Abstract
To evaluate the outcome of amniotic membrane transplantation (AMT) after tumor excision followed by topical interferon alfa-2b (IFNα2b) drops for primary ocular surface squamous neoplasia (OSSN). Twelve eyes of 12 patients with a mean age of 66±10y were included. The average follow-up was 23±10mo. All 12 patients had limbal involvement. Smooth ocular surface and transparent cornea were achieved in all cases. No sign of inflammation, neovascularization, symblepharon or recurrence was noted at the last follow-up. We conclude that AMT with topical IFNα2b drops restores a healthy ocular surface in OSSN without recurrence.
Keywords: amniotic membrane, carcinoma, dysplasia, interferon, ocular surface squamous neoplasia
INTRODUCTION
Ocular surface squamous neoplasia (OSSN) is the most common nonpigmented tumor of the ocular surface[1]. It comprises a range of dysplasia including corneal-conjunctival intraepithelial neoplasia (CIN) and invasive squamous cell carcinoma (SCC)[2].
Amniotic membrane transplantation (AMT) is an effective method for ocular surface reconstruction after wide excision of OSSN[3]. Several topical therapies have also been proved effective in OSSN treatment, including mitomycin C[4], 5-fluorouracil[5], and interferon alfa-2b (IFNα2b)[6]–[7]. In particular, topical IFNα2b has gained appeal for OSSN treatment because of its minimal toxicity[8]. However, the mean tumor resolution time was as long as 12wk by topical IFNα2b treatment for CIN[6]. Furthermore, it was suggested that topical IFNα2b alone not be used in eyes with SCC[6]. To reduce the risk of recurrence after surgery[9], we chose topical IFNα2b as an adjuvant therapy in our clinical practice.
No study has specifically evaluated the outcome of AMT with topical IFNα2b after excision of OSSN. Herein, we retrospectively reviewed our clinical experiences of successfully treating 12 eyes of 12 patients with OSSN.
SUBJECTS AND METHODS
This study was approved by the Ethics Committee of Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology according to the tenets of the Declaration of Helsinki. Twelve patients (12 eyes) with a clinical diagnosis of OSSN undergone AMT with topical IFNα2b after meticulous excision during November 2012 and May 2016 were included. Clinical data concerning age, sex, best corrected visual acuity (BCVA), tumor location, epithelial healing, complications, pathological diagnosis and recurrence were reviewed and analyzed.
Human amniotic membrane (AM) were prepared using previously described method[10]. Topical IFNα2b (1 MIU/mL) was compounded by adding 4 mL of distilled sterile water to 1 mL of 5 MIU/mL IFNα2b (Anfulong, Tianjing Sinobioway Biomedicine, China) and preserved in refrigerator at 4°C.
After topical and peribulbar anesthesia, all tumors were resected intact, using the ‘no touch’ technique with at least 2-mm normal-looking conjunctiva[11]. All surgically resected specimens were sent for pathology diagnosis. A superficial keratectomy or sclerectomy was performed if there was corneal or scleral infiltration. The defects of both conjunctiva and cornea were covered by one sheet of cryopreserved AM with epithelial side facing up, which was then interrupted secured to conjunctiva and episclera with 8/0 vicryl sutures (Ethicon, US) or 10/0 nylon sutures (Alcon, Fort Worth, TX, USA). Topical IFNα2b and antibiotic eye drops were used 4 times daily for 4 and 2wk respectively. The 10/0 nylon sutures were removed 2wk after surgery. All patients were followed up daily for 1wk, weekly for 1mo and then at different intervals after surgery.
RESULTS AND DISCUSSION
Relevant clinical data are summarized in Table 1. There were 12 patients (83.3% males) with a mean age of 66±10 (51 to 79)y. The average follow-up was 23±10 (6 to 40)mo. Complaints at presentation included foreign body sensation in 10 patients (83.3%), eye redness in 8 patients (66.7%), decreased visual acuity in 3 patients (25%), ocular pain in 2 patients (16.7%) and photophobia in 1 patient (8.3%). The mean duration of symptoms before surgery was 16±15 (4 to 60)mo. All 12 patients had limbal involvement. The mean limbal circumference involvement was 5.3±3.6 (1 to 12)h. Neither intraocular nor orbital spread was noted. Pathological diagnosis of CIN (10 eyes) and SCC (2 eyes) was made by pathologist after surgery. During the follow up, neovascularization occurred in 1 eye (8.3%) with 12h limbal involvement in 2wk after surgery (Case 10). The neovascularization regressed at postsurgical 1mo. Visual improvement was evident in 2 patients who had central corneal involvement before surgery. Three patients had vision decreasing due to progression of cataract. Smooth ocular surface and transparent cornea were achieved in all cases. No sign of inflammation, neovascularization, symblepharon and recurrence were noted at the last follow-up.
Table 1. Relevant clinical data.
| Case | Age (a) | Sex | Eye | Pathological diagnosis | Other diagnosis | Clock hours | BCVA |
Follow-up (mo) | |
| Before | After | ||||||||
| 1 | 57 | F | L | CIN | 2 | 20/20 | 20/20 | 40 | |
| 2 | 75 | M | L | SCC | Cataract | 4 | 20/50 | 20/100 | 36 |
| 3 | 60 | M | R | CIN | 1 | 20/20 | 20/20 | 31 | |
| 4 | 76 | F | L | CIN | Cataract | 5.5 | 20/80 | 20/100 | 28 |
| 5 | 62 | M | R | CIN | 5 | 20/25 | 20/25 | 24 | |
| 6 | 79 | M | R | CIN | Cataract | 3 | 20/50 | 20/60 | 24 |
| 7 | 73 | M | L | CIN | 12 | 20/200 | 20/30 | 23 | |
| 8 | 61 | M | R | CIN | AMD | 4 | 20/200 | 20/200 | 22 |
| 9 | 68 | M | L | CIN | 4.5 | 20/25 | 20/25 | 18 | |
| 10 | 51 | M | L | CIN | 12 | 20/25 | 20/20 | 14 | |
| 11 | 79 | M | L | SCC | Cataract | 8 | 20/50 | 20/50 | 12 |
| 12 | 53 | M | L | CIN | 2.5 | 20/20 | 20/20 | 6 | |
AMD: Age-related macular degeneration; BCVA: Best corrected visual acuity; CIN: Corneal-conjunctival intraepithelial neoplasia; SCC: Squamous cell carcinoma.
Case Reports
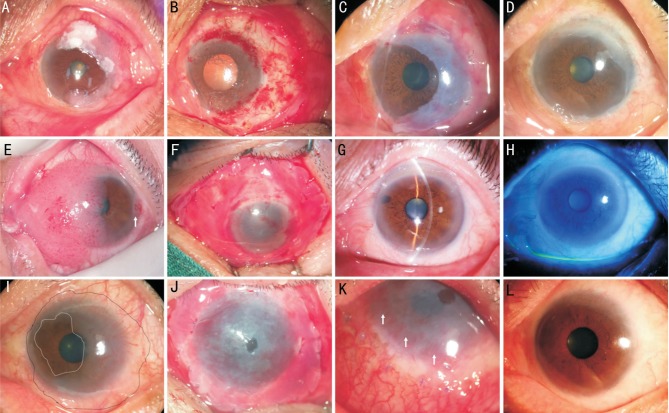
Case 11: A 79-year-old man had complained of foreign body sensation and redness in his left eye for 22mo. Slit lamp examination showed an 8-hour elevated lesion with leukoplakia and corkscrewed feeder vessels on the superior ocular surface, and gelatinous appearance on the temporal and inferior cornea (Figure 1A). The tumor involved conjunctiva, lamellar cornea and sclera were removed (Figure 1B). AM was applied to cover the entire denuded scleral and corneal surface (Figure 1C). A diagnosis of SCC was made after surgery. The ocular surface was stable without recurrence and inflammation at 12mo follow-up (Figure 1D). Case 7: A 73-year-old man had a diffused red mass in his left eye for 26mo. Slit lamp showed a large elevated papiliform tumor with feeder vessels in the nasal half of the ocular surface (Figure 1E) and another small lesion in the temporal limbus (Figure 1E, white arrow). The patient underwent absolute tumor excision and AMT (Figure 1F). A transparent cornea (Figure 1G) and a stable ocular surface (Figure 1H) were perfectly reconstructed without any inflammation or recurrence for 23mo of follow-up. The BCVA improved to 20/30 from 20/200. Case 10: A 51-year-old man presented with a gelatinous tumor involving the 12h limbus (Figure 1I, between white and black dotted line). AMT was performed to cover the entire denuded ocular surface after the tumor were removed (Figure 1J). Neovascularization were noted in the periphery cornea at 2wk after surgery (Figure 1K, white arrows), which regressed automatically within 1mo. A transparent cornea and stable ocular surface were maintained for 14mo follow-up (Figure 1L).
Figure 1. Reconstruction of the ocular surface on patients with OSSNs.
A: Case 11. Slit lamp examination showed an 8-hour-clock elevated lesion with leukoplakia and corkscrewed feeder vessels; B: The tumor was removed; C: AMT was performed; D: The ocular surface was stable at 12mo follow-up; E: Case 7. Slit lamp showed a large elevated papiliform tumor in the nasal half of the ocular surface and another small lesion in the temporal limbus (white arrow); F: The patient underwent absolute tumor excision and AMT; G, H: A transparent cornea and stable ocular surface was achieved at 23mo follow-up; I: Case 10. A patient presented with a gelatinous tumor involving 12-hour-clock limbus (between white and black dotted line); J: AMT was performed to cover the entire denuded ocular surface after tumor removal; K: Neovascularization were noted in the periphery cornea at postoperative 2wk (white arrows); L: A transparent cornea and stable ocular surface were maintained for 14mo follow-up.
AMT is widely applied in reconstruction of ocular surface following generous excision of ocular surface tumors[3]. In this study, topical steroids were not used due to their association with the occurrence of OSSN[12]. All ocular surfaces were stable without any inflammation at the last follow-up. The AM exerted the effect of suppressing inflammation and angiogenesis[13]. Although limbal involvement was present in all 12 eyes and 12-hour limbal excision was performed in 2 patients (Cases 7 and 10), limbal stem cell deficiency was not observed. The AM probably promoted self-renewal of stem cells by creating a noninflamed limbal niche[14].
We also attribute the absent of recurrence to topical IFNα2b drops. IFNα2b was advocated as sole therapy of OSSN[6]–[7] with minimal side effect[8], however, it requires a longer treatment[6] and should not be used alone in eyes with SCC[6]. Adjuvant interferon therapy were suggested in patients with tarsal involvement and recurrent disease[15]. Our preferred method is to prescribe topical IFNα2b (1 MIU/mL) 4 times daily for 1mo after surgery.
As a conclusion, AMT with topical IFNα2b after tumor excision restores a healthy ocular surface in OSSN without complication and recurrence. These finding provides an efficient treatment for OSSN.
Acknowledgments
Foundations: Supported by National Natural Science Foundation of China (No.81300736; No.81370993).
Conflicts of Interest: Xie HT, None; Zhang YY, None; Jiang DL, None; Wu J, None; Wang JS, None; Zhang MC, None.
REFERENCES
- 1.Shields CL, Demirci H, Karatza E, Shields JA. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004;111(9):1747–1754. doi: 10.1016/j.ophtha.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Lee GA, Hirst LW. Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39(6):429–450. doi: 10.1016/s0039-6257(05)80054-2. [DOI] [PubMed] [Google Scholar]
- 3.Palamar M, Kaya E, Egrilmez S, Akalin T, Yagic A. Amniotic membrane transplantation in surgical management of ocular surface squamous neoplasias: long-term results. Eye(Lond) 2014;28(9):1131–1135. doi: 10.1038/eye.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields CL, Naseripour M, Shields JA. Topical mitomycin C for extensive, recurrent conjunctival-corneal squamous cell carcinoma. Am J Ophthalmol. 2002;133(5):601–606. doi: 10.1016/s0002-9394(02)01400-9. [DOI] [PubMed] [Google Scholar]
- 5.Joag MG, Sise A, Murillo JC, Sayed-Ahmed IO, Wong JR, Mercado C, Galor A, Karp CL. Topical 5-Fluorouracil 1% as primary treatment for ocular surface squamous neoplasia. Ophthalmology. 2016;123(7):1442–1448. doi: 10.1016/j.ophtha.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galor A, Karp CL, Chhabra S, Barnes S, Alfonso EC. Topical interferon alpha 2b eye-drops for treatment of ocular surface squamous neoplasia: a dose comparison study. Br J Ophthalmol. 2010;94(5):551–554. doi: 10.1136/bjo.2008.153197. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Shields CL, Shah SU, Kaliki S, Lally SE. Giant ocular surface squamous neoplasia managed with interferon alpha-2b as immunotherapy or immunoreduction. Ophthalmology. 2012;119(5):938–944. doi: 10.1016/j.ophtha.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Nanji AA, Sayyad FE, Karp CL. Topical chemotherapy for ocular surface squamous neoplasia. Curr Opin Ophthalmol. 2013;24(4):336–342. doi: 10.1097/ICU.0b013e3283622a13. [DOI] [PubMed] [Google Scholar]
- 9.Tabin G, Levin S, Snibson G, Loughnan M, Taylor H. Late recurrences and the necessity for long-term follow-up in corneal and conjunctival intraepithelial neoplasia. Ophthalmology. 1997;104(3):485–492. doi: 10.1016/s0161-6420(97)30287-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14(5):473–484. [PubMed] [Google Scholar]
- 11.Shields JA, Shields CL, De Potter P. Surgical management of conjunctival tumors. The 1994 Lynn B. McMahan Lecture. Arch Ophthalmol. 1997;115(6):808–815. doi: 10.1001/archopht.1997.01100150810025. [DOI] [PubMed] [Google Scholar]
- 12.Ramasubramanian A, Shields CL, Sinha N, Shields JA. Ocular surface squamous neoplasia after corneal graft. Am J Ophthalmol. 2010;149(1):62–65. doi: 10.1016/j.ajo.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Tseng SC, Espana EM, Kawakita T, Di Pascuale MA, Li W, He H, Liu TS, Cho TH, Gao YY, Yeh LK, Liu CY. How does amniotic membrane work? Ocul Surf. 2004;2(3):177–187. doi: 10.1016/s1542-0124(12)70059-9. [DOI] [PubMed] [Google Scholar]
- 14.Tseng SCG. HC-HA/PTX3 purified from amniotic membrane as novel regenerative matrix: insight into relationship between inflammation and regeneration. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFh1–ORSFh8. doi: 10.1167/iovs.15-17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galor A, Karp CL, Oellers P, Kao AA, Abdelaziz A, Feuer W, Dubovy SR. Predictors of ocular surface squamous neoplasia recurrence after excisional surgery. Ophthalmology. 2012;119(10):1974–1981. doi: 10.1016/j.ophtha.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]