Abstract
Nonalcoholic fatty liver disease (NAFLD) and resulting nonalcoholic steatohepatitis (NASH) are highly prevalent in the United States, where they are a growing cause of cirrhosis and hepatocellular carcinoma (HCC) and increasingly an indicator for liver transplantation. A Markov model was used to forecast NAFLD disease progression. Incidence of NAFLD was based on historical and projected changes in adult prevalence of obesity and type 2 diabetes mellitus (DM). Assumptions were derived from published literature where available and validated using national surveillance data for incidence of NAFLD‐related HCC. Projected changes in NAFLD‐related cirrhosis, advanced liver disease, and liver‐related mortality were quantified through 2030. Prevalent NAFLD cases are forecasted to increase 21%, from 83.1 million (2015) to 100.9 million (2030), while prevalent NASH cases will increase 63% from 16.52 million to 27.00 million cases. Overall NAFLD prevalence among the adult population (aged ≥15 years) is projected at 33.5% in 2030, and the median age of the NAFLD population will increase from 50 to 55 years during 2015‐2030. In 2015, approximately 20% of NAFLD cases were classified as NASH, increasing to 27% by 2030, a reflection of both disease progression and an aging population. Incidence of decompensated cirrhosis will increase 168% to 105,430 cases by 2030, while incidence of HCC will increase by 137% to 12,240 cases. Liver deaths will increase 178% to an estimated 78,300 deaths in 2030. During 2015‐2030, there are projected to be nearly 800,000 excess liver deaths. Conclusion: With continued high rates of adult obesity and DM along with an aging population, NAFLD‐related liver disease and mortality will increase in the United States. Strategies to slow the growth of NAFLD cases and therapeutic options are necessary to mitigate disease burden. (Hepatology 2018;67:123‐133).
Abbreviations
- DM
type 2 diabetes mellitus
- HCC
hepatocellular carcinoma
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NHANES
National Health and Nutrition Examination Survey
- SEER
Surveillance, Epidemiology, and End Results
Nonalcoholic fatty liver disease (NAFLD) is a growing cause of chronic liver disease globally.1, 2, 3 Characterized by excessive fat deposition in the liver that is not attributable to consumption of alcohol,4 the most common risk factors include obesity, insulin resistance, and the features of metabolic syndrome.5 While other conditions, such as genetic disorders of lipid metabolism, can also cause hepatic fat deposition, they are far less common than excess body weight and features of metabolic syndrome as risk factors for NAFLD.4
NAFLD can manifest as nonalcoholic fatty liver (NAFL) or nonalcoholic steatohepatitis (NASH). NASH has a substantially higher risk of progression to cirrhosis compared to NAFL. The fibrosis stage is important for monitoring the clinical risk of progression to cirrhosis and long‐term liver‐related outcomes and mortality.6 Most liver‐related outcomes occur once cirrhosis has developed, with the exception of hepatocellular carcinoma (HCC) that can occur even in the absence of cirrhosis.7 Thus, the need for health care resources increases substantially with progression to cirrhosis. NASH is rapidly rising as an etiology of end‐stage liver disease and is currently the second most common etiology of HCC requiring liver transplantation.8, 9 Increasing age, obesity, and type 2 diabetes mellitus (DM) have been consistently identified as risk factors for fibrotic progression to cirrhosis.5
There is currently no national public health policy addressing NAFLD. There is thus an unmet need to develop models to define the current and future impact of the disease to drive decision making with respect to research resource allocation, national screening, surveillance strategies, and outcomes assessment. Recent studies have provided an exhaustive assessment of the number of subjects with disease based on published studies of NAFLD and NASH as well as a model of disease and economic burden.10, 11, 12 However, this steady‐state model relied on existing literature on the epidemiology of NAFLD, which is confounded by varying case definitions, methods for assessment, small and heterogeneous populations, and variable analytic approaches. Finally, there is still no dynamic model that defines the health and health care burden of NAFLD over the decades to come.
The objective of this study was to develop a dynamic model of NAFLD to assess the health burden of the disease at a population level that allows a forecast of the health care impact of NAFLD. It is designed to be a “living document” by creating a conceptual framework that can be periodically updated based on emerging data. It is hoped that such a model will provide stakeholders a rationale for allocation of resources for the prevention and treatment of NAFLD.
Materials and Methods
MARKOV MODEL
The details of the Markov model are described in Supporting Sections S1‐S4, and an overview is presented here. The model began with the annual estimated number of incident NAFLD cases, which was back calculated using the change in incidence of obesity and DM in the United States and the estimated prevalence of NAFLD in 2015 (see Supporting Section S4 for details).
Disease progression through 2030 was estimated through METAVIR fibrosis stage and advanced liver disease (Fig. 1), with annual adjustment for all‐cause mortality (background mortality, excess cardiovascular deaths, and liver‐related deaths). Disease progression was simulated by multiplying the total number of cases at a particular stage of the disease by a progression rate to the next stage. Age‐specific fibrosis progression rates were back calculated based on assumptions for the distribution of cases by NASH status and fibrosis stage (Supporting Section S2). A critical step in developing the model was comparison of results to national registry data for liver cancer incidence and mortality.13 Progression rates were modified to ensure that model outputs aligned with reported data for the number of NAFLD‐related HCC cases, HCC deaths, and decompensated cirrhosis cases. Factors influencing rates of progression and regression in the NAFLD population are heterogeneous. For the purpose of the model, progression rates were assumed to be the sum of forward progression minus the rate of regression, which is common among NAFLD cases based on studies of consecutive liver biopsies.14
Figure 1.
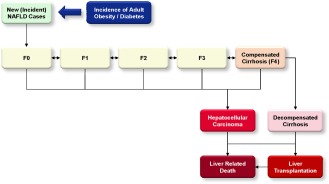
NAFLD Markov Model.
INPUTS
A literature search was used to identify research reporting the prevalence and incidence of NAFLD and NASH, including its stages. Indexed articles available on PubMed and nonindexed sources, such as national data reports, were used. In addition, the Delphi process (Supporting Section S5) was used in which experts were interviewed to identify and obtain consensus around crucial modeling inputs and to validate outputs for current and future disease burden against available empirical data. When input data were unavailable, expert input was used. Ranges were used to capture uncertainty in inputs with relatively larger ranges to imply greater uncertainty. NAFLD/NASH epidemiology data were reported in different years, and modeling was used to calibrate model outputs to time of data collection.
PREVALENCE
There are varied estimates of NAFLD prevalence in the general population. An estimated 17%‐51% of adults have NAFLD.4, 15, 16, 17 Analysis of liver ultrasound data collected between 1988 and 1994 from the Third National Health and Nutrition Examination Survey (NHANES III) reported that 19% of adults have NAFLD,18 while a meta‐analysis of studies from 2006‐2014 estimated a NAFLD prevalence of 24% (20%‐29%) in the general population.10
For this model, it was assumed that 30.0% of individuals aged ≥15 years of age in 2015 experienced NAFLD, with a large majority experiencing steatosis only. With this input, NAFLD prevalence in the total population was estimated at 25.8% after accounting for lower rates among persons aged <15 years, equivalent to approximately 83.1 million Americans with NAFLD.
AGE AND SEX DISTRIBUTION
The age and sex distribution of the NAFLD population was based on data from NHANES III, a national cross sectional survey including over 12,000 adults. This study defined NAFLD as steatosis as measured on ultrasound without evidence of elevated alcohol consumption.18 While prevalence rates are reported for individuals aged <30 years, it was assumed that prevalence would decline among the youngest age groups. The reported ratio of male to female prevalence varied by age group, with the lowest male/female ratio among individuals <30 years (0.94) and the highest among individuals aged 40‐49 years (1.31). The prevalence rates by age were adjusted proportionally to sum to 83.1 million NAFLD cases in 2015 (Fig. 2).
Figure 2.
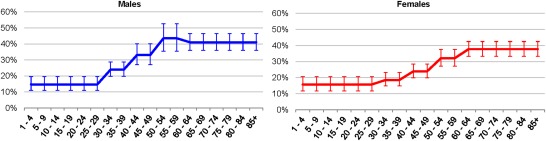
Adjusted prevalence of NAFLD by age group and sex (mean ± 95% confidence interval).
NASH‐STAGE STATUS
The prevalence of NASH was calculated based on published estimates and modeling of fibrosis progression. It was assumed that up to 5% of NAFLD cases without NASH could be NASH regressors, with most NASH regressors still in F0 stage. Therefore, a relatively small number of cases in stages F1‐F4 were assumed to be non‐NASH fatty liver. However, the vast majority of modeled fibrotic cases (F1‐F4) were assumed to be NASH.
Reported estimates show that 3%‐5% of adults in the United States have NASH.4, 16, 19, 20 This model assumes that approximately 20% of NAFLD cases would be classified as NASH in 2015, corresponding to 3% of the adult population. Estimates of NASH prevalence and fibrosis progression rates among NAFLD cases are subject to some uncertainty due to the variable populations studied and the largely retrospective methodology used to assess progression.21 For this analysis, it was estimated that 20%‐25% of those with NAFLD currently have NASH and that about 20% of those with NASH have ≥F3 fibrosis.21, 22, 23, 24, 25 A detailed list of studies reviewed for these estimates is provided in the supplemental documents available online (Supporting Section S6). It was also assumed that it would take 20‐30 years for progression to cirrhosis and the rates of decompensation in those with cirrhosis would be about 3%‐4% annually.26, 27
MORTALITY
Nonliver‐related background mortality rates by age and sex were calculated based on historical and medium fertility variant‐projected estimates for total deaths from the United Nations population database.28 Background rates were adjusted to account for incremental increased mortality related to cardiovascular disease. A range of estimates has been reported for excess mortality among NAFLD cases, with some studies demonstrating little increase and others suggesting significantly elevated cardiovascular mortality.29, 30, 31 A standard mortality ratio of 1.15 (1.00‐1.35) was applied to background mortality rates for all age groups. Liver‐related mortality is more markedly increased in the NAFLD population and was calculated separately as part of disease progression modeling.
TRANSPLANTS
Based on Organ Procurement and Transplantation Network data, there were 6,729 liver transplants performed in the United States in 2014.32 Of these, 619 were classified as fatty‐liver (NASH) cirrhosis.32 In addition, the Organ Procurement and Transplantation Network reports annual transplants classified as cryptogenic (idiopathic) cirrhosis. Of the 215 transplants under this classification in 2014, it was assumed that 42% (90 transplants) were NAFLD‐related cases based on obesity rates among cases with this diagnosis.9 The annual number of transplants was assumed to remain constant after 2014.
Results
NEW NAFLD CASES
The fastest growth in obesity prevalence occurred during 2000‐2002 (Supporting Fig. S4). In comparison, the fastest growth in DM prevalence occurred during 2012‐2014, although at a lower magnitude (Supporting Fig. S4). The increase in DM followed an increase in obesity after a 10‐14‐year delay. The increase in NAFLD cases followed obesity after a 5‐8‐year delay, resulting in a modeled peak NAFLD incidence in 2008 when an estimated 4.17 million new cases occurred (Supporting Fig. S5). Since then, a slowing rate of increase in NAFLD was forecasted, and new cases were estimated to decline to 3.62 million annually. Thus, the total number of NAFLD cases is still increasing but at a lower rate compared to the 2005‐2008 period.
TOTAL NAFLD POPULATION
The total NAFLD population in 2015 was estimated at 83.1 million cases (Fig. 3A), with a prevalence rate of 30.0% among the population aged ≥15 years and 25.8% among all ages. By 2030, the NAFLD population was projected to increase 21% to 100.9 million cases. Prevalence in 2030 is estimated at 33.5% (aged ≥15 years) and 28.4% (all ages). The median age of the NAFLD population was estimated at 50 years of age (2015), increasing to 55 years (2030). During 2015‐2030, there would be 1.2 prevalent NAFLD cases among male individuals for every 1.0 among female individuals.
Figure 3.
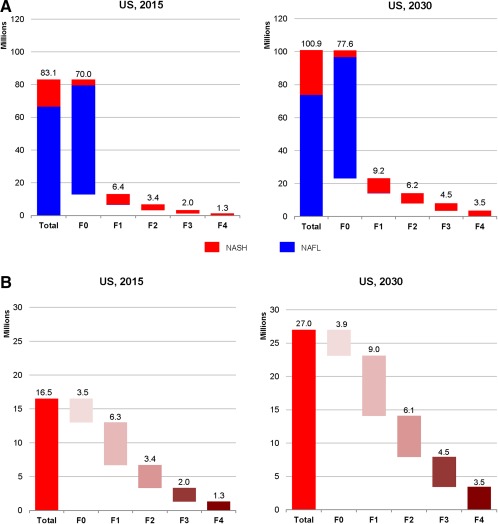
Distribution of the NAFLD and NASH population by fibrosis stage in the United States for 2015 and 2030. (A) NAFLD; (B) NASH.
STEATOSIS‐ONLY POPULATION
The NAFL population classified as steatosis only included those who have not progressed to NASH as well as cases regressed from NASH status. This population increased 11% from 66.6 million cases in 2015 to 73.9 million in 2030 (Fig. 4). The total number of fibrotic (≥F1) cases among the NAFL‐prevalent population was estimated at 113,000 in 2015 (0.17% of steatosis‐only population) and increased to 178,000 cases (0.24% of steatosis‐only population) by 2030 (Fig. 4). Among this group, there were an estimated 2,110 cases with cirrhosis or HCC in 2015, increasing 164% to 5,580 prevalent cases in 2030.
Figure 4.
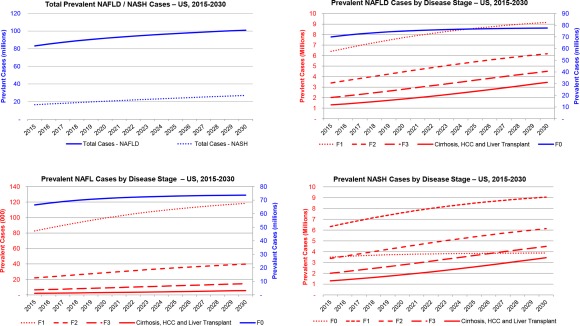
Prevalent NAFLD, NAFL, and NASH cases in the United States, 2015‐2030.
Annual incident NAFL cases were assumed to be the number of individuals developing hepatic steatosis in a given year and not the number of newly diagnosed cases. In 2015, an estimated 3.44 million incident NAFL cases occurred. By 2030, incident NAFL cases decreased 31% to 2.50 million. Peak incident cases occurred in 2008 with 4.17 million annual cases. The gradual decline in new cases is reflective of slowing in the growth of the obese population.33 While the annual number of incident NAFL cases declined since 2008 in the model, the number of prevalent cases and the prevalence rate continued to increase, albeit at a slower rate than in decades past.
NASH POPULATION
The number of NASH cases is projected to increase 63% from 16.52 million cases in 2015 to 27.00 million in 2030 (Fig. 3B). The proportion of NAFLD cases classified as NASH is expected to increase from 20% to 27%, a reflection of disease progression, increasing proportion of diabetic individuals, and an aging population. Among NASH cases in 2015, an estimated 20% have F3/F4 fibrosis or advanced liver disease, encompassing approximately 3.31 million cases (Fig. 4). By 2030, this number is expected to increase over 160% to 7.94 million cases and will account for 29% of NASH cases. Compensated cirrhosis cases among the NASH population increased 163% from 1.16 million cases to 3.05 million during 2015‐2030. Increases were relatively smaller for earlier fibrosis stages; F3 cases increased 124% to 4.49 million cases in 2030, F2 increased 82% (6.13 million in 2030), F1 increased 43% (9.05 million in 2030), and F0 increased only 11% to 3.88 million cases by 2030 (Fig. 4).
DECOMPENSATED CIRRHOSIS
The prevalent number of decompensated cirrhosis cases increases 180% from 134,400 cases in 2015 to 376,100 by 2030. Incident decompensated cirrhosis is forecasted to increase by 168%, from 39,320 cases annually in 2015 to 105,430 cases in 2030, while cumulative incidence during 2015‐2030 was estimated at 1.10 million cases (Fig. 5). The cumulative incidence is much higher than the prevalent population due to the high mortality rate. Liver transplant cases (including previously transplanted minus mortality) were estimated to increase 59% from 4,780 to 7,610 cases during 2015‐2030; however, there is substantial uncertainty surrounding the availability and allocation of donor organs in future years.
Figure 5.
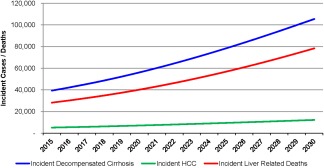
Incident decompensated cirrhosis, HCC, and liver‐related deaths among the prevalent NAFLD population in the United States, 2015‐2030.
HCC
Prevalent HCC cases are expected to increase from 10,100 to 24,900 during 2015‐2030, an increase of 146%, while incident HCC cases are expected to increase by 137% from 5,160 to 12,240 in 2030 (Fig. 5). The cumulative incidence of HCC during 2015‐2030 was estimated at 135,000 cases, much higher than prevalent cases due to high mortality. In 2015, 3,280 incident HCC cases were estimated to have progressed from compensated cirrhosis (64% of total), with the remaining 1,880 incident cases occurring among ≤F3 cases. By 2030, 8,790 incident HCC cases occurred among compensated cirrhotic cases or 72% of the annual incidence, reflecting aging and disease progression.
MODEL VALIDATION
Surveillance, Epidemiology, and End Results (SEER) studies reported rates of HCC13, 34 and provided proportions of HCC attributable to NAFLD1 during 2004‐2009 (range, 13.3%‐20.3%). The studies suggest that over 17,000 NAFLD‐related incident HCC cases occurred during the time period. In comparison, the model predicted 17,720 incident HCC cases during the same time period.
Modeling suggests that the proportion of HCC cases in the United States attributable to NAFLD has increased since 2009 and will likely increase in the coming decades. SEER projects 39,230 incident liver and intrahepatic bile duct cancers in 2016.35 Assuming that 72% of incident cancers are classified as HCC,34 there would be an estimated 28,250 incident HCC cases in 2016. The model predicts 5,510 incident HCC cases in 2016 attributable to NAFLD, approximately 20% of projected total HCC incidence in the United States compared to the 12.6%‐15.8% reported in the literature for 2004‐2009.1 SEER also estimates 27,170 deaths attributable to liver and intrahepatic bile duct cancers in 2016. Using the same assumption for the proportion of HCC, there would be an estimated 19,560 HCC deaths in 2016. The model predicts 4,460 liver‐related deaths among the HCC population in 2016, equivalent to 23% of total projected HCC deaths. A higher proportion of HCC mortality compared to HCC incidence is consistent with studies demonstrating relatively higher mortality among NAFLD‐attributable HCC compared to other etiologies.1
MORTALITY
Total deaths among the NAFLD population in 2015 are estimated at 1.27 million with 85.0% classified as general background, 12.8% as excess cardiovascular, and 2.2% (28,200 deaths) as excess liver‐related deaths. By 2030, total deaths are projected to increase 44% to 1.83 million deaths annually. General background and excess cardiovascular deaths will account for 83.2% and 12.5% of deaths, respectively. Liver deaths are projected at 78,300 in 2030, an increase of 178% from 2015 (Fig. 5). Among an estimated cumulative 24.90 million deaths during 2015‐2030, over 3% (799,900 deaths) will represent excess liver‐related mortality. Deaths attributed to HCC (110,900 deaths) were estimated to account for 14% of liver deaths, whereas decompensated cirrhosis accounted for 85% (683,400 deaths), and the remaining 5,590 liver deaths were projected to occur among the liver transplant population. Among NAFLD cases classified as cirrhotic, HCC, or transplant, a higher proportion of deaths were liver related. In these groups, there were a total of 78,400 deaths in 2015 and 28,200 (36%) were liver related, increasing to 206,300 total deaths in 2030, of which 38% (78,300 deaths) were liver related. If compensated cirrhosis cases were excluded from the estimates, over 80% of all deaths during the time period in this group would be liver related.
MORTALITY IN THE NAFL POPULATION
Among the population with steatosis only, 902,300 total deaths occurred in 2015, increasing 23% to 1,109,600 by 2030. During 2015‐2030, there was a total of 16,335,400 deaths; 2,130,700 were classified as excess cardiovascular attributable to the elevated mortality risk in this population compared to the general United States population, accounting for 13% of all deaths in this population.
It was assumed that all liver‐related deaths occurred among the NASH population. Extrapolation based on estimated rates of disease progression among the steatosis‐only population suggests that <0.2% of liver‐related deaths in 2015 would occur among NAFLD cases without NASH (e.g., NASH regressors).
MORTALITY IN THE NASH POPULATION
In 2015, there were an estimated 370,000 deaths among the NASH population, equivalent to 29% of total NAFLD deaths; this reflects the advanced age and increased rates of liver disease in this population. By 2030, nearly 40% of deaths among NAFLD cases occurred among the NASH population, approximately 716,800 annual deaths. During 2015‐2030, >90% of all deaths in the NASH population was classified as general background or excess cardiovascular.
There were an estimated 28,200 liver‐related deaths among the NASH population (7.6% of total deaths) in 2015, increasing to 78,300 or 10.9% of total deaths in 2030. Excess cardiovascular deaths comprised approximately 13% of overall mortality during 2015‐2030 (1,012,400 total deaths), and the proportion did not change substantially during the time period.
Discussion
This study represents a modeling approach to forecast the current and future burden of disease due to NAFLD in the United States, incorporating real‐world surveillance data for HCC incidence and other outcomes to validate the results. As the national obesity prevalence levels off, it is estimated that the prevalence of NAFLD will also level off. However, the proportion of subjects within the NAFLD population with NASH is likely to continue to rise through 2030 based on the rising prevalence of DM. The proportion of diabetic subjects with NASH is higher than in a general obese population10, 18, 36; thus, the total burden of disease due to NASH will likely rise for the next 15 years.
The current analysis has several potential implications. First, if effective strategies to prevent or treat NASH are not introduced, an exponential increase in mortality related to NASH is expected. This trend is supported by literature documenting the growing contribution of NASH as an etiology for end‐stage liver disease requiring transplantation.1, 9 Liver transplantation, however, is expensive, and many subjects with NASH‐related cirrhosis will not qualify for liver transplantation due to associated comorbidities. Furthermore, the total number of liver transplants that can be performed is limited by the availability of organs.32 The increase in the number of individuals with decompensated cirrhosis may thus swamp the supply of organs and is likely to pose a major public health problem related to organ availability for other liver diseases.
Another important implication of the model is the projected increase in the number of individuals with cirrhosis, especially decompensated cirrhosis due to NASH. It is already well established that the management of cirrhosis is resource intensive and is a major drain on hospital resources, adding to the cost of care.37, 38 The projected rise in the number of patients with decompensated cirrhosis will have a commensurate impact on hospital resources and the overall cost of health care. Depending on the distribution of uninsured or inadequately insured individuals within the population, this is likely to strain the health care budget for such populations. Even in health systems with substantial coverage, such as the Veterans Administration,39 the burden of cirrhosis is substantial and poses a major emotional and financial burden on subjects and their caregivers. These will contribute to a ripple effect magnifying the negative consequences of the increase in number of subjects with cirrhosis due to NASH.
Insurance claim data indicate that NAFLD is already the principal etiology contributing to the burden of HCC.1 Yet another negative consequence of the growing burden of NAFLD is likely to be a further increase in HCC. The output of this model is concordant with projections from the SEER data (Supporting Section S2). While this cancer can theoretically be treated effectively by early identification and liver transplantation, most patients are detected at a stage where liver transplantation is not an option.40 Furthermore, most patients with the disease remain undiagnosed and therefore progress silently until a diagnosis is made due to serendipity or when a major complication occurs. The problem is further heightened by a current lack of effective preventive strategies for HCC in subjects with cirrhosis.40 There is therefore a need to tackle the problem of HCC at multiple levels.
A unique aspect of the current analysis is that it accounts for competing mortality due to background and excess cardiovascular mortality in this population. Despite this, there will clearly be a substantial increase in liver‐related mortality in those afflicted by NASH. A critical factor that is likely to drive the increase in NASH‐related mortality is the increased number and proportion of subjects who will have cirrhosis. This is linked to both the aging of the population, which is a known risk factor for having more advanced disease,5 and the natural progression of the disease toward more advanced stages.4, 14 Importantly, in this study, spontaneous regression was estimated from published literature and back calculation and then applied to the prevalent population in order to generate the most accurate projections for disease progression possible.
As with many models, the utility of the model is linked to the validity of the inputs into the model. The interpretation of this and other models attempting to address the burden of NAFLD are limited by the inability to accurately diagnose steatohepatitis with simple epidemiologic tools. Thus, even in the NHANES data set, the assumptions about NASH are based on a moderately accurate post‐hoc application of liver enzymes and clinical aids in the NAFLD population.22, 41 Similarly, the fibrosis stages in population‐based studies represent best estimates based on the use of clinical aids, such as the Fibrosis‐4, Aspartate Aminotransferase to Platelet Ratio Index, and NAFLD fibrosis scores.21, 42, 43 Studies with histologic assessment are also limited due to ascertainment bias and inherent variability in histologic assessment of NAFLD stage and activity. Moreover, the bulk of the literature on fibrosis progression rates is based on retrospective analysis of various cohorts. The projected increase in DM by the Centers for Disease Control and Prevention is also likely to impact the fibrosis progression rates, given the close link between DM and fibrosis in those with NAFLD.44, 45
Given these uncertainties, we performed an exhaustive literature search (list provided in Supporting Section S6) and used conservative estimates representing the middle ground in the field and paid particular attention to the available data from placebo arms of published, prospective, randomized clinical trials. It is reassuring that a prospective study of transient elastography in the general population demonstrated that of those with DM and steatosis defined by the continuous attenuation parameter, 17.2% had a liver stiffness ≥8 kPA, indicative of clinically significant fibrosis.45 These data are relatively similar to multiple biopsy‐based studies.21, 23, 24, 25 The projections on the prevalence of F3/F4 fibrosis are also aligned with recent assessments from the NHANES data.22 As new data with more accurate estimates of fibrosis progression rates become available, the model will need to be refined.
Another limitation to this analysis relates to uncertainty around projections for NAFLD‐related advanced liver disease, HCC, and mortality. While the number of incident HCC cases and liver transplants attributed to NAFLD is increasing, this is potentially a result of greater recognition and accuracy in classifying such cases. If this is true, then modeling validated using national surveillance data for incident HCC could overstate the increasing trajectory of disease burden and mortality. A follow‐up study of NAFLD cases from the NHANES III cohort for a median of 14.5 years did not find elevated mortality (general, cardiovascular, or liver‐related)46; however, such results are potentially the result of insufficient length of follow‐up.47 Given that cases of advanced liver disease and related mortality form a small portion of the total NAFLD population and that such cases can take many years to experience disease progression, there are notable limitations to follow‐up studies focused on the general NAFLD population. As such, there is uncertainty around projections of future burden of NAFLD‐related advanced liver disease and mortality.
Compared to the model presented by Younossi et al.,12 our model presents similarities and differences. The models differed in the number of disease states as well as disease‐state transition and mortality rates (Supporting Fig. S2). However, both models were calibrated to published data whenever accurate estimates were available. The current model is dynamic, assessing changes over time in NAFLD incidence using obesity and DM trends. This differs from steady‐state models that assume constant transition rates. The models also differ in the estimated total number of NAFLD and NASH cases. This is largely due to the assumption used in the current model of increasing incidence of NAFLD based on substantial increases in adult obesity and DM cases since the collection of NHANES III data, while NAFLD prevalence in the prior model is reflective of data from NHANES III. In addition, the previous model includes progression to NASH as an input, while the current model bases fibrosis progression on NASH prevalence and assumptions for the proportion of NASH in each fibrosis stage. The overall results of both models are consistent; they demonstrate that NAFLD and NASH prevalence, along with resulting advanced liver disease, are increasing over time, highlighting the need for action to mitigate disease burden.
This analysis establishes the substantial burden of liver disease associated with NAFLD/NASH, using national surveillance data for end‐stage liver disease to validate the model. It underscores the need for public health measures to more accurately define disease prevalence, development of surveillance strategies, and eventually preventive and therapeutic approaches to tackle this health care challenge.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29466/suppinfo
Supporting Information 1
Potential conflict of interest: Dr. Younossi consults, advises, and received grants from Gilead and Intercept. He consults for Bristol‐Myers Squibb, Novo Nordisk, Sanofi, and AbbVie. Dr. Loomba consults, advises, and received grants from Gilead and Bristol‐Myers Squibb. He consults and advises Gemphire and Conatus. He consults and received grants from Merck and NGM. He advises and received grants from Galmed, Intecept, Tobira, and Octeta. He consults for Pfizer, Fibrogen, Alnylam, DeuteRx, Zafgen, RuiYi, Shire, Scholar Rock, birdrock, Metacrine, Viking, Receptos, Ionis, Enanta, Celgene, Genkyotex, Nitto Denko, CNI, Boehringer Ingelheim, Eli Lilly, Janssen, KHK, Yuhan, Coh Bar, Allergan, Kowa, Medimmune, and Ph Pharma. He advises Nimbus and Arrowhead. He received grants from Promedior, Kinemed, Adheron, Immuron, Siemens, GE, Genfit, Galectin, Arisaph, Madrigal, and Daiichi‐Sankyo. Dr. Sanyal consults, advises and received grants from Conatus, malinckrodt, Gilead, and Salix. He consults, advises, and owns stock in Hemoshear. He consults and advises Pfizer, Boehringer Ingelheim, Nimbus, Nitto Denko, Lilly, and Ardelyx. He received grants from Novartis, Galectin, Bristol‐Myers Squibb, Merck, and Sequana. He is employed by Sanyal Bio. He received royalties from Elsevier and Uptodate. He owns stock in Exhalenz, Akarna, GenFit, Durect, and Indalo.
Supported by Intercept Pharmaceuticals, Gilead Sciences, and Boehringer Ingelheim. The funders had no role in the study design, data collection, analysis, interpretation of data, or preparation of the manuscript.
REFERENCES
- 1. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723‐1730. [DOI] [PubMed] [Google Scholar]
- 2. Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population‐based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin 2010;26:2183‐2191. [DOI] [PubMed] [Google Scholar]
- 3. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686‐690. [DOI] [PubMed] [Google Scholar]
- 4. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al.; American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology . The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592‐1609. [DOI] [PubMed] [Google Scholar]
- 5. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846‐854. [DOI] [PubMed] [Google Scholar]
- 6. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 7. White DL, Kanwal F, El‐Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol 2012;10:1342‐1359.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 9. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 10. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of non‐alcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 11. Younossi ZM, Henry L. Economic and quality‐of‐life implications of non‐alcoholic fatty liver disease. Pharmacoeconomics 2015;33:1245‐1253. [DOI] [PubMed] [Google Scholar]
- 12. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64:1577‐1586. [DOI] [PubMed] [Google Scholar]
- 13. National Cancer Institute . Surveillance, Epidemiology, and End Results Program. SEER research data (1973‐2013). www.seer.cancer.gov/data. 2016. Accessed August 10, 2016.
- 14. Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta‐analysis of paired‐biopsy studies. Clin Gastroenterol Hepatol 2015;13:643‐654.e1‐e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221‐1231. [DOI] [PubMed] [Google Scholar]
- 16. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263‐2273. [DOI] [PubMed] [Google Scholar]
- 17. Kim YS, Jung ES, Hur W, Bae SH, Choi JY, Song MJ, et al. Noninvasive predictors of nonalcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin Mol Hepatol 2013;19:120‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988‐1994. Am J Epidemiol 2013;178:38‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rinella ME. Will the increased prevalence of nonalcoholic steatohepatitis (NASH) in the age of better hepatitis C virus therapy make NASH the deadlier disease? Hepatology 2011;54:1118‐1120. [DOI] [PubMed] [Google Scholar]
- 20. Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274‐285. [DOI] [PubMed] [Google Scholar]
- 21. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al.; Nonalcoholic Steatohepatitis Clinical Research Network . Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313‐1321. [DOI] [PubMed] [Google Scholar]
- 22. Kabbany MN, Conjeevaram Selvakumar PK, Watt K, Lopez R, Akras Z, Zein N, et al. Prevalence of nonalcoholic steatohepatitis‐associated cirrhosis in the United States: An analysis of National Health and Nutrition Examination Survey data. Am J Gastroenterol 2017;112:581‐587. [DOI] [PubMed] [Google Scholar]
- 23. Ground KE. Liver pathology in aircrew. Aviat Space Environ Med 1982;53:14‐18. [PubMed] [Google Scholar]
- 24. Grant LM, Lisker‐Melman M. Nonalcoholic fatty liver disease. Ann Hepatol 2004;3:93‐99. [PubMed] [Google Scholar]
- 25. Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle‐aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124‐131. [DOI] [PubMed] [Google Scholar]
- 26. Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682‐689. [DOI] [PubMed] [Google Scholar]
- 27. Bhala N, Angulo P, van der Poorten D, Lee E, Hui JM, Saracco G, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. United Nations . Department of Economic Social Affairs Population Division. World population prospects: the 2015 revision. New York: United Nations; 2016. [Google Scholar]
- 29. Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all‐cause mortality and liver‐related mortality in patients with non‐alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017‐3023. [DOI] [PubMed] [Google Scholar]
- 30. Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62(1 Suppl.):S47‐S64. [DOI] [PubMed] [Google Scholar]
- 31. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Nonalcoholic fatty liver disease and risk of incident cardiovascular disease: a meta‐analysis. J Hepatol 2016;65:589‐600. [DOI] [PubMed] [Google Scholar]
- 32. Organ Procurement and Transplantation Network . OPTN data as of October 28, 2016. https://optn.transplant.hrsa.gov/data/. Updated 2016. Accessed November 14, 2016.
- 33. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011‐2012. JAMA 2014;311:806‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Cancer Institute . Surveillance, Epidemiology, and End Results Program. Cancer stat facts: liver and intrahepatic bile duct cancer. http://seer.cancer.gov/statfacts/html/livibd.html. Updated August 2016. Accessed September 22, 2016.
- 36. Portillo‐Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrino Metab 2015;100:2231‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neff GW, Duncan CW, Schiff ER. The current economic burden of cirrhosis. Gastroenterol Hepatol 2011;7:661‐671. [PMC free article] [PubMed] [Google Scholar]
- 38. Whalley S, Puvanachandra P, Desai A, Kennedy H. Hepatology outpatient service provision in secondary care: a study of liver disease incidence and resource costs. Clin Med (Lond) 2007;7:119‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanwal F, Kramer JR, Duan Z, Yu X, White D, El‐Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States cohort of veterans. Clin Gastroenterol Hepatol 2016;14:301‐308.e1‐e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goossens N, Hoshida Y, Song WM, Jung M, Morel P, Nakagawa S, et al. Nonalcoholic steatohepatitis is associated with increased mortality in obese patients undergoing bariatric surgery. Clin Gastroenterol Hepatol 2016;14:1619‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network . Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2009;7:1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut 2010;59:1265‐1269. [DOI] [PubMed] [Google Scholar]
- 44. Gregg EW, Boyle JP, Thompson TJ, Barker LE, Albright AL, Williamson DF. Modeling the impact of prevention policies on future diabetes prevalence in the United States: 2010‐2030. Popul Health Metr 2013;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 2016;63:138‐147. [DOI] [PubMed] [Google Scholar]
- 46. Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non‐alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wattacheril J, Chalasani N. Nonalcoholic fatty liver disease (NAFLD): is it really a serious condition? Hepatology 2012;56:1580‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.29466/suppinfo
Supporting Information 1


