Abstract
Technological advances in glaucoma have challenged the traditional treatment paradigm. Historically incisional surgery has been used in cases of advanced disease and/or uncontrolled intraocular pressures resistant to medical or laser interventions. More recently, perhaps due to advancements in imaging, surgery has been suggested to be beneficial earlier in the treatment paradigm. Despite these trends, surgical manipulation of the tissues and unpredictability of wound healing continue to result in surgical failure. Magnesium is an essential element for human body and plays a critically important role in maintaining the functional and structural integrity of several tissues, including the eye. Due to several of its advantageous properties such as non-toxicity, biodegradability, and high biological compatibility, magnesium alloy has attracted great attention as a novel biomaterial. Biodegradable cardiovascular stents made of magnesium alloy have already been introduced into clinical practice. The purpose of this review is to determine if bioabsorbable magnesium alloys can be utilized as a promising candidate for the development of a new generation of glaucoma surgical assistive devices.
Keywords: glaucoma, glaucoma drainage implant, magnesium alloy, anti-scarring
INTRODUCTION
Magnesium is an essential trace element for human life, and also is one of the most important regulatory cations involved in several biological processes. Magnesium is the second most common cation in the intracellular fluid. Mg2+ plays a crucial role in regulating vascular functions and energy metabolism as well as maintaining water and electrolyte balance[1]–[2]. The level of Mg2+ in the serum ranges from 0.8 to 1.2 mmol/L, with homeostasis being maintained by the kidney and intestine. Hypomagnesemia (serum Mg2+<0.8 mmol/L) has been suggested to be associated with several disorders, such as vascular spasm and arrhythmia. Magnesium deficiency may also lead to cardiovascular, respiratory, and digestive illnesses, as well as abortion, fetal abnormalities and other obstetric diseases[3]. A large number of studies have shown that dietary intake of magnesium can prevent osteoporosis and femoral neck fracture[4]–[7], and help to manage diabetes and coronary artery disease[8]–[9]. Serum Mg2+ levels exceeding 1.2 mmol/L may cause muscular paralysis, respiratory distress and hypotension[10]; however hypermagnesemia is rare due to the efficient excretion of the element in the urine[11].
Glaucoma is the second most common cause of blindness and the leading cause of irreversible worldwide blindness[12]. Surgery is an important treatment option for glaucoma Incisional surgery, with and without glaucoma drainage devices, is a major part of the treatment paradigm when medical options cannot be tolerated and/or fail to reach a pressure that halts progression. Long-term complications are often related to variability in the wound healing process. Devices used adjunctively in surgical glaucoma management represent a foreign body under the conjunctiva, causing fibroblast proliferation. This may be due to the persistent effect of inflammatory mediators and cytokines at the level of the conjunctival-Tenon-episcleral interface resulting in fibrosis and obstruction of the fistula[13]–[14]. There continues to be significant interest in adjunctive approaches to minimize scarring in the subconjunctival space, reducing inflammation, and by slowing or halting the excessive fibrotic healing process.
Bleb scarring is a major contributor to increased intraocular pressure (IOP) and surgical failure. In order to improve the success rate of glaucoma surgery, various intraoperative anti-metabolites have been used to inhibit fibroblast proliferation, such as 5-fluorouracil (5-FU) and mitomycin C (MMC). However, these antimitotic agents interfere indiscriminately with cellular proliferation, and can contribute to a number of postoperative adverse events, including bleb leaks, hypotony, choroidal detachment, endophthalmitis, and keratitis[15]–[16]. There continues to be a significant need for a safe, non-toxic, and effective approach to reduce postoperative scarring and adhesions after glaucoma surgery. The relatively recent introduction of bleb-forming micro-invasive glaucoma surgery has heightened this interest.
Magnesium alloy is a new kind of biodegradable material which possesses many advantageous properties such as high strength, light weight, and high biological compatibility. Additionally, magnesium alloy has been shown to be able to reduce irritation and inflammatory reactions in the body[17]–[20]. We were thus motivated to determine the feasibility of developing a magnesium-based bio-absorbable device for modulation of wound healing associated with glaucoma surgery. Moreover, the possibility that the slow release of Mg2+ by the device could have a local neuroprotective role is an intriguing concept worthy of future investigation.
APPLICATION OF MAGNESIUM ALLOY IN MODERN MEDICINE
The application of magnesium alloy as a bio-absorbable material in clinical practice can be traced back to the early 1900s. The first use of magnesium was reported by Lambotte[21] in 1928, who utilized a plate of pure magnesium with gold-plated steel nails to treat a fracture involving the bones of the lower leg. But the attempt failed as the pure magnesium plate corroded too rapidly in vivo, disintegrating only eight days after surgery and producing a large amount of gas under the skin. In 1938, McBride[22] developed magnesium alloy fixtures which successfully treated 20 cases of fracture without any significant adverse effects, and the fixtures were completely absorbed three months after surgery. In 1944, Troitskii and Tsitrin[23] applied cadmium magnesium alloy as an internal fixation device to secure the bones of 34 consecutive fracture patients. They observed that the fixture could maintain the mechanical integrity up to two months, and it was completely absorbed after 10-12mo. Nine cases were unsuccessful, and these failures were attributed to infection. A hard callous was found around the fracture site; no increase in serum levels of magnesium and no obvious inflammatory reactions to the implant were observed in all of the 34 patients. Znamenskii[24] reported similar results in 1945, where magnesium alloy containing 10% aluminum (Al) was used to treat two patients with gunshot wounds. The magnesium implant and nails were completely absorbed after 4-6wk. These early reports demonstrated that magnesium alloy was a non-toxic biomaterial, and it had the ability to promote bone healing. However, due to its characteristics of hydrogen emission and low corrosion resistance in the electrolytic and aqueous environments of the physiological system, biomaterial research on magnesium alloy was suspended. Thereafter, stainless steel materials were widely applied in bone internal fixation devices. Until recent years, with the use of advanced techniques, more complicated alloy compositions have been introduced. Corrosion protection technologies have effectively reduced the production of hydrogen gas. Interest in the medical use of magnesium alloys has once again increased due to advancements in coatings that alter the corrosive tendency[24].
Numerous in vitro and in vivo studies have focused on the use of magnesium alloys in internal fixation devices for fracture repairs. The corrosion resistance of different types of coated magnesium alloys has been studied in cytological and animal experiments to determine the reduction in hydrogen evolution and tissue compatibility[25]–[26]. In vitro experiments confirmed that there were minimal cytotoxicity and cell apoptosis when fibroblasts and osteoblasts were exposed to various coated magnesium alloys[26]. In vivo experiments found that Mg2+ could enhance bone formation during the degradation process of the implant, and no inflammation was observed[27]–[28]. Witte et al[29]–[30] successfully implanted magnesium alloy stents into the femur of rabbits, and the magnesium implants substantially degraded after three months. Their study also showed that the degradation process of the magnesium alloy stents could promote trabecular bone formation and resorption, without any significant harm to their neighboring tissues. They concluded that even fast-degrading magnesium alloy stents could show favourable biocompatibility thus establishing a more convincing potential role, in musculoskeletal surgery. In addition, Witte et al[31] also carried out an in vitro experiment to investigate the properties of a metallic matrix composite made of magnesium alloy AZ91 as a matrix with hydroxyapatite (HA) particles as reinforcements. The results revealed that the HA particles could stabilize the corrosion rate of the magnesium alloy, and this biodegradable MMC-HA was a cytoompatible biomaterial with adjustable mechanical and corrosive properties. Okazaki[32] developed a novel material containing magnesium, calcium, and phosphate for use as oral implants. They found that magnesium increased the metabolic rate of osteoblasts. Zreiqat et al[33] found that the protein levels were significantly higher in human bone-derived cells cultured on (Mg)-Al2O3 (alumina doped with magnesium ions) compared with those grown on Al2O3 alone. Hunt and Heggarty[34] reported that the magnesium-coated Ti-6Al-4v implant could activate bone cell signal transduction and hence improve protein synthesisand accelerate bone formation. The mechanical and degradation properties of magnesium have been used to develop novel cardiovascular stents, so as to maintain the endothelial function of coronary arteries and to reduce the risk of coronary ischemia and occlusion[35]–[38]. Based on a large number of clinical trials[39]–[40], magnesium coronary stents have been introduced into clinical practice[41].
THE IMPORTANT ROLES OF MG2+ IN THE EYE
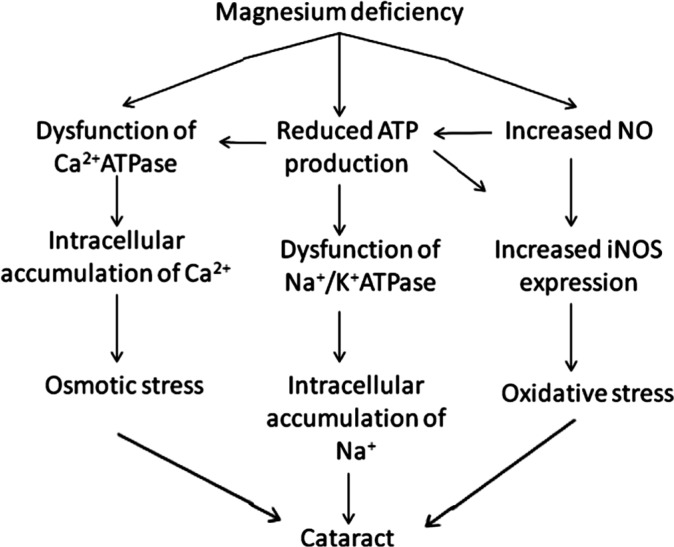
Mg2+ is important for maintaining the structural and functional integrity of several vital ocular tissues such as the cornea; lens and retina. The concentration of magnesium in aqueous humor is 2.97±0.75 mg/100 mL. The magnesium levels in the lens are far higher than those in the anterior chamber and vitreous body. The concentration in the lens periphery is four times greater than the axial regions[42]. Mg2+ plays an extremely important role in maintaining retinal function. The reason for this is because magnesium acts as a co-factor involved in the catalytic function of more than 350 enzymes in the body and regulates neuro-excitability. Membrane associated ATPase functions that are crucial in regulating the intracellular ionic environment, are also magnesium-dependent. As a result, a reduced level of Mg2+ may affect the functions of Na+/K+ ATPase and calcium dependent ATPase, causing an increase in the intracellular concentrations of calcium and sodium as well as a decrease in the potassium[43] concentration. Such ionic imbalances induced by magnesium deficiencymay contribute to the pathogenesis of many eye disorders, such as cataract, corneal, conjunctival, choroidal and retinal diseases[44]–[45].
Maintainence of Corneal Structure and Function
Mg2+ is one of the most important cations in the cornea as it is involved in the metabolism and maintenance of corneal transparency[46]. As early as 1920, Kirkpatrick[47] reported the use of magnesium sulfate in the treatment of keratitis, conjunctivitis, and corneal ulceration. In 1985, Bachman and Wilson[48] performed an animal experiment and found that the epithelial surface of the excised rabbit cornea was maintained best with a buffered solution containing Mg2+, K+, and Ca2+. Hogan et al[49] reported that Mg2+ loss in the corneal stroma was associated with corneal edema. In 2001, Gong et al[50] investigated the effect of magnesium deficiency on the cornea in rats that were fed a low magnesium diet for 3wk. Their findings revealed that magnesium deficiency affected the structural and functional integrity of the cornea.
Keratoconus is defined as a progressive eye disease which causes thinning and fragmentation of membranes, degenerated cells and collagen fibers, swelling of the mitochondria, and biochemical abnormalities in protein synthesis and expansion of central area of the cornea. Thalasselis[51] reported that hypomagnesemia was commonly seen in the serum samples of keratoconus patients, and magnesium deficiency could pathologically affect the integrity of the cornea. Prior studies also showed that magnesium deficiency was associated with a reduced number of microvilli and their irregular arrangement. Vesicular degeneration and swelling of the mitochondria were observed in the cytoplasm of epithelial cells, resulting in abnormal apoptosis of these cells[52]–[53]. Magnesium deficiency was also associated with rupture and fragmentation of the Bowman's layer, which may be an early change leading to keratoconus[54]–[55].
Supporting Lens Metabolism and Preventing Cataract
Cataract is the most common cause of blindness worldwide. It is characterized by progressive lenticular opacities. It is known that cataractous lenses have an abnormal intracellular ionic environment with lower concentrations of potassium and magnesium and higher concentrations of sodium and calcium relative to the cytosol of most cells. The lens membrane has increased permeability in the presence of a cataract[56]. Studies have explored the relationship between magnesium deficiency and cataract. These studies have shown that the alterations in lenticular redox status and ionic imbalances form the basis of the relationship between magnesium deficiency with cataract. It is believed that Mg2+ plays an important role not only in maintaining a low lenticular Ca2+ and Na2+ concentration but also in preserving the lens redox status, which has been shown to reduce lenticular oxidative stress[57]–[58]. Nagai et al[59] reported that the incidence of cataract was significantly lower in Shumiya rats fed 200 mg/L magnesium compared to controls. It was noted that the intracellular Ca2+ level of the lenses was also significantly lower. Based on these findings, they concluded that an appropriate supplementation of magnesium could delay cataract genesis, probably by inhibiting the increase in Ca2+ levels in the lens[60]. After a thorough literature review, Agarwal et al[61] concluded that magnesium supplementation might be of therapeutic value in preventing the onset and progression of cataract.
Oxidative stress is another important contributor to the pathogenesis of cataract. It has been shown in many studies that nitric oxide (NO) could be a risk factor in cataract formation[62]. NO production via inducible nitric oxide synthase (iNOS) causes an oxidative stress response in the lens[63]. Mg2+ has been shown to prevent the increase of NO in the lens which provides protection by inhibiting the nitrosylation of gap junctional proteins and maintaining membrane permeability[64]. It is also known to block iNOS expression and reduce the oxidative stress response. A cytological experiment found that the expression of iNOS was 6 times higher in the lens epithelial cells cultured in the magnesium-deficient medium compared to those cultured in the magnesium-supplemented medium. In addition, Mg2+ was shown to regulate Ca2+ ATPase thus modulating the concentration of Ca2+ in the lens.
Both magnesium deficiency and excessive NO production have been shown to decrease ATP levels[60]. The reduction in adenosine triphosphate ATP levels affects the membrane-associated Ca2+ ATPase and Na+/K+ ATPase, causing ionic imbalance in the lens. Interestingly, the decreased ATP levels in turn may have inhibitory effects on the expression of iNOS[65]. Therefore, it has been speculated that magnesium deficiency may cause an acceleration of the progression of lens opacification (Figure 1).
Figure 1. Magnesium deficiency contributes to the pathogenesis of cataract.
Glaucomatous Neuroprotection
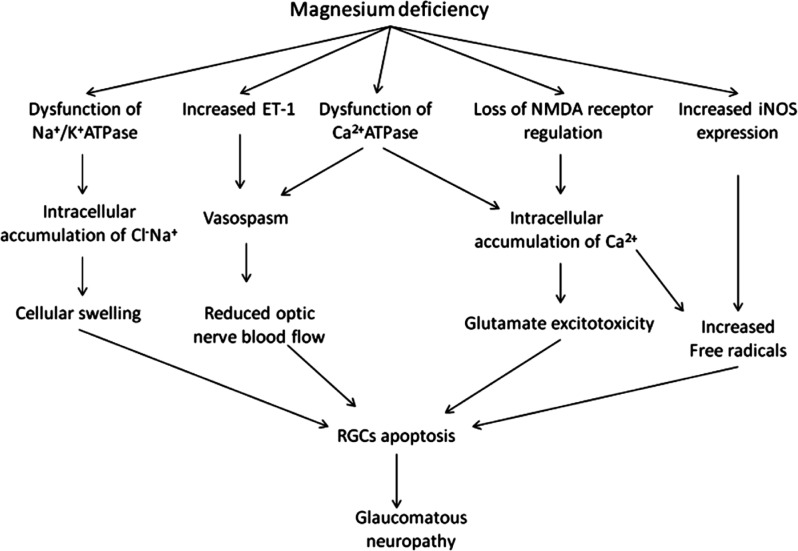
Elevated IOP is not the sole risk factor for chronic glaucomatous neuropathy. In fact, the search for putative non-IOP dependent continues to be of significant interest. Vasomotor dysfunction has also been suggested to contribute to optic neuropathy, by mediating abnormal hemodynamics and oxidative stress[66]. In an epidemiological study, Bonomi et al[67] reported that reduced diastolic perfusion pressure could be an important risk factor for primary open-angle glaucoma. Leske et al[68] reported an association between cardiovascular disease and glaucoma which suggested a vascular role in glaucomatous progression. It has been well-established that Mg2+ can function as a physiological Ca2+ channel blocker which, in turn, prevents ischemia and provides protection to the optic nerve. A large number of studies concerning ocular blood flow and oxidative stress response have confirmed that Mg2+ may regulate the current strength and in activation process of Ca2+ channels[69]–[70]. Even small changes in extracellular Mg2+ levels may have significant effects on vascular tone. Mg2+ has been shown to have a direct vasodilatory effect[71]–[73] while magnesium deficiency has been shown to increase intracellular Ca2+ levels leading to vasoconstriction[74]–[75]. Moreover, as Mg2+ regulates Na+/K+ ATPase, magnesium deficiency is associated with reduced activity of Na+/K+ ATPase and increased intracellular Na+ and Cl− levels causing cellular swelling and apoptosis of retinal ganglion cells (RGCs)[76].
Other studies have demonstrated an association between plasma endothelin-1 (ET-1) levels and normal tension glaucoma (NTG). Patients with NTG in the initial stage of visual field loss demonstrated higher plasma ET-1 levels than those with moderate visual field damage[77]. An increase in extracellular Mg2+ levels inhibits ET-1, which may induce constriction and vasospasm of the ciliary arteries, with reduced blood supply to the optic nerve. Furthermore, ET-1 may affect the functions of axons and astrocytes, and accelerate the apoptosis of RGCs[78]. It has been suggested that the favorable effects of magnesium on the visual field may be attributed to ET-1inhibition, suppressing vasoconstriction, improving the ocular blood flow, and preventing glaucomatous neuropathy[44].
The ion channel of the N-methyl-D-aspartate (NMDA) receptor is calcium dependent and subject to voltage-dependent regulation by Mg2+. Mg2+ can regulate the glutamate-gated ion channel which, in turn, has been shown to prevent excitotoxicity and cell apoptosis[79]–[82]. In the presence of a magnesium deficiency, the toxic effects of glutamate on RGCs are mediated by the over stimulation of NMDA receptors, leading to glutamate excitotoxicity and loss of RGCs in glaucoma patients. Lambuk et al[83] reported that intravitreal Mg2+ could prevent retinal and optic nerve damage induced by NMDA. Moreover, intracellular accumulation of Ca2+ associated with magnesium deficiency has been associated with the production of free radicals[84]–[85].
Magnesium deficiency directly increases the expression of iNOS. Lower levels of Mg2+ have been associated with vasospasm and retinal ischemia which also enhance the expression of iNOS[86], and produce large amounts of free radicals[86]. Numerous studies have shown that Mg2+ plays a neuroprotective role in inhibiting the elevated iNOS activity of neurons in retinal ischemia[87]–[89].
In a study by Gaspar et al[90], 10 glaucoma patients including 6 with primary open angle glaucoma (POAG) and 4 with NTG were administered magnesium 121.5 mg, twice a day for one month. Results showed that magnesium significantly increased the ocular blood flow and improved the peripheral circulation, exerting a beneficial effect on the visual field in glaucoma patients with vasospasm. Aydin et al[91] also reported that in 15 NTG patients who received 300 mg oral magnesium citrate for a month, an improvement in the visual field was observed, but the ocular blood flow remained unchanged. They speculated that mechanisms other than increased ocular blood flow may be responsible for the improvement in the visual field when given oral magnesium therapy. Based on these findings, it was suggested that Mg2+ may play an important role in optic neuroprotection in patients with glaucoma (Figure 2).
Figure 2. Magnesium deficiency contributes to the pathogenesis of glaucomatous neropathy.
THE HISTORY AND PROSPECTS OF USING MAGNESIUM MATERIALS IN GLAUCOMA DRAINAGE SURGERY
Due to the unique properties of magnesium and its satisfactory bio-compatibility, the use of the pure materials was considered as an implant for the treatment of glaucoma in the middle of the 20th century. In 1940, Troncoso[92] utilized pure magnesium implants to increase the aqueous outflow in the treatment of glaucoma. Five years later, Hoff[93] used this technique to treat a patient with neovascular glaucoma who refused enucleation, Twelve hours after operation, the patient's whole anterior chamber was filled with gas and the IOP was significantly increased, resulting in severe pain. The pressure of the gas was relieved by venting with a thin hypodermic needle. The IOP normalized on the sixth postoperative day, but huge bubbles were still found in the anterior chamber and the conjunctiva, which disappeared on the tenth day. Interestingly, before the operation the cornea was opaque at an IOP of 54 mm Hg, but it became absolutely clear when the anterior chamber was full of gas, despite the elevated IOP which varied from 49 to 55. The postoperative IOP was controlled at 1-4mo after surgery, but elevated to 45 mm Hg again at the fifth month. Gonioscopy revealed complete circumferential iridocorneal synechiae. Coarse pigment granules were observed in the dependent part of the angle. Eventually, the eye was removed. Pathological examination revealed a closed filter channel and massive fibroblast proliferation around the incision.
These experiences indicated that pure magnesium had significant adverse effects due to the excessive corrosion when in contact with body fluids with hydrogen gas emission causing treatment failure. Advancements in the area of coating technology, allowed the synthesis of novel coated magnesium alloys with optimized compositions (containing Al, zinc, manganese, calcium, praseodymium, or neodymium) allowing control over the corrosive properties. Coated magnesium alloys have shown significant promise as effective biodegradable materials.
During the last 15y, biodegradable metallic stents have been developed and investigated as alternatives for the currently-used permanent cardiovascular stents[94]–[96]. Traditional cardiovascular stents have significant drawbacks including irritatation and damage to the vascular endothelium, leading to stenosis and blockage[97]–[100]. In order to solve these problems, absorbable magnesium materials have shown success. In vitro studies have demonstrated that magnesium cardiovascular stents can maintain the integrity and function of endothelial cells, as well as reduce inflammation and fibroblast proliferation. In these studies, stents could be fully absorbed within 2-4mo with few complications observed during a two years of follow-up period[38],[40]. Subsequently, a large number of clinical trials were conducted[38]–[39]. In 2007, a prospective multi-center trial in patients with coronary heart disease was published in the Lancet, which showed that biodegradable magnesium stents achieved an immediate angiographic result similar to the result of traditional metal stents and was safely degraded after 4mo[101].
The introduction of magnesium cardiovascular stents has led to interest in how their use can be expanded into other areas of the body. In particular, their potential utility as an adjunctive device in glaucoma surgery is of high interest. The excellent biocompatibility and bio-degradability, have the potential to allow magnesium alloy devices to effectively decrease postoperative irritation, reduce scar formation, improve the success rate of glaucoma surgery, and minimize the long-term effects of permanent implants which tend to produce local complications related to tissue compression. The liberation of Mg2+ cations during the natural degradation process has many potential beneficial effects on the cornea, lens, retina, choroid, and optic nerve.
In conclusion, bio-absorbable coated magnesium alloys may be a very promising candidate for the development of a new generation of glaucoma drainage device.
Acknowledgments
Thank you to the secretaries and staff of Ivey Eye Institute, St. Joseph's Hospital where this research was conducted.
Conflicts of Interest: Li XJ, None; Xie L, None; Pan FS, None; Wang Y, None; Liu H, None; Tang YR, None; Hutnik CML, None.
REFERENCES
- 1.Mubagwa K, Gwanyanya A, Zakharov S, Macianskiene R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch Biochem Biophys. 2007;458(1):73–89. doi: 10.1016/j.abb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Ellahioui Y, Prashar S, Gomez-Ruiz S. A short overview on the biomedical applications of silica, alumina and calcium phosphate-based nanostructured materials. Curr Med Chem. 2016;23(39):4450–4467. doi: 10.2174/0929867323666161024153459. [DOI] [PubMed] [Google Scholar]
- 3.Okuma T. Magnesium and bone strength. Nutrition. 2001;17(7-8):679–680. doi: 10.1016/s0899-9007(01)00551-2. [DOI] [PubMed] [Google Scholar]
- 4.Orchard TS, Larson JC, Alghothani N, Bout-Tabaku S, Cauley JA, Chen Z, LaCroix AZ, Wactawski-Wende J, Jackson RD. Magnesium intake, bone mineral density, and fractures: results from the Women's Health Initiative Observational Study. Am J Clin Nutr. 2014;99(4):926–933. doi: 10.3945/ajcn.113.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farsinejad-Marj M, Saneei P, Esmaillzadeh A. Dietary magnesium intake, bone mineral density and risk of fracture: a systematic review and meta-analysis. Osteoporosis Int. 2016;27(4):1389–1399. doi: 10.1007/s00198-015-3400-y. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference I . The national academies collection: reports funded by national institutes of health. Washington (DC): National Academies Press (US); 1997. [Google Scholar]
- 7.Takeda R, Nakamura T. Effects of high magnesium intake on bone mineral status and lipid metabolism in rats. J Nutr Sci Vitaminol (Tokyo) 2008;54(1):66–75. doi: 10.3177/jnsv.54.66. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien B, Carroll W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: a review. Acta Biomater. 2009;5(4):945–958. doi: 10.1016/j.actbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Mani G, Feldman MD, Patel D, Agrawal CM. Coronary stents: a materials perspective. Biomaterials. 2007;28(9):1689–1710. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294(1-2):1–26. doi: 10.1016/s0009-8981(99)00258-2. [DOI] [PubMed] [Google Scholar]
- 11.Vormann J. Magnesium: nutrition and metabolism. Mol Aspects Med. 2003;24(1-3):27–37. doi: 10.1016/s0098-2997(02)00089-4. [DOI] [PubMed] [Google Scholar]
- 12.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80(5):389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atreides SP, Skuta GL, Reynolds AC. Wound healing modulation in glaucoma filtering surgery. Int Ophthalmol Clin. 2004;44(2):61–106. doi: 10.1097/00004397-200404420-00007. [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro MF, Bhattacharya SS, Schultz GS, Khaw PT. TGF-beta1, -beta2, and -beta3 in vitro: biphasic effects on Tenon's fibroblast contraction, proliferation, and migration. Invest Ophthalmol Vis Sci. 2000;41(3):756–763. [PubMed] [Google Scholar]
- 15.Huan ZG, Leeflang MA, Zhou J, Fratila-Apachitei LE, Duszczyk J. In vitro degradation behavior and cytocompatibility of Mg-Zn-Zr alloys. J Mater Sci Mater Med. 2010;21(9):2623–2635. doi: 10.1007/s10856-010-4111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu CY, Matsuo H, Tomita G, Suzuki Y, Araie M, Shirato S, Tanaka S. Clinical characteristics and leakage of functioning blebs after trabeculectomy with mitomycin-C in primary glaucoma patients. Ophthalmology. 2003;110(2):345–352. doi: 10.1016/S0161-6420(02)01739-6. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann OJ, Bunce C, Matheson MM, Maurino V, Khaw PT, Wormald R, Barton K. Risk factors for development of post-trabeculectomy endophthalmitis. Br J Ophthalmol. 2000;84(12):1349–1353. doi: 10.1136/bjo.84.12.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grillo CA, Alvarez F, Fernandez Lorenzo de Mele MA. Degradation of bioabsorbable Mg-based alloys: Assessment of the effects of insoluble corrosion products and joint effects of alloying components on mammalian cells. Mater Sci Eng C Mater Biol Appl. 2016;58:372–380. doi: 10.1016/j.msec.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Walker J, Shadanbaz S, Woodfield TB, Staiger MP, Dias GJ. Magnesium biomaterials for orthopedic application: a review from a biological perspective. J Biomed Mater Res B Appl Biomater. 2014;102(6):1316–1331. doi: 10.1002/jbm.b.33113. [DOI] [PubMed] [Google Scholar]
- 20.Hanzi AC, Gerber I, Schinhammer M, Loffler JF, Uggowitzer PJ. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg-Y-Zn alloys. Acta Biomater. 2010;6(5):1824–1833. doi: 10.1016/j.actbio.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Lambotte A. The classic. Contribution to conservative surgery of the injured hand. By Dr. A. Lambotte. 1928. Clin Orthop Relat Res. 1987;(214):4–6. [PubMed] [Google Scholar]
- 22.McBride ED. Absorbable metal in bone surgery. J Am Med Assoc. 1938;111(27):2464–2467. [Google Scholar]
- 23.Troitskii VV, Tsitrin DN. The resorbing metallic alloy “Osteosinthezit” as material for fastening broken bone. Khirurgiia. 1944;8:41–44. [Google Scholar]
- 24.Znamenskii MS. Metallic osteosynthesis by means of an apparatus made of resorbing metal. Khirurgiia. 1945;12:60–63. [Google Scholar]
- 25.Zberg B, Uggowitzer PJ, Loffler JF. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat Mater. 2009;8(11):887–891. doi: 10.1038/nmat2542. [DOI] [PubMed] [Google Scholar]
- 26.Gu X, Zheng Y, Cheng Y, Zhong S, Xi T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials. 2009;30(4):484–498. doi: 10.1016/j.biomaterials.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Janning C, Willbold E, Vogt C, Nellesen J, Meyer-Lindenberg A, Windhagen H, Thorey F, Witte F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodelling. Acta Biomater. 2010;6(5):1861–1868. doi: 10.1016/j.actbio.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Yang JX, Cui FZ, Lee IS, Zhang Y, Yin QS, Xia H, Yang SX. In vivo biocompatibility and degradation behavior of Mg alloy coated by calcium phosphate in a rabbit model. J Biomater Appl. 2012;27(2):153–164. doi: 10.1177/0885328211398161. [DOI] [PubMed] [Google Scholar]
- 29.Witte F, Ulrich H, Palm C, Willbold E. Biodegradable magnesium scaffolds: Part II: peri-implant bone remodeling. J Biomed Mater Res A. 2007;81(3):757–765. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 30.Witte F, Ulrich H, Rudert M, Willbold E. Biodegradable magnesium scaffolds: Part 1: appropriate inflammatory response. J Biomed Mater Res A. 2007;81(3):748–756. doi: 10.1002/jbm.a.31170. [DOI] [PubMed] [Google Scholar]
- 31.Witte F, Feyerabend F, Maier P, Fischer J, Stormer M, Blawert C, Dietzel W, Hort N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials. 2007;28(13):2163–2174. doi: 10.1016/j.biomaterials.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 32.Okazaki M. New type scaffold biomaterials with magnesium accelerating osteoblast adhesion and bone formation. J Oral Tissue Eng. 2004;1(1):31–40. [Google Scholar]
- 33.Zreiqat H, Evans P, Howlett CR. Effect of surface chemical modification of bioceramic on phenotype of human bone-derived cells. J Biomed Mater Res. 1999;44(4):389–396. doi: 10.1002/(sici)1097-4636(19990315)44:4<389::aid-jbm4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Hunt J, Heggarty D. Application of microscopic methods for the detection of cell attachment to polymers. Methods Mol Biol. 2004;238:207–216. doi: 10.1385/1-59259-428-x:207. [DOI] [PubMed] [Google Scholar]
- 35.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 36.Bertrand OF, Sipehia R, Mongrain R, Rodes J, Tardif JC, Bilodeau L, Cote G, Bourassa MG. Biocompatibility aspects of new stent technology. J Am Coll Cardiol. 1998;32(3):562–571. doi: 10.1016/s0735-1097(98)00289-7. [DOI] [PubMed] [Google Scholar]
- 37.Heublein B, Rohde R, Kaese V, Niemeyer M, Hartung W, Haverich A. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart. 2003;89(6):651–656. doi: 10.1136/heart.89.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waksman R, Pakala R, Kuchulakanti PK, Baffour R, Hellinga D, Seabron R, Tio FO, Wittchow E, Hartwig S, Harder C, Rohde R, Heublein B, Andreae A, Waldmann KH, Haverich A. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheter Cardiovasc Interv. 2006;68(4) doi: 10.1002/ccd.20727. [DOI] [PubMed] [Google Scholar]
- 39.Zartner P, Cesnjevar R, Singer H, Weyand M. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheter Cardiovasc Interv. 2005;66(4):590–594. doi: 10.1002/ccd.20520. [DOI] [PubMed] [Google Scholar]
- 40.Di Mario C, Griffiths H, Goktekin O, Peeters N, Verbist J, Bosiers M, Deloose K, Heublein B, Rohde R, Kasese V, Ilsley C, Erbel R. Drug-eluting bioabsorbable magnesium stent. J Interv Cardiol. 2004;17(6):391–395. doi: 10.1111/j.1540-8183.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 41.Schranz D, Zartner P, Michel-Behnke I, Akinturk H. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Catheter Cardiovasc Interv. 2006;67(5):671–673. doi: 10.1002/ccd.20756. [DOI] [PubMed] [Google Scholar]
- 42.Mulla A, Massey KL, Kalra J. Vitreous humor biochemical constituents: evaluation of between-eye differences. Am J Forensic Med Pathol. 2005;26(2):146–149. [PubMed] [Google Scholar]
- 43.George GA, Heaton FW. Changes in cellular composition during magnesium deficiency. Biochem J. 1975;152(3):609–615. doi: 10.1042/bj1520609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal R, Iezhitsa L, Agarwal P. Pathogenetic role of magnesium deficiency in ophthalmic diseases. Biometals. 2013 doi: 10.1007/s10534-013-9684-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Hatwal A, Gujral AS, Bhatia RP, Agrawal JK, Bajpai HS. Association of hypomagnesemia with diabetic retinopathy. Acta Ophthalmol (Copenh) 1989;67(6):714–716. doi: 10.1111/j.1755-3768.1989.tb04407.x. [DOI] [PubMed] [Google Scholar]
- 46.Mishima S. Clinical pharmacokinetics of the eye. Proctor lecture. Invest Ophthalmol Vis Sci. 1981;21(4):504–541. [PubMed] [Google Scholar]
- 47.Kirkpatrick H. The use of magnesium sulphate as a local application in inflammation of the conjunctiva and cornea. Br J Ophthalmol. 1920;4(6):281. doi: 10.1136/bjo.4.6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachman WG, Wilson G. Essential ions for maintenance of the corneal epithelial surface. Invest Ophthalmol Vis Sci. 1985;26(11):1484–1488. [PubMed] [Google Scholar]
- 49.Hogan ZS, Brown KL, Ishola A, Gatimu J, Flucker L, Huff JW. Effects of divalent cations on bovine corneal stromal swelling rates. Curr Eye Res. 2008;33(8):677–682. doi: 10.1080/02713680802322605. [DOI] [PubMed] [Google Scholar]
- 50.Gong H, Amemiya T, Takaya K. Retinal changes in magnesium-deficient rats. Exp Eye Res. 2001;72(1):23–32. doi: 10.1006/exer.2000.0928. [DOI] [PubMed] [Google Scholar]
- 51.Thalasselis A. The possible relationship between keratoconus and magnesium deficiency. Ophthalmic Physiol Opt. 2005;25(1):7–12. doi: 10.1111/j.1475-1313.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- 52.Andreassen TT, Simonsen AH, Oxlund H. Biomechanical properties of keratoconus and normal corneas. Exp Eye Res. 1980;31(4):435–441. doi: 10.1016/s0014-4835(80)80027-3. [DOI] [PubMed] [Google Scholar]
- 53.Aktekin M, Sargon MF, Cakar P, Celik HH, Firat E. Ultrastructure of the cornea epithelium in keratoconus. Okajimas Folia Anat Jpn. 1998;75(1):45–53. doi: 10.2535/ofaj1936.75.1_45. [DOI] [PubMed] [Google Scholar]
- 54.Sawaguchi S, Fukuchi T, Abe H, Kaiya T, Sugar J, Yue BY. Three-dimensional scanning electron microscopic study of keratoconus corneas. Arch Ophthalmol. 1998;116(1):62–68. doi: 10.1001/archopht.116.1.62. [DOI] [PubMed] [Google Scholar]
- 55.Pouliquen Y, Graf B, Hamada R, Giraud JP, Offret G. Fibrocytes in keratoconus. Morphological aspect and modification of the extra-cellular space. Study with light and electron microscopy. Arch Ophtalmol Rev Gen Ophtalmol. 1972;32(8):571–586. [PubMed] [Google Scholar]
- 56.Dilsiz N, Olcucu A, Atas M. Determination of calcium, sodium, potassium and magnesium concentrations in human senile cataractous lenses. Cell Biochem Funct. 2000;18(4):259–262. doi: 10.1002/1099-0844(200012)18:4<259::AID-CBF881>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal R, Iezhitsa I, Agarwal P, Spasov A. Magnesium deficiency: does it have a role to play in cataractogenesis? Exp Eye Res. 2012;101:82–89. doi: 10.1016/j.exer.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Agarwal R, Iezhitsa I, Awaludin NA, Ahmad Fisol NF, Bakar NS, Agarwal P, Abdul Rahman TH, Spasov A, Ozerov A, Mohamed Ahmed Salama MS, Mohd Ismail N. Effects of magnesium taurate on the onset and progression of galactose-induced experimental cataract: in vivo and in vitro evaluation. Exp Eye Res. 2013;110:35–43. doi: 10.1016/j.exer.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Nagai N, Fukuhata T, Ito Y. Effect of magnesium deficiency on intracellular ATP levels in human lens epithelial cells. Biol Pharm Bull. 2007;30(1):6–10. doi: 10.1248/bpb.30.6. [DOI] [PubMed] [Google Scholar]
- 60.Nagai N, Ito Y, Inomata M, Shumiya S, Tai H, Hataguchi Y, Nakagawa K. Delay of cataract development in the Shumiya cataract rat by the administration of drinking water containing high concentration of magnesium ion. Biol Pharm Bull. 2006;29(6):1234–1238. doi: 10.1248/bpb.29.1234. [DOI] [PubMed] [Google Scholar]
- 61.Agarwal R, Iezhitsa IN, Agarwal P, Spasov AA. Mechanisms of cataractogenesis in the presence of magnesium deficiency. Magnes Res. 2013;26(1):2–8. doi: 10.1684/mrh.2013.0336. [DOI] [PubMed] [Google Scholar]
- 62.Kao CL, Chou CK, Tsai DC, Hsu WM, Liu JH, Wang CS, Lin JC, Wu CC, Peng CH, Chang CJ, Kao CL, Chiou SH. Nitric oxide levels in the aqueous humor in cataract patients. J Cataract Refract Surg. 2002;28(3):507–512. doi: 10.1016/s0886-3350(01)01102-6. [DOI] [PubMed] [Google Scholar]
- 63.Ornek K, Karel F, Buyukbingol Z. May nitric oxide molecule have a role in the pathogenesis of human cataract? Exp Eye Res. 2003;76(1):23–27. doi: 10.1016/s0014-4835(02)00268-3. [DOI] [PubMed] [Google Scholar]
- 64.Retamal MA, Yin S, Altenberg GA, Reuss L. Modulation of Cx46 hemichannels by nitric oxide. Am J Physiol Cell Physiol. 2009;296(6):C1356–1363. doi: 10.1152/ajpcell.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carafoli E. The Ca2+ pump of the plasma membrane. J Biol Chem. 1992;267(4):2115–2118. [PubMed] [Google Scholar]
- 66.Hulsman CA, Vingerling JR, Hofman A, Witteman JC, de Jong PT. Blood pressure, arterial stiffness, and open-angle glaucoma: the Rotterdam study. Arch Ophthalmol. 2007;125(6):805–812. doi: 10.1001/archopht.125.6.805. [DOI] [PubMed] [Google Scholar]
- 67.Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107(7):1287–1293. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 68.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Wang M, Tashiro M, Berlin JR. Regulation of L-type calcium current by intracellular magnesium in rat cardiac myocytes. J Physiol. 2004;555(Pt 2):383–396. doi: 10.1113/jphysiol.2003.048538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Howarth FC, Levi AJ. Internal free magnesium modulates the voltage dependence of contraction and Ca transient in rabbit ventricular myocytes. Pflugers Arch. 1998;435(5):687–698. doi: 10.1007/s004240050570. [DOI] [PubMed] [Google Scholar]
- 71.Ishiguro S, Matsuyama T, Sakaguchi H, Nishio A. Ex vivo study of the increased sensitivity to NO of endothelium-denuded thoracic aortas isolated from dietary magnesium-deficient rats. Magnes Res. 1997;10(1):21–31. [PubMed] [Google Scholar]
- 72.Laurant P, Touyz RM. Physiological and pathophysiological role of magnesium in the cardiovascular system: implications in hypertension. J Hypertens. 2000;18(9):1177–1191. doi: 10.1097/00004872-200018090-00003. [DOI] [PubMed] [Google Scholar]
- 73.Teragawa H, Kato M, Yamagata T, Matsuura H, Kajiyama G. Magnesium causes nitric oxide independent coronary artery vasodilation in humans. Heart. 2001;86(2):212–216. doi: 10.1136/heart.86.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Barbagallo M, Dominguez LJ, Galioto A, Pineo A, Belvedere M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res. 2010;23(3):131–137. doi: 10.1684/mrh.2010.0214. [DOI] [PubMed] [Google Scholar]
- 75.Soltani N, Keshavarz M, Sohanaki H, Zahedi Asl S, Dehpour AR. Relaxatory effect of magnesium on mesenteric vascular beds differs from normal and streptozotocin induced diabetic rats. Eur J Pharmacol. 2005;508(1-3):177–181. doi: 10.1016/j.ejphar.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Kumar AR, Kurup PA. Inhibition of membrane Na+-K+ ATPase activity: a common pathway in central nervous system disorders. J Assoc Physicians India. 2002;50:400–406. [PubMed] [Google Scholar]
- 77.Sugiyama T, Moriya S, Oku H, Azuma I. Association of endothelin-1 with normal tension glaucoma: clinical and fundamental studies. Surv Ophthalmol. 1995;39(Suppl 1):S49–S56. doi: 10.1016/s0039-6257(05)80073-6. [DOI] [PubMed] [Google Scholar]
- 78.Kaiser HJ, Flammer J, Graf T, Stümpfig D. Systemic blood pressure in glaucoma patients. Graefes Arch Clin Exp ophthalmol. 1993;231(12):677–680. doi: 10.1007/BF00919280. [DOI] [PubMed] [Google Scholar]
- 79.Winterkorn JM. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Surv Ophthalmol. 1995;40(1):83–84. doi: 10.1016/s0039-6257(95)80058-1. [DOI] [PubMed] [Google Scholar]
- 80.McMenimen KA, Dougherty DA, Lester HA, Petersson EJ. Probing the Mg2+ blockade site of an N-methyl-D-aspartate (NMDA) receptor with unnatural amino acid mutagenesis. ACS Chem Biol. 2006;1(4):227–234. doi: 10.1021/cb6000944. [DOI] [PubMed] [Google Scholar]
- 81.Mark LP, Prost RW, Ulmer JL, Smith MM, Daniels DL, Strottmann JM, Brown WD, Hacein-Bey L. Pictorial review of glutamate excitotoxicity: fundamental concepts for neuroimaging. AJNR Am J Neuroradiol. 2001;22(10):1813–1824. [PMC free article] [PubMed] [Google Scholar]
- 82.Decollogne S, Tomas A, Lecerf C, Adamowicz E, Seman M. NMDA receptor complex blockade by oral administration of magnesium: comparison with MK-801. Pharmacol Biochem Behav. 1997;58(1):261–268. doi: 10.1016/s0091-3057(96)00555-2. [DOI] [PubMed] [Google Scholar]
- 83.Lambuk L, Jafri AJ, Arfuzir NN, Iezhitsa I, Agarwal R, Rozali KN, Agarwal P, Bakar NS, Kutty MK, Yusof AP, Krasilnikova A, Spasov A, Ozerov A, Ismail NM. Neuroprotective effect of magnesium acetyltaurate against NMDA-induced excitotoxicity in rat retina. Neurotox Res. 2017;31(1):31–45. doi: 10.1007/s12640-016-9658-9. [DOI] [PubMed] [Google Scholar]
- 84.Carafoli E. Calcium-a universal carrier of biological signals. Delivered on 3 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 2005;272(5):1073–1089. doi: 10.1111/j.1742-4658.2005.04546.x. [DOI] [PubMed] [Google Scholar]
- 85.Blaylock RL. Food additive excitotoxins and degenerative brain disorders. Med Sentinel. 1999;4:212–215. [Google Scholar]
- 86.Sun MH, Pang JH, Chen SL, Han WH, Ho TC, Chen KJ, Kao LY, Lin KK, Tsao YP. Retinal protection from acute glaucoma-induced ischemia-reperfusion injury through pharmacologic induction of heme oxygenase-1. Invest Ophthalmol Vis Sci. 2010;51(9):4798–4808. doi: 10.1167/iovs.09-4086. [DOI] [PubMed] [Google Scholar]
- 87.Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364(6438):626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 88.Suschek CV, Kolb H, Kolb-Bachofen VV. Effects of magnesium dobesilate on nitric oxide synthase activity in endothelial cells. In J Angiol. 1999;8(5):21–24. doi: 10.1007/BF01619845. [DOI] [PubMed] [Google Scholar]
- 89.Sun X, Mei Y, Tong E. Effect of magnesium on nitric oxide synthase of neurons in cortex during early period of cerebral ischemia. J Tongji Med Univ. 2000;20(1) doi: 10.1007/BF02887664. [DOI] [PubMed] [Google Scholar]
- 90.Gaspar AZ, Gasser P, Flammer J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Ophthalmologica. 1995;209(1):11–13. doi: 10.1159/000310566. [DOI] [PubMed] [Google Scholar]
- 91.Aydin B, Onol M, Hondur A, Kaya MG, Ozdemir H, Cengel A, Hasanreisoglu B. The effect of oral magnesium therapy on visual field and ocular blood flow in normotensive glaucoma. Eur J Ophthalmol. 2010;20(1):131–135. doi: 10.1177/112067211002000118. [DOI] [PubMed] [Google Scholar]
- 92.Troncoso MU. Cyclodialysis with insertion of a metal implant in the treatment of glaucoma: a preliminary report. Arch Ophthalmol. 1940;23(2):270–297. [Google Scholar]
- 93.Hoff PHB. Use of troncoso's magnesium implant in cyclodialysis for relief of glaucoma: observations in two cases. Arch Ophthalmol. 1945;33(5):404–405. [Google Scholar]
- 94.Moravej M, Mantovani D. Biodegradable metals for cardiovascular stent application: interests and new opportunities. Int J Mol Sci. 2011;12(7):4250–4270. doi: 10.3390/ijms12074250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Staiger MP, Pietak AM, Huadmai J, Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 96.Zeng R, Dietzel W, Witte F, Hort N, Blawert C. Progress and challenge for magnesium alloys as biomaterials. Adv Eng Mater. 2008;10(8):B3–B14. [Google Scholar]
- 97.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Crdiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 98.Nebeker JR, Virmani R, Bennett CL, Hoffman JM, Samore MH, Alvarez J, Davidson CJ, McKoy JM, Raisch DW, Whisenant BK, Yarnold PR, Belknap SM, West DP, Gage JE, Morse RE, Gligoric G, Davidson L, Feldman MD. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Crdiol. 2006;47(1):175–181. doi: 10.1016/j.jacc.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 99.Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27(23):2784–2814. doi: 10.1093/eurheartj/ehl282. [DOI] [PubMed] [Google Scholar]
- 100.Hofma SH, van der Giessen WJ, van Dalen BM, Lemos PA, McFadden EP, Sianos G, Ligthart JM, van Essen D, de Feyter PJ, Serruys PW. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur Heart J. 2006;27(2):166–170. doi: 10.1093/eurheartj/ehi571. [DOI] [PubMed] [Google Scholar]
- 101.Erbel R, Di Mario C, Bartunek J, Bonnier J, de Bruyne B, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, Ilsley C, Bose D, Koolen J, Luscher TF, Weissman N, Waksman R. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369(9576):1869–1875. doi: 10.1016/S0140-6736(07)60853-8. [DOI] [PubMed] [Google Scholar]