Abstract
An important consideration when probing the function of any neuron is to uncover the source of synaptic input onto the cell, its intrinsic physiology and efferent targets. Over the years, electrophysiological approaches have generated considerable insight into these properties in a variety of cortical neuronal subtypes and circuits. However, as researchers explore neuronal function in greater detail, they are increasingly turning to optical techniques to bridge the gap between local network interactions and behaviour. The application of optical methods has increased dramatically over the past decade, spurred on by the optogenetic revolution. In this review, we provide an account of recent innovations, providing researchers with a primer detailing circuit mapping strategies in the cerebral cortex. We will focus on technical aspects of performing neurotransmitter uncaging and channelrhodopsin‐assisted circuit mapping, with the aim of identifying common pitfalls that can negatively influence the collection of reliable data.
Keywords: cerebral cortex, optogenetics, neural circuits, glutamate uncaging
Introduction
Circuit neuroscientists are faced with a complex problem, namely resolving the dense interconnectivity of the mammalian neocortex. Studies must account for not only the diversity of the constituent cellular components (Molnar & Cheung, 2006; Hattox & Nelson, 2007; Petilla Interneuron Nomenclature et al. 2008; Anastasiades & Butt, 2011; Harris & Shepherd, 2015), but also the vast number of possible synaptic connections. A single cortical column in the rodent contains somewhere in the region of 20,000 neurons, with each cell possessing thousands of synaptic inputs (Meyer et al. 2010; Oberlaender et al. 2012). This provides a significant challenge to our understanding, only slightly simplified by the presence of multiple synapses between connected partners and anatomical limitations to connectivity (Feldmeyer, 2012; Harris & Shepherd, 2015; Markram et al. 2015). Historically both anatomical and physiological studies have contributed significantly to our understanding of cortical circuits (Mountcastle, 1957; Hubel & Wiesel, 1962; Woolsey & Van der Loos, 1970; Gilbert & Wiesel, 1989; Douglas & Martin, 2004). In particular, exploration of the laminar structure of the neocortex has proven particularly fruitful, as individual cell types, axons and dendrites often display layer‐specific distributions (Thomson & Bannister, 2003; Binzegger et al. 2004; Ma et al. 2006; Gonchar et al. 2007; Wimmer et al. 2010; Oberlaender et al. 2012; Hooks et al. 2013; D'Souza et al. 2016).
Recent advances in anatomical tracing techniques have greatly enhanced the specificity with which we can study the organisation of cortical networks (Wickersham et al. 2007, 2013; Kim et al. 2011; Xu & Sudhof, 2013; Wouterlood et al. 2014; Beier et al. 2015; DeNardo et al. 2015). However, electrophysiology remains the gold standard for interrogating cortical circuits, enabling unequivocal determination of functional synaptic connectivity and assessment of the dynamic properties of individual synapses with high temporal resolution. Electrophysiological methods have been applied successfully to resolve synaptic connectivity between small groups of neurons throughout the cortex (Reyes et al. 1998; Thomson & Bannister, 2003; Song et al. 2005; Feldmeyer et al. 2006; Wang et al. 2006; West et al. 2006; Le Be et al. 2007; Silberberg & Markram, 2007; Perin et al. 2011; Jiang et al. 2013, 2015). These studies highlight fundamental principles of synaptic organisation, and identify certain connections that occur at frequencies unexplained by stochastic apposition principles alone (Song et al. 2005; Morishima & Kawaguchi, 2006; Wang et al. 2006; Brown & Hestrin, 2009; Morishima et al. 2011). They have also shown that the probability of connectivity is low in many cases and decreases significantly as a function of distance (Thomson & Bannister, 1998; Song et al. 2005; Morishima & Kawaguchi, 2006). This suggests a need for high‐throughput approaches to map cortical connectivity with cell‐type specificity. Such cell‐type‐specific wiring diagrams will enhance our understanding of cortical circuits, while providing important reference points to compare interareal or cross‐species differences in circuit organisation and ultimately function (Wang et al. 2006; Hooks et al. 2011; Katzel et al. 2011; Harris & Shepherd, 2015).
Optical stimulation methods have emerged as an attractive approach to address this need for high‐throughput assessment of cortical connectivity, enabling rapid and repeated mapping of synaptic inputs emanating from multiple locations across large regions of the cortical network (Dalva & Katz, 1994; Dantzker & Callaway, 2000; Shepherd et al. 2005; Petreanu et al. 2007, 2009; Ashby & Isaac, 2011). Indeed, the array of currently available optical circuit mapping strategies allows researchers to greatly extend studies of cortical connectivity beyond assaying synaptic connections between individual neurons in the local circuit. Such studies include the investigation of long‐range connections routinely severed in reduced acute in vitro preparations (Cruikshank et al. 2007, 2010; Petreanu et al. 2007, 2009; Little & Carter, 2013), as well as the discovery of novel pathways whose scarcity had precluded detection using classical approaches (Pluta et al. 2015), and transient connections restricted to certain periods of development (Anastasiades et al. 2016; Marques‐Smith et al. 2016; Tuncdemir et al. 2016). Optical approaches can also be combined with highly specific genetic tools to facilitate region‐, layer‐ and cell‐type‐specific targeting. Despite the considerable advantages of these methods, rigorous circuit mapping depends upon many technical and practical considerations. The scope of this review is to outline the advances being made in the use of optical techniques to interrogate the structure of neocortical circuits, and highlight examples of best practice.
Optical approaches to studying cortical circuits
Light is an excellent source of excitation as the strength, shape, wavelength and duration of the light beam can be tightly controlled by the experimenter (Callaway & Yuste, 2002; Jerome & Heck, 2011). Moreover, for circuit mapping applications a focused light beam can be rapidly shifted between different sites in the tissue. This laser‐scanning photostimulation (LSPS) method allows relatively fast interrogation of connectivity across a large number of spatially distinct locations (Dantzker & Callaway, 2000; Shepherd et al. 2003). Alternatively, wide‐field illumination can be used to measure the net presynaptic input onto recorded postsynaptic neurons (Little & Carter, 2013; Suter & Shepherd, 2015; McGarry & Carter, 2016). However, because cortical neurons do not normally respond to direct illumination, a method to transduce light into a neural electrochemical signal is required. Photostimulation can be achieved by flash photolysis of caged compounds – where a neurotransmitter molecule (typically the excitatory amino acid glutamate) is bound to a caging moiety via a photoscissile bond, producing an effect via endogenous receptors (Dalva & Katz, 1994; Ellis‐Davies, 2007; Nikolenko et al. 2007). Alternatively, expression of exogeneous light‐ or ligand‐gated ion channels can be used to induce presynaptic excitation (Zemelman et al. 2002; Nagel et al. 2003; Boyden et al. 2005; Szobota et al. 2007; Miesenbock, 2011). These approaches, neurotransmitter uncaging and optogenetics, have yielded considerable information regarding the organisation of cortical networks. However, although these approaches are highly complementary, there are subtle differences in their application which make them better suited to specific circuit mapping questions. Below we outline the different optical methods one can employ to map cortical connectivity, summarising the strengths and weaknesses of each approach.
Neurotransmitter uncaging
Single‐photon uncaging
Early studies mapping cortical connectivity combined single‐photon glutamate uncaging with LSPS to focally stimulate small clusters of neurons across the cortex (Dalva & Katz, 1994; Dantzker & Callaway, 2000; Shepherd et al. 2003). Because ionotropic glutamate receptors are primarily restricted to the soma and dendrites of cortical neurons, glutamate uncaging does not activate en passant axons, making it well suited for mapping the dense, recurrent circuitry of the neocortex. The resolution of the approach is largely dependent upon the point‐spread function of the light source, scattering of light by neuronal tissue, and appropriate calibration to restrict action potentials to the peri‐somatic region (see below). If performed correctly, experimental resolutions in the order of ∼50 μm are readily achievable (Shepherd et al. 2003; Jerome & Heck, 2011; Anastasiades & Butt, 2012), activating somewhere in the region of 30–60 presynaptic neurons with each light pulse (Shepherd et al. 2005).
The sublaminar resolution of single‐photon uncaging makes it well suited to compare interlaminar connectivity within a cortical column (Dantzker & Callaway, 2000; Schubert et al. 2001; Bureau et al. 2004; Shepherd & Svoboda, 2005; Xu & Callaway, 2009; Anderson et al. 2010; Anastasiades & Butt, 2012; Yamawaki & Shepherd, 2015; Anastasiades et al. 2016; Marques‐Smith et al. 2016). Mapping columnar connectivity allows the relative strength and laminar distribution of synaptic inputs to be determined onto postsynaptic neurons in all layers of cortex. Such connectivity matrices can be compared across distinct cytoarchitectonic subdivisions of neocortex to reveal conserved or unique connectivity motifs (Hooks et al. 2011). LSPS can also be used to examine changes in the strength or distribution of synaptic inputs in the same cortical area but under variable experimental conditions. For example, alterations in network activity (Anastasiades & Butt, 2012; Kuhlman et al. 2013; Meng et al. 2015, 2017; Marques‐Smith et al. 2016), changes in gene expression (Bureau et al. 2008; Marques‐Smith et al. 2016; Sun et al. 2016; Rajkovich et al. 2017) or synaptic integration over the course of development (Bureau et al. 2004; Anastasiades & Butt, 2012; Viswanathan et al. 2012; Anastasiades et al. 2016; Marques‐Smith et al. 2016).
One important consideration with glutamate uncaging is the ubiquitous expression of glutamate receptors throughout the mammalian CNS. This presents two immediate issues: first, local connections can be obscured by large direct glutamate responses at recorded neurons (see section on assigning postsynaptic responses). Second, there is no presynaptic cell‐type specificity (Fig. 1 A). However, excitatory and inhibitory conductances can be isolated by adjusting the holding potential of voltage‐clamped postsynaptic neurons to elucidate inhibitory or excitatory afferent input onto a postsynaptic neuron (Roerig & Chen, 2002; Shepherd et al. 2003; Brill & Huguenard, 2009; Xu & Callaway, 2009). Furthermore, uncaging can be combined with numerous methods that allow classification of postsynaptic cell types (Table 1) within both the interneuron (Xu & Callaway, 2009; Apicella et al. 2012) and pyramidal neuron classes (Anderson et al. 2010; Yamawaki & Shepherd, 2015). This approach allows researchers to compare the strength and distribution of synaptic inputs onto distinct populations within the local circuit.
Figure 1. Overview of methods to optically stimulate neuronal populations.
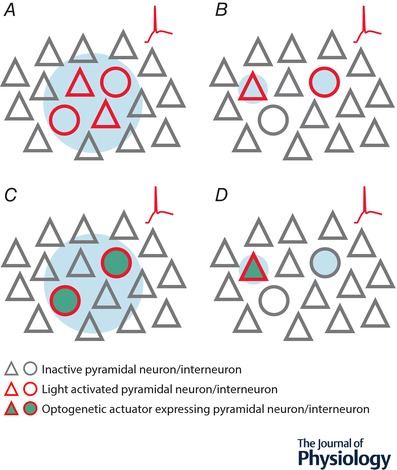
A, single‐photon uncaging indiscriminately activates multiple populations of neurons within the local network. Firing (red trace) is induced in all neurons within the spatial extent of the light beam. B, two‐photon uncaging selectively activates individual neurons with single cell resolution. Firing (red trace) can be induced in target cells, but not adjacent neurons. C, single‐photon optogenetics selectively activates multiple neurons within the local network. Firing (red trace) is induced in all neurons within the spatial extent of the light beam so long as they express the construct encoding the optogenetic actuator. D, two‐photon optogenetics selectively activates individual neurons with single cell resolution. Firing (red trace) can be induced in the target cell, but not adjacent neurons, so long as target neurons express the construct encoding the optogenetic actuator.
Table 1.
Methods to selectively label presynaptic or postsynaptic cells in neural circuits
| Method | Cell types | Presynaptic/postsynaptic | Optogenetic access (Y/N) | Specificity | References |
|---|---|---|---|---|---|
| BAC lines | PYR/IN | Post (mostly) | N (Y with virus) | Various interneuron and projection neuron classes | Gong et al. (2003), and see also www.gensat.org; Xu & Callaway (2009); Apicella et al. (2012); Tang et al. (2015) |
| Cre/Flp drivers | PYR/IN | Pre/Post | Y | Various interneuron and projection neuron classes | Taniguchi et al. (2011); Olsen et al. (2012); Crandall et al. (2015); He et al. (2016) |
| Optogenetic reporters | PYR/IN | Pre | Y | Dependent on driver line | Katzel et al. (2011); Madisen et al. (2012); Anastasiades et al. (2016) |
| Fluorophore reporters | PYR/IN | Post | N | Dependent on driver line | Madisen et al. (2010); Anastasiades et al. (2016) |
| In utero electroporation | PYR/IN | Pre/Post | Y | Dependent on stage of embryogenesis and target location | Petreanu et al. (2007); Petreanu et al. (2009) |
| Viral vector | PYR/IN | Pre/Post | Y | Non‐specific, promoter specific or Cre dependent | Petreanu et al. (2009); Cruikshank et al. (2010); Little & Carter (2013); Dimidschstein et al. (2016) |
| Cre‐off virus | PYR/IN | Pre/Post | Y | Non‐Cre expressing neurons | Saunders et al. (2012); Saunders & Sabatini (2015) |
| Rabies virus | PYR/IN | Pre/Post | Y | Monosynaptically connected neurons | Wickersham et al. (2007); Osakada et al. (2011) |
| Anterograde‐Cre virus | PYR/IN | Pre | Y | Cells receiving input from presynaptic region | Zingg et al. (2017) |
| Retrograde‐Cre virus | PYR (Mostly) | Pre/Post | Y | Projection class | Xu et al. (2016) |
| Retrograde tracer | PYR (Mostly) | Post | N | Projection class | Anderson et al. (2010); Apicella et al. (2012); Little & Carter (2013); McGarry & Carter (2016) |
| TRAP | PYR/IN | Pre/Post | Y | Active neurons | Guenthner et al. (2013) |
Different methods that allow for the labelling and optogenetic manipulation of individual neuronal populations are indicated along with the types of neurons that can be labelled. The suitability of each approach to studying presynaptic inputs or postsynaptic responses along with the ability of each method to provide optogenetic access to specific or broad populations of presynaptic neurons is shown. IN, interneuron; Post, suitable for studying postsynaptic cells; Pre, suitable for studying presynaptic cells; PYR, pyramidal neuron; TRAP, targeted recombination in active populations.
Two‐photon uncaging
Single‐photon uncaging has been used with great success to compare intra‐ and interlaminar connectivity across an entire cortical column. However, it lacks the necessary precision to stimulate a single cell per trial, which requires the greater spatial resolution attained from two‐photon (2‐P) uncaging (Fig. 1 B). This approach is similar to single‐photon uncaging, but requires coincident arrival of two photons in order to release glutamate from the caging moiety (Furuta et al. 1999). 2‐P uncaging utilises a mode‐locked laser light source which drastically increases the probability of coincident multi‐photon excitation at the focal point of the objective (Denk et al. 1990; Denk & Svoboda, 1997; Jerome & Heck, 2011). This provides much greater axial resolution compared to single‐photon techniques, allowing stimulation of single neurons in a defined plane (Furuta et al. 1999). The longer wavelengths used are also advantageous as they have much higher tissue penetrance and lower light scatter than UV lasers used for single‐photon uncaging (Denk & Svoboda, 1997; Nikolenko et al. 2011).
2‐P glutamate uncaging can be used to assay hundreds of potential synaptic connections, providing a relatively high‐throughput method to probe the underlying structure of local cortical networks. For example, 2‐P uncaging has been used to determine connection probability between distinct cell types, as well as the relative convergence or divergence of given inputs onto individual nodes in the network (Ashby & Isaac, 2011; Fino & Yuste, 2011; Packer & Yuste, 2011). As with single‐photon methods, cell‐type specificity is not explicitly achieved by 2‐P stimulation itself (Fig. 1 B). However, it is possible to use fluorescent reporters to label and subsequently target specific presynaptic populations (Fino & Yuste, 2011; Packer & Yuste, 2011). Over the past few years researchers have applied single‐cell 2‐P uncaging to probe the emergence of recurrent connectivity between layer 4 stellate cells in somatosensory cortex (Ashby & Isaac, 2011) and the dense blanket of inhibition mediated by individual interneuron subtypes within superficial (Fino & Yuste, 2011; Packer & Yuste, 2011) and deep layers of neocortex (Packer et al. 2013).
Despite advances in 2‐P uncaging, it is not without limitations. While it can readily be applied to map connectivity within (Ashby & Isaac, 2011) or between layers (Viswanathan et al. 2012), producing single‐cell connectivity matrices across an entire column is challenging due to the vast number of potential connections. The low glutamate yield afforded by 2‐P stimulation is a further issue, resulting in considerable variance in the suprathreshold activation of presynaptic neurons (Nikolenko et al. 2007; Matsuzaki et al. 2008). This is less critical in immature neurons, which possess higher input resistance (Ashby & Isaac, 2011). However, for adult cortical neurons beam‐multiplexing is typically required to evoke action potentials in single neurons (Nikolenko et al. 2007). This involves stimulating at multiple uncaging sites forming a concentric ring around the target neuron to evoke a localised increase in glutamate that is sufficient to drive spiking in the target neuron, but not adjacent cells (Nikolenko et al. 2007). Because of difficulties evoking reliable responses, 2‐P stimulation can be combined with calcium imaging to detect presynaptic firing (Nikolenko et al. 2007). This helps reduce false negatives and ensures reliable assignment of connection probability. An additional consideration is that 2‐P uncaging often requires concentrations of caged compound that either completely (in the case of 4‐methoxy‐7‐nitroindolinyl (MNI)‐glutamate) or partially (for Rubi variants) block GABAergic transmission (Fino et al. 2009). MNI‐glutamate should therefore be avoided for mapping GABAergic connections using 2‐P uncaging. Rubi‐glutamate has been successfully used to map GABAergic connectivity from multiple interneuron subtypes onto pyramidal neurons (Fino & Yuste, 2011; Packer & Yuste, 2011). However, 300 μm Rubi‐glutamate will produce a 50% reduction in inhibitory postsynaptic current amplitude (Fino et al. 2009), which should be taken into account when mapping GABAergic inputs using this approach.
Optogenetics
Over the past decade the application of microbial opsins, most notably Channelrhodopsin‐2 (ChR2) (Nagel et al. 2003; Boyden et al. 2005), to enable neuronal photostimulation has proven a powerful tool for studying cortical connectivity. In contrast to neurotransmitter uncaging, optogenetic approaches can be used to study both local and long‐range inputs. The latter is possible because light‐sensitive opsins are expressed throughout axons and dendrites. Although many axons are severed during preparation of acute in vitro slices, synaptic terminals remain functional and presynaptic release can be evoked using brief pulses of light (Fig. 2 A; Petreanu et al. 2007; Petreanu et al. 2009; Mao et al. 2011). As with glutamate uncaging, optogenetics can be combined with methods to label and target specific postsynaptic neuronal populations. However, one advantage of optogenetics over classical glutamate uncaging is that it can also be combined with a wide range of methods to produce presynaptic input specificity (Table 1).
Figure 2. Strategy to examine long‐range inputs with wide‐field optogenetics.
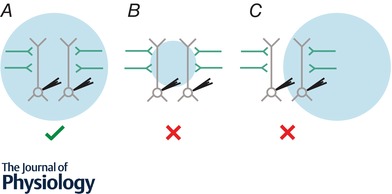
A, presynaptic axons (green) expressing the optogenetic construct are present within cortical network and can be selectively activated by light. Wide‐field illumination must be correctly aligned so the light beam covers the entire extent of the dendritic arbour of both recorded neurons. Input from the presynaptic region expressing the optogenetic actuator can be reliably compared at the two postsynaptic neurons. B, wide‐field illumination is too focused so the light beam only covers a portion of the dendritic arbour of the recorded neurons. Inputs that synapse onto dendrites outside the extent of the light beam are not activated, so total input from the presynaptic region expressing the optogenetic actuator cannot be reliably compared at the two postsynaptic neurons. C, wide‐field illumination is incorrectly aligned as the light beam only covers the dendritic arbour of one of the recorded neurons. Inputs that synapse onto dendrites outside the extent of the light beam are not activated, so inputs onto the leftmost cell cannot be reliably measured.
Long‐range connectivity
To study long‐range circuits, ChR2 is first expressed in a putative presynaptic brain region. After preparation of acute in vitro slices, photosensitive axons are then stimulated via illumination over the postsynaptic structure while recording from target neurons. Optogenetics can therefore confirm the presence or absence of synaptic connectivity between presynaptic structures and postsynaptic neurons located throughout the nervous system (Fig. 2 A). One of the earliest studies to use this approach mapped long‐range connectivity between neocortical pyramidal cells across the corpus callosum (Petreanu et al. 2007). By restricting ChR2 expression to a subset of layer 2/3 neurons using in utero electroporation, it was possible to map the outputs of these cells both within and across hemispheres. This ChR2‐assisted circuit mapping (CRACM) strategy allows inputs to be compared onto target neurons in different layers. While the presence of photoexcitable ChR2 throughout the extent of neurons is an essential part of the utility of this approach, it can make it challenging to assign the origin of recorded synaptic inputs to a specific spatial location (Petreanu et al. 2007; Lewis et al. 2009). The methodology of the approach has been simplified over time, with wide‐field LED illumination reducing the cost and technical requirements. This increased accessibility has yielded numerous studies examining the relative strength of synaptic inputs onto distinct components of the cortical network (Mao et al. 2011; Cruikshank et al. 2012; Hooks et al. 2013; Little & Carter, 2013; Rock & Apicella, 2015; Suter & Shepherd, 2015; McGarry & Carter, 2016).
When performing optogenetic experiments, it is important to account for the considerable variation in ChR2 expression that can occur when using viral vectors, even in slices obtained from the same brain (Mao et al. 2011). This is achieved by normalising input amplitude to a consistent component of the cortical circuit in each slice experiment, for example a pyramidal neuron of a given cell type or located in a specific layer (Mao et al. 2011; Lee et al. 2013; McGarry & Carter, 2016). This normalisation process allows inputs to be compared onto cells of different subtypes (Lee et al. 2013, 2014; Little & Carter, 2013; Yang et al. 2013; Rock & Apicella, 2015; Yamawaki & Shepherd, 2015; McGarry & Carter, 2016), located in different layers (Mao et al. 2011; Hooks et al. 2013; Suter & Shepherd, 2015; Yamawaki & Shepherd, 2015), across experimental conditions (MacAskill et al. 2014; Xue et al. 2014; Rajkovich et al. 2017) or during development (Tuncdemir et al. 2016). To compare inputs between individual cells in a slice, ideally one should use wide‐field illumination, consistent stimulus intensity and focus the light‐beam on the same location across trials. This ensures that equivalent synapses are stimulated to a similar degree across the entire postsynaptic dendrite, allowing direct comparison of input strength for neurons contained within the spatial extent of the light beam (Fig. 2 A–C).
To enhance mechanistic understanding of observed differences in connectivity, optogenetics can be used in a similar manner to classical studies using electrical stimulation. If ChR2‐positive axons are optically stimulated in the presence of extracellular strontium, quantal analysis of evoked, ChR2‐driven mini‐postsynaptic current amplitude and frequency can be performed (Silver, 2003; Little & Carter, 2013; MacAskill et al. 2014). This is possible because strontium interferes with presynaptic release (Goda & Stevens, 1994; Xu‐Friedman & Regehr, 1999), such that asynchronous postsynaptic currents recorded within a defined window after a given synaptic stimulus can largely be attributed to the stimulated axons (Hull et al. 2009). An alternative strategy employs a focused blue laser to limit stimulation to a single axon (Ye et al. 2015; Morgenstern et al. 2016; Del Pino et al. 2017); an approach that is analogous to minimal electrical stimulation (Finnerty et al. 1999; Hull et al. 2009). Additional properties such as AMPA/NMDA ratios (Little & Carter, 2013; McGarry & Carter, 2016), E/I balance (Lee et al. 2014; Xue et al. 2014; Rock & Apicella, 2015; Yamawaki & Shepherd, 2015; McGarry & Carter, 2016) and presynaptic release probability (Little & Carter, 2013; Lee et al. 2014; Crandall et al. 2015; McGarry & Carter, 2016) can also be examined. However, it should be noted that in some cases synaptic currents evoked by optical stimulation have been reported to depress more than those evoked electrically (Zhang & Oertner, 2007; Cruikshank et al. 2010; Schoenenberger et al. 2011; Olsen et al. 2012; Jackman et al. 2014). This disparity could be explained by the slow kinetics of some ChR2 variants broadening the action potential waveform or by the permeability of ChR2 to Ca2+, enhancing its influx directly into synaptic boutons, both of which are situations predicted to artificially inflate release probability (Zhang & Oertner, 2007; Olsen et al. 2012). A detailed study of this issue revealed that the method of ChR2 expression seems to be mainly responsible for the disparities observed, with AAV serotypes influencing synaptic depression in a synapse‐dependent manner (Jackman et al. 2014). Expression of ChR2 by usage of transgenic animals was the method that least altered release probability, when compared to electrical stimulation, followed by AAV9 expression vectors (Jackman et al. 2014).
Subcellular connectivity
Dendritic location has a significant influence over the functional impact of individual synapses (Yuste et al. 1994; Williams & Stuart, 2002). To determine the subcellular location of ChR2‐positive axon terminals, Svoboda and colleagues took advantage of the ability of ChR2 to evoke release of neurotransmitter from presynaptic terminals to determine the subcellular location of afferent input on the postsynaptic dendrite of cortical pyramidal cells (Petreanu et al. 2009). This refinement of their CRACM method was made possible by adding tetrodotoxin (TTX) to block the fast transient sodium channel and the potassium channel blocker 4‐AP to enhance photostimulation‐induced neurotransmitter release. This pharmacological manipulation blocks action potential conductance along the axon, while restoring presynaptic release, effectively restricting photoexcitability to presynaptic terminals (Petreanu et al. 2009). TTX and 4‐AP can therefore be used to ensure postsynaptic responses are monosynaptic (Cruikshank et al. 2010; Little & Carter, 2013). However, when combined with LSPS it is possible for light‐evoked responses to be assigned to specific stimulation sites across the postsynaptic dendrite. Svoboda and colleagues termed this approach subcellular ChR2 assisted circuit mapping (sCRACM) and applied it to study the subcellular distribution of inputs from individual layers, thalamus and motor cortex (Petreanu et al. 2009). sCRACM has subsequently been utilised to study subcellular targeting across numerous inputs and neuronal subtypes (Mao et al. 2011; Hooks et al. 2013; Marlin & Carter, 2014; Suter & Shepherd, 2015). However, a caveat to the sCRACM approach is that electrotonic filtering yields an underestimation of distal input when recorded at the soma (Williams & Stuart, 2002; Williams & Mitchell, 2008; Dembrow et al. 2015). This issue is particularly relevant in large dendritic arbour layer 5 pyramidal neurons, where the approach has been applied most frequently. This is not unique to sCRACM, occurring for synaptic responses evoked using electrical or optogenetic stimulation (Dembrow et al. 2015). Regardless, it remains important to consider dendritic cable properties when interpreting sCRACM data. The attenuation of distal inputs can be reduced slightly through pharmacological blockade of resting conductances (Williams & Mitchell, 2008). Alternatively, input maps can be adjusted to account for dendritic location (Petreanu et al. 2009).
Finally, the resolution of sCRACM (∼60 μm; Petreanu et al. 2009) is insufficient to examine inputs at single spines, where typical interspine distances are ∼1 μm (Konur et al. 2003). To achieve this, optogenetics can be combined with 2‐P calcium imaging to map the dendritic distribution of synaptic inputs at the level of the single spine (Little & Carter, 2012; MacAskill et al. 2012). This approach is particularly useful when examining small neurons with compact dendrites where the lower resolution of sCRACM may limit the ability to discriminate differences in synapse distribution. It also allows researchers to pose more complex questions, such as examining input‐specific synaptic clustering on individual dendritic branches (Gokce et al. 2016).
Local connectivity
Optogenetics can also be used to map local connectivity. This is advantageous as it allows selective stimulation of specific presynaptic cell types within the local network (Fig. 1 C; Katzel et al. 2011; Lee et al. 2013; Pfeffer et al. 2013; Pluta et al. 2015). In addition to microbial opsins, local connectivity can be assessed through selective expression of ligand‐gated ion channels (Anastasiades et al. 2016; Marques‐Smith et al. 2016). In this approach, light‐evoked uncaging or photo‐isomerism causes selective depolarisation of neurons induced to express an optogenetic actuator (Szobota et al. 2007; Miesenbock, 2011). Regardless of the method used, optogenetics can be combined with conditional genetic approaches (Katzel et al. 2011; Olsen et al. 2012; Bortone et al. 2014; Crandall et al. 2015; Pluta et al. 2015) or in utero electroporation (Petreanu et al. 2007; Petreanu et al. 2009) to study layer‐ or cell‐type‐specific inputs. These inputs can be compared within and across layers, in a similar manner to glutamate uncaging and long‐range connectivity. Although axonal expression is advantageous for studying long‐range inputs, it is detrimental to the study of local networks, as it hinders the reliable assignment of inputs to specific sites (Petreanu et al. 2007). To circumvent this problem optogenetic actuators can be restricted to specific subcellular compartments (Lewis et al. 2009; Grubb & Burrone, 2010; Baker et al. 2016), effectively producing responses at the soma but not stimulating distal dendrites or local axons. This approach allows cortical circuits to be mapped in a similar manner to glutamate uncaging, with the spatial location of presynaptic neurons resolved to sublaminar resolutions (Baker et al. 2016).
In theory 2‐P optogenetics can be used to study local connectivity with single‐cell resolution (Fig. 1 D). In practice, as with uncaging, this approach suffers difficulties in generating suprathreshold photocurrents in postsynaptic neurons to drive reliable, time‐locked action potentials necessary to reliably assay connectivity (Packer et al. 2012). Novel stimulation paradigms such as temporal focusing or spiral scanning may help overcome this limitation (Rickgauer & Tank, 2009; Papagiakoumou et al. 2010), while red‐shifted opsin variants with larger single channel conductance at wavelengths typically used for 2‐P stimulation may also enhance the applicability of this approach (Packer et al. 2012; Chaigneau et al. 2016). However, to date the number of studies utilising 2‐P optogenetics are few, and largely limited to technical accounts (Rickgauer & Tank, 2009; Andrasfalvy et al. 2010; Packer et al. 2012; Prakash et al. 2012). We remain hopeful that these recent advances will prove decisive in the success of this potentially powerful technique.
Spatial and temporal calibration
For each of the photostimulation approaches described above, an important initial requirement is the calibration of the light to selectively stimulate the presynaptic input. For interrogation of dense local circuits, such as those found in neocortex, this focuses on controlling the number and spatial extent of light‐evoked action potentials in presynaptic neurons. This applies equally to circuit mapping using optogenetics or glutamate uncaging. For long‐range optogenetic studies, it is essential that the vector used to drive opsin expression is constrained to the presynaptic region of interest. In both cases calibration ensures that the recorded postsynaptic input can be attributed to the spatial location, brain region or cell type interrogated.
Controlling presynaptic firing
For local circuit mapping the photoresponse of the presynaptic population is crucial to correctly interpret experimental data. When using glutamate uncaging, the presynaptic response will vary significantly based on the concentration of caged glutamate, while for optogenetic experiments opsin expression will strongly influence firing. Both factors should be tightly controlled to ensure reliable calibration. The presynaptic population is recorded from first to calibrate a photostimulus that produces reliable, spatially restricted excitation. Recordings should ideally be made in cell‐attached mode, to avoid perturbing the intracellular state of the neuron (Fig. 3 A; Shepherd et al. 2003). Adjustments to the duration, intensity and shape of the light pulse are made until optical stimulation evokes a series of ≤ 3 time‐locked action potentials, spatially restricted to sites adjacent to the soma (Ashby & Isaac, 2011; Anastasiades & Butt, 2012). Evoking multiple (2–3) presynaptic action potentials can compensate for potential failures in synaptic transmission (Dantzker & Callaway, 2000). For 2‐P experiments calibration involves testing for the absence of evoked firing in response to optical stimulation at proximal or distal spines and dendrites (Ashby & Isaac, 2011). For single‐photon experiments the calibration process is repeated across all layers of the cortex creating a response map to the photostimulus (Fig. 3 B). Either the light pulse should be adjusted until firing properties are similar across layers (Anastasiades & Butt, 2012) or differences in presynaptic excitability can be corrected for post hoc (Bureau et al. 2004).
Figure 3. Optimising LSPS laser calibration and event detection.
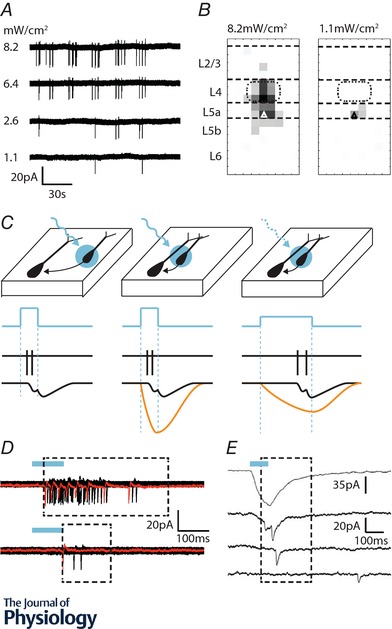
A, loose cell‐attached recordings of action potentials (APs) evoked by glutamate uncaging in a layer 5a pyramidal cell in primary somatosensory cortex at postnatal day 8 (P8). The laser was fired (1 Hz) across a 50 μm resolution grid that covered the depth of a cortical column at this age. Decreasing laser intensity reduced the number of sites at which APs were elicited. B, average maps showing the distribution of points at which APs can be evoked in a recorded layer 5a pyramidal cell (triangle). At high laser power (left) APs can be evoked across layers 4 to 5b as well as at a single point in layer 2/3. Using a low laser power (right) confined action potential generation to sites in the immediate layer 5a, providing good spatial resolution. C, identifying light‐evoked synaptic responses is straight forward for distal presynaptic neurons (left). However targeting presynaptic neurons close to the recorded cell (middle) invariably results in a direct glutamate response (orange line, bottom traces) with an onset locked to the start of the laser pulse. Large direct responses can obscure evoked synaptic responses. Using a long duration, low intensity laser pulse (right) leads to a slower direct glutamate response from which synaptic responses are more readily extracted (see E). D, action potentials evoked from the same neuron upon laser stimulation across the entire depth of the cortex at the two laser intensities shown in B. The monosynaptic event windows (dashed box) begins at the earliest spike and ends 100 ms after the last spike is detected. Top trace recorded at 8.2 mW cm−2, bottom trace, 1.1 mW cm−2. E, example traces recorded from a layer 5a fast spiking (FS) interneuron: top trace, direct glutamate response evoked when the laser was fired at the cell body of the FS interneuron. Despite the careful calibration and slow laser pulse no excitatory postsynaptic current (EPSC) could be extracted from this single spot. Second trace, EPSCs are evoked as a delay from the laser onset and can be extracted from the low amplitude direct response. Third trace, an EPSC evoked from a pyramidal cell distal from the dendritic arbour of the FS cell. Bottom trace, a spontaneous or polysynaptic EPSC that falls outside of the monosynaptic event detection window.
This calibration process ensures the somatic source of synaptic inputs can be confidently assigned to a given stimulation site. When performing calibration experiments, care should be taken to avoid evoking suprathreshold conductances in distal dendrites, particularly the dendritic tufts of pyramidal neurons (Dantzker & Callaway, 2000). We have found that mapping using carefully titrated laser power and long‐duration pulses can effectively limit photostimulation to the immediate soma, even in immature pyramidal cells with relatively simple dendritic arbours (Fig. 3 C; Anastasiades & Butt, 2012). Minimising axo‐dendritic firing is more challenging for optogenetic stimulation, owing to the expression of ChR2 throughout the axon and dendrite (Petreanu et al. 2007). To circumvent this, ChR2 can be restricted to perisomatic regions, using temporally restricted expression (Katzel et al. 2011), or chimeric proteins that contain targeting sequences for Kv2.1 or other subcellularly restricted proteins (Wu et al. 2013; Baker et al. 2016). Alternatively, ‘sculpting’ the photostimulus to create ramped rather than square pulses can help restrict firing to perisomatic stimulation sites (Adesnik & Scanziani, 2010; Pluta et al. 2015) (A. Marques‐Smith, unpublished observations).
Intrinsic physiology, neuronal morphology and glutamate receptor expression can change significantly during development (McCormick & Prince, 1987; Monyer et al. 1994; Picken Bahrey & Moody, 2003; Maravall et al. 2004) or after experimental manipulations (Greenhill et al. 2015; Mowery et al. 2015; Santello & Nevian, 2015), such as sensory deprivation. When comparing input maps across periods of development it is important to use age‐appropriate photostimuli that endeavour to keep presynaptic stimulation levels constant (Anastasiades & Butt, 2012). Similarly, experimental manipulations that might affect the way neurons respond to photostimulation must be controlled for if putative alterations in synaptic input are to be interpreted with any certainty (Shepherd et al. 2003; Del Pino et al. 2017). In such studies, one key consideration is whether the sensitivity of neurons to light varies between control and test samples. To account for differences in presynaptic excitability, it is possible to adjust light intensity to give uniform firing or alternatively scale the afferent input maps post hoc based upon recorded differences in presynaptic spike number (Bureau et al. 2004). Furthermore, for optogenetic experiments it is important to control for variation in expression of optogenetic actuators, within and across conditions (Mao et al. 2011; Arruda‐Carvalho et al. 2017). In essence, the extraneous variable to be controlled is biological, namely presynaptic output, rather than illumination intensity per se.
Assigning postsynaptic responses
A fundamental requirement of circuit mapping is to correctly identify light‐evoked postsynaptic responses. A number of confounds exist that must be controlled for in order for accurate response profiles to be mapped. These responses typically fall into three categories. First, light‐evoked firing may yield polysynaptic activity via distal sites that synapse onto the target cell. Second, across the network there will be a degree of baseline spontaneous activity that must be subtracted from evoked response. Third, ‘direct’ responses occur due to uncaging or opsin stimulation, at the soma or dendrite of recorded neurons, which can obscure local inputs (Fig. 3 C). Polysynaptic activity can be attenuated by standard physiological adjustments, for example, recording at room temperature in artificial cerebrospinal fluid containing high divalent cation concentrations (Shepherd et al. 2003; Jin et al. 2006). Separating direct responses from light‐evoked synaptic input is relatively trivial, as direct responses can be readily distinguished from synaptic responses based on two criteria: (1) the amplitude of the direct response is remarkably constant; (2) direct response onset is coincident with the start of the light pulse. The impact of direct responses can be further reduced by using long, weak light pulses, which are lower in amplitude and decay more slowly, enhancing the ability to distinguish synaptic responses from large direct responses (Fig. 3 C). In contrast, synaptic inputs always occur at a delay from light onset, representing the time taken to fire the presynaptic neuron plus the lag required for synaptic transmission to occur (Fig. 3 C; Shepherd et al. 2003; Nikolenko et al. 2011). Using the data for spike firing from calibration experiments, the monosynaptic detection window is calculated from the onset of the first light‐evoked action potential until a set time after the last or nth spike (typically around 100 ms – Fig. 3 D). The breadth of this detection window accounts for the cell‐to‐cell variability in light‐evoked action potential generation and allows the experimenter to extract and measure the totality (or a very high proportion) of putative light‐evoked postsynaptic currents, while enhancing the ability to distinguish these synaptic events from direct responses (Fig. 3 E; Dantzker & Callaway, 2000; Shepherd et al. 2003; Anastasiades & Butt, 2012). The impact of spontaneous events is reduced by averaging across multiple trials and/or accepting only responses that occur in a set proportion of trials (Ashby & Isaac, 2011; Anastasiades & Butt, 2012; Anastasiades et al. 2016). When recording from cells with high rates of spontaneous synaptic activity, narrowing the postsynaptic detection window (Bendels et al. 2010) or subtracting a value for baseline spontaneous events recorded from trials lacking photostimulation (Dantzker & Callaway, 2000; Xu & Callaway, 2009) can minimise the impact of false positives. For cells with a number of large direct responses, for example neurons with dense dendritic arbours, one can bath apply TTX to the slice perfusate and re‐run the uncaging procedure to produce a map of direct responses (Dantzker & Callaway, 2000; Roerig & Chen, 2002). This approach is also helpful in 2‐P uncaging, and helps rule out false positives where postsynaptic dendrites may run in close proximity to the uncaging site (Nikolenko et al. 2011). These can be subtracted from the synaptic input map to help provide a better estimation of local connectivity.
Ensuring restricted and reliable opsin expression for optogenetics
Expression of optogenetic actuators, such as ChR2, can be achieved in numerous ways. The most common approach – which allows good spatial restriction to ChR2 expression – is to inject a viral vector (typically AAV or lentivirus) encoding the relevant opsin into the presynaptic region of interest. An important step for the investigator is to calibrate the injection volume prior to performing circuit mapping experiments (Arruda‐Carvalho et al. 2017). The expression efficiency of the virus can vary based on serotype (Aschauer et al. 2013), and will also depend upon the duration of expression, so either the injection volume, viral serotype or survival time post‐injection should be adjusted to ensure opsin expression is restricted to the presynaptic structure of interest. Researchers should also check for retrograde transfection, which can occur with AAVs taken up at axon terminals (Aschauer et al. 2013; Rothermel et al. 2013). This is particularly important in the neocortex where many connections between regions are often reciprocal. Retrograde infection may be dependent upon serotype, viral payload (for example Cre‐dependent or ‐independent expression) and duration of expression and occur in a region‐ or projection‐specific manner (Aschauer et al. 2013; Rothermel et al. 2013). It is important that researchers rule out retrograde infection wherever possible and adjust their viral strategy accordingly.
Transgenic reporters (Madisen et al. 2012) and in utero electroporation (Petreanu et al. 2007, 2009; Adesnik & Scanziani, 2010) offer alternatives to viral injections and confer certain advantages and disadvantages. In utero electroporation at defined embryonic time points restricts opsin expression to excitatory projections of individual cortical layers (Petreanu et al. 2007, 2009). However, long‐term expression after in utero electroporation (>40 days), or similar approaches that promote particularly strong opsin expression in neurons, may cause deficits in axonal morphology and synaptic connectivity (Miyashita et al. 2013). Layer‐ or cell‐type‐specific expression can also be achieved using Cre driver lines crossed with optogenetic reporters (Madisen et al. 2012). Reporter lines are advantageous as they help limit variability between experiments by providing consistent expression levels (Madisen et al. 2012; Hooks et al. 2015). However, a potential disadvantage of this approach is that off‐target recombination has been described for some driver lines when crossed with reporters (Hu et al. 2013). For subcortical reporter lines, it should be noted that brain regions typically comprise neurons belonging to multiple subtypes. Some neurons can co‐release multiple neurotransmitters (Tritsch et al. 2012; Saunders et al. 2015), while Cre driver lines may label multiple presynaptic populations (Lammel et al. 2015). Consequently, receptor‐specific pharmacology can be applied to isolate inputs from neurons that utilise a particular neurotransmitter.
Regardless of the approach used, restricted expression methods facilitate mapping intracortical and long‐range connections emanating from distinct cell types or cortical layers (Petreanu et al. 2007, 2009; Olsen et al. 2012; Bortone et al. 2014; Pluta et al. 2015). Cre driver lines combined with optogenetic reporters are particularly useful for studying the connectivity of local interneurons, where it can be assumed that the majority of GABAergic inhibition is from the local circuit (Katzel et al. 2011; Lee et al. 2013; Pfeffer et al. 2013; however, see Basu et al. 2016; He et al. 2016; Rock et al. 2017). This is more problematic for Cre driver lines expressed by broad swaths of neurons – for example the Emx1‐Cre line that captures all projection neurons – as many intracortical projections are recurrent (Mao et al. 2011; Suter & Shepherd, 2015), obscuring regional specificity. However, projection‐specific driver lines can be combined with viral expression of Cre‐dependent opsins to overcome this problem (Olsen et al. 2012; Crandall et al. 2015). Alternatively, more complex viral strategies exist to restrict opsin expression based on specific projection or connection target (Table 1). The tools available to study circuits with optogenetics are considerably more complex than for uncaging and require care to implement them correctly. However, with this complexity comes greatly enhanced specificity, which in turn has yielded new and exciting insight into cortical structure and function.
Concluding remarks
This review has focused on approaches to mapping the structural organisation of the neocortex in a cell type‐, input‐ and layer‐specific fashion. Although the field has progressed rapidly in recent years, technological advances will undoubtedly yield additional possibilities to map cortical connectivity in greater detail. In addition to mapping the structural organisation of cortical networks, circuit mapping has proven adept at uncovering plasticity‐related changes in network architecture (Shepherd et al. 2003; Bureau et al. 2008; Qiu et al. 2011; Marques‐Smith et al. 2016; Rajkovich et al. 2017). It has also been possible to use circuit mapping combined with paired recordings to probe shared inputs amongst reciprocally connected neurons, giving greater insight into the organisation of cortical subnetworks (Yoshimura & Callaway, 2005; Yoshimura et al. 2005; Morgenstern et al. 2016). Recent advances have begun to extract the synaptic connectivity rules that give rise to sensory experience from data recorded in vivo (Ko et al. 2011, 2013). Combining optical circuit mapping of cortical subnetworks with recording of neural activity in vivo will greatly enhance our ability to determine how given neurons form active ensembles and ultimately our functional understanding of neural circuits. Recent advances have begun to bridge the study of circuits in vitro and in vivo by using 2‐P microscopy to combine excitation of putative presynaptic neurons with calcium imaging of postsynaptic responses (Packer et al. 2015). Though this approach is limited to suprathreshold responses, simultaneous whole‐cell patch‐clamp recordings or advances in genetically encoded voltage‐sensitive indicators could open a window into mapping subthreshold connectivity in vivo (Marshall et al. 2016). All‐optical mapping strategies may be especially advantageous in vivo, inasmuch as they circumvent space‐clamp and electrotonic attenuation of distal synapses, issues compounded by the low input resistance of neurons and the challenges involved in obtaining sufficiently low‐access resistance recordings in vivo (Margrie et al. 2002; Williams & Mitchell, 2008 ).
Since early applications of ChR2, the toolkit available to modulate neuronal activity has been growing steadily (Zhang et al. 2011). Tools now exist that provide faster (Gunaydin et al. 2010) or slower (Berndt et al. 2009; Yizhar et al. 2011) on–off kinetics, optical inhibition (Gradinaru et al. 2008) and red shifted activation (Yizhar et al. 2011; Lin et al. 2013; Klapoetke et al. 2014). Many of these have useful application for studying behaviour (Witten et al. 2010; Yizhar et al. 2011; Nieh et al. 2013), but are less practical for studying connectivity. Optical inhibition has been useful for removing certain inputs evoked by a given stimulus, for example feed‐forward inhibition mediated via certain inhibitory neurons (Delevich et al. 2015; Rock & Apicella, 2015). In theory, red shifted opsins should allow for the interrogation of multiple inputs onto a given postsynaptic neuron. In practice, however, the opsin variants available are not sufficiently spectrally distinct to allow isolated multi‐channel stimulation (see, however, Klapoetke et al. 2014; Hooks et al. 2015). In the future, novel optogenetic tools and expression systems will surely provide greater specificity with which to probe cortical networks.
Finally, as we increase our understanding of how individual cell types connect with other neurons, layers or regions of the brain, it will be of interest to uncover the molecular determinants of specific connectivity patterns. Recent advances in single cell genomic analysis (Fuccillo et al. 2015; Usoskin et al. 2015; Zeisel et al. 2015; Foldy et al. 2016; Poulin et al. 2016; Romanov et al. 2017) combined with local circuit mapping methods outlined herein, may offer a reliable, relatively high‐throughput approach to achieve such a goal (Pfeffer et al. 2013). Combining these approaches will provide significant insight into the molecular mechanisms that produce the complex wiring patterns observed within neocortical circuits. It will also provide novel markers, allowing researchers to continue probing the function of cortical microcircuits with ever greater specificity.
Additional information
Competing interests
The authors declare no competing interests in relation to this work.
Author contributions
All authors worked together to conceive the topic of the review, and contributed to writing and editing the text and figure. All authors approved the final version of the manuscript. All persons designated as authors qualify for authorship and all those who qualify for authorship are listed.
Funding
Research in the Butt lab has been supported by the Human Frontiers Science Organisation (CDA0023/2008‐C), the Medical Research Council (MR/K004387/1), BBSRC (BB/P003796/1) and Brain and Behaviour Research Foundation (Narsad grant ref. 19079). P.A. was funded by Imperial College London and A.M.‐S. was the recipient of a Wellcome Trust DPhil scholarship (6362/Z/08/Z).
Acknowledgements
The authors would like to thank our numerous collaborators over the years who have assisted and guided our studies using optical circuit mapping approaches, in particular Profs Gero Miesenböck (Oxford) and Adam Carter (NYU). In addition we would like to acknowledge the input of other members of the research group, namely Daniel Lyngholm, Jacqui Stacey and Cristiana Vagnoni, for their insight, help and enthusiasm.
Biographies
The authors have employed laser scanning photostimulation and optogenetic approaches to probe the earliest circuits in mammalian cerebral cortex. Paul Anastasiades is a postdoctoral associate at the Center for Neural Science, New York University working in the laboratory of Professor Adam Carter. He studies the synaptic organisation and neuromodulation of the Prefrontal Cortex and retains an interest in the development of cortical circuits.

Andre Marques‐Smith received his PhD from the University of Oxford in 2014. During his PhD and first post‐doctoral position, he investigated the development of cortical inhibitory circuits. He is now a researcher at the Sainsbury‐Wellcome Centre (UCL), using high‐density extracellular probes to investigate thalamocortical circuits in behaving rodents.
Simon Butt is an Associate Professor at the University of Oxford with a keen interest in GABAergic interneurons and the emergence of early neocortical circuits involved in sensory perception.
Edited by: Ole Petersen & Tadashi Isa
Contributor Information
Paul G. Anastasiades, Email: pa68@nyu.edu.
Andre Marques‐Smith, Email: a.marques-smith@ucl.ac.uk.
Simon J. B. Butt, Email: simon.butt@dpag.ox.ac.uk.
References
- Adesnik H & Scanziani M (2010). Lateral competition for cortical space by layer‐specific horizontal circuits. Nature 464, 1155–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG & Butt SJ (2011). Decoding the transcriptional basis for GABAergic interneuron diversity in the mouse neocortex. Eur J Neurosci 34, 1542–1552. [DOI] [PubMed] [Google Scholar]
- Anastasiades PG & Butt SJ (2012). A role for silent synapses in the development of the pathway from layer 2/3 to 5 pyramidal cells in the neocortex. J Neurosci 32, 13085–13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG, Marques‐Smith A, Lyngholm D, Lickiss T, Raffiq S, Katzel D, Miesenbock G & Butt SJ (2016). GABAergic interneurons form transient layer‐specific circuits in early postnatal neocortex. Nat Commun 7, 10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CT, Sheets PL, Kiritani T & Shepherd GM (2010). Sublayer‐specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci 13, 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Zemelman BV, Tang J & Vaziri A (2010). Two‐photon single‐cell optogenetic control of neuronal activity by sculpted light. Proc Natl Acad Sci USA 107, 11981–11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella AJ, Wickersham IR, Seung HS & Shepherd GM (2012). Laminarly orthogonal excitation of fast‐spiking and low‐threshold‐spiking interneurons in mouse motor cortex. J Neurosci 32, 7021–7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda‐Carvalho M, Wu WC, Cummings KA & Clem RL (2017). Optogenetic examination of prefrontal‐amygdala synaptic development. J Neurosci 37, 2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S & Rumpel S (2013). Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS One 8, e76310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC & Isaac JT (2011). Maturation of a recurrent excitatory neocortical circuit by experience‐dependent unsilencing of newly formed dendritic spines. Neuron 70, 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CA, Elyada YM, Parra A & Bolton MM (2016). Cellular resolution circuit mapping with temporal‐focused excitation of soma‐targeted channelrhodopsin. Elife 5, e14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A & Siegelbaum SA (2016). Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long‐range inhibition. Science 351, aaa5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC & Luo L (2015). Circuit architecture of VTA dopamine neurons revealed by systematic input‐output mapping. Cell 162, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendels MH, Beed P, Schmitz D, Johenning FW & Leibold C (2010). Detection of input sites in scanning photostimulation data based on spatial correlations. J Neurosci Methods 192, 286–295. [DOI] [PubMed] [Google Scholar]
- Berndt A, Yizhar O, Gunaydin LA, Hegemann P & Deisseroth K (2009). Bi‐stable neural state switches. Nat Neurosci 12, 229–234. [DOI] [PubMed] [Google Scholar]
- Binzegger T, Douglas RJ & Martin KA (2004). A quantitative map of the circuit of cat primary visual cortex. J Neurosci 24, 8441–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortone DS, Olsen SR & Scanziani M (2014). Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron 82, 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G & Deisseroth K (2005). Millisecond‐timescale, genetically targeted optical control of neural activity. Nat Neurosci 8, 1263–1268. [DOI] [PubMed] [Google Scholar]
- Brill J & Huguenard JR (2009). Robust short‐latency perisomatic inhibition onto neocortical pyramidal cells detected by laser‐scanning photostimulation. J Neurosci 29, 7413–7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP & Hestrin S (2009). Intracortical circuits of pyramidal neurons reflect their long‐range axonal targets. Nature 457, 1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM & Svoboda K (2004). Precise development of functional and anatomical columns in the neocortex. Neuron 42, 789–801. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GM & Svoboda K (2008). Circuit and plasticity defects in the developing somatosensory cortex of FMR1 knock‐out mice. J Neurosci 28, 5178–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM & Yuste R (2002). Stimulating neurons with light. Curr Opin Neurobiol 12, 587–592. [DOI] [PubMed] [Google Scholar]
- Chaigneau E, Ronzitti E, Gajowa MA, Soler‐Llavina GJ, Tanese D, Brureau AY, Papagiakoumou E, Zeng H & Emiliani V (2016). Two‐photon holographic stimulation of ReaChR. Front Cell Neurosci 10, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall SR, Cruikshank SJ & Connors BW (2015). A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron 86, 768–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Ahmed OJ, Stevens TR, Patrick SL, Gonzalez AN, Elmaleh M & Connors BW (2012). Thalamic control of layer 1 circuits in prefrontal cortex. J Neurosci 32, 17813–17823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ & Connors BW (2007). Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci 10, 462–468. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Urabe H, Nurmikko AV & Connors BW (2010). Pathway‐specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65, 230–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalva MB & Katz LC (1994). Rearrangements of synaptic connections in visual cortex revealed by laser photostimulation. Science 265, 255–258. [DOI] [PubMed] [Google Scholar]
- Dantzker JL & Callaway EM (2000). Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci 3, 701–707. [DOI] [PubMed] [Google Scholar]
- Delevich K, Tucciarone J, Huang ZJ & Li B (2015). The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci 35, 5743–5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Pino I, Brotons‐Mas JR, Marques‐Smith A, Marighetto A, Frick A, Marin O & Rico B (2017). Abnormal wiring of CCK+ basket cells disrupts spatial information coding. Nat Neurosci 20, 784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembrow NC, Zemelman BV & Johnston D (2015). Temporal dynamics of L5 dendrites in medial prefrontal cortex regulate integration versus coincidence detection of afferent inputs. J Neurosci 35, 4501–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo LA, Berns DS, DeLoach K & Luo L (2015). Connectivity of mouse somatosensory and prefrontal cortex examined with trans‐synaptic tracing. Nat Neurosci 18, 1687–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH & Webb WW (1990). Two‐photon laser scanning fluorescence microscopy. Science 248, 73–76. [DOI] [PubMed] [Google Scholar]
- Denk W & Svoboda K (1997). Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron 18, 351–357. [DOI] [PubMed] [Google Scholar]
- Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo L, Xu Q, Liu R, Lu C, Chu J, Grimley JS, Krostag AR, Kaykas A, Avery MC, Rashid MS, Baek M, Jacob AL, Smith GB, Wilson DE, Kosche G, Kruglikov I, Rusielewicz T, Kotak VC, Mowery TM, Anderson SA, Callaway EM, Dasen JS, Fitzpatrick D, Fossati V, Long MA, Noggle S, Reynolds JH, Sanes DH, Rudy B, Feng G & Fishell G (2016). A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci 19, 1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ & Martin KA (2004). Neuronal circuits of the neocortex. Annu Rev Neurosci 27, 419–451. [DOI] [PubMed] [Google Scholar]
- D'Souza RD, Meier AM, Bista P, Wang Q & Burkhalter A (2016). Recruitment of inhibition and excitation across mouse visual cortex depends on the hierarchy of interconnecting areas. Elife 5, e19332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis‐Davies GC (2007). Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods 4, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D (2012). Excitatory neuronal connectivity in the barrel cortex. Front Neuroanat 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer D, Lubke J & Sakmann B (2006). Efficacy and connectivity of intracolumnar pairs of layer 2/3 pyramidal cells in the barrel cortex of juvenile rats. J Physiol 575, 583–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty GT, Roberts LS & Connors BW (1999). Sensory experience modifies the short‐term dynamics of neocortical synapses. Nature 400, 367–371. [DOI] [PubMed] [Google Scholar]
- Fino E, Araya R, Peterka DS, Salierno M, Etchenique R & Yuste R (2009). RuBi‐glutamate: two‐photon and visible‐light photoactivation of neurons and dendritic spines. Front Neural Circuits 3, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E & Yuste R (2011). Dense inhibitory connectivity in neocortex. Neuron 69, 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Darmanis S, Aoto J, Malenka RC, Quake SR & Sudhof TC (2016). Single‐cell RNAseq reveals cell adhesion molecule profiles in electrophysiologically defined neurons. Proc Natl Acad Sci USA 113, E5222–E5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuccillo MV, Foldy C, Gokce O, Rothwell PE, Sun GL, Malenka RC & Sudhof TC (2015). Single‐cell mRNA profiling reveals cell‐type‐specific expression of neurexin isoforms. Neuron 87, 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T, Wang SS, Dantzker JL, Dore TM, Bybee WJ, Callaway EM, Denk W & Tsien RY (1999). Brominated 7‐hydroxycoumarin‐4‐ylmethyls: photolabile protecting groups with biologically useful cross‐sections for two photon photolysis. Proc Natl Acad Sci USA 96, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD & Wiesel TN (1989). Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci 9, 2432–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y & Stevens CF (1994). Two components of transmitter release at a central synapse. Proc Natl Acad Sci USA 91, 12942–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce O, Bonhoeffer T & Scheuss V (2016). Clusters of synaptic inputs on dendrites of layer 5 pyramidal cells in mouse visual cortex. Elife 5, e09222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q & Burkhalter A (2007). Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front Neuroanat 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME & Heintz N (2003). A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425, 917–925. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Thompson KR & Deisseroth K (2008). eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol 36, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill SD, Juczewski K, de Haan AM, Seaton G, Fox K & Hardingham NR (2015). Adult cortical plasticity depends on an early postnatal critical period. Science 349, 424–427. [DOI] [PubMed] [Google Scholar]
- Grubb MS & Burrone J (2010). Channelrhodopsin‐2 localised to the axon initial segment. PLoS One 5, e13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenthner CJ, Miyamichi K, Yang HH, Heller HC & Luo L (2013). Permanent genetic access to transiently active neurons via TRAP: targeted recombination in active populations. Neuron 78, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K & Hegemann P (2010). Ultrafast optogenetic control. Nat Neurosci 13, 387–392. [DOI] [PubMed] [Google Scholar]
- Harris KD & Shepherd GM (2015). The neocortical circuit: themes and variations. Nat Neurosci 18, 170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattox AM & Nelson SB (2007). Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol 98, 3330–3340. [DOI] [PubMed] [Google Scholar]
- He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, Kelly SM, Krugikov I, Wu P, Chen Y, Gong L, Hou Y, Osten P, Rudy B & Huang ZJ (2016). Strategies and tools for combinatorial targeting of gabaergic neurons in mouse cerebral cortex. Neuron 91, 1228–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Hires SA, Zhang YX, Huber D, Petreanu L, Svoboda K & Shepherd GM (2011). Laminar analysis of excitatory local circuits in vibrissal motor and sensory cortical areas. PLoS Biol 9, e1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Lin JY, Guo C & Svoboda K (2015). Dual‐channel circuit mapping reveals sensorimotor convergence in the primary motor cortex. J Neurosci 35, 4418–4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, Mao T, Gutnisky DA, Yamawaki N, Svoboda K & Shepherd GM (2013). Organization of cortical and thalamic input to pyramidal neurons in mouse motor cortex. J Neurosci 33, 748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Cavendish JZ & Agmon A (2013). Not all that glitters is gold: off‐target recombination in the somatostatin‐IRES‐Cre mouse line labels a subset of fast‐spiking interneurons. Front Neural Circuits 7, 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1962). Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol 160, 106–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Isaacson JS & Scanziani M (2009). Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci 29, 9127–9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Beneduce BM, Drew IR & Regehr WG (2014). Achieving high‐frequency optical control of synaptic transmission. J Neurosci 34, 7704–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome J & Heck DH (2011). The age of enlightenment: evolving opportunities in brain research through optical manipulation of neuronal activity. Front Syst Neurosci 5, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Shen S, Cadwell CR, Berens P, Sinz F, Ecker AS, Patel S & Tolias AS (2015). Principles of connectivity among morphologically defined cell types in adult neocortex. Science 350, aac9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL & Zhu JJ (2013). The organization of two new cortical interneuronal circuits. Nat Neurosci 16, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Prince DA & Huguenard JR (2006). Enhanced excitatory synaptic connectivity in layer V pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci 26, 4891–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzel D, Zemelman BV, Buetfering C, Wolfel M & Miesenbock G (2011). The columnar and laminar organization of inhibitory connections to neocortical excitatory cells. Nat Neurosci 14, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhao T, Petralia RS, Yu Y, Peng H, Myers E & Magee JC (2011). mGRASP enables mapping mammalian synaptic connectivity with light microscopy. Nat Methods 9, 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey‐Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine‐Paton M, Wong GK & Boyden ES (2014). Independent optical excitation of distinct neural populations. Nat Methods 11, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Cossell L, Baragli C, Antolik J, Clopath C, Hofer SB & Mrsic‐Flogel TD (2013). The emergence of functional microcircuits in visual cortex. Nature 496, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Hofer SB, Pichler B, Buchanan KA, Sjostrom PJ & Mrsic‐Flogel TD (2011). Functional specificity of local synaptic connections in neocortical networks. Nature 473, 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konur S, Rabinowitz D, Fenstermaker VL & Yuste R (2003). Systematic regulation of spine sizes and densities in pyramidal neurons. J Neurobiol 56, 95–112. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X & Trachtenberg JT (2013). A disinhibitory microcircuit initiates critical‐period plasticity in the visual cortex. Nature 501, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg EE, Foldy C, Wall NR, Beier K, Luo L & Malenka RC (2015). Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron 85, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Be JV, Silberberg G, Wang Y & Markram H (2007). Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb Cortex 17, 2204–2213. [DOI] [PubMed] [Google Scholar]
- Lee AT, Gee SM, Vogt D, Patel T, Rubenstein JL & Sohal VS (2014). Pyramidal neurons in prefrontal cortex receive subtype‐specific forms of excitation and inhibition. Neuron 81, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kruglikov I, Huang ZJ, Fishell G & Rudy B (2013). A disinhibitory circuit mediates motor integration in the somatosensory cortex. Nat Neurosci 16, 1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TL Jr, Mao T, Svoboda K & Arnold DB (2009). Myosin‐dependent targeting of transmembrane proteins to neuronal dendrites. Nat Neurosci 12, 568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Knutsen PM, Muller A, Kleinfeld D & Tsien RY (2013). ReaChR: a red‐shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci 16, 1499–1508.23995068 [Google Scholar]
- Little JP & Carter AG (2012). Subcellular synaptic connectivity of layer 2 pyramidal neurons in the medial prefrontal cortex. J Neurosci 32, 12808–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JP & Carter AG (2013). Synaptic mechanisms underlying strong reciprocal connectivity between the medial prefrontal cortex and basolateral amygdala. J Neurosci 33, 15333–15342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH & Agmon A (2006). Distinct subtypes of somatostatin‐containing neocortical interneurons revealed in transgenic mice. J Neurosci 26, 5069–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Cassel JM & Carter AG (2014). Cocaine exposure reorganizes cell type‐ and input‐specific connectivity in the nucleus accumbens. Nat Neurosci 17, 1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Little JP, Cassel JM & Carter AG (2012). Subcellular connectivity underlies pathway‐specific signaling in the nucleus accumbens. Nat Neurosci 15, 1624–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsaki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE & Zeng H (2012). A toolbox of Cre‐dependent optogenetic transgenic mice for light‐induced activation and silencing. Nat Neurosci 15, 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES & Zeng H (2010). A robust and high‐throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13, 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Kusefoglu D, Hooks BM, Huber D, Petreanu L & Svoboda K (2011). Long‐range neuronal circuits underlying the interaction between sensory and motor cortex. Neuron 72, 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravall M, Stern EA & Svoboda K (2004). Development of intrinsic properties and excitability of layer 2/3 pyramidal neurons during a critical period for sensory maps in rat barrel cortex. J Neurophysiol 92, 144–156. [DOI] [PubMed] [Google Scholar]
- Margrie TW, Brecht M & Sakmann B (2002). In vivo, low‐resistance, whole‐cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch 444, 491–498. [DOI] [PubMed] [Google Scholar]
- Markram H, Muller E, Ramaswamy S, Reimann MW, Abdellah M, Sanchez CA, Ailamaki A, Alonso‐Nanclares L, Antille N, Arsever S, Kahou GA, Berger TK, Bilgili A, Buncic N, Chalimourda A, Chindemi G, Courcol JD, Delalondre F, Delattre V, Druckmann S, Dumusc R, Dynes J, Eilemann S, Gal E, Gevaert ME, Ghobril JP, Gidon A, Graham JW, Gupta A, Haenel V, Hay E, Heinis T, Hernando JB, Hines M, Kanari L, Keller D, Kenyon J, Khazen G, Kim Y, King JG, Kisvarday Z, Kumbhar P, Lasserre S, Le Be JV, Magalhaes BR, Merchan‐Perez A, Meystre J, Morrice BR, Muller J, Munoz‐Cespedes A, Muralidhar S, Muthurasa K, Nachbaur D, Newton TH, Nolte M, Ovcharenko A, Palacios J, Pastor L, Perin R, Ranjan R, Riachi I, Rodriguez JR, Riquelme JL, Rossert C, Sfyrakis K, Shi Y, Shillcock JC, Silberberg G, Silva R, Tauheed F, Telefont M, Toledo‐Rodriguez M, Trankler T, Van Geit W, Diaz JV, Walker R, Wang Y, Zaninetta SM, DeFelipe J, Hill SL, Segev I & Schurmann F (2015). Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492. [DOI] [PubMed] [Google Scholar]
- Marlin JJ & Carter AG (2014). GABA‐A receptor inhibition of local calcium signaling in spines and dendrites. J Neurosci 34, 15898–15911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques‐Smith A, Lyngholm D, Kaufmann AK, Stacey JA, Hoerder‐Suabedissen A, Becker EB, Wilson MC, Molnar Z & Butt SJ (2016). A transient translaminar GABAergic interneuron circuit connects thalamocortical recipient layers in neonatal somatosensory cortex. Neuron 89, 536–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Li JZ, Zhang Y, Gong Y, St‐Pierre F, Lin MZ & Schnitzer MJ (2016). Cell‐type‐specific optical recording of membrane voltage dynamics in freely moving mice. Cell 167, 1650–1662.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis‐Davies GC & Kasai H (2008). Three‐dimensional mapping of unitary synaptic connections by two‐photon macro photolysis of caged glutamate. J Neurophysiol 99, 1535–1544. [DOI] [PubMed] [Google Scholar]
- McCormick DA & Prince DA (1987). Post‐natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol 393, 743–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry LM & Carter AG (2016). Inhibitory gating of basolateral amygdala inputs to the prefrontal cortex. J Neurosci 36, 9391–9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Kao JP, Lee HK & Kanold PO (2015). Visual deprivation causes refinement of intracortical circuits in the auditory cortex. Cell Rep 12, 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Kao JP, Lee HK & Kanold PO (2017). Intracortical circuits in thalamorecipient layers of auditory cortex refine after visual deprivation. eNeuro 4, ENEURO.0092‐17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HS, Wimmer VC, Oberlaender M, de Kock CP, Sakmann B & Helmstaedter M (2010). Number and laminar distribution of neurons in a thalamocortical projection column of rat vibrissal cortex. Cereb Cortex 20, 2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G (2011). Optogenetic control of cells and circuits. Annu Rev Cell Dev Biol 27, 731–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Shao YR, Chung J, Pourzia O & Feldman DE (2013). Long‐term channelrhodopsin‐2 (ChR2) expression can induce abnormal axonal morphology and targeting in cerebral cortex. Front Neural Circuits 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z & Cheung AF (2006). Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res 55, 105–115. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B & Seeburg PH (1994). Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540. [DOI] [PubMed] [Google Scholar]
- Morgenstern NA, Bourg J & Petreanu L (2016). Multilaminar networks of cortical neurons integrate common inputs from sensory thalamus. Nat Neurosci 19, 1034–1040. [DOI] [PubMed] [Google Scholar]
- Morishima M & Kawaguchi Y (2006). Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci 26, 4394–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima M, Morita K, Kubota Y & Kawaguchi Y (2011). Highly differentiated projection‐specific cortical subnetworks. J Neurosci 31, 10380–10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB (1957). Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol 20, 408–434. [DOI] [PubMed] [Google Scholar]
- Mowery TM, Kotak VC & Sanes DH (2015). Transient hearing loss within a critical period causes persistent changes to cellular properties in adult auditory cortex. Cereb Cortex 25, 2083–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P & Bamberg E (2003). Channelrhodopsin‐2, a directly light‐gated cation‐selective membrane channel. Proc Natl Acad Sci USA 100, 13940–13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Kim SY, Namburi P & Tye KM (2013). Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res 1511, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolenko V, Fino E & Yuste R (2011). Two‐photon mapping of neural circuits. Cold Spring Harb Protoc 2011, pdb.top111. [DOI] [PubMed] [Google Scholar]
- Nikolenko V, Poskanzer KE & Yuste R (2007). Two‐photon photostimulation and imaging of neural circuits. Nat Methods 4, 943–950. [DOI] [PubMed] [Google Scholar]
- Oberlaender M, de Kock CP, Bruno RM, Ramirez A, Meyer HS, Dercksen VJ, Helmstaedter M & Sakmann B (2012). Cell type‐specific three‐dimensional structure of thalamocortical circuits in a column of rat vibrissal cortex. Cereb Cortex 22, 2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H & Scanziani M (2012). Gain control by layer six in cortical circuits of vision. Nature 483, 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B & Callaway EM (2011). New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71, 617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, McConnell DJ, Fino E & Yuste R (2013). Axo‐dendritic overlap and laminar projection can explain interneuron connectivity to pyramidal cells. Cereb Cortex 23, 2790–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Peterka DS, Hirtz JJ, Prakash R, Deisseroth K & Yuste R (2012). Two‐photon optogenetics of dendritic spines and neural circuits. Nat Methods 9, 1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Russell LE, Dalgleish HW & Hausser M (2015). Simultaneous all‐optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat Methods 12, 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM & Yuste R (2011). Dense, unspecific connectivity of neocortical parvalbumin‐positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31, 13260–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagiakoumou E, Anselmi F, Begue A, de Sars V, Gluckstad J, Isacoff EY & Emiliani V (2010). Scanless two‐photon excitation of channelrhodopsin‐2. Nat Methods 7, 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin R, Berger TK & Markram H (2011). A synaptic organizing principle for cortical neuronal groups. Proc Natl Acad Sci USA 108, 5419–5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature Group , Ascoli GA, Alonso‐Nanclares L, Anderson SA, Barrionuevo G, Benavides‐Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo‐Rodriguez M, Wang Y, West DC & Yuste R (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci 9, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A & Svoboda K (2007). Channelrhodopsin‐2‐assisted circuit mapping of long‐range callosal projections. Nat Neurosci 10, 663–668. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM & Svoboda K (2009). The subcellular organization of neocortical excitatory connections. Nature 457, 1142–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ & Scanziani M (2013). Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 16, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken Bahrey HL & Moody WJ (2003). Early development of voltage‐gated ion currents and firing properties in neurons of the mouse cerebral cortex. J Neurophysiol 89, 1761–1773. [DOI] [PubMed] [Google Scholar]
- Pluta S, Naka A, Veit J, Telian G, Yao L, Hakim R, Taylor D & Adesnik H (2015). A direct translaminar inhibitory circuit tunes cortical output. Nat Neurosci 18, 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Tasic B, Hjerling‐Leffler J, Trimarchi JM & Awatramani R (2016). Disentangling neural cell diversity using single‐cell transcriptomics. Nat Neurosci 19, 1131–1141. [DOI] [PubMed] [Google Scholar]
- Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ & Deisseroth K (2012). Two‐photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat Methods 9, 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S, Anderson CT, Levitt P & Shepherd GM (2011). Circuit‐specific intracortical hyperconnectivity in mice with deletion of the autism‐associated Met receptor tyrosine kinase. J Neurosci 31, 5855–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovich KE, Loerwald KW, Hale CF, Hess CT, Gibson JR & Huber KM (2017). Experience‐dependent and differential regulation of local and long‐range excitatory neocortical circuits by postsynaptic Mef2c. Neuron 93, 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P & Sakmann B (1998). Target‐cell‐specific facilitation and depression in neocortical circuits. Nat Neurosci 1, 279–285. [DOI] [PubMed] [Google Scholar]
- Rickgauer JP & Tank DW (2009). Two‐photon excitation of channelrhodopsin‐2 at saturation. Proc Natl Acad Sci USA 106, 15025–15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C & Apicella AJ (2015). Callosal projections drive neuronal‐specific responses in the mouse auditory cortex. J Neurosci 35, 6703–6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C, Zurita H, Lebby S, Wilson CJ & Apicella AJ (2017). Cortical circuits of callosal GABAergic neurons. Cereb Cortex (in press; https://doi.org/10.1093/cercor/bhx025). [DOI] [PubMed] [Google Scholar]
- Roerig B & Chen B (2002). Relationships of local inhibitory and excitatory circuits to orientation preference maps in ferret visual cortex. Cereb Cortex 12, 187–198. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Zeisel A, Bakker J, Girach F, Hellysaz A, Tomer R, Alpar A, Mulder J, Clotman F, Keimpema E, Hsueh B, Crow AK, Martens H, Schwindling C, Calvigioni D, Bains JS, Mate Z, Szabo G, Yanagawa Y, Zhang MD, Rendeiro A, Farlik M, Uhlen M, Wulff P, Bock C, Broberger C, Deisseroth K, Hokfelt T, Linnarsson S, Horvath TL & Harkany T (2017). Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat Neurosci 20, 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Brunert D, Zabawa C, Diaz‐Quesada M & Wachowiak M (2013). Transgene expression in target‐defined neuron populations mediated by retrograde infection with adeno‐associated viral vectors. J Neurosci 33, 15195–15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M & Nevian T (2015). Dysfunction of cortical dendritic integration in neuropathic pain reversed by serotoninergic neuromodulation. Neuron 86, 233–246. [DOI] [PubMed] [Google Scholar]
- Saunders A, Granger AJ & Sabatini BL (2015). Corelease of acetylcholine and GABA from cholinergic forebrain neurons. Elife 4, e06412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A, Johnson CA & Sabatini BL (2012). Novel recombinant adeno‐associated viruses for Cre activated and inactivated transgene expression in neurons. Front Neural Circuits 6, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders A & Sabatini BL (2015). Cre activated and inactivated recombinant adeno‐associated viral vectors for neuronal anatomical tracing or activity manipulation. Curr Protoc Neurosci, Unit 1.24, https://doi.org/10.1002/0471142301.ns0124s72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger P, Scharer YP & Oertner TG (2011). Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol 96, 34–39. [DOI] [PubMed] [Google Scholar]
- Schubert D, Staiger JF, Cho N, Kotter R, Zilles K & Luhmann HJ (2001). Layer‐specific intracolumnar and transcolumnar functional connectivity of layer V pyramidal cells in rat barrel cortex. J Neurosci 21, 3580–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Pologruto TA & Svoboda K (2003). Circuit analysis of experience‐dependent plasticity in the developing rat barrel cortex. Neuron 38, 277–289. [DOI] [PubMed] [Google Scholar]
- Shepherd GM, Stepanyants A, Bureau I, Chklovskii D & Svoboda K (2005). Geometric and functional organization of cortical circuits. Nat Neurosci 8, 782–790. [DOI] [PubMed] [Google Scholar]
- Shepherd GM & Svoboda K (2005). Laminar and columnar organization of ascending excitatory projections to layer 2/3 pyramidal neurons in rat barrel cortex. J Neurosci 25, 5670–5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G & Markram H (2007). Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 53, 735–746. [DOI] [PubMed] [Google Scholar]
- Silver RA (2003). Estimation of nonuniform quantal parameters with multiple‐probability fluctuation analysis: theory, application and limitations. J Neurosci Methods 130, 127–141. [DOI] [PubMed] [Google Scholar]
- Song S, Sjostrom PJ, Reigl M, Nelson S & Chklovskii DB (2005). Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol 3, e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ikrar T, Davis MF, Gong N, Zheng X, Luo ZD, Lai C, Mei L, Holmes TC, Gandhi SP & Xu X (2016). Neuregulin‐1/ErbB4 signaling regulates visual cortical plasticity. Neuron 92, 160–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter BA & Shepherd GM (2015). Reciprocal interareal connections to corticospinal neurons in mouse M1 and S2. J Neurosci 35, 2959–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szobota S, Gorostiza P, Del Bene F, Wyart C, Fortin DL, Kolstad KD, Tulyathan O, Volgraf M, Numano R, Aaron HL, Scott EK, Kramer RH, Flannery J, Baier H, Trauner D & Isacoff EY (2007). Remote control of neuronal activity with a light‐gated glutamate receptor. Neuron 54, 535–545. [DOI] [PubMed] [Google Scholar]
- Tang JC, Rudolph S, Dhande OS, Abraira VE, Choi S, Lapan SW, Drew IR, Drokhlyansky E, Huberman AD, Regehr WG & Cepko CL (2015). Cell type‐specific manipulation with GFP‐dependent Cre recombinase. Nat Neurosci 18, 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB & Huang ZJ (2011). A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71, 995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM & Bannister AP (1998). Postsynaptic pyramidal target selection by descending layer III pyramidal axons: dual intracellular recordings and biocytin filling in slices of rat neocortex. Neuroscience 84, 669–683. [DOI] [PubMed] [Google Scholar]
- Thomson AM & Bannister AP (2003). Interlaminar connections in the neocortex. Cereb Cortex 13, 5–14. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB & Sabatini BL (2012). Dopaminergic neurons inhibit striatal output through non‐canonical release of GABA. Nature 490, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B & Fishell G (2016). Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron 89, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling‐Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S & Ernfors P (2015). Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nat Neurosci 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Bandyopadhyay S, Kao JP & Kanold PO (2012). Changing microcircuits in the subplate of the developing cortex. J Neurosci 32, 1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Markram H, Goodman PH, Berger TK, Ma J & Goldman‐Rakic PS (2006). Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci 9, 534–542. [DOI] [PubMed] [Google Scholar]
- West DC, Mercer A, Kirchhecker S, Morris OT & Thomson AM (2006). Layer 6 cortico‐thalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb Cortex 16, 200–211. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Finke S, Conzelmann KK & Callaway EM (2007). Retrograde neuronal tracing with a deletion‐mutant rabies virus. Nat Methods 4, 47–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickersham IR, Sullivan HA & Seung HS (2013). Axonal and subcellular labelling using modified rabies viral vectors. Nat Commun 4, 2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR & Mitchell SJ (2008). Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11, 790–798. [DOI] [PubMed] [Google Scholar]
- Williams SR & Stuart GJ (2002). Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science 295, 1907–1910. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Bruno RM, de Kock CP, Kuner T & Sakmann B (2010). Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cereb Cortex 20, 2265–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C & Deisseroth K (2010). Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA & Van der Loos H (1970). The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17, 205–242. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Bloem B, Mansvelder HD, Luchicchi A & Deisseroth K (2014). A fourth generation of neuroanatomical tracing techniques: exploiting the offspring of genetic engineering. J Neurosci Methods 235, 331–348. [DOI] [PubMed] [Google Scholar]
- Wu C, Ivanova E, Zhang Y & Pan ZH (2013). rAAV‐mediated subcellular targeting of optogenetic tools in retinal ganglion cells in vivo. PLoS One 8, e66332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Krabbe S, Grundemann J, Botta P, Fadok JP, Osakada F, Saur D, Grewe BF, Schnitzer MJ, Callaway EM & Luthi A (2016). Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell 167, 961–972.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W & Sudhof TC (2013). A neural circuit for memory specificity and generalization. Science 339, 1290–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X & Callaway EM (2009). Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J Neurosci 29, 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu‐Friedman MA & Regehr WG (1999). Presynaptic strontium dynamics and synaptic transmission. Biophys J 76, 2029–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV & Scanziani M (2014). Equalizing excitation‐inhibition ratios across visual cortical neurons. Nature 511, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N & Shepherd GM (2015). Synaptic circuit organization of motor corticothalamic neurons. J Neurosci 35, 2293–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Carrasquillo Y, Hooks BM, Nerbonne JM & Burkhalter A (2013). Distinct balance of excitation and inhibition in an interareal feedforward and feedback circuit of mouse visual cortex. J Neurosci 33, 17373–17384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Mostajo‐Radji MA, Brown JR, Rouaux C, Tomassy GS, Hensch TK & Arlotta P (2015). Instructing perisomatic inhibition by direct lineage reprogramming of neocortical projection neurons. Neuron 88, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P & Deisseroth K (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y & Callaway EM (2005). Fine‐scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci 8, 1552–1559. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y, Dantzker JL & Callaway EM (2005). Excitatory cortical neurons form fine‐scale functional networks. Nature 433, 868–873. [DOI] [PubMed] [Google Scholar]
- Yuste R, Gutnick MJ, Saar D, Delaney KR & Tank DW (1994). Ca2+ accumulations in dendrites of neocortical pyramidal neurons: an apical band and evidence for two functional compartments. Neuron 13, 23–43. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Munoz‐Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, Rolny C, Castelo‐Branco G, Hjerling‐Leffler J & Linnarsson S (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single‐cell RNA‐seq. Science 347, 1138–1142. [DOI] [PubMed] [Google Scholar]
- Zemelman BV, Lee GA, Ng M & Miesenbock G (2002). Selective photostimulation of genetically chARGed neurons. Neuron 33, 15–22. [DOI] [PubMed] [Google Scholar]
- Zhang F, Vierock J, Yizhar O, Fenno LE, Tsunoda S, Kianianmomeni A, Prigge M, Berndt A, Cushman J, Polle J, Magnuson J, Hegemann P & Deisseroth K (2011). The microbial opsin family of optogenetic tools. Cell 147, 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP & Oertner TG (2007). Optical induction of synaptic plasticity using a light‐sensitive channel. Nat Methods 4, 139–141. [DOI] [PubMed] [Google Scholar]
- Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW & Zhang LI (2017). AAV‐mediated anterograde transsynaptic tagging: mapping corticocollicular input‐defined neural pathways for defense behaviors. Neuron 93, 33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


