonates (Table 6).
Table 1.
Sociodemographic and clinical characteristics of 129 patients with invasive candidiasis who were admitted in King Fahad Medical City, Riyadh, Kingdom of Saudi Arabia, between January 2010 and January 2015.
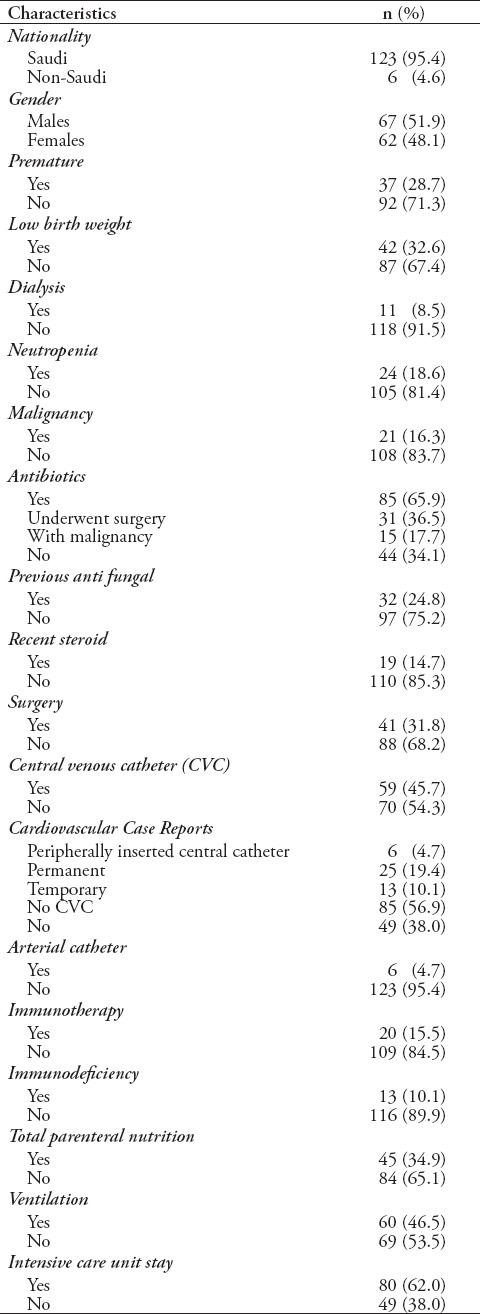
Table 2.
Clinical and laboratory characteristics of Candida (n=129) age 2.4±1.41 years.
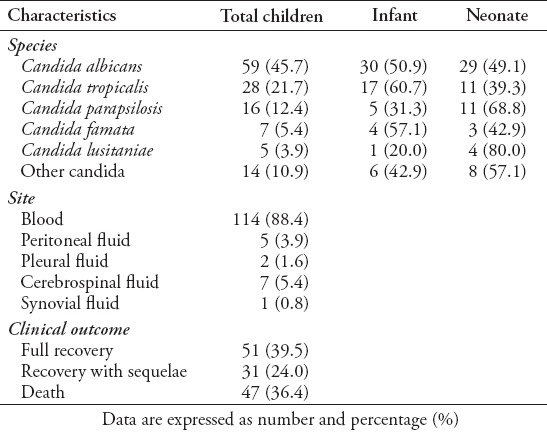
Table 3.
Univariate analysis of patient characteristics versus survival status (n=129).
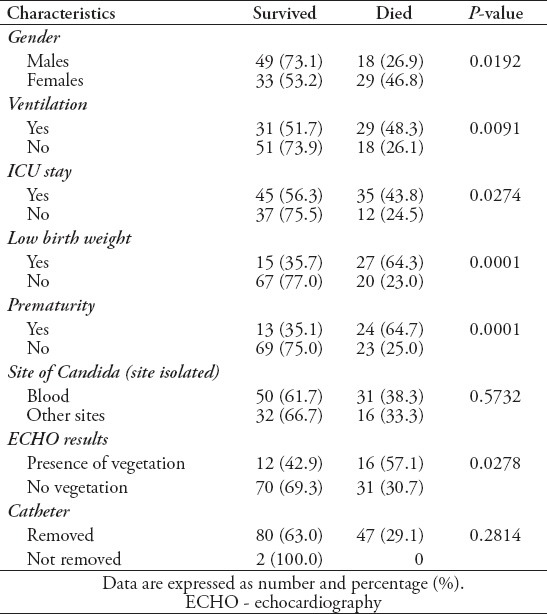
Table 4.
Multivariate analysis of patient characteristics versus survival status (n=129).
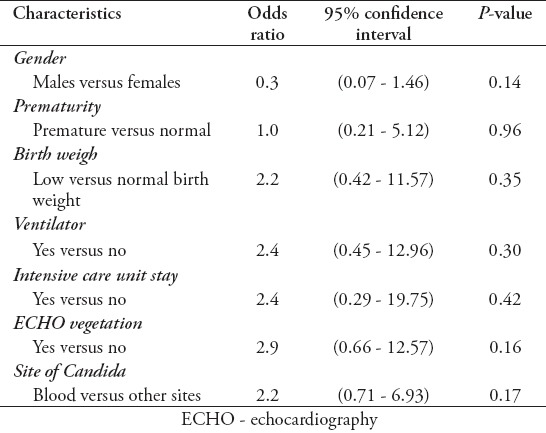
Table 6.
Mortality by Candida species (n=129).
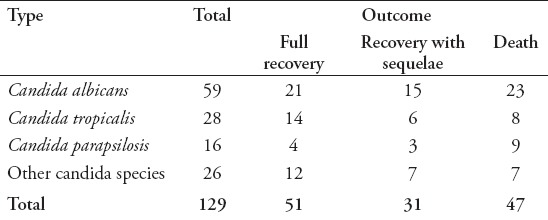
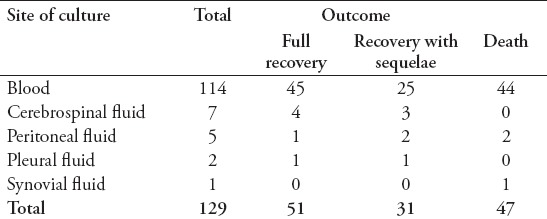
Table 7.
Mortality by site of blood culture (n=129).
Discussion
Our review included 129 pediatric patients with invasive candidiasis from a tertiary medical center representing the largest series in the pediatric age group in Saudi Arabia. The study has investigated the mortality rate and identified the predictive risk factors in the study group. We found that gender, prolonged ICU stay, blood candidemia and ventilation support were the major risk factors for invasive candidiasis and associated with high mortality rate. Coexistence of multiple risk factors in females and in patients with C. parapsilosis have increased the mortality rates within these subgroups.
Candida species are a major contributor to morbidity and mortality in hospitalized children. Data from the National Healthcare Safety Network have identified Candida spp. as the second most common cause, behind coagulase negative staphylococci, of central line-associated bloodstream infection in hospitalized patients in the US.21 Among hospitalized children, fungi are the second most common pathogen identified in the setting of sepsis22,23 and are the leading infectious cause of death in children with cancer24,25 or following an organ or hematopoietic stem cell transplant.26,27 The all-cause mortality associated with pediatric candidiasis exceeds 15%, with an attributable mortality of 10%.28 Prospective multi-center studies have been conducted to examine pediatric invasive candidiasis in specific regions of the world,17–19 but additional research is needed to fill important knowledge gaps in pediatric candidiasis.
While aspects of pediatric and adult invasive candidiasis are similar, numerous studies have revealed critical differences in host factors, pharmacokinetics, and outcomes in children that underscore the importance of dedicated pediatric studies.28-33 Candidiasis disproportionately affects critically ill children. In 2000, approximately 50% of the candidemia cases in children in the US occurred in patients in an ICU setting.28 Moreover, Candidiasis among ICU patients is associated with higher mortality rates.33,34 In 2010, Zaoutis et al35 derived a clinical prediction rule for candidemia among pediatric ICU patients. The single center model determined that the presence of a central venous catheter, recent exposure to certain antimicrobial agents (vancomycin, anti-anaerobic agents), recent exposure to parenteral nutrition, and underlying malignancy resulted in a predictive probability for candidemia of 46% (95% CI: 19%-75%).
Candida albicans (48.3%) was the most common species, but collectively the non-albicans Candida species predominated (51.6%) (Table 2), which is comparable to the results of the largest multi-center, multi-national study of pediatric candidiasis, in which a total of 441 episodes of invasive candidiasis were identified in 423 patients from 30 participating sites (20 in the US and 10 international).36 Of the 449 Candida isolates recovered from the 441 episodes, C. albicans (40%) was the most common species, but collectively the non-albicans Candida species predominated (60%). Similarly, the largest and most contemporary retrospective review from a single pediatric center (patients aged 6 months to 18 years old) found that C. albicans was the most common isolate (44.2%), followed by C. parapsilosis (23.9%).37 The only difference between our study and the previously mentioned studies, as well as other studies, is that C. tropicalis was the second most common Candida species that was identified in patients with invasive candidiasis. Nevertheless, we stress the importance of separately analyzing pediatric and neonatal patients in future studies, as well as the need for multinational collaboration for a more accurate description of species distribution.
This study showed that most non-albicans species included C. tropicalis (23%) and C. parapsilosis (13.1%). Candida famata (5.7%) was more common in our study than C. lusitaniae (4.1%) and C. glabrata (2.5%) (Table 2). Interestingly, none of the previous local studies have addressed this issue. A study by Omrani et al38 conducted in the same region showed the following distribution: C. albicans was the most common species (38.7%), followed by C. tropicalis (18.9%), and C. glabrata (16.3%).
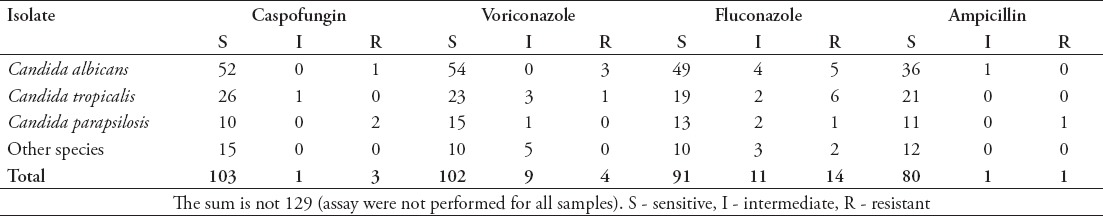
This study revealed good in vitro susceptibility rates of Candida species to the available antifungal medication in our medical city; 78% of isolates were susceptible to fluconazole, 88.7% were susceptible to voriconazole, 96.3% were susceptible to caspofungin, and 97.6% were susceptible to amphotericin B (Table 5). This susceptibility pattern in our institution is similar to that described in a review by Omrani et al,38 which reported good susceptibility patterns to all antifungal medications.
Table 5.
Antifungal susceptibility of Candida species (n=129).
Our study assessed the clinical prognostic outcome of invasive candidiasis in the Kingdom of Saudi Arabia. The overall mortality in this study is considered low compared to those in local, regional, and international reports, and mortality was calculated at the end of therapy. A similar study of predictors and outcomes of Candida infection of the bloodstream in the region reported a mortality rate of 50%,2 while another international study of Candida infections reported a high crude mortality of 46-75%, which reflected the severe underlying illnesses of the infected patients.5
In conclusion, pediatric candidiasis continues to change with respect to its epidemiology and risk factors in specific patient populations. In general, invasive candidiasis in the Kingdom of Saudi Arabia has a poor clinical prognostic outcome. This study has revealed significant proportion of death within females, patients with candida isolated from blood, those on ventilators and those with prolonged ICU stay as well. One of the limitations is the retrospective nature of the study and the dependence on one site data. However, the findings highlighted the common predictors that may guide early prophylactic intervention and the need for further prospective studies. Only through detailed multi-center studies will the medical community be able to best define the risks and optimal strategies for early diagnosis
Footnotes
References
- 1.Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr, Calandra TF, Edwards JE, Jr, et al. Clinical practice guidelines for the management of candidiasis:2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al Thaqafi AH, Farahat FM, Al Harbi MI, Al Amri AF, Perfect JR. Predictors and outcomes of Candida bloodstream infection: eight-year surveillance, Western Saudi Arabia. Int J Infect Dis. 2014;21:5–9. doi: 10.1016/j.ijid.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence. 2014;5:161–169. doi: 10.4161/viru.26187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dornbusch HJ, Manzoni P, Roilides E, Walsh TJ, Groll AH. Invasive fungal infections in children. Pediatr Infect Dis J. 2009;28:734–737. doi: 10.1097/INF.0b013e3181b076b1. [DOI] [PubMed] [Google Scholar]
- 5.Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, et al. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One. 2014;9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alothman AF, Al-Musawi T, Al-Abdely HM, Salman JA, Almaslamani M, Yared N, et al. Clinical practice guidelines for the management of invasive Candida infections in adults in the Middle East region: Expert panel recommendations. J Infect Public Health. 2014;7:6–19. doi: 10.1016/j.jiph.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical Practice Guideline for the Management of Candidiasis:2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et al. ESCMID*guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18:19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 9.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT) Clin Microbiol Infect. 2012;18:53–67. doi: 10.1111/1469-0691.12041. [DOI] [PubMed] [Google Scholar]
- 10.Bow EJ, Evans G, Fuller J, Laverdière M, Rotstein C, Rennie R, et al. Canadian clinical practice guidelines for invasive candidiasis in adults. Can J Infect Dis Med Microbiol. 2010;21:e122–e150. doi: 10.1155/2010/357076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, et al. ESCMID*guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. 2012;18:38–52. doi: 10.1111/1469-0691.12040. [DOI] [PubMed] [Google Scholar]
- 12.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 13.Garey KW, Rege M, Pai MP, Mingo DE, Suda KJ, Turpin RS, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 14.Fraser A, Paul M, Almanasreh N, Tacconelli E, Frank U, Cauda R, et al. Benefit of appropriate empirical antibiotic treatment: thirty-day mortality and duration of hospital stay. Am J Med. 2006;119:970–976. doi: 10.1016/j.amjmed.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–3645. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54:1739–1746. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 17.Steinbach WJ, Roilides E, Berman D, Hoffman JA, Groll AH, Bin-Hussain I, et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr Infect Dis J. 2012;31:1252–1257. doi: 10.1097/INF.0b013e3182737427. [DOI] [PubMed] [Google Scholar]
- 18.Santolaya ME, Alvarado T, Queiroz-Telles F, Colombo AL, Zurita J, Tiraboschi IN, et al. Active surveillance of candidemia in children from Latin America: a key requirement for improving disease outcome. Pediatr Infect Dis J. 2014;33:e40–e44. doi: 10.1097/INF.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 19.Pemán J, Cantón E, Linares-Sicilia MJ, Roselló EM, Borrell N, Ruiz-Pérez-de-Pipaon MT, et al. Epidemiology and antifungal susceptibility of bloodstream fungal isolates in pediatric patients: a Spanish multicenter prospective survey. J Clin Microbiol. 2011;49:4158–4163. doi: 10.1128/JCM.05474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Zone diameter interpretive standards, corresponding minimal inhibitory concentration (MIC) interpretive breakpoints, and quality control limits for antifungal disk diffusion susceptibility testing of yeasts. Informational supplement, CLSI document M27-S3. Wayne (USA): Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 21.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 22.Wisplinghoff H, Seifert H, Tallent SM, Bischoff T, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in pediatric patients in United States hospitals: epidemiology, clinical features and susceptibilities. Pediatr Infect Dis J. 2003;22:686–691. doi: 10.1097/01.inf.0000078159.53132.40. [DOI] [PubMed] [Google Scholar]
- 23.Watson RS, Carcillo JA, Linde-Zwirble WT, Clermont G, Lidicker J, Angus DC. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 24.Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. 2004;22:4384–4393. doi: 10.1200/JCO.2004.01.191. [DOI] [PubMed] [Google Scholar]
- 25.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91:1068–1075. [PubMed] [Google Scholar]
- 26.Martin SR, Atkison P, Anand R, Lindblad AS. Studies of pediatric liver transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr Transplant. 2004;8:273–283. doi: 10.1111/j.1399-3046.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 27.Profumo RJ. Pediatric liver transplant recipients: mortality analysis over 20 years. J Insur Med. 2006;38:3–8. [PubMed] [Google Scholar]
- 28.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–1239. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 29.Pfaller MA, Diekema DJ, Jones RN, Messer SA, Hollis RJ. Trends in antifungal susceptibility of Candida spp. isolated from pediatric and adult patients with bloodstream infections: SENTRY antimicrobial surveillance program, 1997 to 2000. J Clin Microbiol. 2002;40:852–856. doi: 10.1128/JCM.40.3.852-856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krcmery V, Laho L, Huttova M, Ondrusova A, Kralinsky K, Pevalova L, et al. Aetiology, antifungal susceptibility, risk factors and outcome in 201 fungaemic children: data from a 12-year prospective national study from Slovakia. J Med Microbiol. 2002;51:110–116. doi: 10.1099/0022-1317-51-2-110. [DOI] [PubMed] [Google Scholar]
- 31.Hope WW, Seibel NL, Schwartz CL, Arrieta A, Flynn P, Shad A, et al. Population pharmacokinetics of micafungin in pediatric patients and implications for antifungal dosing. Antimicrob Agents Chemother. 2007;51:3714–3719. doi: 10.1128/AAC.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blyth CC, Chen SC, Slavin MA, Serena C, Nguyen Q, Marriott D, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123:1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 33.Zaoutis TE, Coffin SE, Chu JH, Heydon K, Zhao H, Greves HM, et al. Risk factors for mortality in children with candidemia. Pediatr Infect Dis J. 2005;24:736–739. doi: 10.1097/01.inf.0000172938.76561.8e. [DOI] [PubMed] [Google Scholar]
- 34.Singhi SC, Reddy TC, Chakrabarti A. Candidemia in a pediatric intensive care unit. Pediatr Crit Care Med. 2004;5:369–374. doi: 10.1097/01.pcc.0000123550.68708.20. [DOI] [PubMed] [Google Scholar]
- 35.Zaoutis TE, Prasad PA, Localio AR, Coffin SE, Bell LM, Walsh TJ, et al. Risk factors and predictors for candidemia in pediatric intensive care unit patients: implications for prevention. Clin Infect Dis. 2010;51:e38–e45. doi: 10.1086/655698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palazzi DL, Arrieta A, Castagnola E, Halasa N, Hubbard S, Brozovich AA, et al. Candida speciation, antifungal treatment and adverse events in pediatric invasive candidiasis: results from 441 infections in a prospective, multi-national study. Pediatr Infect Dis J. 2014;33:1294–1296. doi: 10.1097/INF.0000000000000431. [DOI] [PubMed] [Google Scholar]
- 37.Dutta A, Palazzi DL. Candida non-albicans versus Candida albicans fungemia in the non-neonatal pediatric population. Pediatr Infect Dis J. 2011;30:664–668. doi: 10.1097/INF.0b013e318213da0f. [DOI] [PubMed] [Google Scholar]
- 38.Omrani AS, Makkawy EA, Baig K, Baredhwan AA, Almuthree SA, Elkhizzi NA, et al. Ten-year review of invasive Candida infections in a tertiary care center in Saudi Arabia. Saudi Med J. 2014;35:821–826. [PubMed] [Google Scholar]


