Abstract
AIM
To present the rationale, design, methodology, and the baseline data of the Beijing Desheng Diabetic Eye Study (BDDES), and to determine the prevalence of diabetic retinopathy (DR) and possible risk factors in patients with type 2 diabetes mellitus (T2DM) in an urban community of Beijing, China.
METHODS
Community-based prospective cohort study of persons diagnosed with T2DM aged 30y or older. The main variables of interest are the presence and progression of DR as determined by the standardized ETDRS grading of seven fields fundus photographs. The presence and severity of DR were analyzed for possible correlations to non-genetic and genetic dispositions.
RESULTS
A total of 1438 participants with data available for analysis, the prevalence of any DR was 35.4%. The prevalence of mild non-proliferative diabetic retinopathy (NPDR), moderate NPDR, severe NPDR, and proliferative diabetic retinopathy was 27.7%, 2.6%, 0.5% and 4.5%, respectively. By multiple logistic regression analysis, risk factors for the presence of any DR included male (P=0.031), lower income level (P=0.011), lower education background (P=0.022), longer duration of diabetes (P=0.001), younger age at diabetic onset (P=0.001), higher systolic blood pressure (P=0.007), higher glycosylated hemoglobin A1c levels (P=0.001), high albuminuria (P=0.03), and use of insulin (P<0.001). For vision-threatening DR, four factors were significant: younger age at diabetic onset (P<0.001), higher systolic blood pressure (P=0.042), high albuminuria (P<0.001), and use of insulin (P<0.001).
CONCLUSION
The BDDES is the first large-scale ongoing cohort study of a Chinese urban population of persons with type 2 diabetes. Using standardized grading system comparable to large cohort studies from western populations, our baseline data shows that the prevalence of DR and major risk factors in this Chinese ethnic population are comparable to that found in the western population studies.
Keywords: type 2 diabetes mellitus, diabetic retinopathy, risk factors, cohort study
INTRODUCTION
The rapid increase in persons with diabetes in both developing and developed countries is now one of the greatest public health concerns[1]. Diabetic retinopathy (DR) is the most common microvascular complications of diabetes and the leading cause of legal blindness in working-aged people. The occurrence of DR is estimated to grow from 126.6 million in 2010 to 191 million by 2030[2]. To our knowledge, the epidemiology of DR has largely been studied in western populations (Table 1)[3]–[13]. In China, the most of studies on DR from Chinese populations relied either on simple direct ophthalmoscope to detect DR without use of photographic grading of retinal images[14] or two 45° digital fundus photographs centered on the optic disc and fovea[15]–[19]. Having comparable methods for DR screening is essential to compare data across different populations. The Beijing Desheng Diabetic Eye Study (BDDES) is the first large-scale ongoing cohort study on DR in a Chinese urban diabetic population that uses standard seven fields imaging of the fundus with the Early Treatment of Diabetic Retinopathy Study (ETDRS) grading system comparable to studies in western populations. This article aims to present the rationale, study design, methodology and baseline data of the study.
Table 1. Summary of prevalence studies of diabetic retinopathy.
| Years conducted | Name of study | Population | Sample age (a) | Sample size | Prevalence | Photograph | Classification |
| 1980-1982 | WESDR[3] | American | ≥30 | 1370 | 28.8% (5y); 77.8% (15y) | Fields 1-7 | ETDRS |
| 1985-1987 | SAHS[4] | American | ≥40 | 351 | 36.2% | Fields 1-7 | ETDRS |
| 1984-1988 | SLVDS[5] | Mexican American/non Hispanic whites | ≥40 | 360 | 35.3% | Fields 1,2,4 | ETDRS |
| 1988-1990 | BDES[6] | White | ≥40 | 410 | 35.1% | Fields 1-7 | ETDRS |
| 1988-1992 | BES[7] | Black | 40-84 | 615 | 28.5% | Fields 1-2 | ETDRS |
| 1992-1994 | BMES[8] | White | ≥50 | 252 | 29% | Fields 1-5 | ETDRS |
| 1999-2000 | Proyecto VER[9] | Mexican American | ≥40 | 1044 | 44.3% | Fields 1,2,4 | ETDRS |
| 2000-2003 | LALES[10] | Latinos | ≥40 | 1217 | 45.8% | Fields 1-7 | ETDRS |
| 2004 | SiMES[11] | Malay | 40-79 | 757 | 35% | Fields 1-2 | ETDRS |
| 2003-2005 | CURES[12] | Indian | ≥20 | 1636 | 17.6% | Four-field | ETDRS |
| 2003-2005 | SN-DREAMS[13] | Indian | ≥40 | 1816 | 18% | Four-field | ETDRS |
WESDR: Wisconsin Epidemiologic Study of Diabetic Retinopathy; SAHS: San Antonio Heart study; SLVDS: San Luis Valley Diabetes Study; BES: Barbados Eye Study; BDES: Beaver Dam Eye Study; BMES: Blue Mountains Eye Study; LALES: Los Angeles Latino Eye Study; SiMES: Singapore Malay Eye Study; CURES: Chennai Urban Rural Epidemiology Study Eye Study; SN-DREAMS: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study; ETDRS: Early Treatment Diabetic Retinopathy Study grading system.
SUBJECTS AND METHODS
Study Design
The BDDES is a community-based cohort study mainly designed to gain a better understanding of the natural progression of DR in patients with type 2 diabetes mellitus (T2DM). The patients were recruited in Desheng Community of urban Beijing and will be followed-up at 5 and 10y interval. The study protocol was approved by the Ethics Committee of Beijing Tongren Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants before their enrollment.
Specific Objectives
The specific objectives of the cohort study is 1) to assess the prevalence, incidence and progression rate of DR; 2) to identify risk factors (clinical, sociodemographic, anthropomorphic, and biochemical) for the development and progression of DR; 3) to build a gene bank for analysis of sequence variants or polymorphisms in the candidate genes (for example, vascular endothelial growth factor gene, the receptor for advanced glycosylation end products gene, the protein kinase C-β gene, erythropoietin gene and the apolipoprotein E gene) are associated with DR in the Chinese patients with T2DM; 4) to build a bank of plasma/serum material for future studies and to identify the association between non-genetic factors and DR in the Chinese patients with T2DM.
Study Population, Inclusion and Exclusion Criteria
Eligible patients were recruited between November 2009 and June 2012 from a typical urban community in Beijing with a relative stable population of 116 000 predominantly of Han Chinese Ethnicity (98%). Persons with known diabetes of age group 30y or older were recruited with the help of posters, pamphlets, and phone calls to person's home.
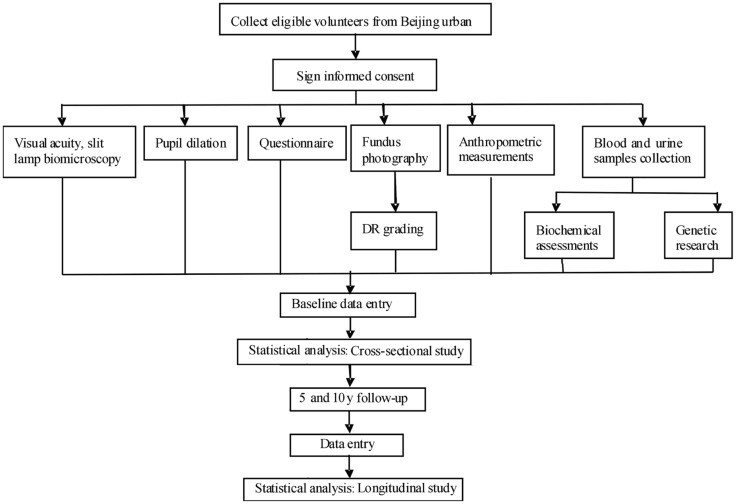
Diabetes was defined as either a history of physician diagnosed T2DM and being treated with insulin, oral hypoglycemic or diet only, or fasting plasma glucose (FPG) of 7.0 mmol/L (126 mg/dL) or more in at least two previous examination, or casual plasma glucose of 11.1 mmol/L (200 mg/dL) or above. The duration of diabetes was defined as the interval between the first diagnosis of diabetes by internists and enrollment into the present study. Patient with severe media opacity or shallow anterior chamber/angle-closure glaucoma precluding mydriasis were excluded. The workflow was presented in Figure 1.
Figure 1. The workflow of the Beijing Desheng Diabetic Eye Study.
Calculation of Sample Size
The target sample size for the study cohort was calculated to achieve an adequate precision around estimates of progression rate of DR and to allow for risk factor analyses. Previous studies[20]–[21] have reported the progression rate of DR is about 30%. Considering an acceptable 95% confidence interval (Z1-α/2=1.96) and precision of 0.05 (d), a target sample size of approximately 323 patients with DR would be needed 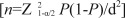 . Assuming 30% of the persons with diabetes having DR[22], 1077 subjects with diabetes would need to be recruited for the target sample size. In order to adjust for possible dropouts, a further 20% sample was planned. Finally, the total calculated target sample size was about 1292 patients with T2DM.
. Assuming 30% of the persons with diabetes having DR[22], 1077 subjects with diabetes would need to be recruited for the target sample size. In order to adjust for possible dropouts, a further 20% sample was planned. Finally, the total calculated target sample size was about 1292 patients with T2DM.
Clinical and Laboratory Examinations, Questionnaire Survey
Visual acuity and refraction
Uncorrected visual acuity was determined by using an ETDRS chart projector with tumbling E letters monocularly (right eye followed by left eye) at a distance of 4 m. The number of E letters recognized was recorded. If the patient read less than 20 E letters in the chart at 4 m, vision was examined at one meter; if the patient failed to read the largest E letters at one meter, perception of hand movement was observed; if hand movement still cannot be perceptible, light perception was observed, which was recorded as present or absent. Auto-refractometer (Canon Co., Japan) was used to get the patient's refraction monocularly (right eye followed by left eye). Corrected visual acuity tests were examined with pinhole and spectacle, and measurements were the same as the above mentioned.
Slit-lamp examination
Slit-lamp biomicroscopy (Topcon, PS-62E, Japan) was performed by ophthalmologists before pupil dilation. Any abnormalities in the anterior segment of the eye were recorded (e.g. corneal disease, iris abnormalities, in particular, new vessels on the iris). The angle and depth of the anterior chamber were evaluated to determine the risk of occlusion upon dilation. The examiner assessed the angle by viewing it nasally and temporally with a narrow beam directed at an angle of about 45 degrees. All participants included in the study had their pupils dilated using 0.5% tropicamide and 5% phenylephrine (Santen Pharmaceutical, Osaka, Japan) after an examination of the anterior chamber angle of the eye. When the examiner was in doubt about the chamber angle, a senior examiner was consulted. For participants with narrow angles, once pupil dilation was performed, they were cautioned about acute angle closure symptoms, and their chamber depth was measured again after the end of examination. In addition, follow-up with a telephone call was made on the next day to inquire about acute symptoms.
Fundus photography
At least 6 mm pupil dilatation was insured before taking fundus photography. Seven standard fields of 30° color fundus photographs with stereoscopic images of optic disc and macula were taken monocularly (right eye followed by left eye) by using a digital fundus camera (Zeiss Visucam Pro, Oberkochen, Germany). The seven fields were named as follows: field 1, the center of the optic disc; field 2, the center of the macula; field 3, temporal to the macula; field 4, temporal superior; field 5, temporal inferior; field 6, nasal superior; and field 7, nasal inferior (Figure 2). Digital fundus images were stored as uncompressed JPEG files. No image manipulation was done before or during grading.
Figure 2. The standard of 7 fields fundus photography.
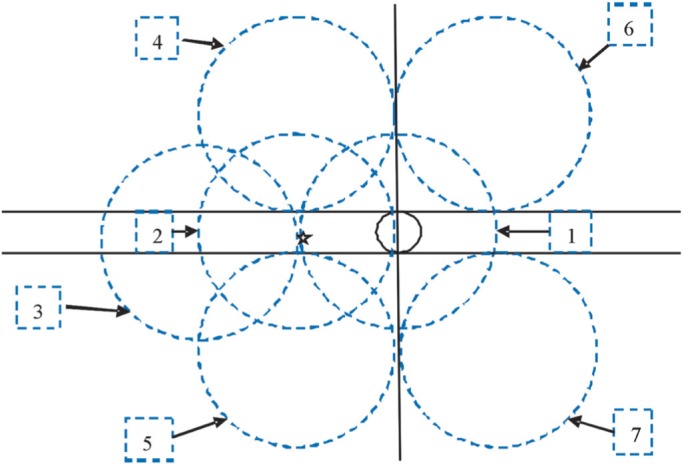
Black solid circle indicates optic disc, pentagon indicates the center of macular, seven blue-dotted circles indicate the location of each field respectively.
Grading of Diabetic Retinopathy
The fundus photographs were graded at the University of Wisconsin Fundus Photographic Reading Center by the same ophthalmologist (Yang XF) in a masked manner according to the modified ETDRS standard classification protocol. DR was considered present if any characteristic lesions as defined by the ETDRS severity scale were present: microaneurysms, hemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, venous beading and new vessels. The level of retinopathy for each eye was determined and the classification for each individual was based on the score of worse eye. For each eye, a retinopathy severity score was assigned according to the modified scale of ETDRS as follows: no DR (NDR, level 10) or any DR (levels 14 and above). Any DR was further divided into mild non-proliferative diabetic retinopathy (NPDR) (levels 14-35), moderate NPDR (levels 43 through 47), severe NPDR (level 53), and proliferative diabetic retinopathy (PDR) (level>60). Macular edema was defined as retinal thickening in the macular area according to the ETDRS classification protocol and further divided into those with or without clinical significant macular edema (CSME). CSME was considered present when the macular edema was present within 500 µm of the foveal center or focal photocoagulation scars were present. Vision-threatening DR (VTR) was defined as the presence of severe NPDR, PDR, or CSME. Quality of image grading in relation to reproducibility was assessed by regrading 5% of the eyes by a senior grader at the University of Wisconsin Fundus Photographic Reading Center. Exact agreement on retinopathy level and presence of macular edema were 86% and 61.9% respectively, and weighted Kappa was 0.82[23]. These statistics are in agreement with published reproducibility from the reading center[24].
Lens photography and grading of lens opacities
After taking fundus photos, the same digital camera was used to photograph the lens. The ideal image ensured that caruncles can be identified, with a clear view of corneas, iris texture and conjunctival blood vessels. Grading of lens opacities was performed by an ophthalmologist according to the Lens Opacities Classification System III (LOCS III). Cortical and posterior subcapsular opacities, nuclear opalescence and color were graded by visual comparison with a standard set of photographs (LOCS chart III, Leo T. Chylack, Harvard Medical School, Boston, MA, USA).
Anthropometric measurement
Anthropometric parameters included body weight and height, waist and hip circumference. Height and weight were measured with subjects in light clothing and not wearing shoes by a trained observer. Body mass index (BMI, kg/m2) was calculated as the ratio between weight and the square of height of the participant. Waist-to-hip ratio (WHR) was calculated as waist circumference divided by hip circumference.
Blood pressure
Blood pressure measurements were secured by undertaking three measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP) in resting status with 5min apart.
Blood sample
Every patient who agreed to participate in the study was informed to be fasting for at least 8h prior to blood drawing. Overnight fasting venous blood samples were collected for measurements of C-reactive protein, creatinine, uric acid, FPG, lipid profile (total cholesterol, triglycerides, high-density and low-density lipoprotein cholesterol), glycosylated hemoglobin A1c (HbA1c) and fasting insulin on the same day. For quality control, random tests of 10% of blood samples were undertaken. A whole blood sample collected in tube without anticoagulants was stood at room temperature for 30min for coagulation and then was centrifuged at 3500 revolutions per minute (rpm) for 15min for serum obtaining. Another whole blood sample collected in tube with EDTA anticoagulant was centrifuged at 3000 rpm for 10min for plasma obtaining. All samples were protected from exposure to direct light during processing. Blood sample with EDTA anticoagulation was also collected for genomic DNA, which was isolated from venous blood leukocytes using a genomic DNA extraction and purification kit (TIANamp Swab DNA Kit, Tiangen Biotech, Beijing, China). Serum/plasma and DNA samples were aliquoted and stored at -80°C for later uses.
Urine sample
A first-void, mid-stream morning spot urine sample was collected and sent for analysis at the Beijing Dian Clinical Laboratory. Albuminuria was measured by immunonephelometry (Roche/Cobas C501 analyzer, Ibaraki, Japan), and high albuminuria was defined as ≥20 mg/L.
Questionnaire
A detailed interviewer-administered questionnaire regarding relevant basic information, lifestyle factors and medical history was administered. Basic information included birth date, marital status, sex, monthly income, education and occupation. Female participants were additionally asked questions of fertility histories. The lifestyle factors included smoking history, tea-drinking, diet habits, and medical history included systemic medical and surgical history, current medications, and family history of diseases. Persons currently smoking more than one cigarette/cigar/pipe a day for at least one year were classified as current smokers. Persons currently not smoking but with a prior smoking history were classified as ex-smokers. Persons who never smoked were classified as never smokers. Participants recruited between January 2010 and January 2011 were asked to record their three meals a day for three days.
Follow-up visits
Five and ten years' follow-up study were scheduled. Follow-up visits will include ophthalmologic examinations (corrected visual acuity, slit-lamp biomicroscopy, dilated digital lens and fundus photography), anthropometric measurements and biochemical investigations (glycosylated hemoglobin, lipid profile, urea and creatinine, and microalbuminuria).
Quality Control
Quality control procedures were implemented throughout the study to ensure that the final dataset was as accurate and complete as possible. All staff took part in a comprehensive training course prior to getting involved in the study. The course included a full spectrum of education to ensure that all investigators have knowledge of the study process, purposes, data forms, and technical skills needed to conduct the protocol in a scientifically sound manner.
Before the participants leaving the examination center, the entire questionnaire was checked for any deficiencies. The collected data was scrutinized manually before input into the computer. If the forms were not filled in completely, the concerned person was consulted to fill in the missing data or clarify an inconsistent data.
Data Entry and Statistical Analysis
Epidata, which has the function of double checks to avoid wrong entries, was used for the data entry. All data were independently entered into the database by two individuals using Epidata software 3.1 (The Epidata Association, Odense, Denmark). Statistical analysis was performed using the R statistical analysis package (http://www.r-project.org/). The data were presented as numbers (percentage) for categorical variables, mean±standard deviation (SD) for normally distributed variables, and the median (interquartile range) for skewed distributed variables. Prevalence estimates for all outcomes were performed for the overall sample. For risk factors analysis, t-tests, analysis of variance (ANOVA), and linear regressions models were used for continuous variables and Chi-squared test or Fisher's exact test for categorical variables. Multiple logistic regression was performed to control for effects of age, gender, and other potential confounders. Statistic results were expressed as P-value, odds ratio (OR) and 95% confidence intervals (CI). Statistical significance was set at P<0.05.
RESULTS
A total of 1466 patients with T2DM were recruited between November 2009 and June 2012. After excluding 28 subjects with non-gradable fundus photographs, 1438 (98.1%) participants were available for further study.
Table 2 summarizes the baseline characteristics of the 1438 subjects. The average (±SD) age was 65.04±8.25y, ranging from 33 to 86y. There were 575 (40%) males and 863 (60%) females. Of the 1438 participants, 1332 (92.6%) were self-identified Han people, 462 (32.1%) had a monthly income of less than 2000 CNY, 1272 (88.5%) had completed middle school or above, and 1280 (89.0%) were married. Compared to the NDR group, subjects in the any DR group were more likely to be male (P=0.004), had lower monthly income level (P=0.01), lower education background (P=0.004), younger age at diabetic onset (P<0.001), longer duration of diabetes (P<0.001), and higher levels of SBP (P<0.001), FPG (P<0.001), HbA1c (P<0.001), albuminuria (P<0.001) and creatinine (P=0.005). Moreover, patients with DR were more likely to be smoker (P=0.007) and user of insulin (P<0.001).
Table 2. Baseline characteristics of participants in the Beijing Desheng Diabetic Eye Study.
| Characteristics | All | NDR | Any DR | P |
| Number | 1438 | 929 | 509 | |
| Age (a) | 65.04±8.25 | 65.24±8.25 | 64.68±8.26 | 0.22 |
| Male, n (%) | 575 (40.0) | 346 (37.2) | 229 (45.0) | 0.004 |
| Nationality, n (%) | 0.07 | |||
| Han | 1332 (92.6) | 852 (91.7) | 480 (94.3) | |
| Minority | 106 (7.4) | 77 (8.3) | 29 (5.7) | |
| Monthly income (CNY), n (%) | 0.01 | |||
| <2000 | 462 (32.1) | 272 (29.3) | 190 (37.3) | |
| 2000-2999 | 655 (45.5) | 431 (46.4) | 224 (44.0) | |
| 3000-3999 | 185 (12.9) | 130 (14.0) | 55 (10.8) | |
| ≥4000 | 130 (9.0) | 91 (9.8) | 39 (7.7) | |
| Education, n (%) | 0.004 | |||
| Lower than primary school | 166 (11.5) | 100 (10.8) | 66 (13.0) | |
| Middle/high school | 860 (59.8) | 536 (57.7) | 324 (63.7) | |
| College and above | 412 (28.7) | 293 (31.5) | 119 (23.4) | |
| Marital, n (%) | 0.39 | |||
| Single | 10 (0.7) | 7 (0.8) | 3 (0.6) | |
| Married | 1280 (89.0) | 817 (87.9) | 463 (91.0) | |
| Divorced | 10 (0.7) | 7 (0.8) | 3 (0.6) | |
| Widow/widower | 138 (9.6) | 98 (10.5) | 40 (7.9) | |
| Age of diabetic onset (a) | 55.62±10.70 | 57.67±8.96 | 51.90±12.48 | <0.001 |
| Duration of diabetes (y) | 9.42±8.60 | 7.58±6.31 | 12.78±10.89 | <0.001 |
| Use of insulin, n (%) | 347 (24.1) | 136 (14.6) | 211 (41.5) | <0.001 |
| Smoking status, n (%) | 0.007 | |||
| Current smoking | 202 (14.0) | 114 (12.3) | 88 (17.3) | |
| Ex-smoking | 270 (18.8) | 167 (18.0) | 103 (20.2) | |
| Never smoking | 960 (66.8) | 646 (69.5) | 314 (61.7) | |
| BMI (kg/m2) | 25.63±3.62 | 25.56±3.59 | 25.76±3.67 | 0.30 |
| WHR | 0.92±0.07 | 0.92±0.07 | 0.92±0.06 | 0.18 |
| SBP (mm Hg) | 135.08±16.68 | 133.56±16.21 | 137.85±17.19 | <0.001 |
| DBP (mm Hg) | 78.14±9.86 | 77.78±9.66 | 78.80±10.19 | 0.06 |
| FPG (mmol/L) | 7.78±2.56 | 7.33±2.14 | 8.62±3.01 | <0.001 |
| HbA1c, n (%) | <0.001 | |||
| <7.0% | 877 (61.0) | 638 (68.7) | 239 (47.0) | |
| ≥7.0% | 465 (32.3) | 235 (25.3) | 230 (45.2) | |
| High albuminuria, n (%) | 233 (15.5) | 114 (12.3) | 109 (21.4) | <0.001 |
| CRP (mg/dL) | 0.12 (0.05,0.24) | 0.12 (0.05,0.24) | 0.11 (0.05,0.23) | 0.36 |
| Creatinine (mg/dL) | 66.00 (57.00,78.00) | 65.00 (56.00,76.00) | 68.00 (58.00,81.00) | 0.005 |
| Uric acid (µmol/L) | 285.00 (238.00,342.00) | 286.40 (239.00,341.15) | 280.00 (234.00,344.00) | 0.38 |
| TG (mmol/L) | 1.39 (0.94,2.02) | 1.41 (0.97,2.04) | 1.34 (0.90,1.98) | 0.12 |
| TC (mmol/L) | 4.95 (4.26,5.65) | 4.93 (4.29,5.60) | 5.01 (4.21,5.77) | 0.16 |
| HDL-C (mmol/L) | 1.19 (1.03,1.40) | 1.20 (1.03,1.39) | 1.18 (1.02,1.42) | 0.69 |
| LDL-C (mmol/L) | 2.99 (2.43,3.60) | 2.97 (2.45,3.93) | 3.03 (2.40,3.66) | 0.49 |
The data were expressed as numbers (%) for categorical variables, mean±SD for normally distributed variables, and the median (interquartile range) for skewed distributed variables. NDR: No diabetic retinopathy; any DR: Any diabetic retinopathy; CNY: China Yuan; BMI: Body mass index; WHR: Waist and hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; FPG: Fasting plasma glucose; HbA1c: Glycosylated hemoglobin A1c; CRP: C-reactive protein; TG: Triglycerides; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol. Differences in baseline characteristics of patients in the NDR and any DR groups were compared using independent sample t-tests or Mann-Whitney U test for continuous variables as appropriate, and Chi-square test or Fisher's exact test for categorical variables as appropriate. P<0.05 was considered statistically significant.
As shown in Table 3, DR was diagnosed in 509 (35.4%) cases, among which mild NPDR was detected in 399 (27.7%) cases, moderate NPDR in 38 (2.6%) cases, severe NPDR in 7 (0.5%) cases, and PDR in 65 (4.5%) cases. When the patients were stratified by duration of diabetes, the prevalence of any DR and PDR was 20.4% and 0.8% in persons who had diabetes for less than five years, and the prevalence of any DR and PDR was 60.4% and 14.9% in persons who had diabetes for 15y or more.
Table 3. The presence and severity of diabetic retinopathy in the Beijing Desheng Diabetic Eye Study.
| Grading of DR | Subjects | Subjects by diabetic duration (y) |
||
| 0-4 | 5-14 | ≥15 | ||
| NDR | 929 (64.6) | 398 (79.6) | 425 (63.4) | 106 (39.6) |
| Any DR | 509 (35.4) | 102 (20.4) | 245 (36.6) | 162 (60.4) |
| Mild NPDR | 399 (27.7) | 93 (18.6) | 206 (30.7) | 100 (37.3) |
| Moderate NPDR | 38 (2.6) | 4 (0.8) | 15 (2.2) | 19 (7.1) |
| Severe NPDR | 7 (0.5) | 1 (0.2) | 3 (0.4) | 3 (1.1) |
| PDR | 65 (4.5) | 4 (0.8) | 21 (3.1) | 40 (14.9) |
| Total | 1438 (100) | 500 (100) | 670 (100) | 268 (100) |
DR: Diabetic retinopathy; NDR: No diabetic retinopathy; NPDR: Non-proliferative diabetic retinopathy; PDR: Proliferative diabetic retinopathy.
n (%)
Table 4 shows the influencing factors on the presence of any DR and VTR by multiple logistic regression analysis. Factors for the presence of DR included male (OR 1.44, 95% CI: 1.03-2.00, P=0.031), lower income level (OR 1.49, 95% CI: 1.10-2.03, P=0.011), lower education background (OR 1.43, 95% CI: 1.06-2.00, P=0.022), longer duration of diabetes (OR 2.31, 95% CI: 1.50-3.54, P<0.001), younger age at diabetic onset (OR 1.86, 95% CI: 1.02-3.41, P=0.044), higher SBP (OR 1.46, 95% CI: 1.11-1.93, P=0.007), higher HbA1c levels (OR 1.63, 95% CI: 1.23-2.16, P=0.001), high albuminuria (OR 1.45, 95% CI: 1.04-2.02, P=0.03), and use of insulin (OR 2.54, 95% CI: 1.85-3.48, P<0.001). For VTR, four factors were significant: younger age at diabetic onset (OR 6.55, 95% CI: 2.37-18.13, P<0.001), higher SBP (OR 1.99, 95% CI: 1.03-3.85, P=0.042), high albuminuria (OR 4.81, 95% CI: 2.46-9.39, P<0.001), and use of insulin (OR 10.04, 95% CI: 4.42-22.81, P<0.001).
Table 4. Risk factors for diabetic retinopathy by multiple logistic regression analysis in the Beijing Desheng Diabetic Eye Study.
| Variable | Any DR |
VTR |
||||
| OR | 95% CI | P | OR | 95% CI | P | |
| Sex (M vs F) | 1.44 | 1.03-2.00 | 0.031 | 1.28 | 0.59-2.76 | 0.53 |
| Monthly income (CNY) | ||||||
| ≥2000 | 1.00 | 1.00 | ||||
| <2000 | 1.49 | 1.10-2.03 | 0.011 | 2.07 | 0.99-4.33 | 0.05 |
| Education | ||||||
| College and above | 1.00 | 1.00 | ||||
| High school and below | 1.43 | 1.06-2.00 | 0.022 | 1.34 | 0.58-3.12 | 0.50 |
| Duration of diabetes (y) | ||||||
| 0-4 | 1.00 | 0.001 | 1.00 | 0.12 | ||
| 5-9 | 1.58 | 1.12-2.22 | 0.01 | 0.63 | 0.14-2.91 | 0.56 |
| 10-14 | 1.80 | 1.24-2.62 | 0.002 | 2.32 | 0.66-8.14 | 0.19 |
| ≥15 | 2.31 | 1.50-3.54 | <0.001 | 2.28 | 0.62-8.43 | 0.22 |
| Age of diabetic onset (a) | ||||||
| ≥50 | 1.00 | 0.001 | 1.00 | <0.001 | ||
| 40-49 | 1.85 | 1.33-2.57 | <0.001 | 3.96 | 1.78-8.81 | 0.001 |
| <40 | 1.86 | 1.02-3.41 | 0.044 | 6.55 | 2.37-18.13 | <0.001 |
| SBP (mm Hg) | ||||||
| <140 | 1.00 | 1.00 | ||||
| ≥140 | 1.46 | 1.11-1.93 | 0.007 | 1.99 | 1.03-3.85 | 0.042 |
| HbA1c (%) | ||||||
| <7.0 | 1.00 | 1.00 | ||||
| ≥7.0 | 1.63 | 1.23-2.16 | 0.001 | 0.87 | 0.44-1.70 | 0.68 |
| High albuminuria (Y vs N) | 1.45 | 1.04-2.02 | 0.03 | 4.81 | 2.46-9.39 | <0.001 |
| Use of insulin (Y vs N) | 2.54 | 1.85-3.48 | <0.001 | 10.04 | 4.42-22.81 | <0.001 |
Data were presented as OR, 95% CI, and P-values obtained by multiple logistic regression analysis. The presence of any DR or VTR was taken as dependent variable. Independent variables entered in the model included established risk factors of DR and variables with P≤0.20 in the univariate analysis: age of diabetic onset (<40y, 40-49y, ≥50y), durations of diabetes (0-4y, 5-9y, 10-14y, ≥15y), monthly income lower than 2000 CNY (Yes or No), education lower than high school (Yes or No), BMI (<25 kg/m2, ≥25 kg/m2), SBP (<140 mm Hg, ≥140 mm Hg), DBP (<90 mm Hg, ≥90 mm Hg), HbA1c (≤7%, >7%), high albuminuria (Yes or No), CRP (continuous), creatinine (continuous), TG (continuous), TC (continuous), and use of insulin (Yes or No). DR: Diabetic retinopathy; VTR: Vision-threatening diabetic retinopathy; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; HbA1c: Glycosylated hemoglobin A1c; CRP: C-reactive protein; TG: Triglycerides; TC: Total cholesterol. P<0.05 was considered statistically significant.
DISCUSSION
The BDDES is designed as a community-based cohort study mainly aiming to gain a better understanding of the natural progression of DR amongst persons with T2DM in an urban Chinese population and to identify possible risk factors. A community-based cohort with relatively large sample size of T2DM patients was successfully recruited, and continued follow-up study will provide insights into the early prevention of DR for patients at high risk.
Studies of persons with diabetes in China have shown that the prevalence of DR varies from 18.0% to 43.1%[15]–[17],[19]. Whether these are real differences or due to variation in diagnostic technique are unclear. This study is the first study that reports prevalence rates using a standard ETDRS strategy for fundus photography and DR grading, making it possible to compare the differences of DR prevalence, incidence and risk factors between Chinese and other ethnic groups. The prevalence of any DR in the BDDES (35.4%) was comparable to that in patients aged 40 and above in the San Antonio Heart study (36.2%)[4] and Beaver Dam Eye Study (35.1%)[6], but relatively lower than that in the Los Angeles Latino Eye Study (45.8%)[10] and higher than that in Urban Slums (15.37%)[25] and the island of Funen, Denmark (21.1%)[26]. In patients with diabetes for less than five years, the prevalence of any DR and PDR was 20.4% and 0.8% in the BDDES cohort, relatively lower than that reported in WESDR (28.8% and 2.0%, respectively)[3]. In patients with diabetes for 15 or more years, the prevalence of any DR in the BDDES cohort (60.4%) was still lower than that in WESDR (77.8%), but the prevalence of PDR in the BDDES (14.9%) was similar to that in WESDR (15.5%)[3]. Ethic and environment factors may be accountable partly for this discrepancy. However, the main risk factors for DR including longer duration of diabetes, younger age at diabetic diagnosis, higher HbA1c levels, higher systolic BP, use of insulin, presence of proteinuria are consistent with previous studies.
Strengths of the study mainly include the following aspects. Firstly, participants in the present study were recruited from a relatively stabilized population in a same community of urban Beijing, which may reduce the potential influence of environments and increase the follow-up. Secondly, using a standardized ETDRS photographic method and grading protocol of DR, which have been recognized as the golden standard for clinical trial studies, may allow comparison across populations and facilitate academic exchanges. Thirdly, a cohort with relatively large sample size of diabetic patients is expected to offer insights into incidence and progression of DR in this Chinese population. Fourthly, the establishment of serum, plasma and DNA bank may help explore the genetic and non-genetic risk/protective factors for the development and progression of DR.
The present study has inherent limitations. There could be a potential selection bias since the recruitment of participants was randomized and restricted to primarily self-reported T2DM on their convenience. Estimates of the relationship of risk factors and DR thus may not be generalized to the population as a whole. However, comparison of basic characteristics to the Beijing Community Diabetes Study (BCDS)[27], a contemporaneous prospective multi-center study conducted in 15 Beijing urban communities, showed similar age (65.04±8.25y in current study vs 63.9±9.9y in BCDS), sex ratio (P=0.97), and rate of insulin treatment (P=0.09), suggesting that the BDDES cohort may be representative to some extent.
In summary, the BDDES is the first large-scale ongoing cohort study of a Chinese urban population of persons with type 2 diabetes. Using standardized grading system comparable to large cohort studies from western populations our baseline data shows that the prevalence of DR and main risk factors in this Chinese ethnic population are comparable to that found in the western population studies.
Acknowledgments
Authors' contributions: Li YY: data acquisition, analysis and interpretation, drafting of manuscript. Yang XF and Gu H: data acquisition, analysis and interpretation, critical revision of manuscript. Snellingen T, Liu XP and Liu NP: conception, design, data acquisition, analysis and interpretation, critical revision of manuscript. All authors read and approved the final manuscript. The authors thank Ronald R Danis, James L. Reimers and Ashwini Narkar, staff at the Fundus Photograph Reading Center, Department of Ophthalmology and Visual Sciences, University of Wisconsin, for their important contributions on the diabetic retinopathy grading and quality control.
Foundations: Supported by the Beijing Natural Science Foundation (No.7131007); the Norwegian Research Council (No.180419/D15/1k).
Conflicts of Interest: Li YY, None; Yang XF, None; Gu H, None; Liu XP, None; Snellingen T, None; Liu NP, None.
REFERENCES
- 1.Coda A, Sculley D, Santos D, Girones X, Acharya S. Exploring the effectiveness of smart technologies in the management of type 2 diabetes mellitus. J Diabetes Sci Technol. 2017 doi: 10.1177/1932296817711198. 1932296817711198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng Y, He M, Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60(5):428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Fong D, Stern MP, Pugh JA, Hazuda HP, Patterson JK, van Heuven WA, Klein R. Diabetic retinopathy in Mexican Americans and non-Hispanic whites. Diabetes. 1988;37(7):878–884. doi: 10.2337/diab.37.7.878. [DOI] [PubMed] [Google Scholar]
- 5.Hamman RF, Mayer EJ, Moo-Young GA, Hildebrandt W, Marshall JA, Baxter J. Prevalence and risk factors of diabetic retinopathy in non-Hispanic whites and Hispanics with NIDDM. San Luis Valley Diabetes Study. Diabetes. 1989;38(10):1231–1237. doi: 10.2337/diab.38.10.1231. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Moss SE, Linton KL. The Beaver Dam Eye Study. Retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology. 1992;99(1):58–62. doi: 10.1016/s0161-6420(92)32011-1. [DOI] [PubMed] [Google Scholar]
- 7.Leske MC, Wu SY, Hyman L, Li X, Hennis A, Connell AM, Schachat AP. Diabetic retinopathy in a black population: the Barbados Eye Study. Ophthalmology. 1999;106(10):1893–1899. doi: 10.1016/s0161-6420(99)90398-6. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Smith W, Wang JJ, Attebo K. Prevalence of diabetic retinopathy in an older community. The Blue Mountains Eye Study. Ophthalmology. 1998;105(3):406–411. doi: 10.1016/S0161-6420(98)93019-6. [DOI] [PubMed] [Google Scholar]
- 9.West SK, Klein R, Rodriguez J, Muñoz B, Broman AT, Sanchez R, Snyder R, Proyecto VER. Diabetes and diabetic retinopathy in a Mexican-American population: Proyecto VER. Diabetes Care. 2001;24(7):1204–1209. doi: 10.2337/diacare.24.7.1204. [DOI] [PubMed] [Google Scholar]
- 10.Varma R, Torres M, Peña F, Klein R, Azen SP, Los Angeles Latino Eye Study Group Prevalence of diabetic retinopathy in adult Latinos: the Los Angeles Latino eye study. Ophthalmology. 2004;111(7):1298–1306. doi: 10.1016/j.ophtha.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, Lim SC, Tai ES, Mitchell P. Prevalence and riskfactors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11):1869–1875. doi: 10.1016/j.ophtha.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 12.Pradeepa R, Anitha B, Mohan V, Ganesan A, Rema M. Risk factors for diabetic retinopathy in a South IndianType 2 diabetic population--the Chennai Urban Rural Epidemiology Study (CURES) Eye Study 4. Diabet Med. 2008;25(5):536–542. doi: 10.1111/j.1464-5491.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 13.Raman R, Rani PK, Gnanamoorthy P, Sudhir RR, Kumaramanikavel G, Sharma T. Association of obesity withdiabetic retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS Report no. 8) Acta Diabetol. 2010;47(3):209–215. doi: 10.1007/s00592-009-0113-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu DP, Molyneaux L, Chua E, Wang YZ, Wu CR, Jing H, Hu LN, Liu YJ, Xu ZR, Yue DK. Retinopathy in a Chinese population with type 2 diabetes: factors affecting the presence of this complication at diagnosis of diabetes. Diabetes Res Clin Pract. 2002;56(2):125–131. doi: 10.1016/s0168-8227(01)00349-7. [DOI] [PubMed] [Google Scholar]
- 15.Peng JJ, Zou HD, Wang WW, Fu J, Shen BJ, Xu X, Zhang X, Zhao NQ, Yu YF. The application study of community-based tele-screening system for diabetic retinopathy in Beixinjing blocks, Shanghai. Zhonghua Yan Ke Za Zhi. 2010;46(3):258–262. [PubMed] [Google Scholar]
- 16.Xie XW, Xu L, Wang YX, Jonas JB. Prevalence and associated factors of diabetic retinopathy. The Beijing Eye Study 2006. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1519–1526. doi: 10.1007/s00417-008-0884-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang FH, Liang YB, Zhang F, Wang JJ, Wei WB, Tao QS, Sun LP, Friedman DS, Wang NL, Wong TY. Prevalence of diabetic retinopathy in rural China: the Handan Eye Study. Ophthalmology. 2009;116(3):461–467. doi: 10.1016/j.ophtha.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Wei WB, Yuan MX, Yuan SY, Wan G, Zheng YY, Li YB, Wang S, Xu L, Fu HJ, Zhu LX, Pu XL, Zhang JD, Du XP, Li YL, Ji Y, Gu XN, Li Y, Pan SF, Cui XL, Bai W, Chen YJ, Wang ZM, Zhu QS, Gao Y, Liu DY, Ji YT, Yang Z, Jonas JB. Prevalence and risk factors for diabetic retinopathy: the Beijing Communities Diabetes Study 6. Retina. 2012;32(2):322–329. doi: 10.1097/IAE.0b013e31821c4252. [DOI] [PubMed] [Google Scholar]
- 19.Pan CW, Wang S, Qian DJ, Xu C, Song E. Prevalence, awareness, and risk factors of diabetic retinopathy among adults with known type 2 diabetes mellitus in an urban community in China. Ophthalmic Epidemiol. 2017;24(3):188–194. doi: 10.1080/09286586.2016.1264612. [DOI] [PubMed] [Google Scholar]
- 20.Cikamatana L, Mitchell P, Rochtchina E, Foran S, Wang JJ. Five-year incidence and progression of diabetic retinopathy in a defined older population: the Blue Mountains Eye Study. Eye (Lond) 2007;21(4):465–471. doi: 10.1038/sj.eye.6702771. [DOI] [PubMed] [Google Scholar]
- 21.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6y from diagnosis. Diabetologia. 2001;44(2):156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 22.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang XF, Deng Y, Gu H, Lim A, Snellingen T, Liu XP, Wang NL, Domalpally A, Danis R, Liu NP. C-reactive protein and diabetic retinopathy in Chinese patients with type 2 diabetes mellitus. Int J Ophthalmol. 2016;9(1):111–118. doi: 10.18240/ijo.2016.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li HK, Hubbard LD, Danis RP, Esquivel A, Florez-Arango JF, Ferrier NJ, Krupinski EA. Digital versus film Fundus photography for research grading of diabetic retinopathy severity. Invest Ophthalmol Vis Sci. 2010;51(11):5846–5852. doi: 10.1167/iovs.09-4803. [DOI] [PubMed] [Google Scholar]
- 25.Sunita M, Singh AK, Rogye A, Sonawane M, Gaonkar R, Srinivasan R, Natarajan S, Stevens FCJ, Scherpbier AJJA, Kumaramanickavel G, McCarty C. Prevalence of diabetic retinopathy in urban Slums: The Aditya Jyot Diabetic Retinopathy in Urban Mumbai Slums Study-Report 2. Ophthalmic Epidemiol. 2017;24(5):303–310. doi: 10.1080/09286586.2017.1290258. [DOI] [PubMed] [Google Scholar]
- 26.Larsen MB, Henriksen JE, Grauslund J, Peto T. Prevalence and risk factors for diabetic retinopathy in 17 152 patients from the island of Funen, Denmark. Acta Ophthalmol. 2017;95(8):778–786. doi: 10.1111/aos.13449. [DOI] [PubMed] [Google Scholar]
- 27.Yang GR, Yuan SY, Fu HJ, Wan G, Zhu LX, Yuan MX, Lv YJ, Zhang JD, Du XP, Li YL, Ji Y, Zhou L, Li Y, Beijing Community Diabetes Study G Influence of educational attainments on long term glucose control and morbid events in patients with type 2 diabetes receiving integrated care from 15 China urban communities: The Beijing Community Diabetes Study 11. Prim Care Diabetes. 2015;9(6):473–481. doi: 10.1016/j.pcd.2015.03.005. [DOI] [PubMed] [Google Scholar]