Abstract
Antibiotics are added to semen extenders to take care of heavy microbial load, however, their continuous use poses a constant threat of developing antibiotic resistance by the common microbes present in the semen. Our hypothesis was that natural honey, having antibacterial activity and rich in fructose could replace the use of antibiotics and fructose in the semen extender. Twenty-four ejaculates from six crossbred rams were obtained and extended with tris-based extender without (control) and with honey at 2.5% (T1), 5% (T2) and 7% (T3). Sperm quality was measured in terms of percentage sperm motility, live sperm count, intact acrosome and hypo-osmotic swelling test (HOST) reacted spermatozoa. The semen samples at post-thaw were also evaluated for total viable count (colony forming units/ml). At post-thaw, control exhibited significantly (P<0.05) higher sperm motility in comparison to T2 and T3. The percent of live sperm count, intact acrosome and HOST reacted spermatozoa were significantly higher (P<0.05) for control than all other treatment groups at post-thaw. Among treatment groups, T1 maintained significantly higher (P<0.05) percentage of live sperm count, intact acrosome and HOST reacted spermatozoa than T2 and T3. The total viable count at post-thaw was significantly lower (P<0.05) for control than all the treatment groups. In conclusion, honey cannot be used as an alternative to antibiotics to take care of heavy microbial load in semen, however, levels up to 2.5% may be supplemented to semen as an energy source.
Key Words: Antibiotics, Colony forming units, Cryopreservation, Honey, Ram
Introduction
Artificial insemination (AI) with frozen semen has been the most widely used reproductive technique to extend the use of superior sires to bring about rapid genetic improvement in livestock species (El-Sheshtawy et al., 2016 ▶). However, its success is related to the quality of semen used (Hossain et al., 2011 ▶). The microbials gain access to the raw semen from the reproductive tract during collection and processing (Althouse and Lu, 2005 ▶). If left unchecked, their presence can severely affect quality through competition for nutrients with the spermatozoa or through production of harmful products (Catry et al., 2010 ▶; Morrell and Wallgren, 2014 ▶). Higher bacterial load in the extended semen results in reduced sperm viability and motility, increases in the proportion of spermatozoa with defective acrosome (Althouse et al., 2000 ▶; Ortega-Ferrusola et al., 2009 ▶; Kustar and Althouse, 2016 ▶). In addition, there may be early embryonic death, endometritis or systemic ailment in the animal to be inseminated with such contaminated frozen semen (Maes et al., 2008 ▶). Therefore, antibiotics are added to the semen extender to limit the growth of bacterial contamination in the ejaculate prolonging the shelf life of semen and reducing the transmission of pathogenic microbes into the female tract (Morrel and Wallgren, 2011 ▶; Schulze et al., 2016 ▶). However, their continuous use could be detrimental as the bacterial population may gradually become resistant, which may lead to deterioration of sperm quality and disease in the female (Schulze et al., 2016 ▶). It has been recently reported that more than 90% of the bacteria isolated from the extended semen sample are resistant to the routinely used antibiotics in the semen extender (Bolarin, 2011 ▶; Bussalleu and Torner, 2013 ▶). Therefore, agents or strategies other than antibiotics may prove useful in limiting microbial population in semen (Morrel and Wallgren, 2011 ▶). Physical removal of the bacteria by Single Layer Centrifugation (SLC) (Morrell and Wallgren, 2014 ▶) and inclusion of antimicrobial peptides (AMP) in semen diluents (Bussaleu et al., 2017 ▶; Schulze et al., 2016 ▶) have been tried in order to limit the microbial population in the semen with limited success. Physical removal of bacteria either by SLC or density gradient centrifugation (DGC) is a tedious and stressful process affecting the keeping quality of spermatozoa over a long period of time. Further, inclusion of AMPs in diluents have been found cytotoxic at higher concentration and lower concentrations were not effective in controlling microbial growth (Schulze et al., 2016 ▶). Moreover, some AMPs have shown good promise in maintaining viability but could not maintain progressive motility (Bussalleu et al., 2017 ▶) which is the most fundamental for sucessful fertilization. Therefore, supplementation of an agent in the extender that can overcome the threat of antibiotic resistance and at the same time would maintain the sperm motility by taking care of the bacterial load in the semen may prove a very useful strategy for successful preservation of semen for years to come. To this, honey, as a natural product has been studied as a supplement with different aims like antioxidant, cryoprotectant, and energy source for improvement of post-thaw semen quality in bull (El-Sheshtawy et al., 2014; Yimer et al., 2015 ▶), ram (Jerez-Ebenesperger et al., 2015 ▶), buck (Olayemi et al., 2011 ▶), buffalo bull (El-Nattat et al., 2016 ▶) and human (Tartibian and Maleki, 2012 ▶; Fakhrildin et al., 2014 ▶). Honey also possesses antibacterial activity (Molan and Russell, 1988 ▶; Aljady et al., 2000 ▶; Taormina et al., 2001 ▶; Al-Waili, 2004 ▶; Zoheir et al., 2015 ▶) besides containing high concentration of D-glucose and D-fructose (Nayik et al., 2015 ▶) as an energy source. The antibacterial activity of honey is attributed to the presence of bee defensin-1 and methylglyoxal (Molan, 1992 ▶; Kwakman and Zaat, 2012 ▶), presence of high content of sugars and more importantly to the generation of hydrogen peroxide when diluted due to conversion of glucose into hydrogen peroxide by glucose oxidase enzyme present in all types of honey (Aurongzeb and Azim, 2011 ▶). Thus its inclusion in the semen extenders may replace the use of fructose and antibiotics in the extender. This is a new kind of work in which suitability of honey as a replacement to antibiotics and fructose has been studied.
Materials and Methods
Chemicals and honey
Chemicals used were purchased from Merck (India Pvt Ltd.) and Himedia (India Pvt Ltd.). Natural pure honey harvested aseptically in a sterilized container was procured from State Agricultural Department (Srinagar, J&K, India).
Selection of animals, collection and cryo-preservation of semen
Crossbred rams (n=6) with good health condition were randomly selected during the breeding season and maintained under isomanagerial conditions throughout this study. Twenty-four ejaculates were collected in the morning by artificial vagina method. Bacterial contamination was minimized by thorough washing of the prepuce and trimming of preputial hair if any before semen collection on each day. Each collected semen sample was observed for consistency, volume, colour and presence of any foreign body. Normal ejaculates were initially diluted with the tris buffer (1:1) for recording of initial motility. Ejaculates that showed ≥70% initial sperm motility were pooled (total 6 pools) to eliminate the individual variation. For pre-freeze quality check, a portion of the pooled sample was taken in a separate tube and mixed with tris extender. The rest of the sample was quickly divided into four equal aliquots labelled as control, 2.5% (T1), 5% (T2) and 7% (T3). These aliquots were subsequently extended with tris extender without or with different levels of natural honey (Table 1). Natural pure sterile honey was first mixed with a portion of tris buffer and this mixture was then added to the final extender. The samples were then immediately placed in an ice chest. It took about 50 min for the semen samples to attain 4-5 °C and was considered as cooling. The distance between the divisional laboratory and frozen semen station is nearly 40 km and the time taken from the initial processing at the divisional laboratory to reach at the frozen semen station was about 3 h. The time excluding the period of cooling was included in the total equilibration period of 4 h and the amount of time left for equilibration was completed in cold handling cabinet at the station itself (Banday et al., 2017 ▶). After equilibration, the semen samples were loaded into French mini straws (0.25 ml) with an automatic filling and sealing machine (IMV Technologies, France). The concentration of sperm in each straw was set at 150 million. Vapour freezing of loaded straws was achieved in a Biological Programmable Freezer (IMV Technologies, France) with inside pressure maintained at 3 Bar. The freezing rate was set as: -5°C/min (from +4°C to -10°C), -40°C/min (from -10°C to -100°C) and -20°C/min (from -100°C to -140°C). The straws were then plunged deep in liquid nitrogen.
Table 1.
Composition of tris extender for control and treatments
| Components | Tris extender |
|||
|---|---|---|---|---|
| Control | T1 | T2 | T3 | |
| Tris buffer | 73 ml | 70.5 ml | 68 ml | 66 ml |
| Fructose | 1.25 g | - | - | - |
| Egg yolk | 20 ml | 20 ml | 20 ml | 20 ml |
| Glycerol | 7 ml | 7 ml | 7 ml | 7 ml |
| Penicillin G sodium | 80000 IU | - | - | - |
| Streptomycin | 100 mg | - | - | - |
| Honey | - | 2.5 ml | 5 ml | 7 ml |
Pre-freeze sperm quality evaluation
Sperm motility and live sperm count
A small drop (100 μL of diluted semen sample) was placed on a clean grease free slide kept on a biotherm stage and a cover slip was then placed on the drop. The slide was examined under ×400 magnification (phase contrast microscope, Nikon Eclipse E200, Japan). Three readings of three trained individuals were taken and an average value was considered as a final value of the sperm motility. The procedure for live sperm count by eosin nigrosin method is discussed elsewhere (Banday et al., 2017 ▶). After staining, a thin smear was prepared, air dried immediately as soon as possible and then observed at ×1000 magnification (phase contrast microscope, Nikon Eclipse E200, Japan). Colourless sperm were considered as live and completely or partially stained were considered as dead. A total of 200 spermatozoa were counted and live sperm count percentage was determined.
Acrosomal integrity and hypo-osmotic swelling test (HOST)
The acrosomal integrity is generally evaluated by Giemsa staining method. The method is described in detail in our previous publication (Lone et al., 2012 ▶). The stained smear is observed at ×1000 magnification (Phase contrast microscope, Nikon Eclipse E200, Japan) to determine spermatozoa with percentage intact acrosome (PIA). Two hundred spermatozoa were counted randomly in different fields and PIA was determined. HOST was performed to assess the functional integrity of sperm plasma membrane. For complete procedure refer to Banday et al. (2017). ▶ After incubation, a small drop (15 µL) of sample was placed on a clean grease free slide, then a cover slip was put on top and it was immediately examined at ×400 magnification (Phase contrast microscope, Nikon Eclipse E200, Japan) for determination of HOST reacted spermatozoa. HOST reacted spermatozoa were identified by having highly coiled tails. Two hundred sperm were counted and percentage of HOST reacted spermatozoa was determined.
Post-thaw sperm quality evaluation
After a week, the straws were thawed individually in warm water bath maintained at 37°C for 30 s. The sperm quality was measured as in the case of pre-freeze. In addition to this, total viable count (CFU/ml) was also measured in post-thaw semen samples.
Total viable count (CFU/ml)
The microbial load of thawed semen samples from the control and treatment groups was determined as per the standard method described by Shukla (2005) ▶. Briefly, the semen straws were thawed in sterile water maintained at 37°C for 30 s. From each treatment, two straws were thawed to obtain semen volume of 0.5 ml. Each semen sample (0.5 ml) was diluted in 4.5 ml sterile normal saline. Ten (10) fold serial dilutions were made up to 10-4 dilutions and then 0.1 ml of sample from each dilution was inoculated in duplicate on sterile petri plates containing plate count agar. The petri plates were incubated for 24 h at 37°C followed by the subsequent examination of colony forming units. The plates were examined and the colonies were counted. Plates with 30-300 colonies were only considered and colony forming unit (CFU/ml) was determined as:
Statistical analysis
The percentage data of each group for sperm motility, live sperm count, intact acrosome, HOST reacted spermatozoa were first transformed to arc-sin data and then analyzed with one way ANOVA. However, non-transformed data of total viable count of treatment and control groups was also analyzed by one way ANOVA. Post hoc analysis was done by Tukey’s HSD test. However, the difference in sperm quality between pre-freeze and post-thaw in each group for each parameter was analysed by paired t-test. All the statistical analysis was done by using SPSS, statistics-20. P-value ≤0.05 was considered significant. The data are presented in the tables as mean±SEM.
Results
Sperm motility and live sperm count
The sperm motility percentage at post-thaw for T1 was non-significantly (P>0.05) lower than control, but significantly (P<0.05) higher than other treatment groups. The sperm motility percentage declined significantly (P<0.05) from pre-freeze to post-thaw in all the treatment and control groups. The percentage of live sperm count at post-thaw for T1 was significantly (P<0.05) lower than control but significantly higher (P<0.05) than T2 and T3. The percentage of live sperm count declined significantly (P<0.05) from pre-freeze to post-thaw in all the treatment and control groups (Table 2).
Table 2.
Effect of different levels of honey on percentage of sperm motility and live sperm count (mean±SEM) of ram during cryopreservation
| Treatments | Sperm motility (%) |
Live sperm count (%) |
||
|---|---|---|---|---|
| Pre-freeze | Post-thaw | Pre-freeze | Post-thaw | |
| Control | 80.83 ± 1.53A | 55.83 ± 1.53aB | 85.08 ± 1.42A | 65.08 ± 2.00aB |
| T1 | 80.83 ± 1.53A | 52.50 ± 1.11aB | 85.08 ± 1.42A | 57.25 ± 1.25bB |
| T2 | 80.83 ± 1.53A | 37.50 ± 1.11bB | 85.08 ± 1.42A | 47.41 ± 1.55cB |
| T3 | 80.83 ± 1.53A | 15.00 ± 1.29cB | 85.08 ± 1.42A | 30.08 ± 1.26dB |
Means with different superscripts in a column
() row differ significantly (P<0.05)
Acrosomal integrity and hypo-osmotic swelling test
The PIA at post-thaw were significantly higher (P<0.05) for control than all the other treatment groups. Among the treatment groups, T1 had significantly higher (P<0.05) PIA than T2 and T3 at post-thaw. The PIA declined significantly (P<0.05) from pre-freeze to post-thaw in all the treatment and control groups. The percentage of HOST reacted spermatozoa at post-thaw was significantly higher (P<0.05) for control than all the other treatments. Among the treatment groups, T1 had significantly higher (P<0.05) number of HOST reacted spermatozoa than T2. The percentage of HOST reacted spermatozoa declined significantly (P<0.05) from pre-freeze to post-thaw in control and all the treatment
groups (Table 3).
Table 3.
Effect of different levels of honey on PIA and HOST reacted ram spermatozoa (mean±SEM) of ram during cryopreservation
| Treatments | Intact acrosome (%) |
HOST reacted spermatozoa (%) |
||
|---|---|---|---|---|
| Pre-freeze | Post-thaw | Pre-freeze | Post-thaw | |
| Control | 93.16 ± 0.40A | 80.75 ± 0.62aB | 79.91 ± 1.34A | 60.33 ± 1.42aB |
| T1 | 93.16 ± 0.40A | 76.50 ± 0.88bB | 79.91 ± 1.34A | 51.83 ± 2.27bB |
| T2 | 93.16 ± 0.40A | 70.83 ± 1.06cB | 79.91 ± 1.34A | 42.91 ± 0.85cB |
| T3 | 93.16 ± 0.40A | 67.75 ± 1.18dB | 79.91 ± 1.34A | 19.41 ± 0.85dB |
() Means with different superscripts in a column
() row differ significantly (P<0.05)
Total viable count (CFU/ml)
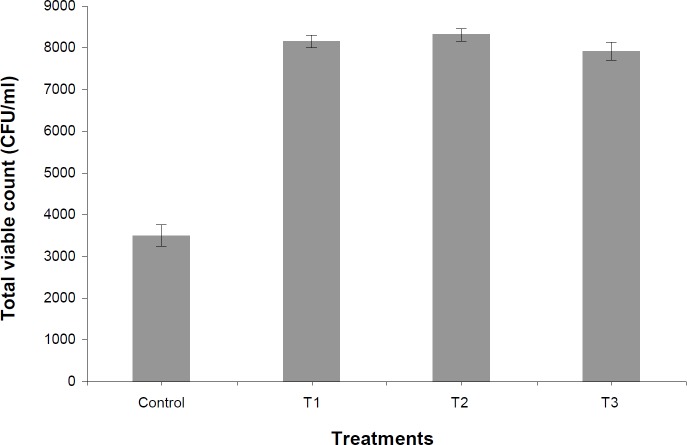
At post-thaw, the total viable count was significantly lower (P<0.05) in control group (3500 ± 259) than all treatment groups (Fig. 1). Among treatment groups, T3 (7916 ± 219) maintained non-significantly (P>0.05) lower number of colonies than T2 (8316 ± 151) and T1 (8150 ± 158).
Fig. 1.
Effect of different levels of honey on total viable count (CFU/ml) of ram semen at post thaw
Discussion
Scientists nowadays are making efforts to search for an alternative to antibiotics so as to stem the use of antibiotics in semen extenders. Honey, a naturally occurring product has potent antibacterial activity (Zoheir et al., 2015 ▶) and is a good source of energy to spermatozoa owing to the presence of glucose and fructose (Al-Waili, 2004 ▶). The percentage of sperm motility at post-thaw was significantly higher (P<0.05) for control than T2 (honey 5%) and T3 (honey 7%), but non-significantly higher than T1 (honey 2.5%). However, among treatment groups, T1 maintained significantly higher sperm motility and live sperm count at post-thaw than T2 and T3. The higher sperm motility in control may be ascribed to the presence of antibiotics which have inhibited the microbial growth. Microorganisms present in the semen reduce sperm motility, longevity and acrosomal integrity by directly competing with spermatozoa for nutrients or by the production of toxic products (Catry et al., 2010 ▶; Morrell and Wallgren, 2014 ▶). The results could not be compared due to lack of literature on this aspect. The authors have not come across any published report where honey has been used as an alternative to antibiotics. However, honey has been used as a cryoprotectant and a source of fructose in the semen extender for cryopreservation (Olayemi et al., 2011 ▶; Yimer et al., 2015 ▶). In the present study, T1 resulted in significantly higher sperm motility and live sperm count as compared to T2 and T3. The decrease in sperm motility and live sperm count at post-thaw in T2 and T3 group may be attributed to the higher concentration of honey which might have created an excessive hyperosmotic extracellular environment and subsequently led to an excessive dehydration of the sperm, resulting in their death (Yimer et al., 2015 ▶). Honey at 2.5% level might have maintained nearly isotonic environment around the sperm and provided energy as it is a rich source of fructose and glucose. The results of the present study are in close agreement with the study of El-Nattat et al. (2016) ▶ who also obtained better post-thaw sperm motility and viability with honey at the rate of 2% as compared to 3%, 4% and 5%. El-Sheshtawy et al. (2014) ▶ also reported better post-thaw sperm motility and live sperm count at 1% level for cryopreserved bull sperm and at 3% level for chilled bull semen and others (Yimer et al., 2015 ▶) also obtained better post-thaw sperm quality at 2.5% level as compared to 5% and 10%. However, our results are in disagreement with those of Fakhrildin et al. (2014) ▶ who reported better post-thaw sperm quality at 10% level. The reason could be the resilience of human sperm to high osmotic environments.
The percentage of intact acrosome and HOST reacted spermatozoa at post-thaw was significantly higher for control than T1, T2 and T3 group at post-thaw. Higher number of HOST reacted spermatozoa and spermatozoa with intact acrosome in control may be due to the presence of fructose and antibiotics in the extender. The higher number of PIA recorded in control is in concurrence with the previous studies in ram (Paulenz et al., 2002 ▶; Gundogan, 2009 ▶; Bohlooli et al., 2012 ▶; Rakha, 2013 ▶) and buck semen (Islam et al., 2006 ▶). However, among the treatment groups, T1 maintained better acrosomal integrity as compared to T2 and T3. Higher number of sperm with intact acrosome in T1 may be due to sugar content in the honey, as additions of sugars have been found to have a beneficial effect on acrosomal integrity (El-Sheshtawy et al., 2016 ▶). The lower values of PIA in T2 and T3 may be due to high concentration of honey, possibly resulting in osmotic shock and disruption in plasma membrane integrity.
The total viable count at post-thaw were significantly lower for control than T1, T2 and T3, reflecting the role of antibiotics in the semen extender. Honey in any of the treatment groups studied could not take care of heavy microbial load in the semen and this may have led to decease in the sperm quality in the treatment groups as compared to control. The results could not be compared due to lack of available literature on this aspect. This appears to be the first report, where honey has been studied as an alternative to antibiotics and fructose in the semen extender. The permissible limit of colony forming units in frozen bull semen is 5000 CFU/ml as per OIE guidelines and minimum standard protocol for production of bovine semen (NDDB, 2012 ▶). However, such standard is lacking in ram semen as AI is not routinely practiced in sheep. Therefore, a greater number of studies need to be carried out on the maximum permissible limit of CFU/ml in frozen ram semen. In conclusion, natural honey could not take care of heavy microbial load in the semen, therefore, there are no benefits to its use as an alternative to antibiotics. However, levels up to 2.5% were not detrimental to crossbred ram spermatozoa, and hence may be used as an energy source in the semen extender.
Acknowledgements
We would like to thank the In Charge Mountain Research Centre for Sheep and Goat for Experimental Animals and Frozen Semen Station, Ranbirbagh for providing cryopreservation facilities for this research trial. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest that might inappropriately influence or bias their work.
References
- Aljady AM, Kamaruddin MY, Jamal AM, Mohd Yassim MY. Biochemical study on the efficacy of Malaysian honey on inflicted wounds: an animal model. Med. J. Islamic World Acad. Sci. 2000;13:125–132. [Google Scholar]
- Althouse GC, Kuster CE, Clark SG, Weisiger RM. Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology. 2000;53:1167–1176. doi: 10.1016/S0093-691X(00)00261-2. [DOI] [PubMed] [Google Scholar]
- Althouse GC, Lu KG. Bacteriospermia in extended porcine semen. Theriogenology. 2005;63:573–584. doi: 10.1016/j.theriogenology.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Al-Waili NS. Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J. Med. Food. 2004;7:210–222. doi: 10.1089/1096620041224139. [DOI] [PubMed] [Google Scholar]
- Aurongzeb M, Azim MK. Antimicrobial properties of natural honey: a review of literature. Pak. J. Biochem. Mol. Biol. 2011;44:118–124. [Google Scholar]
- Banday MN, Lone FA, Rasool F, Rashid M, Shikari A. Use of antioxidants reduce lipid peroxidation and improve quality of crossbred ram sperm during its cryopreservation. Cryobiology. 2017;74:25–30. doi: 10.1016/j.cryobiol.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Bohlooli S, Codden F, Jang JP, Razzaghzadeh S, Bozoglu S. The effect of different extenders on post-thaw sperm viability, motility and membrane integrity in cryopreserved semen of Zandi ram. J. Basic. Appl. Sci. Res. 2012;2:1120–1123. [Google Scholar]
- Bolarin Guillen A. Bacteriology in porcine semen. Avances en Tecnología Porcina (España) 2011;8:20–30. [Google Scholar]
- Bussalleu E, Sancho S, Briz MD, Yeste M, Bonet S. Do antimicrobial peptides PR-39, PMAP-36 and PMAP-37 have any effect on bacterial growth and quality of liquid-stored boar semen? Theriogenology. 2017;89:235–243. doi: 10.1016/j.theriogenology.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Bussalleu E, Torner E. Quality improvement of boar seminal doses. In: Bonet S, Casas I, Holt WV, Yeste M, editors. Boar reproduction: fundamentals and new biotechnological trends. 1st Edn. Springer: Heidelberg; 2013. pp. 517–550. [Google Scholar]
- Catry B, Van Duijkeren E, Pomba MC, Greko C, Moreno MA, Pyorala S, Torneke K. Reflection paper on MRSA in food-producing and companion animals, epidemiology and control options for human and animal health. Epidemiol. Infect. 2010;138:626–644. doi: 10.1017/S0950268810000014. [DOI] [PubMed] [Google Scholar]
- El-Nattat WS, El-Sheshtawy RI, El-Batawy KA, Shahba MI, El-Seadawy IE. Preservability of buffalo bull semen in tris-citrate extender enriched with bee’s honey. J. Innov. Pharm. Biol. Sci. 2016;3:180–185. [Google Scholar]
- El-Sheshtawy RI, El-Badry DA, Gamal A, El-Nattat WS, Almaaty AMA. Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters. Asian Pac. J. Reprod. 2016;5:331–334. [Google Scholar]
- El-Sheshtawy RI, El-Nattat WS, Sabra HA, Ali AH. Effect of honey solution on semen preservability of local breeds of cattle bulls. World Appl. Sci. J. 2014;32:2076–2078. [Google Scholar]
- Fakhrildin MB, Rana MR, Alsaadi AR. Honey supplementation to semen-freezing medium improves human sperm parameters post-thawing. J. Family Reprod. Health. 2014;8:27–31. [PMC free article] [PubMed] [Google Scholar]
- Gundogan M. Short term preservation of ram semen with different extenders. Kafkas Üniversitesi Veteriner Fakültesi Dergis. 2009;15:429–435. [Google Scholar]
- Hossain M, Johannisson A, Wallgren M, Nagy S, Siqueira AP, Rodriguez-Martinez H. Flow cytometry for the assessment of animal sperm integrity and functionality, state of the art. Asian J. Androl. 2011;13:406–419. doi: 10.1038/aja.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam R, Ahmed K, Deka BC. Effect of holding and washing on the quality of goat semen. Small Rumin. Res. 2006;66:51–57. [Google Scholar]
- Jerez-Ebensperger RA, Luno V, Olaciregui M, Gonzalez N, de Blas I, Gil L. Effect of pasteurized egg yolk and rosemary honey supplementation on quality of cryopreserved ram semen. Small Rumin. Res. 2015;130:153–156. [Google Scholar]
- Kuster CE, Althouse GC. The impact of bacteriospermia on boar sperm storage and reproductive performance. Theriogenology. 2016;85:21–26. doi: 10.1016/j.theriogenology.2015.09.049. [DOI] [PubMed] [Google Scholar]
- Kwakman PH, Zaat SA. Antibacterial com-ponents of honey. IUBMB life. 2012;64:48–55. doi: 10.1002/iub.578. [DOI] [PubMed] [Google Scholar]
- Lone FA, Islam R, Khan MZ, Sofi KA. Effect of different egg yolk-based extenders on the quality of ovine cauda epididymal spermatozoa during storage at 4°C. Reprod. Domest. Anim. 2012;47:257–262. doi: 10.1111/j.1439-0531.2011.01847.x. [DOI] [PubMed] [Google Scholar]
- Maes D, Nauwynck H, Rijsselaere T, Mateusen B, Vyt P, de Kruif A, Van Soom A. Diseases in swine transmitted by artificial insemination: an overview. Theriogenology. 2008;70:1337–1345. [Google Scholar]
- Molan PC. The antibacterial activity of honey 2—Variation in the potency of the antibacterial activity. Bee World. 1992;73:59–76. [Google Scholar]
- Molan PC, Russell KM. Non-peroxide anti-bacterial activity in some New Zealand honeys. J. Apic. Res. 1988;27:62–67. [Google Scholar]
- Morrell JM, Wallgren M. Removal of bacteria from boar ejaculates by Single Layer Centrifugation can reduce the use of antibiotics in semen extenders. Anim. Reprod. Sci. 2011;123:64–69. doi: 10.1016/j.anireprosci.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Morrell JM, Wallgren M. Alternatives to antibiotics in semen extenders, a review. Pathogens. 2014;3:934–946. doi: 10.3390/pathogens3040934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayik GA, Dar BN, Nanda V. Physico-chemical, rheological and sugar profile of different unifloral honeys from Kashmir valley of India. Arab. J. Chem. 2015 (In press) https://doi.org/10.1016/j.arabjc.2015.08. 017. [Google Scholar]
- NDDB. National Dairy Plan Phase I. Manual on. Semen Production. Project Implementation Plan. Vol. IV C. Project Management Unit. (Located in NDDB) 2012. Available from: http://www.nddb.coop/ndpi/English/AboutNDPI/Manuals-Guidelines/PDFDocuments/PIP-Vol-IV-C-Manual-on-Semen-Production.pdf.
- Olayemi FO, Adeniji DA, Oyeyemi MO. Evaluation of sperm motility and viability in honey-included egg yolk based extenders. Global Vet. 2011;7:19–21. [Google Scholar]
- Ortega-Ferrusola C, Gonzsalez-Fernandez L, Muriel A, Macias-Garcia B, Rodriguez-Martinez H, Tapia JA, Pena FJ. Does the microbial flora in the ejaculate affect the freezeability of stallion sperm? Reprod. Domest. Anim. 2009;44:518–522. doi: 10.1111/j.1439-0531.2008.01267.x. [DOI] [PubMed] [Google Scholar]
- Paulenz H, Soderquist L, Perez-Pe R, Berg KA. Effect of different extenders and storage temperatures on sperm viability of liquid ram semen. Theriogenology. 2002;57:823–836. doi: 10.1016/s0093-691x(01)00683-5. [DOI] [PubMed] [Google Scholar]
- Rakha BA, Hussain I, Akhter S, Ullah N, Aandrabi SM, Ansari MS. Evaluation of tris-citric acid, skim milk and sodium citrate extenders for liquid storage of Punjab Urial (Ovis vignei punjabiensis) spermatozoa. Reprod. Biol. 2013;13:238–242. doi: 10.1016/j.repbio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Schulze M, Dathe M, Waberski D, Muller K. Liquid storage of boar semen: current and future perspectives on the use of cationic antimicrobial peptides to replace antibiotics in semen extenders. Theriogenology. 2016;85:39–46. doi: 10.1016/j.theriogenology.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Shukla MK. Correlation of microbial load of cryo-preserved semen with quality of neat and cryopreserved murrah buffalo bull semen. Buffalo Bull. 2005;24:84–87. [Google Scholar]
- Taormina PJ, Niemira BA, Beuchat LR. Inhibitory activity of honey against foodborne pathogens as influenced by the presence of hydrogen peroxide and level of antioxidant power. Int. J. Food Microbiol. 2001;69:217–225. doi: 10.1016/s0168-1605(01)00505-0. [DOI] [PubMed] [Google Scholar]
- Tartibian B, Maleki BH. The effects of honey supplementation on seminal plasma cytokines, oxidative stress biomarkers, and antioxidants during 8 weeks of intensive cycling training. J. Androl. 2012;33:449–461. doi: 10.2164/jandrol.110.012815. [DOI] [PubMed] [Google Scholar]
- Yimer N, Muhammad N, Sarsaifi K, Rosnina Y, Wahid H, Khumran AM, Kaka A. Effect of honey supplementation into Tris extender on cryopreservation of bull spermatozoa. MJAS. 2015;18:47–54. [Google Scholar]
- Zoheir KMA, Harisa GI, Abo-Salem OM, Ahmad SF. Honey bee is a potential antioxidant against cyclophosphamide-induced genotoxicity in albino male mice. Pak. J. Pharm. Sci. 2015;28:973–981. [PubMed] [Google Scholar]