Abstract
Objective
This 24‐week, phase IIb, double‐blind study was undertaken to evaluate the efficacy and safety of mavrilimumab (a monoclonal antibody to granulocyte–macrophage colony‐stimulating factor receptor α) and golimumab (a monoclonal antibody to tumor necrosis factor [anti‐TNF]) in patients with rheumatoid arthritis (RA) who have had an inadequate response to disease‐modifying antirheumatic drugs (DMARDs) (referred to as DMARD‐IR) and/or inadequate response to other anti‐TNF agents (referred to as anti‐TNF–IR).
Methods
Patients with active RA and a history of DMARD‐IR (≥1 failed regimen) or DMARD‐IR (≥1 failed regimen) and anti‐TNF–IR (1–2 failed regimens) were randomized 1:1 to receive either mavrilimumab 100 mg subcutaneously every other week or golimumab 50 mg subcutaneously every 4 weeks alternating with placebo every 4 weeks, administered concomitantly with methotrexate. The primary end points were the American College of Rheumatology 20% improvement (ACR20), 50% improvement, and 70% improvement response rates at week 24, percentage of patients achieving a Disease Activity Score in 28 joints using C‐reactive protein level (DAS28‐CRP) of <2.6 at week 24, percentage of patients with a score improvement of >0.22 on the Health Assessment Questionnaire (HAQ) disability index (DI) at week 24, and safety/tolerability measures. This study was not powered to formally compare the 2 treatments.
Results
At week 24, differences in the ACR20, ACR50, and ACR70 response rates between the mavrilimumab treatment group (n = 70) and golimumab treatment group (n = 68) were as follows: in all patients, −3.5% (90% confidence interval [90% CI] −16.8, 9.8), −8.6% (90% CI −22.0, 4.8), and −9.8% (90% CI −21.1, 1.4), respectively; in the anti‐TNF–IR group, 11.1% (90% CI −7.8, 29.9), −8.7% (90% CI −28.1, 10.7), and −0.7% (90% CI −18.0, 16.7), respectively. Differences in the percentage of patients achieving a DAS28‐CRP of <2.6 at week 24 between the mavrilimumab and golimumab groups were −11.6% (90% CI −23.2, 0.0) in all patients, and −4.0% (90% CI −20.9, 12.9) in the anti‐TNF–IR group. The percentage of patients achieving a >0.22 improvement in the HAQ DI score at week 24 was similar between the treatment groups. Treatment‐emergent adverse events were reported in 51.4% of mavrilimumab‐treated patients and 42.6% of golimumab‐treated patients. No deaths were reported, and no specific safety signals were identified.
Conclusion
The findings of this study demonstrate the clinical efficacy of both treatments, mavrilimumab at a dosage of 100 mg every other week and golimumab at a dosage of 50 mg every 4 weeks, in patients with RA. Both regimens were well‐tolerated in patients who had shown an inadequate response to DMARDs and/or other anti‐TNF agents.
Granulocyte–macrophage colony‐stimulating factor (GM‐CSF) is a proinflammatory cytokine that plays a central role in rheumatoid arthritis (RA) pathogenesis through its effects on the activation, differentiation, and survival of macrophages, dendritic cells, and neutrophils 1, 2, 3, 4. This knowledge, taken together with the observation that GM‐CSF and its receptor, GM‐CSFRα, are up‐regulated in synovial tissue and circulating mononuclear cells from patients with RA 5, 6, 7, supports targeting of the GM‐CSF pathway as a potential therapeutic approach. Mavrilimumab, a fully human monoclonal antibody that targets GM‐CSFRα, is designed to modulate the activation, differentiation, and survival of macrophages and neutrophils, thereby reducing cell numbers in inflammatory lesions 8. The efficacy of mavrilimumab has previously been demonstrated and was well‐tolerated in patients with RA who have had an inadequate response to disease‐modifying antirheumatic drugs (DMARDs) 9, 10, 11 (herein referred to as DMARD‐IR). However, mavrilimumab has not been evaluated in patients with RA who have had an inadequate response to anti–tumor necrosis factor (anti‐TNF) agents (herein referred to as anti‐TNF–IR).
The use of TNF antagonists in RA has substantially improved outcomes in patients 12. However, analysis of the Consortium of Rheumatology Researchers of North America registry, a database of North American RA patients, indicates that 80% of patients do not achieve a Disease Activity Score in 28 joints using erythrocyte sedimentation rate (DAS28‐ESR) of <2.6 13 within 12 months of initiating anti‐TNF treatment 14. Therapies targeting other mechanisms involved in the pathogenesis of RA (inhibition of T cell costimulation [abatacept]; the B cell–restricted surface antigen CD20 [rituximab]; the interleukin‐6 receptor [tocilizumab]; and JAK kinase [tofacitinib]) are also beneficial 15, 16. Nonetheless, ~30–40% of patients receiving treatment with an approved biologic agent do not achieve a 20% improvement response based on the American College of Rheumatology improvement response criteria (ACR20) 17, 18, 19, highlighting a continued unmet need for therapies with an alternative mechanism of action.
Against this background, GM‐CSF inhibition is a plausible and novel potential option for the management of RA. In this phase IIb study, we sought to evaluate the efficacy and safety of mavrilimumab in patients with a history of DMARD‐IR and anti‐TNF–IR. The study included a detailed assessment of patients’ pulmonary function, because inhibition of GM‐CSF signaling by mavrilimumab has the potential to affect the clearance of surfactant by alveolar macrophages; anti–GM‐CSF autoantibodies are responsible for ~90% of cases of pulmonary alveolar proteinosis, a rare disease resulting from the accumulation of lung surfactant due to the development of autoantibodies that inhibit GM‐CSF signaling 20, 21. For contextual relevance, we included a parallel treatment arm in which a TNF antagonist, golimumab, was evaulated. Direct comparison of these agents will form the basis of a dedicated biomarker program, enabling the elucidation of differential mechanisms of action for these agents, each of which targets different mediators of inflammation.
Patients and methods
Study design. The study was designed as a phase II multicenter, exploratory, double‐blind, randomized, parallel‐group study in patients with RA (known as EARTH EXPLORER 2), conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. It was approved by the appropriate institutional review boards or independent ethics committees at each site. All patients provided written informed consent.
Patients ages 18–80 years with adult‐onset RA who had at least moderately active disease, defined as a DAS28 using C‐reactive protein (DAS28‐CRP) of ≥3.2 at screening and a DAS28‐ESR of ≥3.2 on day 1, and who had at least 4 swollen joints at screening and on day 1 and were considered to have a history of either DMARD‐IR (≥1 failed regimen) or DMARD‐IR (≥1 failed regimen) and anti‐TNF–IR (1–2 failed regimens; excluding golimumab) were enrolled. Patients who experienced treatment failure with a TNF antagonist were defined as those who had experienced an inadequate response, safety issue, or intolerance to 1 or 2 anti‐TNF agents other than golimumab, given for at least 3 months, with the last dose having been administered at least 8 weeks prior to the first study dose. Key exclusion criteria included a history of, or current presence of, inflammatory joint disease or other systemic autoimmune disorder other than RA. In addition, patients were excluded if they had ever received biologic therapies (other than a TNF antagonist) for RA or had received sulfasalazine, azathioprine, cyclosporine, or D‐penicillamine within ≤28 days of day 1 and leflunomide ≤12 weeks before day 1.
Patients were randomized using an interactive voice recognition system/interactive web recognition system (IVRS/IWRS), which proceeded once the investigators had confirmed the patients’ eligibility for the study. Patients were randomized (by IVRS/IWRS) in a 1:1 ratio to receive either mavrilimumab subcutaneously (SC) 100 mg every other week or golimumab SC 50 mg every 4 weeks (starting at week 0) alternating with placebo every 4 weeks (starting at week 2; used to maintain the treatment blind), for 24 weeks. Randomization was performed in an equal ratio across the treatment arms and stratified by number of prior anti‐TNF failures (0, 1, or 2). Patients in the DMARD‐IR group were in the anti‐TNF stratum of 0.
The choice of mavrilimumab dosage was based on previous efficacy and safety data from a phase IIa study 9 in which mavrilimumab 100 mg every other week was used as the largest dosage, as supported by pharmacokinetic/pharmacodynamic modeling of the same data. There were no data available on mavrilimumab dosages of >100 mg every other week at the time that this study was initiated. Therefore, mavrilimumab 100 mg every other week was selected. The golimumab dosage selected for this study (50 mg SC every 4 weeks) was based on the approved dosage recommended in the prescribing information.
All patients received their randomized study drug for 24 weeks concomitantly with a stable dosage of methotrexate (7.5–25 mg/week). For their dosage to be considered stable, patients should have received the same dosage of methotrexate for at least 4 weeks before screening. Stable dosages of oral corticosteroids (prednisone ≤7.5 mg/day), analgesics, and nonsteroidal antiinflammatory drugs were permitted during the course of the study. After week 12, patients who had not responded adequately to the blinded study treatment (defined as <20% improvement in both the swollen joint count and the tender joint count compared with day 1) were eligible to enter an open‐label extension (OLE) study (ClinicalTrials.gov identifier 01712399). After week 24, all patients were eligible for screening to enter the long‐term OLE study.
The primary population for efficacy analyses was the modified intent‐to‐treat (mITT) population, including all randomized patients who had received any study drug. The safety population included all patients who had received any study drug and had safety data available.
Efficacy assessments. The primary efficacy end points were the ACR20 improvement response rate as well as the 50% and 70% improvement response rates at week 24, the percentage of patients with a DAS28‐CRP of <2.6 at week 24, and the percentage of patients with a score improvement of >0.22 in the Health Assessment Questionnaire (HAQ) disability index (DI) 22 at week 24.
Secondary efficacy end points (assessed at weeks 1, 2, 4, 8, 12, 16, 20, and 24) included the following: change from baseline in the DAS28‐CRP, percentage of patients with a European League Against Rheumatism improvement response 23 based on the DAS28‐CRP, percentage of patients with a DAS28‐CRP of <3.2, percentage of patients with a DAS28‐ESR of <2.6, the ACR20, ACR50, and ACR70 response rates, change from baseline in the CRP and ESR, response rates based on achievement of a Clinical Disease Activity Index (CDAI) score of ≤2.8 24, and response rates based on achievement of a Simplified Disease Activity Index (SDAI) score of ≤3.3 25.
Immunogenicity assessments. The immunogenicity potential of mavrilimumab (measured as the presence of any antidrug antibody response) was determined using a validated electrochemiluminescence method. Serum samples for determination of the antidrug antibody response were obtained predose on day 1, and at weeks 0, 4, 12, 20, and 24.
Safety assessments. Adverse events (AEs) and serious AEs (SAEs) were graded by investigators according to their severity and relationship to the study drug. In addition, investigators assessed the relationship between SAEs and protocol procedures. Laboratory measurements, vital signs, pulmonary safety tests (including spirometry, testing for diffusing capacity for carbon monoxide of the lung, chest radiography, and dyspnea score), and the change from baseline scores were summarized by treatment group and time point. Adjudication of lung function abnormalities and pulmonary AEs, to identify potential cases of pulmonary alveolar proteinosis, was performed by an independent pulmonary expert committee whose members were blinded with regard to the treatment group.
Statistical analysis. The sample size was selected to estimate the difference between the mavrilimumab and golimumab treatment groups with a stated degree of accuracy. It was not selected to detect differences with statistical significance (and no power analyses were carried out). With a sample size of 60 patients per treatment group, the difference between the upper limit of the 90% confidence interval (90% CI) and the estimate of the treatment difference ranged between 9% and 15% for the primary end points. For each of the primary end points, responses were analyzed by applying a test of treatment difference in the proportion of responders, using logistic regression. The number of TNF antagonist failures was used as a categorical covariate, and results were reported with 2‐sided 90% CIs; the standard error of the difference in proportions was estimated using the delta method 26. Patients who withdrew from treatment for any reason (including entering the OLE), those who started a new medication for RA, or those for whom the methotrexate dosage was increased were imputed as nonresponders for all subsequent assessments. AE and other safety data were summarized with descriptive statistics.
Results
Demographic and baseline clinical characteristics of the patients. The study was conducted at 36 sites in 13 European and South American countries, with patients randomized and evaluated between March 2013 (first patient randomized) and February 2015 (last patient, last visit). In total, 215 patients were screened, with 138 randomized to 1 of the 2 treatment groups (68 to receive golimumab, 70 to receive mavrilimumab); 14 patients discontinued treatment (3 in the golimumab group, 11 in the mavrilimumab group), and 124 patients (89.9%) completed the study (Figure 1). All randomized patients were included in both the mITT and safety populations.
Figure 1.
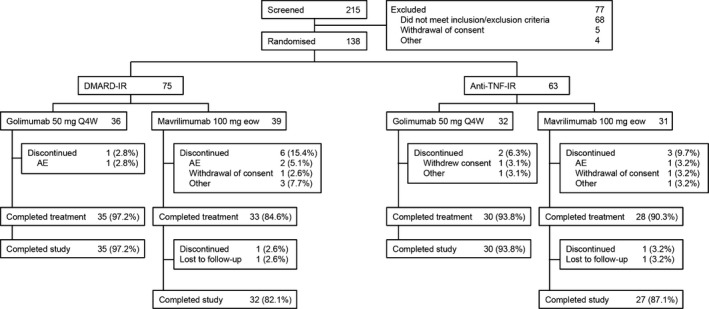
Disposition of patients in the randomized phase IIb study of the efficacy and safety of mavrilimumab and golimumab in patients with rheumatoid arthritis. DMARD‐IR = inadequate response to disease‐modifying antirheumatic drugs; anti‐TNF–IR = inadequate response to anti–tumor necrosis factor agents; Q4W = every 4 weeks; eow = every other week; AE = adverse event.
Baseline demographics and disease characteristics were similar between the treatment groups (Table 1), with the following exceptions: for patients in the mavrilimumab treatment group compared with the golimumab group, disease duration was shorter (5.8 years versus 7.6 years) and the baseline CRP concentration was higher (8.3 mg/liter versus 6.5 mg/liter). Across both treatment groups, a higher level of disease activity was observed at baseline in the anti‐TNF–IR stratum compared with the DMARD‐IR population, in terms of longer duration of RA, more frequent use of corticosteroids, higher disease activity according to the DAS28‐CRP, a higher baseline HAQ DI score, and higher baseline concentrations of CRP. An imbalance in regional distribution of recruitment between the 2 strata was noted, with more patients in the anti‐TNF–IR stratum than in the DMARD‐IR stratum recruited in South America (~56% versus ~11%).
Table 1.
Demographic and baseline clinical characteristics of the patients with rheumatoid arthritis (RA)a
| Anti‐TNF–IR | DMARD‐IR | Overall population | ||||
|---|---|---|---|---|---|---|
| Mavrilimumab 100 mg every other week (n = 31) | Golimumab 50 mg every 4 weeks (n = 32) | Mavrilimumab 100 mg every other week (n = 39) | Golimumab 50 mg every 4 weeks (n = 36) | Mavrilimumab 100 mg every other week (n = 70) | Golimumab 50 mg every 4 weeks (n = 68) | |
| Demographics | ||||||
| Age, mean ± SD years | 50.2 ± 13.3 | 46.9 ± 10.5 | 50.2 ± 13.3 | 52.5 ± 11.8 | 50.2 ± 13.3 | 49.9 ± 11.4 |
| Female, no. (%) | 28 (90.3) | 28 (87.5) | 28 (71.8) | 29 (80.6) | 56 (80.0) | 57 (83.8) |
| Race, no. (%) | ||||||
| White | 26 (83.9) | 25 (78.1) | 36 (92.3) | 32 (88.9) | 62 (88.6) | 57 (83.8) |
| American Indian or native Alaskan | 5 (16.1) | 7 (21.9) | 2 (5.1) | 2 (5.6) | 7 (10.0) | 9 (13.2) |
| Asian | 0 (0.0) | 0 (0.0) | 1 (2.6) | 1 (2.8) | 1 (1.4) | 1 (1.5) |
| Black or African American | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (2.8) | 0 (0.0) | 1 (1.5) |
| Region of enrollment, no. (%) | ||||||
| Europe | 12 (38.7) | 16 (50.0) | 35 (89.7) | 32 (88.9) | 47 (67.1) | 48 (70.6) |
| South America | 19 (61.3) | 16 (50.0) | 4 (10.3) | 4 (11.1) | 23 (32.9) | 20 (29.4) |
| Weight, mean ± SD kg | 72.9 ± 14.3 | 76.3 ± 17.4 | 73.1 ± 17.3 | 74.0 ± 19.1 | 73.0 ± 16.0 | 75.1 ± 18.8 |
| Body mass index, mean ± SD kg/m2 | 28.2 ± 5.5 | 29.2 ± 5.4 | 26.6 ± 2.2 | 26.5 ± 5.1 | 27.3 ± 5.3 | 27.8 ± 5.4 |
| Baseline clinical characteristics | ||||||
| Duration of RA, median years | 6.0 | 8.4 | 5.4 | 6.8 | 5.8 | 7.6 |
| Methotrexate use | ||||||
| Total no. (%) | 31 (100) | 32 (100) | 39 (100) | 36 (100) | 70 (100) | 68 (100) |
| Dosage, mean ± SD mg/week | 15.9 ± 3.8 | 14.1 ± 3.7 | 15.5 ± 4.1 | 15.3 ± 3.9 | 15.6 ± 4.0 | 14.7 ± 3.8 |
| <12.5 mg/week, no. (%) | 3 (9.7) | 10 (31.3) | 7 (17.9) | 5 (13.9) | 10 (14.3) | 15 (22.1) |
| ≥12.5−<20 mg/week, no. (%) | 21 (67.7) | 19 (59.4) | 22 (56.4) | 24 (66.7) | 43 (61.4) | 43 (63.2) |
| ≥20–25 mg/week, no. (%) | 7 (22.6) | 3 (9.4) | 10 (25.6) | 7 (19.4) | 17 (24.3) | 10 (14.7) |
| Corticosteroid use | ||||||
| Total no. (%) | 22 (71.0) | 22 (68.8) | 19 (48.7) | 18 (50.0) | 41 (58.6) | 40 (58.8) |
| Dosage, mean ± SD mg/day | 5.4 ± 1.2 | 4.8 ± 1.7 | 5.3 ± 1.2 | 5.4 ± 1.0 | 5.4 ± 1.1 | 5.1 ± 1.5 |
| <5 mg/day, no. (%) | 0 (0.0) | 3 (13.6) | 1 (5.3) | 0 (0.0) | 1 (2.4) | 3 (7.5) |
| ≥5 mg/day, no. (%) | 22 (100) | 19 (86.4) | 18 (94.7) | 18 (100) | 40 (97.6) | 37 (92.5) |
| Previous anti‐TNF failures, no. (%) | ||||||
| 0 (DMARD‐IR population) | 0 (0.0) | 0 (0.0) | 39 (100) | 36 (100) | 39 (55.7) | 36 (52.9) |
| 1 (anti‐TNF–IR population) | 27 (87.1) | 30 (93.8) | 0 (0.0) | 0 (0.0) | 27 (38.6) | 30 (44.1) |
| 2 (anti‐TNF–IR population) | 4 (12.9) | 2 (6.3) | 0 (0.0) | 0 (0.0) | 4 (5.7) | 2 (2.9) |
| Anti‐TNF failure, no. (%)b | ||||||
| Adalimumab | 13 (41.9) | 7 (21.9) | 0 (0.0) | 0 (0.0) | 13 (18.6) | 7 (10.3) |
| Certolizumab pegol | 7 (22.6) | 7 (21.9) | 0 (0.0) | 0 (0.0) | 7 (10.0) | 7 (10.3) |
| Etanercept | 10 (32.3) | 15 (46.9) | 0 (0.0) | 0 (0.0) | 10 (14.3) | 15 (22.1) |
| Infliximab | 5 (16.1) | 6 (18.8) | 0 (0.0) | 0 (0.0) | 5 (7.1) | 6 (8.8) |
| Rheumatoid factor–positive, no. (%) | 24 (77.4) | 24 (75.0) | 29 (74.4) | 29 (80.6) | 53 (75.7) | 53 (77.9) |
| ACPA‐positive, no. (%) | 23 (74.2) | 22 (68.8) | 24 (61.5) | 30 (83.3) | 47 (67.1) | 52 (76.5) |
| DAS28‐CRP, mean ± SD | 6.1 ± 0.8 | 6.0 ± 0.6 | 5.6 ± 1.1 | 5.5 ± 0.9 | 5.8 ± 1.0 | 5.7 ± 0.8 |
| DAS28‐ESR, mean ± SD | 6.8 ± 0.7 | 6.8 ± 0.6 | 6.4 ± 1.0 | 6.3 ± 0.9 | 6.6 ± 0.9 | 6.5 ± 0.8 |
| HAQ DI score, mean ± SD | 1.9 ± 0.5 | 1.8 ± 0.5 | 1.4 ± 0.6 | 1.4 ± 0.5 | 1.6 ± 0.6 | 1.6 ± 0.5 |
| Swollen joint count, mean ± SD | 16.1 ± 8.4 | 16.2 ± 7.1 | 12.5 ± 4.9 | 12.9 ± 5.4 | 14.1 ± 6.9 | 14.5 ± 6.4 |
| Tender joint count, mean ± SD | 25.9 ± 13.6 | 28.9 ± 14.6 | 24.4 ± 12.4 | 21.4 ± 12.1 | 25.0 ± 12.9 | 24.9 ± 13.7 |
| Geometric mean CRP, mg/liter | 10.9 | 7.0 | 6.7 | 6.1 | 8.3 | 6.5 |
| Geometric mean ESR, mm/hour | 39.9 | 38.6 | 36.2 | 35.5 | 37.8 | 36.9 |
Anti‐TNF–IR = inadequate response to anti–tumor necrosis factor agents; DMARD‐IR = inadequate response to disease‐modifying antirheumatic drugs; ACPA = anti–citrullinated protein antibody; DAS28‐CRP = Disease Activity Score in 28 joints using C‐reactive protein level; DAS28‐ESR = DAS28 using erythrocyte sedimentation rate; HAQ DI = Health Assessment Questionnaire disability index.
The prior anti‐TNF treatment had failed because there was a lack of initial efficacy (n = 14) or loss of efficacy (n = 48), the medication was given only in the clinical study (n = 1), or an adverse event occurred (n = 3). Some patients had previously experienced treatment failure with 2 anti‐TNF agents; if the reasons for failure were different for the 2 TNFs, both reasons were included.
Efficacy. Overall population (week 24). At week 24, ACR20, ACR50, and ACR70 improvement responses in disease activity were observed in 62.0%, 34.8%, and 16.1% of patients, respectively, among those receiving mavrilimumab 100 mg every other week, compared with 65.6%, 43.4%, and 25.9% of patients, respectively, among those receiving golimumab 50 mg every 4 weeks. Differences in the ACR20, ACR50, and ACR70 response rates between the mavrilimumab and golimumab treatment groups were −3.5% (90% CI −16.8, 9.8), −8.6% (90% CI −22.0, 4.8), and −9.8% (90% CI −21.1, 1.4), respectively.
A DAS28‐CRP of <2.6 was achieved at week 24 by 17.4% of the mavrilimumab‐treated patients and 29.0% of the golimumab‐treated patients, with a difference between treatment groups of −11.6% (90% CI −23.2, 0.0). Evidence of low disease activity (a DAS28‐CRP of <3.2) at week 24 was observed in 28.9% of the mavrilimumab‐treated patients and 40.6% of the golimumab‐treated patients. A higher percentage of patients in the golimumab treatment group (69.0%) than in the mavrilimumab treatment group (58.7%) were classified as responders (HAQ DI score improvement of >0.22) 27; the difference in the HAQ DI response between the 2 treatment groups was −10.3% (90% CI −23.7, 3.0). The mean ± SEM change from baseline in the HAQ DI score was −0.44 ± 0.07 in the mavrilimumab group and −0.64 ± 0.07 in the golimumab group.
The percentages of patients with a CDAI score of ≤2.8 at week 24 were 5.7% among those receiving mavrilimumab 100 mg every other week and 17.6% among those receiving golimumab 50 mg every 4 weeks. An SDAI score of ≤3.3 was reported in 7.2% of patients receiving mavrilimumab and 18.9% of those receiving golimumab.
Although treatment differences in the ACR20 response rate and the DAS28‐CRP were consistent within regions, differences in response rates were observed between the geographic regions of Europe and South America, irrespective of the drug allocation and stratum. For example, higher response rates were observed in patients from South America compared with those from Europe, both in the mavrilimumab treatment group (ACR20 response rate, 78.3% versus 53.2%; adjusted change in the DAS28‐CRP, mean ± SEM −2.47 ± 0.298 versus −1.30 ± 0.272) and in the golimumab treatment group (ACR20 response rate, 85.0% versus 58.3%; adjusted change in the DAS28‐CRP, mean ± SEM −2.86 ± 0.315 versus −1.56 ± 0.275).
Anti‐TNF–IR population (week 24). Of the patients within the anti‐TNF–IR group (n = 63), the percentage achieving an ACR20 response at week 24 was 72.3% (22 of 31 patients) in the mavrilimumab treatment group compared with 61.2% (20 of 32 patients) in the golimumab treatment group (Figure 2A), a difference of 11.1% (90% CI −7.8, 29.9). The percentage of ACR50 or ACR70 responders was similar between treatment groups, with a difference between the mavrilimumab and golimumab treatment groups of −8.7% (90% CI −28.1, 10.7) for the ACR50 response rate and −0.7% (90% CI −18.0, 16.7) for the ACR70 response rate (Figure 2A). Similarly, the percentages of patients achieving a DAS28‐CRP of <2.6, improvement in the HAQ DI score of >0.22, and a DAS28‐CRP of <3.2 at week 24 were similar between the treatment groups (Figures 2B−D).
Figure 2.
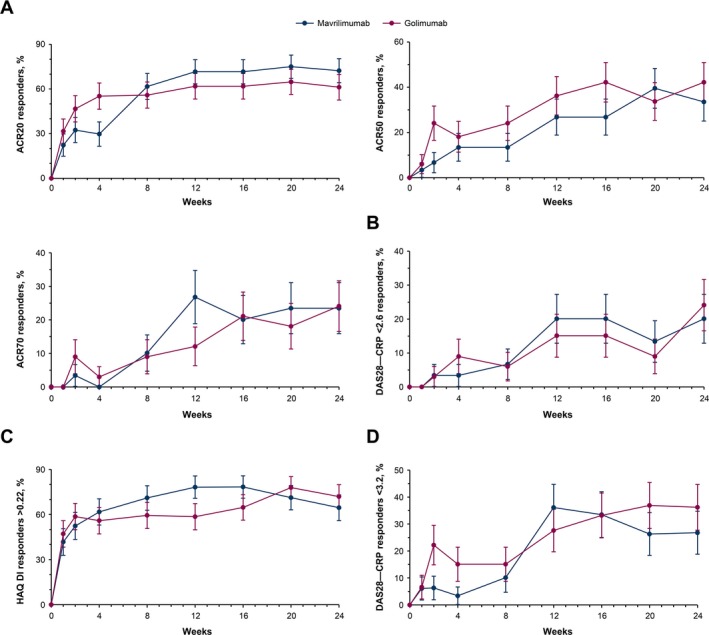
Response to mavrilimumab compared to golimumab over 24 weeks of treatment in patients with a history of an inadequate response to anti–tumor necrosis factor agents. Responses were measured based on the percentage of patients with a response according to the American College of Rheumatology criteria for 20% improvement (ACR20), 50% improvement, and 70% improvement (A), the percentage of patients with a Disease Activity Score in 28 joints using C‐reactive protein level (DAS28‐CRP) of <2.6 (B), the percentage of patients with a score improvement of >0.22 on the Health Assessment Questionnaire (HAQ) disability index (DI) (C), and the percentage of patients with a DAS28‐CRP of <3.2 (D). Results are shown as the mean ± SEM.
At week 24 in the anti‐TNF–IR population, the adjusted change from baseline in the DAS28‐CRP was a mean ± SEM –1.99 ± 0.3 in those receiving mavrilimumab 100 mg every other week compared with –2.24 ± 0.3 in those receiving golimumab 50 mg every 4 weeks, with a difference between the mavrilimumab and golimumab groups of −4.0% (90% CI −20.9, 12.9). The percentage of patients with a CDAI score of ≤2.8 at week 24 was 6.7% in the mavrilimumab 100 mg every other week group compared with 9.0% in the golimumab 50 mg every 4 weeks group. An SDAI score of ≤3.3 was achieved at week 24 by 10.1% of mavrilimumab‐treated patients and 12.1% of golimumab‐treated patients.
Of the 6 patients who had previously experienced treatment failure with 2 TNF antagonists, an ACR20 improvement response was achieved by 2 of the 3 patients treated with mavrilimumab. In contrast, none of the 3 patients treated with golimumab achieved an ACR20 response.
DMARD‐IR population (week 24). The ACR20, ACR50, and ACR70 response rates, the percentage of patients achieving a HAQ DI score improvement of >0.22 27, and the percentage of patients achieving a DAS28‐CRP of <2.6 and DAS28‐CRP of <3.2 at week 24 in the DMARD‐IR stratum are shown in Figures 3A–D. Differences in the ACR20, ACR50, and ACR70 response rates between the mavrilimumab‐treated patients and the golimumab‐treated patients were −15.6% (90% CI −33.8, 2.6), −8.5% (90% CI −27.1, 10.0), and −17.5% (90% CI −32.2, −2.9), respectively. The adjusted change from baseline in the DAS28‐CRP was a mean ± SEM –1.89 ± 0.2 in those receiving mavrilimumab 100 mg every other week compared with –2.27 ± 0.2 in those receiving golimumab 50 mg every 4 weeks, with a difference between the mavrilimumab‐treated and golimumab‐treated patients of −17.9% (90% CI −34.0, −1.9).
Figure 3.
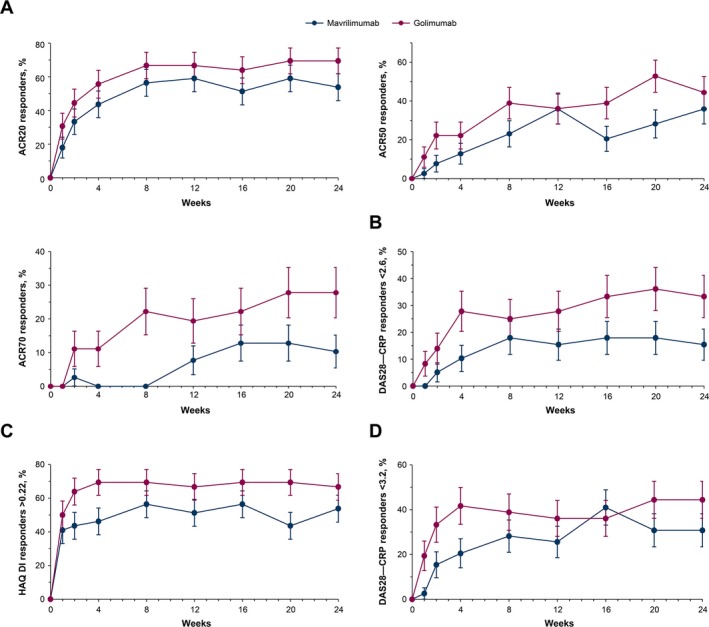
Response to mavrilimumab compared to golimumab over 24 weeks of treatment in patients with a history of inadequate response to disease‐modifying antirheumatic drugs. Responses were measured based on the percentage of patients with response according to the American College of Rheumatology criteria for 20% improvement (ACR20), 50% improvement, and 70% improvement (A), the percentage of patients with a Disease Activity Score in 28 joints using C‐reactive protein level (DAS28‐CRP) of <2.6 (B), the percentage of patients with a score improvement of >0.22 on the Health Assessment Questionnaire (HAQ) disability index (DI) (C), and the percentage of patients with a DAS28‐CRP of <3.2 (D). Results are shown as the mean ± SEM.
Response rates based on achievement of a CDAI score of ≤2.8 at week 24 in the DMARD‐IR stratum were 5.1% in the mavrilimumab 100 mg every other week group and 25.0% in the golimumab 50 mg every 4 weeks group. The percentage of patients with an SDAI of ≤3.3 at week 24 was 5.1% among patients treated with mavrilimumab and 25.0% among those treated with golimumab.
Disease markers. In the overall population, normalization of the serum CRP concentration and ESR was demonstrated in both the mavrilimumab treatment group and the golimumab treatment group. At week 24, the geometric mean serum CRP concentrations were 4.6 mg/liter in the mavrilimumab‐treated patients and 4.4 mg/liter in the golimumab‐treated patients. The geometric mean ESR values were 23.4 mm/hour in the mavrilimumab‐treated patients and 22.4 mm/hour in those treated with golimumab. Serum CRP concentrations and the ESR over time, in both the anti‐TNF–IR and DMARD‐IR populations, are provided in Figures 4A–D. In the anti‐TNF–IR population, treatment with mavrilimumab resulted in a sustained decrease in the geometric mean serum CRP concentration up to 24 weeks. In contrast, after an initial reduction in concentration, the geometric mean serum CRP concentrations increased to the values observed at baseline or above baseline in patients receiving golimumab. In the same anti‐TNF–IR population, the geometric mean serum ESR declined after both treatments, but started to increase toward baseline values at later time points, particularly in the golimumab‐treated patients. For patients in the DMARD‐IR population, treatment with either mavrilimumab or golimumab resulted in a decrease from baseline in the geometric mean CRP concentrations and ESR.
Figure 4.
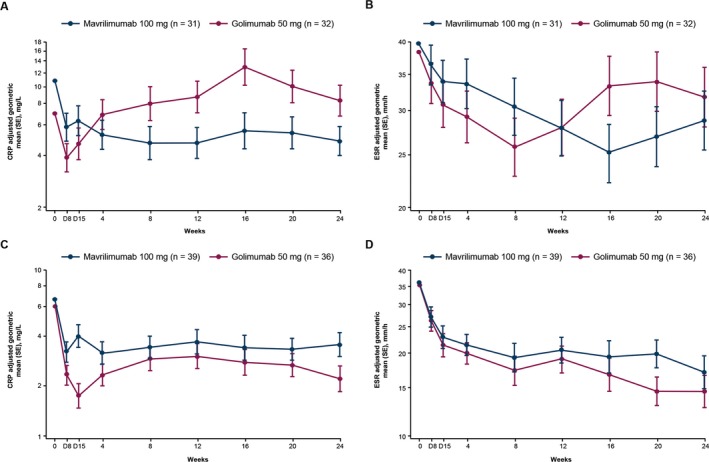
Adjusted geometric mean C‐reactive protein (CRP) levels and adjusted geometric mean erythrocyte sedimentation rate (ESR) over time in patients with a history of an inadequate response to anti–tumor necrosis factor agents (A and B) and patients with an inadequate response to disease‐modifying antirheumatic drugs (C and D). Results are shown as the mean ± SEM.
Immunogenicity. The overall incidence of antidrug antibodies in the mavrilimumab‐treated patients with available immunogenicity data was 3.1% (2 of 64 patients; 1 in the DMARD‐IR stratum and 1 in the anti‐TNF–IR stratum).
Safety. Treatment‐emergent AEs (TEAEs) in the overall population are summarized in Table 2, including the most common TEAEs occurring in ≥3% of patients and any treatment‐emergent SAEs (TESAEs). Two TESAEs were considered by the investigators to be related to the study treatment. Both of these events, Pneumocystis jirovecii pneumonia and “lung disorder” (Medical Dictionary for Regulatory Activities preferred term), were reported in the golimumab treatment group. P jirovecii pneumonia, observed in a 69‐year‐old male patient, was classified as severe and developed 8 days after administration of the tenth golimumab dose. Golimumab was withdrawn, and the patient recovered, with sequelae. The “lung disorder” was observed in a patient who presented with lung abnormalities detected on a chest radiograph at week 24 (end of treatment visit). Analysis of the subsequent biopsy sample raised suspicion of a diagnosis of “granulomatous process—possible sarcoidosis.”
Table 2.
TEAEs occurring in ≥3% of patients in either treatment group, and TESAEs in the overall population
| Mavrilimumab 100 mg every other week (n = 70) | Golimumab 50 mg every 4 weeks (n = 68) | |
|---|---|---|
| Any TEAE, no. (%) reporting ≥1 event | 36 (51.4) | 29 (42.6) |
| TEAEs in ≥3% of patients in any group, no. (%) | ||
| Headache | 3 (4.3) | 2 (2.9) |
| Hepatic enzyme levels increaseda | 3 (4.3) | 2 (2.9) |
| Hypertension | 3 (4.3) | 1 (1.5) |
| Nasopharyngitits | 4 (5.7) | 1 (1.5) |
| Upper respiratory tract infection | 3 (4.3) | 2 (2.9) |
| Viral upper respiratory tract infection | 3 (4.3) | 2 (2.9) |
| TEAEs considered by investigators to be related to the study drug, no. (%) reporting ≥1 event | 12 (17.1) | 11 (16.2) |
| TEAEs resulting in permanent discontinuation of the study drug, no. (%) reporting ≥1 event | 3 (4.3) | 1 (1.5) |
| TEAEs resulting in interruption of the study drug, no. (%) reporting ≥1 event | 7 (10.0) | 4 (5.9) |
| TESAEs, no. (%) | ||
| Gastroduodenitis | 0 (0.0) | 1 (1.5) |
| Hepatic enzyme levels increaseda | 0 (0.0) | 1 (1.5) |
| Lung disorderb | 0 (0.0) | 1 (1.5) |
| Parathyroid tumor, benign | 1 (1.4) | 0 (0.0) |
| Peptic ulcer | 0 (0.0) | 1 (1.5) |
| Pneumocystis jirovecii pneumoniab | 0 (0.0) | 1 (1.5) |
| Vertebrobasilar insufficiency | 1 (1.4) | 0 (0.0) |
Investigator‐reported treatment‐emergent adverse event (TEAE) (i.e., there was no defined threshold).
Treatment‐emergent serious adverse event (TESAE) considered by the investigators to be related to the study drug.
No deaths were reported in either treatment group. Moreover, no significant safety signals, including those related to pulmonary events, were identified during the study (pulmonary function test results are shown in Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.40323/abstract).
Discussion
This phase IIb study was designed to evaluate the clinical effects and tolerability of the anti–GM‐CSFRα monoclonal antibody mavrilimumab in a mixed population of biologic agent–naive and biologic agent–experienced patients with active RA. It was planned to support the design and potential progression of mavrilimumab to phase III development, which has yet to be initiated. It was not powered to provide a direct comparison of mavrilimumab and golimumab.
The selection of the mavrilimumab dosage of 100 mg every other week for this study was based on efficacy, safety, pharmacokinetic, and pharmacodynamic data from the phase IIa study 9, in which it was the highest dosage evaluated. In addition, the benefit:risk ratio of higher dosages of mavrilimumab was yet to be proven. However, since the initiation of the current study, the phase IIb study (EARTH EXPLORER 1) demonstrated that a mavrilimumab dosage of 150 mg every other week was more efficacious than 100 mg every other week in patients with a history of DMARD‐IR 11.
In the overall population, the efficacy of both mavrilimumab (100 mg every other week) and golimumab (50 mg every 4 weeks) was demonstrated, resulting in improved disease‐related physical function. However, the overall study population was heterogeneous in terms of medication history (0, 1, or 2 anti‐TNF failures). Therefore, it is important to interpret these data with caution. The efficacy of mavrilimumab and golimumab differed between the anti‐TNF–IR and DMARD‐IR populations. In the DMARD‐IR group, golimumab yielded numerically higher response rates compared with mavrilumab. Although the current results with regard to the efficacy of mavrilimumab are consistent with those previously reported by Burmester et al 11, evidence from that same study indicated that the higher dosage of 150 mg every other week had greater efficacy; therefore, a mavrilimumab dosage of 100 mg every other week may be suboptimal for clinical efficacy. However, despite the suboptimal dosage used in this study, mavrilimumab demonstrated efficacy that was similar to that of golimumab in the anti‐TNF–IR population. The individual DMARD‐IR and anti‐TNF–IR strata each had a greater degree of homogeneity, but the relatively low numbers of patients per stratum and treatment group limit the interpretability and generalizability of the results. Therefore, larger studies are required to fully evaluate whether a second anti‐TNF agent is efficacious in an anti‐TNF–IR population, and to determine the optimal mavrilimumab dosage for the same population.
Although there are limited data available, several studies have suggested that switching to a different anti‐TNF agent may be associated with clinical benefit in patients with RA 28, 29, 30. Alternatively, therapies with a different mechanism of action may prove to be an effective treatment option. To our knowledge, no randomized controlled study has compared the efficacy of an anti‐TNF agent with that of an experimental non–TNF antagonist agent in patients with a history of anti‐TNF–IR. We have noted that studies have been conducted in patients with a history of DMARD‐IR and in patients who have never received treatment with a biologic agent.
Subgroup analysis of the ACR20 response rate and DAS28‐CRP at week 24 by geographic region demonstrated higher response rates both with mavrilimumab and with golimumab in patients from South America compared with those from Europe. However, the importance of this observation remains to be determined. These regional variations may be observed to a greater extent in the anti‐TNF–IR population than in the DMARD‐IR population, since the percentage of patients with a history of anti‐TNF–IR was higher among those recruited at sites in South America. Interestingly, the ACR response rates observed in patients both from South America and from Europe in the current study were substantially higher than those previously reported in the phase III golimumab (GO‐AFTER) study, in which a similar cohort of patients was evaluated 30. For example, in the current study, the ACR20 response rates at week 24 among patients in the anti‐TNF–IR stratum who were randomized to receive golimumab 50 mg every 4 weeks were 81.2% in those from South America and 43.8% in those from Europe. In contrast, the ACR20 response rate reported in patients receiving golimumab 50 mg every 4 weeks and concomitant DMARDs in the total GO‐AFTER study population was 34.0% 30.
Individual biomarkers (serum CRP concentrations and ESR) were decreased in both treatment groups by week 24. However, the observed reduction in the CRP level with golimumab treatment appeared to be transient in patients within the anti‐TNF–IR stratum, with concentrations returning to baseline values by week 16. We have not found any studies in the anti‐TNF–IR RA population in which the CRP data have been presented in a manner similar to that in our study. One study showed that switching to rituximab provided greater clinical benefit than switching to a second TNF inhibitor in patients with RA in whom treatment with a TNF inhibitor had previously failed 29. In that study, the patients’ clinical characteristics were measured 6 months after study initiation. At the 6‐month time point, there was no difference in the CRP levels between the rituximab group and the alternative TNF antagonist group. However, the DAS28‐CRP and ESR were significantly lower in the rituximab group than in the alternative TNF antagonist group 29.
Mavrilimumab and golimumab were generally well tolerated in the present study. The percentage of patients experiencing any TEAE was higher in the mavrilimumab treatment group (51.4% versus 42.6% in the golimumab treatment group). However, the majority of TEAEs were mild and not considered to be related to the study drug. The incidence of TESAEs and the incidence of TEAEs leading to drug discontinuation were low, and no laboratory abnormality signals were observed with mavrilimumab. No pulmonary safety signals were identified in the patients following the adjudication of lung function abnormalities and pulmonary AEs by independent experts. Notably, following treatment with mavrilimumab, no serious pulmonary TEAEs and no suspected or confirmed cases of pulmonary alveolar proteinosis were identified. Overall, the safety profiles of mavrilimumab and golimumab observed in this study were similar to those reported in previous studies 9, 10, 31.
The study was limited by not being statistically powered to directly compare the efficacy of mavrilimumab and golimumab. In addition, the population was heterogeneous. Although stratification allowed analysis of more homogeneous populations, the relatively low numbers of patients per stratum and per treatment group limited the interpretability and generalizability of the results. However, the strength of the study lies in its novel design. Because of the inclusion of an active comparator as a reference arm and a mixed population of patients with or without prior exposure to a biologic agent, this study offers an effective approach to investigating new biologic agents in a manner that reflects the current treatment landscape. Any new drug for the treatment of RA would enter a field of biologics and would be compared to other drugs rather than to placebo.
In summary, mavrilimumab at a dosage of 100 mg every other week was efficacious and well‐tolerated both in patients with a history of DMARD‐IR and in patients with a history of anti‐TNF–IR. Recently published data demonstrated that administration of mavrilimumab at a dosage of 150 mg every other week achieved greater efficacy than a dosage of 100 mg every other week in patients with DMARD‐IR 10, suggesting that a higher dosage of mavrilimumab (at least 150 mg every other week) may be expected to achieve improved benefit in RA patients with a history of an inadequate response to anti‐TNF agents. However, this hypothesis requires confirmation. In summary, these data demonstrate that meaningful benefits can be obtained by targeting the GM‐CSF receptor in a broad population of patients with active RA.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Weinblatt had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Weinblatt, McInnes, Kremer, Miranda, Guo, Ryan, Godwood, Close, Burmester.
Acquisition of data
Miranda, Vencovsky, Guo, Ryan, Godwood, Albulescu, Close, Burmester.
Analysis and interpretation of data
Weinblatt, McInnes, Kremer, Miranda, Vencovsky, Guo, White, Ryan, Godwood, Albulescu, Close, Burmester.
Role of the study sponsor
AstraZeneca/MedImmune facilitated the study design and reviewed and approved the manuscript prior to submission. The authors independently collected the data and were involved in drafting the article, critically reviewing it for important intellectual content, and making substantial contributions to the analysis and interpretation of the data. All of the authors had the final decision to submit the manuscript for publication. AstraZeneca/MedImmune funded writing assistance for the manuscript, which was provided by Katie Alexander, PhD (QXV Comms, Macclesfield, UK; an Ashfield Company, part of UDG Healthcare) in accordance with the Guidelines for Good Publication Practice for Communicating Company‐Sponsored Medical Research (http://www.ismpp.org/gpp3). Publication of this article was contingent upon approval by AstraZeneca/MedImmune.
Supporting information
Acknowledgments
The authors would like to thank the study investigators, patients, and the AstraZeneca/MedImmune Study Group.
Supported by AstraZeneca/MedImmune.
Dr. Weinblatt has received consulting fees and/or honoraria from AbbVie, Amgen, Novartis, Roche, GlaxoSmithKline, Merck, Samsung, Crescendo Bioscience, and AstraZeneca (less than $10,000 each) and Bristol‐Myers Squibb, Lilly, Pfizer, and UCB (more than $10,000 each), and has received research funding from Amgen, Bristol‐Myers Squibb, Crescendo Bioscience, Sanofi, and UCB. Dr. McInnes has received consulting fees, speaking fees, and/or honoraria from MedImmune, Janssen, Novartis, Roche, Pfizer, AstraZeneca, AbbVie, UCB, and Bristol‐Myers Squibb (less than $10,000 each). Dr. Kremer has received consulting fees, speaking fees, and/or honoraria from AbbVie, Bristol‐Myers Squibb, Lilly, Novartis, and Pfizer (less than $10,000 each). Dr. Miranda has received fees for realization of clinical trials from MedImmune, Roche, Jannsen, Sanofi Aventis Abbott, Merck Serono, and Amgen (more than $10,000 each). Dr. Vencovsky has received consulting fees, speaking fees, and/or honoraria from Biogen, Pfizer, UCB, AbbVie, Bristol‐Myers Squibb, MSD, Novartis, MedImmune, and Eli Lilly (less than $10,000 each). Drs. Guo, White, Godwood, and Close own stock in AstraZeneca. Dr. Ryan owns stock in AstraZeneca/MedImmune. Dr. Albulescu owns stock in MedImmune. Dr. Burmester has received honoraria from MedImmune (less than $10,000)
References
- 1. Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum 1996;39:115–24. [DOI] [PubMed] [Google Scholar]
- 2. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011;365:2205–19. [DOI] [PubMed] [Google Scholar]
- 3. Di Franco M, Gerardi MC, Lucchino B, Conti F. Mavrilimumab: an evidence based review of its potential in the treatment of rheumatoid arthritis. Core Evid 2014;9:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reynolds G, Gibbon JR, Pratt AG, Wood MJ, Coady D, Raftery G, et al. Synovial CD4+ T‐cell‐derived GM‐CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann Rheum Dis 2016;75:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiehn C, Wermann M, Pezzutto A, Hufner M, Heilig B. Plasma GM‐CSF concentrations in rheumatoid arthritis, systemic lupus erythematosus and spondyloarthropathy. Z Rheumatol 1992;51:121–6. [PubMed] [Google Scholar]
- 6. Bell AL, Magill MK, McKane WR, Kirk F, Irvine AE. Measurement of colony‐stimulating factors in synovial fluid: potential clinical value. Rheumatol Int 1995;14:177–82. [DOI] [PubMed] [Google Scholar]
- 7. Greven DE, Cohen ES, Gerlag DM, Campbell J, Woods J, Davis N, et al. Preclinical characterisation of the GM‐CSF receptor as a therapeutic target in rheumatoid arthritis. Ann Rheum Dis 2015;74:1924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Minter RR, Cohen ES, Wang B, Liang M, Vainshtein I, Rees G, et al. Protein engineering and preclinical development of a GM‐CSF receptor antibody for the treatment of rheumatoid arthritis. Br J Pharmacol 2013;168:200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burmester GR, Weinblatt ME, McInnes IB, Porter D, Barbarash O, Vatutin M, et al. Efficacy and safety of mavrilimumab in subjects with rheumatoid arthritis. Ann Rheum Dis 2013;72:1445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burmester G, McInnes IB, Kremer JM, Miranda P, Korkosz M, Vencovsky J, et al. Efficacy and safety/tolerability of mavrilimumab, a human GM‐CSFRα monoclonal antibody in patients with rheumatoid arthritis [abstract]. Arthritis Rheumatol 2014;66 Suppl 10:S1231. [Google Scholar]
- 11. Burmester GR, McInnes IB, Kremer J, Miranda P, Korkosz M, Vencovsky J, et al. A randomised phase 2b study of mavrilimumab, a novel GM‐CSF receptor α monoclonal antibody, in the treatment of rheumatoid arthritis. Ann Rheum Dis 2017;76:1020–30. [DOI] [PubMed] [Google Scholar]
- 12. Rubbert‐Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther 2009;11 Suppl 1:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 14. Furst DE, Pangan AL, Harrold LR, Chang H, Reed G, Kremer JM, et al. Greater likelihood of remission in rheumatoid arthritis patients treated earlier in the disease course: results from the Consortium of Rheumatology Researchers of North America registry. Arthritis Care Res (Hoboken) 2011;63:856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emery P. Optimizing outcomes in patients with rheumatoid arthritis and an inadequate response to anti‐TNF treatment. Rheumatology (Oxford) 2012;51 Suppl 5:22–30. [DOI] [PubMed] [Google Scholar]
- 16. Lundquist LM, Cole SW, Sikes ML. Efficacy and safety of tofacitinib for treatment of rheumatoid arthritis. World J Orthop 2014;5:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti–tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35–45. [DOI] [PubMed] [Google Scholar]
- 18. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. [DOI] [PubMed] [Google Scholar]
- 19. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 20. Borie R, Danel C, Debray MP, Taille C, Dombret MC, Aubier M, et al. Pulmonary alveolar proteinosis. Eur Respir Rev 2011;20:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juvet SC, Hwang D, Waddell TK, Downey GP. Rare lung disease II: pulmonary alveolar proteinosis. Can Respir J 2008;15:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum 1980;23:137–45. [DOI] [PubMed] [Google Scholar]
- 23. Van Gestel AM, Prevoo ML, van 't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis: comparison with the preliminary American College of Rheumatology and the World Health Organization/International League Against Rheumatism criteria. Arthritis Rheum 1996;39:34–40. [DOI] [PubMed] [Google Scholar]
- 24. Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005;7:R796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, et al. A Simplified Disease Activity Index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003;42:244–57. [DOI] [PubMed] [Google Scholar]
- 26. Ge M, Durham LK, Meyer RD, Xie W, Thomas N. Covariate‐adjusted difference in proportions from clinical trials using logistic regression and weighted risk differences. Drug Information Journal 2011;45:481–93. [Google Scholar]
- 27. Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient's perspective. J Rheumatol 1993;20:557–60. [PubMed] [Google Scholar]
- 28. Furst DE, Gaylis N, Bray V, Olech E, Yocum D, Ritter J, et al. Open‐label, pilot protocol of patients with rheumatoid arthritis who switch to infliximab after an incomplete response to etanercept: the opposite study. Ann Rheum Dis 2007;66:893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smolen JS, Kay J, Doyle M, Landewe R, Matteson EL, Gaylis N, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumor necrosis factor α inhibitors: findings with up to five years of treatment in the multicenter, randomized, double‐blind, placebo‐controlled, phase 3 GO‐AFTER study. Arthritis Res Ther 2015;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smolen JS, Kay J, Doyle MK, Landewe R, Matteson EL, Wollenhaupt J, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO‐AFTER study): a multicentre, randomised, double‐blind, placebo‐controlled, phase III trial. Lancet 2009;374:210–21. [DOI] [PubMed] [Google Scholar]
- 31. Kay J, Fleischmann R, Keystone E, Hsia EC, Hsu B, Mack M, et al. Golimumab 3‐year safety update: an analysis of pooled data from the long‐term extensions of randomised, double‐blind, placebo‐controlled trials conducted in patients with rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis. Ann Rheum Dis 2015;74:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


