Abstract
Key points
Cardiac hypertrophy following endurance‐training is thought to be due to hypertrophy of existing cardiomyocytes.
The benefits of endurance exercise on cardiac hypertrophy are generally thought to be short‐lived and regress to sedentary levels within a few weeks of stopping endurance training.
We have now established that cardiomyocyte hyperplasia also plays a considerable role in cardiac growth in response to just 4 weeks of endurance exercise in juvenile (5–9 weeks of age) rats.
The effect of endurance exercise on cardiomyocyte hyperplasia diminishes with age and is lost by adulthood.
We have also established that the effect of juvenile exercise on heart mass is sustained into adulthood.
Abstract
The aim of this study was to investigate if endurance training during juvenile life ‘reprogrammes’ the heart and leads to sustained improvements in the structure, function, and morphology of the adult heart. Male Wistar Kyoto rats were exercise trained 5 days week−1 for 4 weeks in either juvenile (5–9 weeks of age), adolescent (11–15 weeks of age) or adult life (20–24 weeks of age). Juvenile exercise training, when compared to 24‐week‐old sedentary rats, led to sustained increases in left ventricle (LV) mass (+18%; P < 0.05), wall thickness (+11%; P < 0.05), the longitudinal area of binucleated cardiomyocytes (P < 0.05), cardiomyocyte number (+36%; P < 0.05), and doubled the proportion of mononucleated cardiomyocytes (P < 0.05), with a less pronounced effect of exercise during adolescent life. Adult exercise training also increased LV mass (+11%; P < 0.05), wall thickness (+6%; P < 0.05) and the longitudinal area of binucleated cardiomyocytes (P < 0.05), despite no change in cardiomyocyte number or the proportion of mono‐ and binucleated cardiomyocytes. Resting cardiac function, LV chamber dimensions and fibrosis levels were not altered by juvenile or adult exercise training. At 9 weeks of age, juvenile exercise significantly reduced the expression of microRNA‐208b, which is a known regulator of cardiac growth, but this was not sustained to 24 weeks of age. In conclusion, juvenile exercise leads to physiological cardiac hypertrophy that is sustained into adulthood long after exercise training has ceased. Furthermore, this cardiac reprogramming is largely due to a 36% increase in cardiomyocyte number, which results in an additional 20 million cardiomyocytes in adulthood.
Keywords: exercise physiology, cardiac hypertrophy, developmental programming
Key points
Cardiac hypertrophy following endurance‐training is thought to be due to hypertrophy of existing cardiomyocytes.
The benefits of endurance exercise on cardiac hypertrophy are generally thought to be short‐lived and regress to sedentary levels within a few weeks of stopping endurance training.
We have now established that cardiomyocyte hyperplasia also plays a considerable role in cardiac growth in response to just 4 weeks of endurance exercise in juvenile (5–9 weeks of age) rats.
The effect of endurance exercise on cardiomyocyte hyperplasia diminishes with age and is lost by adulthood.
We have also established that the effect of juvenile exercise on heart mass is sustained into adulthood.
Introduction
Programming occurs when environmental factors induce physiological changes on growth and development that have long‐term consequences on an organism. In light of this, it is now well recognized that an adverse fetal environment can lead to cardiovascular adaptations within the fetus, that in turn programme for an increased risk of cardiovascular disease in adulthood. For example, our group, and others, have found that restriction of growth in prenatal life induced by uteroplacental insufficiency or inadequate nutrition adversely impacts on later cardiovascular health including increased rates of hypertension (Vickers et al. 2000; Wlodek et al. 2007, 2008; Black et al. 2012), myocardial insulin resistance (Tsirka et al. 2001) and lower cardiomyocyte number (Corstius et al. 2005; Bensley et al. 2010; Black et al. 2012). Although the adverse cardiac programming effects of a poor in utero environment are now well established, to date little progress has been made relating to factors that can positively programme long‐term cardiovascular health. Importantly, in this regard, findings from recent studies (Waring et al. 2015; Wadley et al. 2016) suggest that enhanced physical activity in early life may have the potential to lead to long‐term cardiovascular benefits.
There have been many studies demonstrating beneficial impacts of exercise on the structure and function of the heart, resulting in physiological cardiac hypertrophy (Hickson et al. 1979, 1983; Kemi et al. 2004; Bocalini et al. 2010). However, up until recently, it was considered that these beneficial effects were only temporary and lost several weeks after training was ceased (Hickson et al. 1979, 1983; Kemi et al. 2004; Bocalini et al. 2010). Notably, the exercise interventions in the majority of these studies were conducted in adulthood (Hickson et al. 1979, 1983; Kemi et al. 2004; Bocalini et al. 2010). In contrast, recent studies (Waring et al. 2015; Wadley et al. 2016) suggest that exercise training specifically during juvenile and perhaps adolescent life may lead to persistent beneficial effects to cardiac structure. This likely relates to the potential to stimulate cardiomyocyte proliferation and/or recruitment of cardiac stem/progenitor cells during this period, but not in adulthood. In support of this idea, our recent studies showed a sustained 10% increase in heart mass of adult rats (24 weeks old) who underwent just 4 weeks of endurance training in juvenile life (5–9 weeks of age) (Wadley et al. 2016). In another study, there was a significant increase in cardiomyocyte number and capillary density in rats 4 weeks after they had completed a vigorous exercise training programme during adolescence, even though the exercise‐induced cardiac hypertrophy had regressed (Waring et al. 2015). Based on these important findings, the aim of the present study was to systematically explore the longer‐term effects of endurance training in rats during specific life course windows on cardiac function and structure including cardiomyocyte growth and gene expression. To address this aim, male rats were examined at a time when exercise training had ceased for a sustained period and they were sedentary. Furthermore, we utilized the techniques of: 2D‐transthoracic echocardiography (TTE) to examine in vivo cardiac structure and function, stereology to estimate cardiomyocyte number, and confocal microscopy combined with image analysis to measure cardiomyocyte size and levels of cardiac fibrosis. We hypothesized that exercise training in juvenile or adolescent life would lead to increases in heart mass, function and cardiomyocyte number that would be sustained into adult life. To investigate the molecular processes responsible for cardiac programming following juvenile exercise, we also investigated the insulin‐like growth factor‐1 (IGF‐1)–Akt pathway, which is a key regulator of physiological cardiac hypertrophy (Bostrom et al. 2010), along with several cardiac enriched microRNA species that are known regulators of cardiomyocyte growth and proliferation (Care et al. 2007; Liu et al. 2015; Ramasamy et al. 2015).
Methods
Ethical approval
All experimental procedures were approved by The University of Melbourne Animal Experimentation Ethics Sub‐Committee (reference no. 1212675) and conducted in accordance with the National Health and Medical Research Council Australian code for the care and use of animals for scientific purposes.
Animals
Male Wistar‐Kyoto (WKY) rats, aged 4 weeks, were obtained from the Australian Resource Centre (Murdoch, WA, Australia). Rats were randomly allocated to four treatment groups: sedentary; juvenile exercise (trained from 5–9 weeks of age); adolescent exercise (trained from 11–15 weeks of age) and adult exercise (trained from 20–24 weeks of age). All four treatment groups were killed at 24 weeks of age. In addition, groups of sedentary and juvenile exercise rats were also killed at 9 weeks of age. A total of 77 animals were used for echocardiography and cardiomyocyte analysis. A further 55 animals were used for enzymatic, protein and RNA analysis. Animal numbers in each group are listed in all table and figure legends. All animals were killed by an overdose of anaesthetic with an intraperitoneal injection of Ilium Xylazil‐20 (30 mg kg−1) and ketamine (100 mg kg−1) (Wlodek et al. 2007).
Training
Exercise training involved treadmill running 5 days per week for 4 weeks for up to one hour per day, as described previously (Laker et al. 2011; Wadley et al. 2016). For the endurance training protocol, running duration progressively increased from 20 up to 60 min, with the treadmill speed set at 15 m min−1 for the first week and 20 m min−1 thereafter (Laker et al. 2011; Wadley et al. 2016). The exercise training protocol was the same for all rats allocated to either the juvenile, adolescent or adult exercise training groups, such that all exercise groups performed the same absolute workload at their specific exercise training periods of life.
Body weights were measured each day (5 days week−1) during the exercise training periods for all animals. Twenty‐four‐hour food intake was measured at 7, 13, 16 and 21 weeks of age. The Comprehensive Lab Animal Monitoring System and the controlling software (CLAMS; Columbus Instruments, Columbus, OH, USA) was used to measure oxygen consumption (), carbon dioxide production (), RER (respiratory exchange ratio) and heat for all rats at 18 weeks of age.
2D‐transthoracic echocardiography (TTE)
At 9 or 24 weeks of age, TTE was performed for all rats 24 h after the last bout of exercise. Rats were anaesthetized using 2.5% isoflurane and spontaneously ventilated via a nose cone. Animals were placed on a heated pad in the semi‐left lateral position with upright tilt, suitable for echocardiographic examination. Pulse rate, oxygen saturation and temperature were continuously monitored. After shaving, sequential examination of the left (LV) and right ventricle (RV) was performed using a Vivid E9 unit equipped with an i13‐L (5.9–14.1 MHz) linear array transducer (GE Healthcare, Waukesha, WI, USA). LV measurements were made in the parasternal long axis (PLAX) and short axis (PSAX) views, and RV measurements in the apical four‐chamber (4C) and aortic short‐axis views (AoSAX). Examination was in accordance with the American Society of Echocardiography Guidelines, ensuring technical accuracy of repeated measurement (Lang et al. 2005). LV morphology was assessed for interventricular septal (IVS) wall and posterior wall (PW) thickness, LV internal diameter at diastole (LVIDd), LV internal diameter at systole (LVIDs), LV end‐diastolic chamber area (LVAd), LV end‐systolic chamber area (LVAs) and relative wall thickness (RWT = 2PWD/LVIDd). The right ventricle (RV) was similarly examined for chamber size and wall thickness. Cardiac systolic function was quantified by estimation of fractional shortening (FS = LVIDd‐LVIDs) and fractional area change (FAC = LVAd‐LVAs). Ejection fraction (EF) was measured at the LV and RV outflow tracts (LVOT and RVOT respectively) via estimation of stoke volume using pulsed wave Doppler (Teichholz et al. 1976; Quinones et al. 1981).
Tissue preparation
Twenty‐four hours after echocardiography was performed, 9‐ or 24‐week‐old rats were killed and hearts collected. Hearts were either perfusion‐fixed for histological analysis or frozen in liquid nitrogen for RNA and protein analysis. Perfusion fixation was performed as previously described (Stacy et al. 2009; Black et al. 2012; Master et al. 2014) in a retrograde fashion via the abdominal aorta with 4% paraformaldehyde in 0.1 mol l−1 phosphate buffer. Once fixation was complete, the heart was excised, weighed and stored in 10% Neutral Buffer Formalin (NBF) until later stereological analysis (Stacy et al. 2009; Bensley et al. 2010; Black et al. 2012; Master et al. 2014). In rats that were not subject to perfusion fixation, hindlimb muscles (gastrocnemius, soleus and extensor digitorum longus), dorsal fat and liver were rapidly excised and weighed. Since the main focus of the study was on the effects of juvenile exercise, only the sedentary, juvenile exercise and adult exercise groups were further assessed for RNA and protein analysis. For the RNA and protein analysis, whole heart was rapidly excised, washed with cold saline and weighed. The LV was then separated and rapidly frozen in liquid nitrogen. The LV was crushed into a powder in liquid nitrogen and then stored at −80°C for later analysis (Master et al. 2014; Wadley et al. 2016).
Stereological analysis
All tissue blocks were coded by an independent investigator so that the stereological and image analyses were performed in a blinded manner, to remove any bias during analyses. The total number of cardiomyocyte nuclei in the LV of each heart was determined using an optical dissector/fractionator approach in the Haematoxylin‐stained glycolmethacrylate embedded sections as previously published (Corstius et al. 2005; Stacy et al. 2009; Bensley et al. 2010). This well‐established approach is currently among the best available to estimate cardiomyocyte number, as the technique involves unbiased sampling of the entire ventricular wall, and the cardiomyocyte counts are not influenced by tissue swelling or shrinkage during processing. The cardiomyocyte nuclei are easily distinguished from the other cell type nuclei, due to their distinctive large oval‐shaped, lightly staining nuclei. An Olympus BX51 microscope fitted with a motorized stage (Olympus, Tokyo, Shinjuku‐ku, Japan) was employed and counting of cardiomyocyte nuclei was performed using the Computer Aided Stereological Toolbox stereological program (Olympus, Alberstand, Denmark).
Confocal microscopy
Paraffin embedded sections were incubated overnight in a solution of wheat germ agglutinin‐Alexa Fluor 488 (Invitrogen, Melbourne, VIC, Australia) to stain cell membranes and 4ʹ6‐diamidino‐2‐phenylindole, dihydrochloride (DAPI) to stain cell nuclei. The proportion of mononucleated and binucleated cardiomyocytes in the LV was determined in the 40 μm fluorescently labelled paraffin sections using confocal microscopy (Lim et al. 2010; Black et al. 2012; Master et al. 2014). Utilizing three‐dimensional software (Imaris Version 6.1/6.2, Bitplane, AG, Zurich, Switzerland), the number of nuclei within at least 200 cardiomyocytes in each heart were examined. The total number of cardiomyocytes within the LV was subsequently determined from the initial cardiomyocyte nuclei counts by adjusting for the relative proportions of mononucleated and multinucleated cardiomyocytes. The cell boundaries of cardiomyocytes, were traced for assessment of longitudinal and cross‐sectional area (NIS‐Elements, Nikon, Kawasaki, Kanagawa, Japan).
Assessment of cardiac fibrosis
Paraffin‐embedded tissues were used to assess the levels of fibrosis (interstitial, perivascular and reparative) within the myocardium and viewed under 20× magnification and the proportion of interstitial and perivascular collagen was quantified using image analysis, as previously described (Bensley et al. 2010; Black et al. 2012; Master et al. 2014).
Enzyme activity and immunoblotting
For enzyme activity and immunoblotting, approximately 30 mg of tissue was homogenized as previously described (Wadley et al. 2016) in ice‐cold lysis buffer (20 μL buffer (mg muscle)−1; 50 mmol L−1 Tris at pH 7.5 containing 1 mmol L−1 EDTA, 10% vol/vol glycerol, 1% vol/vol Triton X‐100, 50 mmol L−1 NaF, 5 mmol L−1 Na4P2O7, 1 mmol L−1 DTT, 1 mmol L−1 PMSF, and 5 μl mL−1 protease inhibitor cocktail (P8340; Sigma, St Louis, MO, USA). Tissue lysates were then incubated on ice for 20 min and then spun at 10,000 g for 20 min at 4°C. The protein concentration of the homogenate was determined using the bicinchoninic acid protein assay (Pierce, Rockford, IL, USA) and BSA was used as the standard (Wadley et al. 2016).
Cytochrome c oxidase (COX) activity was assayed at room temperature by measuring the decrease in absorbance at 550 nm corresponding to the oxidation of ferrocytochrome c by COX using a commercially available kit (Sigma) (Wadley & McConell, 2007).
Total lysates were solubilized in Laemmli sample buffer. Equal amounts of total protein (30 μg) were separated on TGX stain‐free gels (Bio‐Rad Laboratories, NSW, Australia) and electrotransfer of proteins from the gel to PVDF membranes was performed as previously described (Wadley et al. 2016). The mouse monoclonal antibody for phospho‐Akt Ser473 (pSer473 Akt), rabbit polyclonal antibodies for Akt, phospho‐SRF Ser103 (pSer103 SRF) and SRF were all from Cell Signalling Technology (Herts, UK). The rabbit polyclonal Rhoa antibody was from Santa Cruz (Santa Cruz Biotechnologies, Inc., Dallas, TX, USA). Binding was detected with IRDye 680‐conjugated anti‐mouse IgG (Molecular Probes, Eugene, OR, USA) or IRDye 800‐conjugated anti‐rabbit IgG (Rockland, Gilbertsville, PA, USA) fluorescent antibodies via infrared detection (Odyssey Imaging system, LI‐COR Biosciences, Lincoln, NE, USA). Samples were normalized to the total protein content of each well using stain‐free imaging. A two point standard curve (e.g. 20 μg and 40 μg of total protein from one sample) was run on every membrane to confirm that a 100% increase in protein loaded resulted in a 100% increase in signal intensity for all proteins of interest (Wadley et al. 2016).
Real‐time RT‐PCR
Total RNA was extracted from frozen heart with Trizol and silica‐membrane RNeasy spin columns and DNase on‐column digestion (Qiagen miRNeasy kit), as described previously (Wadley et al. 2016). RNA integrity was verified and concentration determined spectrophotometrically (Nanodrop 1000 Spectrophotometer, Thermo Fisher Scientific, MA, USA). To measure mRNA levels, first‐strand cDNA was generated from 1 μg RNA using a commercially available kit (Applied Biosystems, Carlsbad, CA, USA). Real‐time PCR using SYBR Green chemistry was carried out using a Stratagene MX3000 thermal cycler as previously described (Wadley et al. 2016). Primer sequences are given in Table 1. Cebpb was measured using the TaqMan Gene Expression Assay (Rn00824635_s1, Applied Biosystems, Melbourne, VIC, Australia). Gene expression data was normalized to the expression of the ‘housekeeping’ gene, ribosomal 18 s (r18s).
Table 1.
Rat primers and sequences for real‐time RT‐PCR
| Primers | ||
|---|---|---|
| Gene | Forward 5′–3′ | Reverse 5′–3′ |
| Actb | GACGGAATTTACGGCTCAACAT | AATTAGGAAAGTTGAGCCAATAATTACG |
| Bcl‐2 | GAGGGGCTACGAGTGGGATA | CGGTAGCGACGAGAGAAGTC |
| Bax | GCTGCAGAGGATGATTGCTGA | TAGAAAAGGGCAACCACCCG |
| Foxo1 | AAGAGTTAGTGAGCAGGCAAC | CAGAGCACAGGCAGTACACA |
| IGF‐1 | CAGTTCGTGTGTGGACCAAG | TCAGCGGAGCACAGTACATC |
| Gata4 | CTCTTCGTCCTTCGCTGGAG | GACTGGTCTCGAACACCCTG |
| Myh6 | AAGAGTGACAGGATGACGGA | GTCACCGTCTTGCCGTTTTC |
| Nkx2.5 | CCTCGGATTTCACACCCACA | CCGAGGCATCAGGTTAGGTC |
| Nppa | GAAGCGGGGGCGGCACTTAG | TTCGGTACCGGAAGCTGTTGCA |
| Nppb | AACAATCCACGATGCAGAAGC | GGCGCTGTCTTGAGACCTAA |
| Rhoa | GGTTTATGTGCCCACGGTGT | TACCGGCTCCTGCTTCATTTT |
| r18s | GCATGGCCGTTCTTAGTTGG | TGCCAGAGTCTCGTTCGTTA |
| miRNA analysis | |
|---|---|
| Assay name | Sequence |
| U6 snRNA | GTGCTCGCTTCGGCAGCACATATACTAAAATTGGAACGATACAGAGAAGATTAGCATGGCCCCTGCGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTT |
| snoRNA234 | CTTTTGGAACTGAATCTAAGTGATTTAACAAAAATTCGTCACTACCACTGAGA |
| miR‐1 | UGGAAUGUAAAGAAGUAUGUAU |
| miR‐30e | UGUAAACAUCCUUGACUGGAAG |
| miR‐99b | CACCCGUAGAACCGACCUUGCG |
| miR‐125b | ACAAGUCAGGCUCUUGGGACCU |
| miR‐133b | UUUGGUCCCCUUCAACCAGCUA |
| miR‐204 | UUCCCUUUGUCAUCCUAUGCCU |
| miR‐208b | AAGCUUUUUGCUCGCGUUAU |
| miR‐222 | CUCAGUAGCCAGUGUAGAUCCU |
This table presents the genes and miRNAs of interest with probe sequences and assay numbers. Actb, beta‐actin; Foxo1, Forkhead Box O1; Gata4, GATA transcription factor 4; Myh6, myosin heavy chain 6; Nkx2.5, Nk2 homeobox 5; Nppa, natriuretic peptide A; Nppb, brain natriuretic peptide; r18s, ribosomal 18 s; Rhoa, Ras homolog gene family, member A; Bcl2, Bcl2‐associated X protein; Bax, B‐cell lymphoma 2; IGF‐1, insulin‐like growth factor 1.
The cardiac enriched miRNA species miR‐1, miR‐30e, miR‐99b, miR‐125b, miR‐133b, miR‐204, miR‐208b and miR‐222 were chosen based on their established roles in the regulation of cardiac hypertrophy and proliferation (Eulalio et al. 2012; Sirish et al. 2012; Liang et al. 2015; Tian et al. 2015) and their differential expression following exercise‐induced cardiac hypertrophy (Care et al. 2007; Fernandes et al. 2011; Liu et al. 2015; Ramasamy et al. 2015). For miRNA analysis, RNA was reverse transcribed using the Taqman microRNA Reverse Transcription kit (Applied Biosystems, Melbourne, VIC, Australia). miRNA‐specific primers are given in Table 1. Real‐time PCR using Taqman chemistry was carried out using a Stratagene MX3000 thermal cycler. sno234 and U6 were used as the endogenous controls for the samples at 9 weeks and 24 weeks of age, respectively.
Statistical analysis
All data are presented as means ± SD and were analysed using GraphPad Prism 6.01 (GraphPad Software, Inc. La Jolla, CA, USA). The statistical significance was set at P < 0.05. Analysis of 9‐week‐old rats (sedentary and juvenile exercise groups) was compared using a Student's unpaired t test. Analysis of 24‐week‐old rats (sedentary, juvenile exercise, adolescent exercise and adult exercise) was compared using one‐way ANOVA. If this revealed significance, differences between treatment groups were located with Newman‐Keuls post hoc analysis.
Results
Body weight, and growth, respiratory measurements and spontaneous physical activity levels are not altered with juvenile exercise training
There were no significant differences in body weight following juvenile and adult endurance exercise training at any age compared to the sedentary groups (Table 2). At 24 weeks of age the adolescent exercise group had a ∼3% higher body weight compared to all other groups (P < 0.05, Table 2). Measurements for 24 h food intake did not reveal any significant differences for any of the treatment groups at any age (data not shown).
Table 2.
Body weight, respiratory and physical activity analysis
| Sedentary | Juvenile exercise | Adolescent exercise | Adult exercise | ||
|---|---|---|---|---|---|
| Body weight (g) | |||||
| At week age 7 | 181 ± 15 | 177 ± 18 | 194 ± 26 | 199 ± 23 | |
| At week age 13 | 316 ± 18 | 316 ± 15 | 314 ± 27 | 323 ± 19 | |
| At week age 16 | 351 ± 21 | 363 ± 14 | 364 ± 27 | 356 ± 22 | |
| At week age 18 | 370 ± 19 | 379 ± 17 | 384 ± 27 | 372 ± 22 | |
| At week age 21 | 390 ± 21 | 396 ± 12 | 409 ± 21 | 393 ± 27 | |
| At week age 24 | 415 ± 19 | 414 ± 22 | 428 ± 21* | 409 ± 19 | |
| Respiratory measurements | |||||
| (ml kg h−1) | Light cycle | 1065 ± 305 | 1098 ± 105 | 1145 ± 114 | 1098 ± 158 |
| Dark cycle | 1263 ± 87 | 1310 ± 92 | 1385 ± 112 | 1296 ± 147 | |
| (ml kg h−1) | Light cycle | 1003 ± 93 | 1022 ± 111 | 1087 ± 114 | 1024 ± 171 |
| Dark cycle | 1222 ± 67 | 1270 ± 87 | 1357 ± 97 | 1256 ± 150 | |
| RER (/) | Light cycle | 0.94 ± 0.02 | 0.93 ± 0.02 | 0.95 ± 0.02 | 0.92 ± 0.03 |
| Dark cycle | 0.97 ± 0.02 | 0.97 ± 0.01 | 0.98 ± 0.02 | 0.97 ± 0.02 | |
| Heat (kcal h−1) | Light cycle | 1.90 ± 0.22 | 2.06 ± 0.19 | 2.17 ± 0.17* | 2.00 ± 0.25 |
| Dark cycle | 2.27 ± 0.18 | 2.48 ± 0.14* | 2.65 ± 0.17* | 2.38 ± 0.22 | |
| Physical activity | |||||
| X‐TOT (counts) | Light cycle | 27.4 ± 8.2 | 28.5 ± 7.8 | 27.3 ± 10.0 | 26.1 ± 7.4 |
| Dark cycle | 42.8 ± 10.4 | 47.8 ± 13.1 | 48.9 ± 12.2 | 43.3 ± 12.4 | |
| X‐Ambulatory (counts) | Light cycle | 7.3 ± 2.0 | 7.2 ± 2.0 | 7.2 ± 2.1 | 7.3 ± 2.3 |
| Dark cycle | 12.5 ± 3.2 | 13.5 ± 3.6 | 14.5 ± 3.8 | 12.8 ± 4.2 | |
| Z‐TOT (counts) | Light cycle | 9.7 ± 3.3 | 10.8 ± 3.7 | 9.7 ± 3.5 | 9.9 ± 2.8 |
| Dark cycle | 21.0 ± 4.9 | 26.0 ± 9.2 | 25.2 ± 6.6 | 21.1 ± 5.5 | |
Body weight measurements were recorded for sedentary (n = 20), juvenile exercise (n = 22), adolescent exercise (n = 14) and adult exercise (n = 13) rats on daily basis. Oxygen consumption (), carbon dioxide production (), and respiratory exchange ratio (RER) were measured at 18 weeks of age (in 9–14 rats per group) as no groups were doing any exercise during this period. During the light and dark cycles, there was no significant difference in or in the juvenile exercise or adult exercise groups compared to the sedentary groups. was significantly higher only in the adolescence exercise group during the dark cycle. RER remained unchanged throughout the 24 h period, regardless of light–dark cycle. Heat production was significantly higher for the juvenile and adolescent exercise groups in the dark cycle compared to other groups. Heat production during the light cycle was significantly higher in the adolescent exercise group compared to the other groups. Total activity during the light and dark cycles was also assessed with infra‐red beams. There were no significant differences between the experimental groups for total activity (X‐beam), ambulation (X‐ambulatory) and rearing (Z‐beam). Data presented as means ± SD. * P < 0.05 vs. sedentary.
Heat production was significantly higher in the juvenile and adolescence exercise groups during the dark cycle compared to sedentary and adult rats (Table 2). However, spontaneous physical activity levels were not significantly different between any of the groups in either dark or light cycles (Table 2).
2D‐transthoracic echocardiography
Juvenile exercise increases left ventricle mass and wall thickness
At 9 weeks of age, when compared to sedentary rats, juvenile exercise significantly increased the LV mass by 25% (Fig. 1 A). Posterior wall thickness (PWT), relative wall thickness (RWT) and septal thickness significantly increased by 17%, 11% and 7%, respectively (Fig. 1 B–D). When measured at 24 weeks of age, the LV mass and wall thicknesses of rats who completed juvenile exercise was still significantly higher by ∼18% (Fig. 1 A–D). Rats who completed adolescent or adult exercise also had significantly increased, by 10–20%, LV mass and wall thicknesses (Fig. 1 A–D) compared to the sedentary group.
Figure 1. Juvenile and adolescent exercise training leads to sustained increases in left ventricle (LV) mass (A) and wall thicknesses (B–D) in the adult heart.
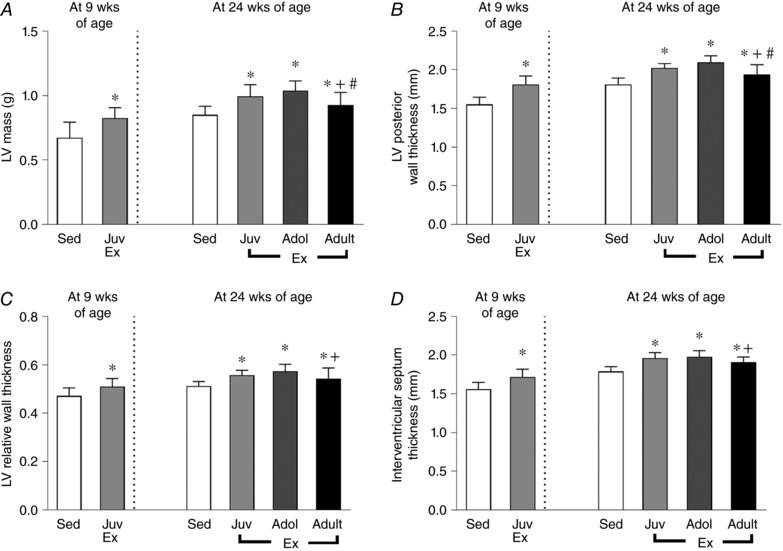
Short‐term (4 weeks) endurance training was completed during juvenile (Juv Ex, 5–9 weeks old), adolescent (Adol, 11–15 weeks old) and adult (Adult Ex, 20–24 weeks old) life. LV mass and wall thicknesses measured with 2D‐transthoracic echocardiography. Data presented as means ± SD. 9‐week‐old rats: sedentary n = 15, juvenile n = 15. 24‐week‐old rats: sedentary n = 9, juvenile n = 11, adolescent n = 14, adult n = 13. Significant differences are indicated: * P < 0.05 vs. sedentary, # P < 0.05 vs.juveniles, + P < 0.05 vs. adolescents. 9‐week‐old rats were compared using an unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis.
Heart function, LV chamber dimensions and fibrosis levels are normal with no adverse effects with exercise training
When compared to sedentary rats, juvenile exercise significantly increased LV internal diameter at diastole (LVIDd) and systole (LVIDs) by 7% and 10%, respectively at 9 weeks of age (Table 3). At 24 weeks of age, the LVIDs was 12% higher for the juvenile exercise training group (Table 3, P < 0.05) whereas the LVIDd was not significantly different between other exercise groups (Table 3). There was no significant difference in collagen content of the left ventricular myocardium in all treatment groups (Table 3). Echocardiographic measurements for the right ventricle did not reveal any significant changes in the structure or function of the right ventricle in any of the experimental groups at 9 or 24 weeks of age (Table 4).
Table 3.
LV 2D‐Transthoracic echocardiography and fibrosis measurements
| 9 weeks old | 24 weeks old | |||||
|---|---|---|---|---|---|---|
| Sedentary | Juvenile exercise | Sedentary | Juvenile exercise | Adolescent exercise | Adult exercise | |
| LV/body weight ratio (mg g−1) | 2.94 ± 0.46 | 3.87 ± 0.34* | 2.14 ± 0.18 | 2.36 ± 0.16* | 2.43 ± 0.14* | 2.32 ± 0.19* |
| LVIDd (mm) | 6.62 ± 0.62 | 7.01 ± 0.36* | 7.03 ± 0.26 | 7.21 ± 0.28 | 7.28 ± 0.24 | 7.09 ± 0.51 |
| LVIDs (mm) | 3.27 ± 0.45 | 3.61 ± 0.33* | 3.43 ± 0.35 | 3.86 ± 0.20* | 3.74 ± 0.32 | 3.58 ± 0.26 |
| LVAd (mm) | 3.79 ± 0.74 | 3.96 ± 0.43 | 4.52 ± 0.61 | 4.84 ± 0.77 | 4.73 ± 0.70 | 4.62 ± 0.69 |
| LVAs (mm) | 0.87 ± 0.29 | 0.90 ± 0.16 | 1.22 ± 0.30 | 1.27 ± 0.28 | 1.36 ± 0.46 | 1.29 ± 0.21 |
| FAC (%) | 77 ± 4 | 77 ± 3 | 73 ± 4 | 75 ± 3 | 75 ± 5 | 72 ± 3 |
| FS (%) | 51 ± 6 | 48 ± 4 | 52 ± 3 | 46 ± 2* | 48 ± 4 | 50 ± 3 |
| EF (%) | 86 ± 4 | 83 ± 3 | 87 ± 3 | 83 ± 2 | 84 ± 4 | 85 ± 2 |
| Interstitial/reparative fibrosis (% collagen) | NA | NA | 1.84 ± 0.61 | 1.56 ± 0.23 | 1.49 ± 0.52 | 1.85 ± 0.59 |
| Perivascular fibrosis ratio of Adventitia:lumen | NA | NA | 0.27 ± 0.12 | 0.37 ± 0.13 | 0.34 ± 0.09 | 0.29 ± 0.10 |
LV, left ventricle; LVIDd and LVIDs, LV internal diameter at diastole and systole; LVAd and LVAs, mid‐papillary LV area at diastole and systole; FAC, fractional area change; FS, fractional shortening; EF, ejection fraction; NA, not assessed. Data presented as means ± SD. For echocardiography: 9‐week‐old rats, sedentary n = 15, juvenile n = 15; 24‐week‐old rats, sedentary n = 9, juvenile n = 11, adolescent n = 14, adult n = 13. For fibrosis, n = 8 per group. 9‐week‐old rats were compared using a Student's unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis. * P < 0.05 vs. sedentary.
Table 4.
2D‐Echocardiography of right ventricle
| 9 weeks old | 24 weeks old | |||||
|---|---|---|---|---|---|---|
| Sedentary | Juvenile exercise | Sedentary | Juvenile exercise | Adolescent exercise | Adult exercise | |
| RVEDA (mm) | 3.86 ± 0.63 | 3.80 ± 0.43 | 4.60 ± 0.63 | 4.28 ± 1.02 | 4.38 ± 0.82 | 4.44 ± 0.75 |
| RVFAC (%) | 0.57 ± 0.07 | 0.57 ± 0.07 | 0.53 ± 0.11 | 0.53 ± 0.10 | 0.57 ± 0.08 | 0.55 ± 0.10 |
| RVWT (mm) | 0.45 ± 0.04 | 0.48 ± 0.04 | 0.60 ± 0.10 | 0.59 ± 0.11 | 0.55 ± 0.07 | 0.57 ± 0.11 |
| PA (mm) | 2.37 ± 0.26 | 2.41 ± 0.09 | 2.76 ± 0.45 | 2.69 ± 0.30 | 2.56 ± 0.09 | 2.56 ± 0.17 |
| PAAT (ms) | 28.3 ± 4.8 | 29.1 ± 5.0 | 29.7 ± 2.8 | 29.7 ± 2.9 | 30.6 ± 3.9 | 30.7 ± 3.2 |
Examination the right ventricle (RV) did not reveal any significant change in structure or function for any of the experimental groups both in 9‐week‐old and 24‐week‐old hearts. RVEDA, RV end‐diastolic area; RVFAC, RV fractional area change; PA, pulmonary artery diameter; RVWT, RV free wall thickness; PAAT, pulmonary valve flow acceleration time. 9‐week‐old rats: sedentary n = 15, juvenile n = 15. 24‐week‐old rats: sedentary n = 9, juvenile n = 11, adolescent n = 14, adult n = 13. Data presented as means ± SD.
Juvenile exercise training did not significantly alter resting cardiac function in rats at 9 weeks of age as assessed by fractional shortening (FS), fractional area change (FAC) or ejection fraction (EF) (Table 3). At 24 weeks of age, FAC and EF were also not significantly different between any of the exercise treatment groups (Table 3). However, juvenile exercise significantly reduced FS by ∼6% compared to the sedentary rats (Table 3), although this is still considered to be within the normal range (Waring et al. 2012).
Postmortem tissue weights and heart protein content
Dorsal body fat, liver and hindlimb muscle mass were not significantly different between the exercise and sedentary groups at any age (Table 5). Consistent with our previous study (Wadley et al. 2016), only adult exercise significantly increased total protein content in the heart.
Table 5.
Postmortem weight of tissues and heart protein content and COX activity
| 9 weeks old | 24 weeks old | ||||
|---|---|---|---|---|---|
| Sedentary | Juvenile exercise | Sedentary | Juvenile exercise | Adult exercise | |
| Heart protein content (% wet weight) | 16.2 ± 1.3 | 16.1 ± 1.8 | 16.2 ± 1.2 | 16.5 ± 0.8 | 17.7 ± 1.5*, # |
| Heart COX activity (μmol min (g protein)−1) | 532 ± 223 | 822 ± 235* | 445 ± 222 | 463 ± 117 | 649 ± 169*, # |
| Hindlimb muscle weight (g) | 1.95 ± 0.16 | 1.88 ± 0.16 | 3.73 ± 0.30 | 3.85 ± 0.18 | 3.72 ± 0.23 |
| Dorsal fat (g) | 0.86 ± 0.17 | 0.74 ± 0.25 | 4.97 ± 0.46 | 4.32 ± 0.52 | 4.74 ± 0.77 |
| Dorsal fat/body weight (ratio) | 0.41 ± 0.17 | 0.40 ± 0.13 | 1.22 ± 0.42 | 1.04 ± 0.37 | 1.27 ± 0.21 |
| Liver weight (g) | 6.59 ± 0.58 | 6.52 ± 0.50 | 9.97 ± 0.49 | 10.68 ± 0.74 | 10.26 ± 0.75 |
| Liver/body weight (ratio) | 3.48 ± 0.40 | 3.55 ± 0.28 | 2.48 ± 0.84 | 2.85 ± 0.16 | 2.75 ± 0.13 |
Hindlimb muscle weight is the combined weight of the soleus, EDL and gastrocnemius. Data presented as means ± SD (n = 11 per group). 9‐week‐old rats were compared using an unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis. * P < 0.05 vs. Sedentary; # P < 0.05 vs. Juvenile exercise.
Cytochrome c oxidase (COX) activity was measured as a marker of mitochondrial protein content. Compared to sedentary rats, COX activity was significantly higher in juvenile exercised rats at 9 weeks of age and adult exercised rats at 24 weeks of age (Table 5). Juvenile exercise did not lead to sustained increases in COX activity at 24 weeks of age (Table 5).
Juvenile exercise leads to a sustained increase in the number and nuclearity of cardiomyocytes in the heart
At 9 weeks of age, juvenile exercise increased LV cardiomyocyte number by 40% compared to sedentary rats (Fig. 2 A) and this increase was sustained to 24 weeks of age (36% higher, P < 0.05; Fig. 2 A) when compared to rats that remained sedentary throughout life. LV cardiomyocyte number was not significantly different in the adolescent exercise group compared to the sedentary or the adult exercise groups (Fig. 2 A). In the juvenile exercise group there was also a sustained increase in the proportion of mononucleated cardiomyocytes and a concomitant reduction in the proportion of binucleated cardiomyocytes compared to the other experimental groups. At 9 weeks of age, the hearts of juvenile exercise rats contained double the proportion of mononucleated cardiomyocytes compared to the sedentary heart (14% vs. 8%, respectively, Fig. 2 B). Accordingly, the proportion of binucleated cardiomyocytes in the 9‐week‐old juvenile exercise hearts was significantly lower (86% vs. 92% respectively, Fig. 2 C) compared to the sedentary group. At 24 weeks of age, the proportion of binucleated cardiomyocytes in the juvenile exercise group remained significantly lower than the sedentary group (89% vs. 94% respectively, Fig. 2 C) and accordingly, the proportion of mononucleated cardiomyocytes in the juvenile exercise heart was significantly higher compared to the sedentary group (11% vs. 6%, respectively, P < 0.05, Fig. 2 B). In absolute terms, juvenile exercise resulted in an additional ∼4.5 million mononucleated and 16.0 million binucleated cardiomyocytes, respectively at 24 weeks of age. Exercise during adolescence also increased the proportion of mononucleated cardiomyocytes compared to the sedentary (9% vs. 6%, respectively, P < 0.05, Fig. 2 B) and adult exercise groups, but not to the same extent as juvenile exercise. Furthermore, adult exercise did not alter the proportion of mononucleated or binucleated cardiomyocytes compared with the sedentary controls (Fig. 2 B and C).
Figure 2. Juvenile exercise training leads to sustained increases in cardiomyocyte number (A) and proportion of mononucleated cardiomyocytes (B) in the adult heart, with a less pronounced effect of exercise during adolescent life, and a concomitant reduction in the proportion of binucleated cardiomyocytes (C).
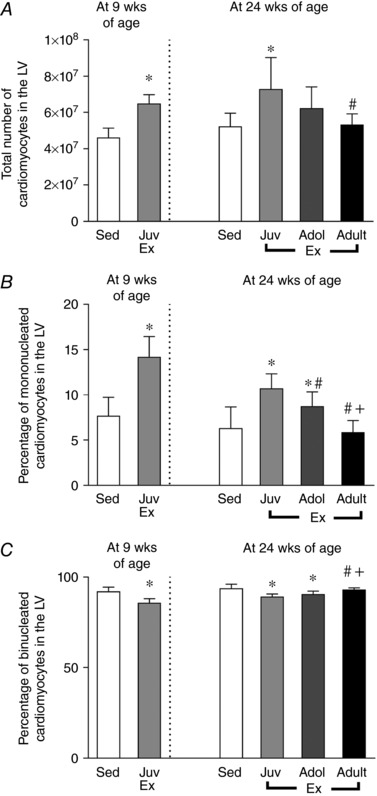
Short‐term (4 weeks) endurance training during juvenile (Juv Ex, 5–9 weeks old), adolescent (Adol, 11–15 weeks old) and adult (Adult Ex, 20–24 weeks old) life. Data presented as means ± SD. n = 8 per group. 9‐week‐old rats were compared using an unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis. Significant differences are indicated: * P < 0.05 vs. sedentary, # P < 0.05 vs. juveniles, + P < 0.05 vs. adolescents.
Juvenile exercise leads to sustained cardiomyocyte hypertrophy
Following classification according to nuclearity, cardiomyocyte hypertrophy of mononucleated and binucleated cardiomyocytes was assessed according to longitudinal area. At 9 weeks of age, juvenile exercise significantly increased the longitudinal area of binucleated, but not mononucleated cardiomyocytes when compared to the sedentary rats (Fig. 3 A and B). Furthermore, the increase in longitudinal area of binucleated cardiomyocytes following juvenile exercise was sustained into adulthood at 24 weeks of age (P < 0.05, Fig. 3 B). At 24 weeks of age, all of the exercise groups displayed significantly increased longitudinal areas of binucleated cardiomyocytes when compared to the sedentary group (Fig. 3 B). Also, adolescent exercise increased the longitudinal area of mononucleated cardiomyocytes compared to all other groups (Fig. 3 C).
Figure 3. Juvenile exercise training leads to sustained increases in the longitudinal area of binucleated (B), but not mononucleated (A) cardiomyocytes.
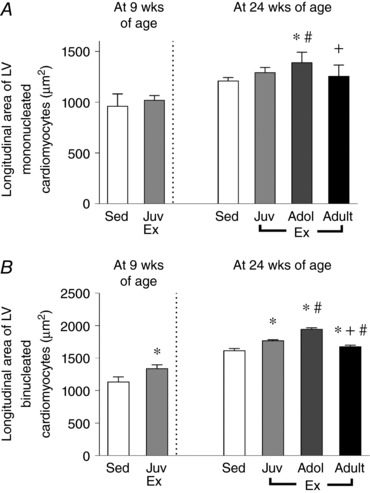
Short‐term (4 weeks) endurance training during juvenile (Juv Ex, 5–9 weeks old), adolescent (Adol, 11–15 weeks old) and adult (Adult Ex, 20–24 weeks old) life. Data presented as means ± SD. n = 8 per group. 9‐week‐old rats were compared using an unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis. Significant differences are indicated: * P < 0.05 vs. sedentary, # P < 0.05 vs. juveniles, + P < 0.05 vs. adolescents.
Juvenile exercise training and miRNA expression
Because several miRNA species are known to regulate cardiac hypertrophy and cardiomyocyte proliferatio, we measured the expression of several muscle enriched miRNA species to investigate if they were differentially expressed by juvenile exercise in the heart. At 9 weeks of age, the expression of miR‐208b was significantly reduced following juvenile exercise training compared to the sedentary group (Fig. 4 A). When compared to sedentary rats, juvenile exercise did not alter the expression of any of the other miRNA species measured such as miR‐1, miR‐133b or miR‐222 (Fig. 4 B–D, respectively) or miR‐30e, miR‐99b, miR‐125b and miR‐204 (data not shown).
Figure 4. At 9 weeks of age, juvenile exercise training significantly reduces the expression of miR‐208b (A), but not miR‐222, miR‐1 or miR‐133b (B–D).
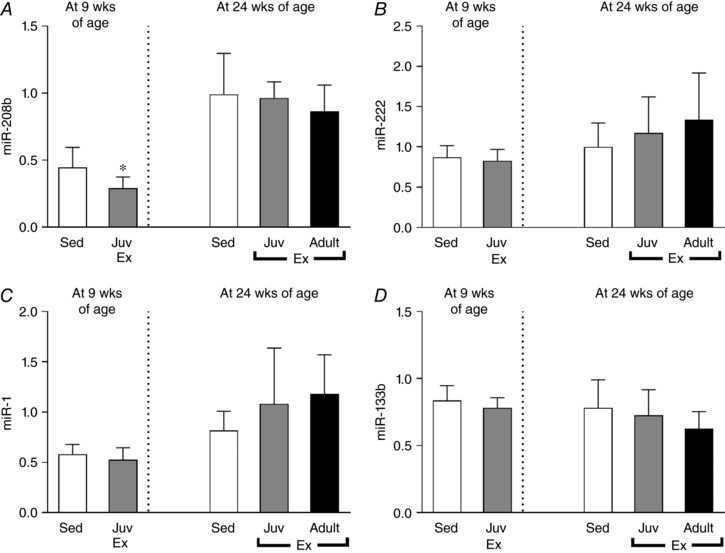
Data presented as means ± SD. n = 11 per group. Short‐term (4 weeks) endurance training during juvenile (Juv Ex, 5–9 weeks old) and adult (Adult Ex, 20–24 weeks old) life. 9‐week‐old rats were compared using an unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis. Significant differences are indicated: * P < 0.05 vs. sedentary.
Neither juvenile nor adult exercise significantly altered the mRNA expression of genes with known functions in cardiac hypertrophy, cardiomyocyte proliferation and/or differentiation (Bostrom et al. 2010) such as Igf‐1, Myh6, Gata4, Nkx2.5, Foxo1 or Cebpb (Fig. 5 A–F, respectively) or genes known to regulate apoptosis such as Bcl‐2 and Bax (Fig. 5 G and H). However, juvenile exercise when measured at 9 weeks of age significantly altered genes known to regulate cardiac hypertrophy with reduced Rhoa and Nppa, and increased Nppb mRNA expression (Fig. 6 A–D, respectively).
Figure 5. Juvenile exercise training does not alter the mRNA expression of genes that regulate cardiac growth or the apoptosis markers Bcl‐2 and Bax .
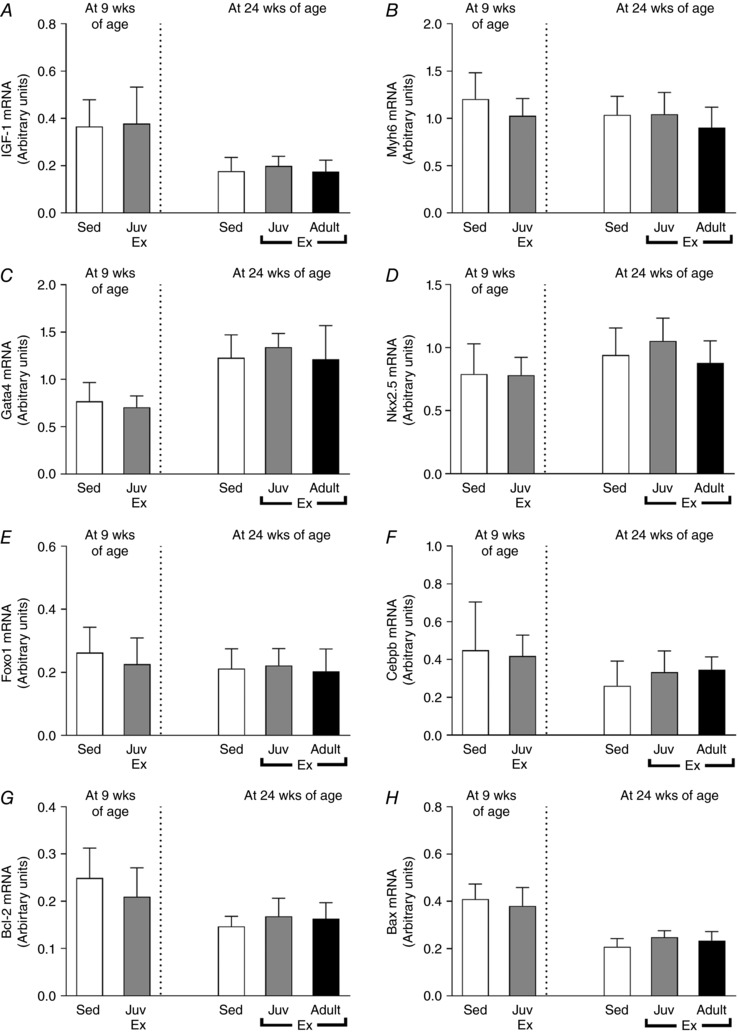
Data presented for the following genes: Igf‐1 (A), Myh6 (B), Gata4 (C), Nkx2.5 (D), Foxo1 (E) and Cebpb (F), Bcl‐2 (G) and Bax (H). Data presented as means ± SD. n = 11 per group. Short‐term (4 weeks) endurance training during juvenile (Juv Ex, 5–9 weeks old) and adult (Adult Ex, 20–24 weeks old) life. 9‐week‐old rats were compared using an unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis.
Figure 6. At 9 weeks of age juvenile exercise training alters the mRNA expression of Rhoa (A), Nppa (C) and Nppb (D), but not Actb (B).
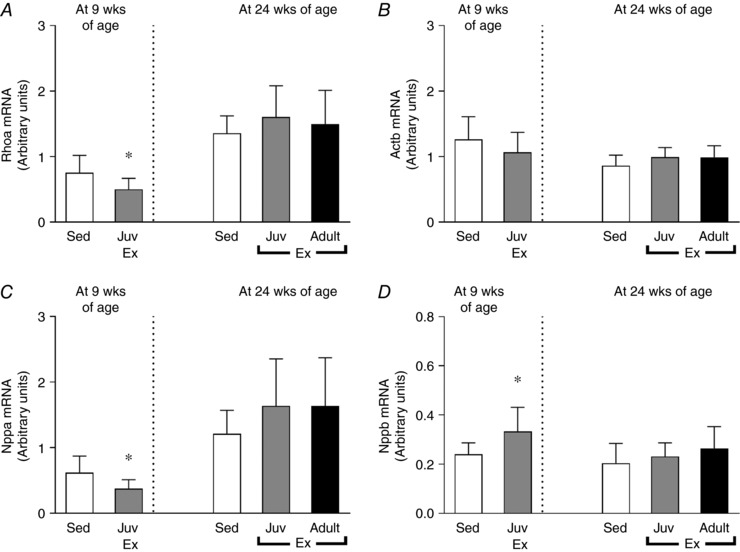
Data presented as means ± SD. n = 11 per group. 9‐week‐old rats were compared using an unpaired t test. 24‐week‐old rats were compared using one‐way ANOVA with Newman‐Keuls post hoc analysis. * P < 0.05 vs. sedentary.
The phosphorylation and protein abundance of Akt and SRF were not significantly altered by juvenile exercise at any age or following adult exercise (Fig. 7), nor was Rhoa protein abundance (Fig. 7).
Figure 7. Neither juvenile nor adult exercise altered the phosphorylation and protein abundance of Akt or SRF or protein abundance of Rhoa.
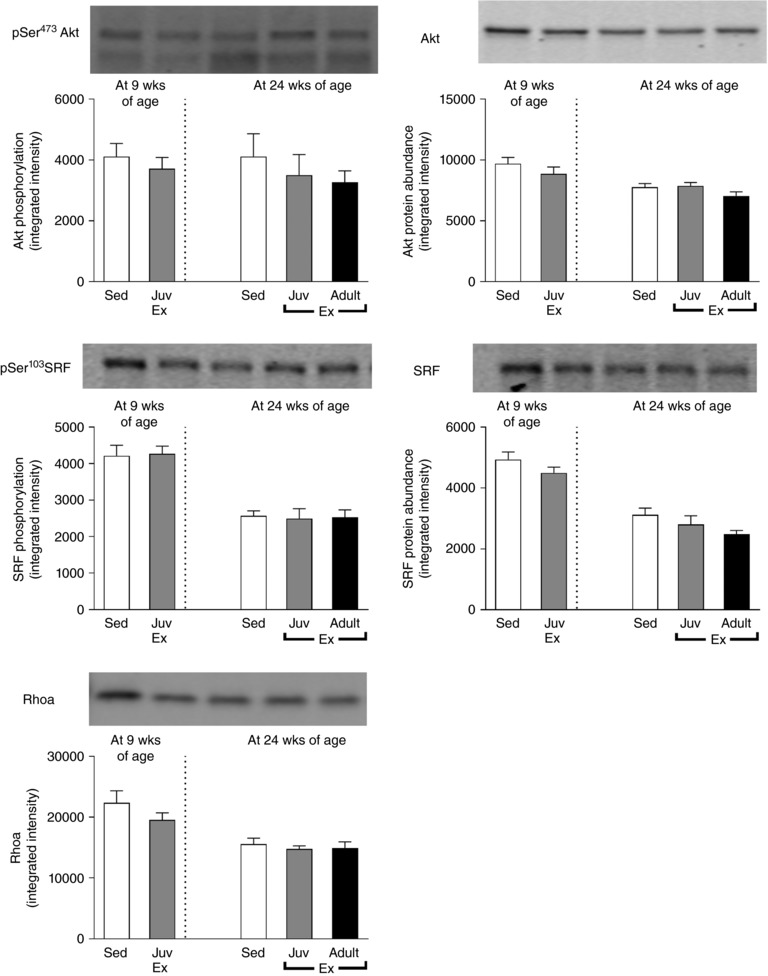
Data presented as means ± SD. n = 9–10 per group.
Discussion
The findings of this study clearly demonstrate that the mammalian heart can be positively programmed by exercise training in juvenile life leading to sustained benefits to cardiac structure and functional reserve, even after exercise training has ceased. In particular, a few weeks of moderate intensity endurance training during juvenile life can lead to sustained and physiologically relevant increases in heart mass, LV wall thickness, cardiomyocyte number and size in adulthood. Also, the ability of endurance training to increase cardiomyocyte number appears to diminish with age, with the effect being completely lost by adulthood.
It is well established that heart mass typically increases following endurance exercise training in humans and in animal models and this is considered to be the result of physiological hypertrophy (Hickson et al. 1979, 1983; Kemi et al. 2004; Bocalini et al. 2010; Arbab‐Zadeh et al. 2014). The findings from the present study support this concept, with an increase in cardiac mass (as a result of concentric hypertrophy) in all the exercise training groups, without any increases in cardiac fibrosis. Unfortunately, however, when exercise programmes are initiated in adulthood the cardiac hypertrophy regresses when exercise training ceases (Hickson et al. 1979, 1983; Kemi et al. 2004; Bocalini et al. 2010). Hence, for long‐term benefits to heart health, the exercise programmes need to be continued long‐term, which many people find difficult. As a consequence, until recently it was generally considered that the beneficial effects of exercise on the heart were temporary, as the cardiac hypertrophy usually regresses back to sedentary levels 2–6 weeks after training has ceased (Hickson et al. 1979, 1983; Kemi et al. 2004; Bocalini et al. 2010). However, our findings do not support this notion and suggest that an exercise intervention programme in rats, when initiated in juvenile life, can have permanent beneficial effects on cardiac structure and cell number. Importantly, in the present study, when the exercise training programme was undertaken in juvenile life (equivalent to childhood or adolescence in humans) the left ventricle of the heart remained enlarged in adulthood, even though the rats had been sedentary for a prolonged period of time (15 weeks or equivalent to ∼10 years in humans (Quinn, 2005)). Furthermore, although the relationship between the ages of humans and rodents is not linear (Quinn, 2005), it is important to note that a 24‐week‐old rat is well past sexual maturity and, whilst not in senescence, it is considered an adult. Accompanying the increase in left ventricular size there were increases in cardiomyocyte number and size. Hence our findings demonstrate that an early life exercise programme can lead to a sustained increase in cardiac size and in the complement of cardiomyocytes (functional reserve), thus potentially rendering the hearts better equipped for the structural and functional challenges in adult life. Our findings also suggest that a juvenile exercise programme may have the potential to reverse programmed cardiomyocyte deficits such as occurs following intrauterine growth restriction (Corstius et al. 2005; Bensley et al. 2010; Black et al. 2012). To our knowledge, no previous study has reported such sustained changes following endurance training on the heart.
Some studies suggest that adolescence may also be a life‐course period for therapeutic cardiac intervention (Waring et al. 2015). However, the findings from the present study are equivocal in this regard. Waring et al. (2015) previously demonstrated that cardiomyocyte number significantly increased by 7% in adolescent rats following 4 weeks of training at a much higher exercise intensity (80–85% ) than that employed in the present study (∼65% ); an effect Waring et al. (2015) found was sustained by 4 weeks of detraining. Also, in their model, there was regression of the cardiac hypertrophy after a 4 week sedentary period (Waring et al. 2015). Furthermore, although we found no statistical difference in the total number of cardiomyocytes in the rats from the adolescence exercise training group compared with the sedentary controls, there was a significant increase in the proportion of mononucleated cardiomyocytes following adolescent exercise. Collectively, this suggests that the impact of endurance training on the programming of cardiomyocyte number/nuclearity diminishes with age. Nevertheless, in our model, further studies are required to explore the potential benefits of exercise training during adolescence on the complement of cardiomyocytes in the adult heart.
Until recently, the general dogma was that all cardiomyocytes exited the cell cycle in the perinatal period. However, recent studies clearly demonstrate that although the proliferative capacity of cardiomyocytes is markedly reduced after birth, there remains a low level of cardiomyocyte proliferation postnatally up until adulthood (Beltrami et al. 2001; Mollova et al. 2013; Senyo et al. 2013). Hence, cardiomyocyte proliferation likely plays a role in postnatal physiological hypertrophy. Of particular relevance to the present findings, recent studies demonstrate that childhood is the period when the postnatal levels of cardiomyocyte proliferation are highest in the human heart and in animal models (Beltrami et al. 2001; Mollova et al. 2013; Senyo et al. 2013) and thus appear amenable to positive environmental stimuli such as exercise.
Generally in the rat heart, mononucleated cardiomyocytes are considered to be capable of proliferation, whereas binucleated cardiomyocytes are thought to have exited the cell cycle and to be terminally differentiated (Paradis et al. 2014). Hence, in the present study, the significant increase in the proportion of mononucleated cardiomyocytes in the adult heart following the juvenile exercise training programme, and to a lesser extent following the adolescent exercise training programme, support the concept that these early life exercise interventions are able to increase the proportion of proliferating cardiomyocytes within the myocardium. In contrast, this was not the case in the adult exercise group, with no difference in the proportion of mononucleated cardiomyocytes relative to the sedentary group. The increase in the number of mononucleated cardiomyocytes in the hearts of the rats from the juvenile and adolescence exercise training groups may have arisen from the existing cardiomyocyte population or from cardiac stem cells. Reports of an increase in c‐kit positive endogenous cardiac stem‐progenitor cells following endurance exercise training (Waring et al. 2012; Leite et al. 2015) supports the latter.
In addition to the increase in cardiomyocyte number following the juvenile exercise training programme, we also found an increase in cardiomyocyte hypertrophy. However, analysis of longitudinal area suggests that most of the cardiomyocyte hypertrophy occurs in the binucleated cardiomyocytes. Indeed, at 9 weeks of age juvenile exercise only increased the longitudinal area of the binucleated cardiomyocytes. This suggests that the mononucleated cardiomyocytes have a relatively normal size and that even if they are newly formed, they have not undergone hypertrophy. Thus, juvenile exercise training leads to a sustained increase in heart mass and LV wall thickness in adulthood via an increase in the number of mononucleated and binucleated cardiomyocytes and also by hypertrophy of existing binucleated cardiomyocytes.
The present study found resting cardiac function was maintained following juvenile exercise as measured by 2D‐transthoracic echocardiography (TTE). Although TTE is accurate and used widely by the field to assess cardiac structure and function (Watson et al. 2004; Stuckey et al. 2008), magnetic resonance imaging (MRI) techniques, although less widely available, do offer more accurate and reproducible measures of cardiac function (Stuckey et al. 2008; Mads Dam et al. 2015) and may provide further insights in future studies.
In our study, we targeted a number of cardiac enriched miRNAs to examine if they could be playing a role in the sustained increase in cardiomyocyte number following juvenile exercise. This was based on the established roles of miRNA species in the regulation of cardiac hypertrophy (Eulalio et al. 2012; Sirish et al. 2012; Liang et al. 2015; Tian et al. 2015) and their differential expression following exercise‐induced cardiac hypertrophy (Care et al. 2007; Fernandes et al. 2011; Liu et al. 2015; Ramasamy et al. 2015). The present study found miR‐208b was significantly reduced in the larger hearts of juvenile exercised rats at 9 weeks of age. Consistent with this, cardiac miR‐208b levels are known to be downregulated following endurance exercise (Soci et al. 2016). However, it is unlikely miR‐208b is responsible for the sustained increase in cardiomyocyte number since its current known function in the heart is the regulation of myosin heavy chain expression (Soci et al. 2016; Zhou et al. 2017) and myh6 mRNA levels were unaltered in the present study.
We also examined some established molecular pathways of cardiac growth to determine if they could explain the sustained effects of juvenile exercise on cardiac mass. Cardiomyocyte hypertrophy and renewal following endurance training in young adult mice has previously been shown to be dependent on a reduction in the expression of the transcription factor, CCAAT/enhancer‐binding protein beta (Cebpb) which induces downstream induction of the cardiac‐specific transcription factors including Nkx2.5 and Gata4 (Bostrom et al. 2010). This downregulation of cardiac Cebpb following endurance training in mice most likely occurs through the upstream activation of the IGF–Akt–SRF pathway (Bostrom et al. 2010). However, the endurance training protocol used in the present study did not alter any aspects of this pathway including Igf‐1 mRNA expression, Akt and SRF phosphorylation, or mRNA expression of Cebpb, Nkx2.5 or Gata4. Further studies are now required to establish the molecular mechanisms for how juvenile exercise leads to sustained increases in heart mass and cardiomyocyte number.
Overall, our findings suggest that the juvenile period is a life‐course window that can potentially be targeted for exercise training interventions, with benefits to cardiac health that last into young adulthood. In addition to the benefits of exercise on the structure of the heart, evidence in humans is beginning to emerge demonstrating that exercise initiated early in life also leads to persistent benefits associated with lower rates of type 2 diabetes and hypertension in adulthood (Fernandes & Zanesco, 2010). Furthermore, aerobic fitness in adolescent life is independently associated with a lower risk of myocardial infarction (MI) in adult life (Hogstrom et al. 2014). These human studies, combined with the rodent findings from the present study provide a strong rationale to examine if the benefits of juvenile exercise for the adult male rat heart will also translate to female rats and then also to humans. Should our findings translate to humans they would provide support for exercise training programmes in school curricula and suggest that there are long‐term cardiac benefits of exercise training to all children, even if the children do not continue with regular exercise in adulthood. Nevertheless, whilst the effects of endurance training on the heart in the present study are promising, resistance training is likely to have different effects (Spence et al. 2011) and may require investigation.
In conclusion, just 4 weeks of endurance training in juvenile life induces a sustained increase in heart mass in adulthood, increasing LV wall thickness, and maintaining LV chamber size and cardiac function. Furthermore, this increase in heart mass is due, at least in part to a sustained increase in the number of mononucleated and binucleated cardiomyocytes and also to hypertrophy of binucleated cardiomyocytes. Importantly, our findings demonstrate that exercise‐induced cardiac growth is mediated by cardiomyocyte hyperplasia and hypertrophy when the exercise training occurs during juvenile life and by cardiomyocyte hypertrophy alone when initiated in adulthood.
Additional information
Competing interests
None to declare.
Author contributions
All animal exercise and tissue collection experiments were performed in the laboratory of M.E.W. Stereological analysis and confocal microscopy was performed in the laboratory of M.J.B. Enzyme activity, immunoblotting and real‐time RT‐PCR analysis was conducted in the laboratory of G.D.W. Conception and design of the experiment: Y.A., M.J.B., M.E.W. and G.D.W. Acquisition and analysis of data: Y.A and G.D.W. Interpretation of data: Y.A., M.J.B., M.E.W., P.F.S., A.P.R. and G.D.W. Drafting the article and revising it critically for intellectual content: Y.A., M.J.B., M.E.W., P.F.S., A.P.R. and G.D.W. All authors approve the final version of the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was supported by a grant from Deakin University (Deakin University Central Research Grant Scheme: RM29488). Y.A. was supported by a Deakin University International Postgraduate Scholarship.
Translational perspective
Until recently, the number of cardiomyocytes in the mammalian heart was thought to be fixed shortly after birth and exercise‐induced cardiac hypertrophy was only due to hypertrophy of existing cardiomyocytes. We have now established that cardiomyocyte hyperplasia (increased cell number) also plays a considerable role in cardiac growth in response to just 4 weeks of exercise in juvenile (5–9 weeks of age) rats. This effect of exercise on hyperplasia diminishes with age and is lost by adulthood. Also, until now, the benefits of endurance exercise on cardiac hypertrophy was generally thought to be short‐lived and regress to sedentary levels within few weeks of stopping training. However, we have established that the effect of juvenile exercise is sustained well into adulthood. Although endurance exercise is beneficial to heart health at any stage of life, the novel implications of our research for humans is that if children are encouraged to be active early in life then this could augment cardiovascular health later in life, as well as promoting a healthy active lifestyle.
Acknowledgements
The authors would like to thank Monash Micro Imaging Centre.
Edited by: Scott Powers & Anne McArdle
This is an Editor's Choice article from the 15 January 2018 issue.
References
- Arbab‐Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams‐Huet B, Haykowsky MJ & Levine BD (2014). Cardiac remodeling in response to 1 year of intensive endurance training. Circulation 130, 2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Urbanek K, Kajstura J, Yan S‐M, Finato N, Bussani R, Nadal‐Ginard B, Silvestri F, Leri A, Beltrami CA & Anversa P (2001). Evidence that human cardiac myocytes divide after myocardial infarction. N Eng J Med 344, 1750–1757. [DOI] [PubMed] [Google Scholar]
- Bensley JG, Stacy VK, De Matteo R, Harding R & Black MJ (2010). Cardiac remodelling as a result of pre‐term birth: implications for future cardiovascular disease. Eur Heart J 31, 2058–2066. [DOI] [PubMed] [Google Scholar]
- Black MJ, Siebel AL, Gezmish O, Moritz K & Wlodek ME (2012). Normal lactational environment restores cardiomyocyte number after uteroplacental insufficiency: implications for the preterm neonate. Am J Physiol Regul Integr Comp Physiol 302, R1101–R1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocalini DS, Carvalho EV, de Sousa AF, Levy RF & Tucci PJ (2010). Exercise training‐induced enhancement in myocardial mechanics is lost after 2 weeks of detraining in rats. Eur J Appl Physiol 109, 909–914. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A & Spiegelman BM (2010). C/EBPbeta controls exercise‐induced cardiac growth and protects against pathological cardiac remodeling. Cell 143, 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang M‐L, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MVG, Hoydal M, Autore C, Russo MA, Dorn GW, Ellingsen O, Ruiz‐Lozano P, Peterson KL, Croce CM, Peschle C & Condorelli G (2007). MicroRNA‐133 controls cardiac hypertrophy. Nat Med 13, 613–618. [DOI] [PubMed] [Google Scholar]
- Corstius HB, Zimanyi MA, Maka N, Herath T, Thomas W, Van Der Laarse A, Wreford NG & Black MJ (2005). Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res 57, 796–800. [DOI] [PubMed] [Google Scholar]
- Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S & Giacca M (2012). Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381. [DOI] [PubMed] [Google Scholar]
- Fernandes RA & Zanesco A (2010). Early physical activity promotes lower prevalence of chronic diseases in adulthood. Hypertens Res 33, 926–931. [DOI] [PubMed] [Google Scholar]
- Fernandes T, Hashimoto NY, Magalhaes FC, Fernandes FB, Casarini DE, Carmona AK, Krieger JE, Phillips MI & Oliveira EM (2011). Aerobic exercise training‐induced left ventricular hypertrophy involves regulatory MicroRNAs, decreased angiotensin‐converting enzyme‐angiotensin II, and synergistic regulation of angiotensin‐converting enzyme 2‐angiotensin (1‐7). Hypertension 58, 182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson RC, Galassi TM & Dougherty KA (1983). Repeated development and regression of exercise‐induced cardiac hypertrophy in rats. J Appl Physiol (1985) 54, 794–797. [DOI] [PubMed] [Google Scholar]
- Hickson RC, Hammons GT & Holloszy JO (1979). Development and regression of exercise‐induced cardiac hypertrophy in rats. Am J Physiol Heart Circ Physiol 236, H268–H272. [DOI] [PubMed] [Google Scholar]
- Hogstrom G, Nordstrom A & Nordstrom P (2014). High aerobic fitness in late adolescence is associated with a reduced risk of myocardial infarction later in life: a nationwide cohort study in men. Eur Heart J 35, 3133–3140. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Haram PM, Wisloff U & Ellingsen O (2004). Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation 109, 2897–2904. [DOI] [PubMed] [Google Scholar]
- Laker RC, Gallo LA, Wlodek ME, Siebel AL, Wadley GD & McConell GK (2011). Short‐term exercise training early in life restores deficits in pancreatic beta‐cell mass associated with growth restriction in adult male rats. Am J Physiol 301, E931–940. [DOI] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS & Stewart WJ (2005). Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18, 1440–1463. [DOI] [PubMed] [Google Scholar]
- Leite CF, Lopes CS, Alves AC, Fuzaro CS, Silva MV, Oliveira LF, Garcia LP, Farnesi TS, Cuba MB, Rocha LB, Rodrigues V Jr, Oliveira CJ & Dias da Silva VJ (2015). Endogenous resident c‐Kit cardiac stem cells increase in mice with an exercise‐induced, physiologically hypertrophied heart. Stem Cell Res 15, 151–164. [DOI] [PubMed] [Google Scholar]
- Liang D, Li J, Wu Y, Zhen L, Li C, Qi M, Wang L, Deng F, Huang J, Lv F, Liu Y, Ma X, Yu Z, Zhang Y & Chen YH (2015). miRNA‐204 drives cardiomyocyte proliferation via targeting Jarid2. Int J Cardiol 201, 38–48. [DOI] [PubMed] [Google Scholar]
- Lim K, Zimanyi MA & Black MJ (2010). Effect of maternal protein restriction during pregnancy and lactation on the number of cardiomyocytes in the postproliferative weanling rat heart. Anat Rec (Hoboken) 293, 431–437. [DOI] [PubMed] [Google Scholar]
- Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Bostrom P, Che L, Zhang C, Spiegelman BM & Rosenzweig A (2015). miR‐222 is necessary for exercise‐induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab 21, 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mads Dam V, Asger A, Thomas Krarup A, Sofie A, Sarah H, Stine A, Steffen R & Jens Erik N‐K (2015). Limitations and pitfalls in measurements of right ventricular stroke volume in an animal model of right heart failure. Physiol Meas 36, 925–937. [DOI] [PubMed] [Google Scholar]
- Master JS, Zimanyi MA, Yin KV, Moritz KM, Gallo LA, Tran M, Wlodek ME & Black MJ (2014). Transgenerational left ventricular hypertrophy and hypertension in offspring after uteroplacental insufficiency in male rats. Clin Exp Pharmacol Physiol 41, 884–890. [DOI] [PubMed] [Google Scholar]
- Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park S‐Y, Silberstein LE, dos Remedios CG, Graham D, Colan S & Kühn B (2013). Cardiomyocyte proliferation contributes to heart growth in young humans. Proc Natl Acad Sci USA 110, 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis AN, Gay MS & Zhang L (2014). Binucleation of cardiomyocytes: the transition from a proliferative to a terminally differentiated state. Drug Discov Today 19, 602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn R (2005). Comparing rat's to human's age: how old is my rat in people years? Nutrition 21, 775–777. [DOI] [PubMed] [Google Scholar]
- Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL Jr, Ribeiro LG & Miller RR (1981). A new, simplified and accurate method for determining ejection fraction with two‐dimensional echocardiography. Circulation 64, 744–753. [DOI] [PubMed] [Google Scholar]
- Ramasamy S, Velmurugan G, Shanmugha Rajan K, Ramprasath T & Kalpana K (2015). MiRNAs with apoptosis regulating potential are differentially expressed in chronic exercise‐induced physiologically hypertrophied hearts. PloS One 10, e0121401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin‐Kern JL, Lechene CP & Lee RT (2013). Mammalian heart renewal by pre‐existing cardiomyocytes. Nature 493, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirish P, Lopez JE, Li N, Wong A, Timofeyev V, Young JN, Majdi M, Li RA, Chen HS & Chiamvimonvat N (2012). MicroRNA profiling predicts a variance in the proliferative potential of cardiac progenitor cells derived from neonatal and adult murine hearts. J Mol Cell Cardiol 52, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soci UP, Fernandes T, Barauna VG, Hashimoto NY, Mota GF, Rosa KT, Irigoyen MC, Phillips MI & de Oliveira EM (2016). Epigenetic control of exercise training‐induced cardiac hypertrophy by miR‐208. Clin Sci (Lond) 130, 2005–2015. [DOI] [PubMed] [Google Scholar]
- Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, Watson P, Oxborough D, George KP & Green DJ (2011). A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol 589, 5443–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy V, De Matteo R, Brew N , Sozo F, Probyn ME, Harding R & Black MJ (2009). The influence of naturally occurring differences in birthweight on ventricular cardiomyocyte number in sheep. Anat Rec (Hoboken) 292, 29–37. [DOI] [PubMed] [Google Scholar]
- Stuckey DJ, Carr CA, Tyler DJ & Clarke K (2008). Cine‐MRI versus two‐dimensional echocardiography to measure in vivo left ventricular function in rat heart. NMR Biomed 21, 765–772. [DOI] [PubMed] [Google Scholar]
- Teichholz LE, Kreulen T, Herman MV & Gorlin R (1976). Problems in echocardiographic volume determinations: echocardiographic‐angiographic correlations in the presence of absence of asynergy. Am J Cardiol 37, 7–11. [DOI] [PubMed] [Google Scholar]
- Tian Y, Liu Y, Wang T, Zhou N, Kong J, Chen L, Snitow M, Morley M, Li D, Petrenko N, Zhou S, Lu M, Gao E, Koch WJ, Stewart KM & Morrisey EE (2015). A microRNA‐Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med 7, 279ra238–279ra238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka AE, Gruetzmacher EM, Kelley DE, Ritov VH, Devaskar SU & Lane RH (2001). Myocardial gene expression of glucose transporter 1 and glucose transporter 4 in response to uteroplacental insufficiency in the rat. J Endocrinol 169, 373–380. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL & Gluckman PD (2000). Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol 279, E83–E87. [DOI] [PubMed] [Google Scholar]
- Wadley G, Laker R, McConell G & Wlodek M (2016). Endurance training in early life results in long‐term programming of heart mass in rats. Physiol Rep 4, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadley GD & McConell GK (2007). Effect of nitric oxide synthase inhibition on mitochondrial biogenesis in rat skeletal muscle. J Appl Physiol (1985) 102, 314–320. [DOI] [PubMed] [Google Scholar]
- Waring CD, Henning BJ, Smith AJ, Nadal‐Ginard B, Torella D & Ellison GM (2015). Cardiac adaptations from 4 weeks of intensity‐controlled vigorous exercise are lost after a similar period of detraining. Physiol Rep 3, e12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring CD, Vicinanza C, Papalamprou A, Smith AJ, Purushothaman S, Goldspink DF, Nadal‐Ginard B, Torella D & Ellison GM (2012). The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 35, 2722–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson LE, Sheth M, Denyer RF & Dostal DE (2004). Baseline echocardiographic values for adult male rats. J Am Soc Echocardiogr 17, 161–167. [DOI] [PubMed] [Google Scholar]
- Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA & Moritz KM (2007). Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18, 1688–1696. [DOI] [PubMed] [Google Scholar]
- Wlodek ME, Westcott K, Siebel AL, Owens JA & Moritz KM (2008). Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int 74, 187–195. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Schotterl S, Backes D, Brunner E, Hahn JK, Ionesi E, Aidery P, Sticht C, Labeit S, Kandolf R, Gawaz M & Gramlich M (2017). Inhibition of miR‐208b improves cardiac function in titin‐based dilated cardiomyopathy. Int J Cardiol 230, 634–641. [DOI] [PubMed] [Google Scholar]


