Abstract
Background
Implementation of noninvasive prenatal testing (NIPT) as a highly accurate aneuploidy screening test has raised questions around whether the high uptake may result in more terminations of pregnancies and fewer births of children with Down syndrome (DS).
Aim
The aim of the study was to investigate the impact of NIPT on termination and live birth rates for DS.
Methods
Literature reporting pregnancy outcomes following NIPT was reviewed. Termination rates were calculated for women with a high‐risk NIPT result for DS. Two audits of pregnancy outcomes where NIPT indicated DS were conducted in the United Kingdom and Singapore.
Results
Fourteen studies from the United States, Asia, Europe, and the United Kingdom were included in the review. Live births of children with DS were reported in 8 studies. Termination rates following NIPT were unchanged or decreased when compared to termination rates prior to the introduction of NIPT. Audits found 15 of 43 women in the United Kingdom and 2 of 6 in Singapore continued pregnancies following a high‐risk NIPT result.
Conclusions
Termination rates following the detection of DS by NIPT are unchanged or decreased compared to historical termination rates. Impact on live birth rates may be minimal in settings where termination rates fall. Population‐based studies are required to determine the true impact.
Short abstract
WHAT IS ALREADY KNOWN ABOUT THIS TOPIC?
Noninvasive prenatal testing (NIPT) has been shown to be a highly accurate prenatal screening test for DS and is being implemented widely throughout the world.
Introduction of NIPT has increased the prenatal detection of DS with a significantly reduced invasive testing rate, but the impact on rates of termination of pregnancy and the number of children born with DS is not yet known.
WHAT DOES THIS STUDY ADD?
Introduction of NIPT has a variable effect on termination rates for DS, but rates have remained unchanged or decreased when compared to termination rates reported prior to the introduction of NIPT, with many parents using NIPT for information and continuing pregnancies when results show a high risk of DS.
Practical and emotional support structures are needed for these families.
Where termination rates fall NIPT may have a minimal impact on live birth rates for DS.
Monitoring at population levels is required for a more accurate assessment of live birth rates.
1. INTRODUCTION
Noninvasive prenatal testing (NIPT) for aneuploidy based on analysis of cell‐free DNA (cfDNA) in the maternal plasma became available in the private sector in 2011 and is now being offered widely throughout the world.1 NIPT is a highly accurate screening test that can be used from 10 weeks in pregnancy to detect Down syndrome (DS) (Trisomy 21) with high sensitivity (99%) and specificity (99.5%).2 However, NIPT is not diagnostic, and confirmation of a positive result by invasive testing (chorionic villus sampling or amniocentesis) is recommended.3 NIPT has been shown to be accurate in both the high‐risk and general pregnancy populations,4 and the use of NIPT as a screening test has been endorsed by professional bodies from several countries who encourage provision of pretest and posttest counseling to support informed choice.3, 5 NIPT has a much greater sensitivity than traditional screening methods such as the combined test that measures nuchal translucency and maternal serum biochemistry and a growing number of studies have confirmed that the introduction of NIPT into the screening pathway has significantly reduced the need for invasive testing.6, 7, 8, 9, 10
Overall, key stakeholders, such as pregnant women and health professionals, are very positive about the introduction of NIPT and highlight benefits such as safety, accuracy, and the detection of pregnancies affected with DS that would have been missed with traditional screening.11, 12, 13, 14 One of the questions raised in the literature about the widespread implementation of NIPT for DS is whether it will lead to an increase in the number of parents seeking prenatal testing and termination of pregnancy with a resultant significant decrease in the number of children born with DS.11, 12, 15, 16, 17 Research with pregnant women11, 12 and parents of children with DS16, 17 identified concerns that fewer children being born with DS could result in a reduction of social supports and resources for children with disabilities and a society that is less tolerant of people who have children with disabilities. Furthermore, these key stakeholder groups felt that a less tolerant society could increase feelings of pressure for pregnant women to have testing and subsequently terminate an affected pregnancy.11, 12, 16
Interest in and uptake of NIPT is high,7 making it likely that the number of parents opting for prenatal testing for DS will grow as many parents who would not have previously opted for prenatal testing because of the risk of miscarriage would be willing to have NIPT.11, 18 It is not yet clear, however, whether the increase in the numbers of women having prenatal testing will directly result in more terminations of pregnancy. There will be wide variation in the number of children born with DS between and even within countries depending on attitudes to prenatal testing, disability, and termination that are influenced by religious, social, and cultural settings, costs of prenatal testing and access to termination of pregnancy. Ultimately, we will see the impact of NIPT on the number of children born with DS from long‐term population‐based studies comparing live birth rates before and after the introduction of NIPT. These population‐based studies are crucial as they reflect the number of children born to parents who have chosen to continue the pregnancy after a prenatal diagnosis of DS as well as the number of children born to parents who decided not to have prenatal diagnosis and then had a baby with DS.
Several studies conducted prior to the introduction of NIPT have reported the number of children born with DS compared to expected numbers based on the prevalence of DS in the general population. These figures vary between countries: in the United States there were 30% fewer individuals with DS (2007),19 50% fewer in the Netherlands (2015),20 48% fewer in England and Wales (2008),21 55% fewer in Australia (2004),22 94% fewer in Taiwan (2010),23 and 55% fewer in China (2011).24 As the numbers of older women giving birth have grown, the numbers of pregnancies affected with DS have also increased. However, in England and Wales21 and in Europe25 the live birth rate remained relatively unchanged between 1990 and 2009 even though prenatal screening and diagnosis became more common over the same period. In contrast, in the United States, the live birth rate has increased since the early 1990s through to 2007.19 One small regional study conducted in the Hampton Roads area of Virginia reported that NIPT has not affected the number of children born with DS in this area.7 More time is needed to see the impact of NIPT on the live birth rate more widely and conclusively.
While we await definitive population‐based studies, there are several lines of evidence available to us now that can help assess the impact the introduction of NIPT might ultimately have on the numbers of parents choosing to continue their pregnancy following a prenatal diagnosis of DS. Here we examine the literature to look at reports of pregnancy outcomes following NIPT. We also describe 2 new audits of NIPT services in clinical practice that were conducted in England and in Singapore.
What is already known about this topic?
Noninvasive prenatal testing (NIPT) has been shown to be a highly accurate prenatal screening test for DS and is being implemented widely throughout the world.
Introduction of NIPT has increased the prenatal detection of DS with a significantly reduced invasive testing rate, but the impact on rates of termination of pregnancy and the number of children born with DS is not yet known.
What does this study add?
Introduction of NIPT has a variable effect on termination rates for DS, but rates have remained unchanged or decreased when compared to termination rates reported prior to the introduction of NIPT, with many parents using NIPT for information and continuing pregnancies when results show a high risk of DS.
Practical and emotional support structures are needed for these families.
Where termination rates fall NIPT may have a minimal impact on live birth rates for DS.
Monitoring at population levels is required for a more accurate assessment of live birth rates.
2. METHODS
2.1. Literature review
We reviewed the published literature reporting the number of live births of children with DS following screening with NIPT. A search of English‐language articles from the time NIPT entered clinical practice (January 1, 2011, to September 25, 2017) was conducted. We searched the PubMed electronic database using the following search terms: “cell free fetal DNA,” “NIPT” or “Non‐invasive prenatal test*” or “noninvasive prenatal test*” or “NIPD” or “non‐invasive prenatal diagnosis” or “noninvasive prenatal diagnosis.” A manual search of the reference lists of included studies and relevant original and review articles was also performed. Publications were included if they described data on numbers of women having NIPT for DS and provided information on pregnancy outcomes, such as live births, pregnancy termination, fetal demise, or stillbirths. Studies that described modelled data were excluded. The search identified 1726 articles. Titles and abstracts were examined by 1 reviewer (M.H.), and full‐text articles were obtained for 87 potentially relevant articles. Sixteen articles describing 14 studies met the inclusion criteria.8, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40 Formal quality appraisal of individual studies was not undertaken as the data sought generally comprised only a small component of the overall study. Data describing the study setting and the pregnancy outcomes were independently extracted by 2 reviewers (M.H., A.B.). A narrative synthesis of studies was then performed. Termination rates were calculated as a proportion of all pregnancies with a high‐risk NIPT result (excluding false positives and negatives). This includes cases confirmed with invasive testing and those confirmed at birth without confirmatory invasive testing. Where additional data were available, termination rates were calculated for women going directly to invasive testing.
A separate search was conducted to identify published termination of pregnancy rates prior to introduction of NIPT. Termination rates were sought for each of the countries where the studies included in the review were conducted. The PubMed electronic database was searched using the following terms: “country name” AND “Down* syndrome” AND “termination” or “abortion” or “live‐birth rates.”
2.2. Audit of pregnancy outcomes following NIPT as a clinical service in Singapore
NIPT has been offered at the National University Hospital Singapore since April 2014. NIPT is offered to all patients as an out‐of‐pocket test, with no subsidies. Invasive testing is recommended for confirmation of a high‐risk NIPT result. Other criteria for offering invasive diagnostic testing include a previous affected pregnancy, a high‐risk combined screening test, advanced maternal age, and structural abnormalities. A retrospective audit of pregnancy outcomes for women who chose to have NIPT or went straight to invasive testing without prior NIPT from April 1, 2014, to January 31, 2017, was conducted (DSRB number 2016/00253). Termination rates were calculated as a proportion of all pregnancies that had a high‐risk NIPT result (false positives and negatives excluded). Termination rates were also calculated for women going directly to invasive testing without NIPT.
2.3. Audit of pregnancy outcomes following NIPT offered as a clinical service in the United Kingdom
Following the development and validation of a cfDNA sequencing protocol by our NHS service laboratory (North East Thames Regional Genetics Service), NIPT was initially offered as part of a research trial in 8 UK maternity units, which demonstrated that NIPT could be successfully offered as a contingent test without increasing costs in the NHS.35 One weakness of this study was that there was potentially more pretest counseling offered by the research team than might be available in clinical practice. To evaluate informed choice in routine maternity care the study was extended and NIPT was offered by local maternity staff trained to discuss NIPT in North Thames units to women with combined test risk of ≥1/150 from March 1, 2015, to October 31, 2016. Invasive testing to confirm a high‐risk NIPT result was recommended. An audit of pregnancy outcomes was conducted (registered at Great Ormond Street Hospital NHS Foundation Trust as a service evaluation). The laboratory database of NIPT tests performed was reviewed, and a request was made to each referring hospital to give details of the pregnancy outcomes of all women who chose to have NIPT. Termination rates were calculated as a proportion of all pregnancies with a high‐risk NIPT result (excluding false positives and negatives).
3. RESULTS
3.1. Literature review of pregnancy outcomes following NIPT
Fourteen studies were included in the review, 8 were prospective8, 30, 31, 32, 34, 35, 36, 37, 39 and 6 were retrospective audits.26, 27, 28, 29, 33, 38, 40 Most studies were conducted in the United States (n = 5),8, 28, 29, 31, 33 however, 2 studies from the United Kingdom35, 36, 37 and China,32, 40 and individual studies from Hong Kong,26, 27 Taiwan,30 the Netherlands,34 Spain,38 and France39 were also identified. The 14 studies were diverse in their objectives and study design. Taking a broad overview of objectives, 4 studies reviewed the experience of offering NIPT at a single centre, looking at factors such as patient characteristics and uptake of NIPT and invasive testing,28, 29, 31, 38 one study was questionnaire based across 4 centres and explored women's views about NIPT and factors influencing decision making, including views on termination of pregnancy.8 One study looked at the utility of using NIPT for twin pregnancies.39 Four studies aimed to examine NIPT performance,26, 27, 30, 32, 40 and 3 explored the impact of implementing NIPT as part of state supported health care services.34, 35, 36, 37 Only 1 study had the explicit aim of looking at clinical outcomes and patient choices following NIPT, including continuing an affected pregnancy or opting for termination of pregnancy.33
Pregnancy outcome data from each of the studies are summarised in Table 1. A significant proportion of pregnancies where NIPT indicated a high risk of DS resulted in live births of infants with DS in each of the studies from the United States, the United Kingdom, and the Netherlands; however, there were no live births in the studies from China, Hong Kong, Taiwan, France and Spain. It is important, however, to consider that the numbers reported in some studies were very small. Notably, in 7 studies8, 29, 31, 33, 35, 37, 40 there were women with NIPT results indicating that the baby had a high risk of having DS who declined the offer of invasive testing to confirm the NIPT result.
Table 1.
Summary of studies reporting outcomes of pregnancies following NIPT
| Citation | Country | Study Design | NIPT Offered To | NIPT Tests Performed | Pregnancies with NIPT Suggesting DS | NIPT Confirmed by Invasive Testing | Termination | Miscarriage/Fetal Demise | Live Birth of Children with DS | Termination ratea |
|---|---|---|---|---|---|---|---|---|---|---|
| Lau et al26, 27 | Hong Kong | Retrospective audit at 1 centre (Aug 2011‐Feb 2013) | All | 1982 | 23 | 23 | 23 | 0 | 0 | 100% |
| Pettit et al28 | United States | Retrospective audit at 1 centre (May‐Dec 2012) | High riskb | 206 | 8 | Not reported | 5 | 0 | 3 | 63% |
| Vahanian et al29 | United States | Retrospective audit at 1 centre (Mar‐Jul 2012) | High riskb | 93 | 2 | 1 | 0 | 0 | 2 | 0% |
| Shaw et al30 | Taiwan | Prospective study at 11 centres (June‐Dec 2012) | Very high‐risk (>1:30 or NT >3) or low‐risk (<1:1500) women having IPD | 201 | 11 | 11 | 11 | 0 | 0 | 100% |
| Beamon et al31 | United States | Prospective study at 1 centre (Jan‐Sept 2012) | High riskb | 208 | 5 | 4 | 3 | 0 | 2 | 60% |
| Tiller et al8 | United States | Prospective study at 4 centres (Mar‐May 2013) | High riskb | 200 | 5 | 3 | 2 | 1 | 2 | 40% |
| Song et al32 | China | Prospective study at 1 centre (May 2012‐Aug 2013) | High riskb | 212 | 3 | 2 | 2 | 1 | 0 | 67% |
| Dobson et al33 | United States | Retrospective audit at 2 centres (Mar 2012‐Dec 2014) | High riskb | Not reported (105 singletons/9 twins included in audit) |
Singletons: 53 Twins: 5 |
Singletons: 38 (includes 3 false positive) Twins: not reported |
Singletons: 32 Twins: 2 |
Singletons: 3 Twins: 2 |
Singletons: 14 (1 lost to follow‐up) Twins: 1 |
Singletons: 64% Twins: 40% |
| Oepkes et al34 | The Netherlands | Prospective study at 21 centres (April‐September 2014) | High risk (≥1:200) | 1211 | 31 | 31 (includes 2 false positive) | 25 | 2 | 2 | 86% |
| Chitty et al35 | United Kingdom | Prospective study at 8 centres (Nov 2013‐Feb 2015) | High risk (≥1:150) offered NIPT or IPD/intermediate risk (1:101‐1:2500) offered NIPT | 2494 |
NIPT: 44 IPD: 29 |
36 (includes 1 false positive) |
NIPT: 30 IPD: 27 |
NIPT: 3 IPD: 0 |
NIPT: 10 IPD: 2 |
NIPT: 70% IPD: 93% Overall: 79% |
| Gil et al36, 37 | United Kingdom | Prospective study at 2 centres (Oct 2013‐Feb 2015) | High risk (≥1:100) offered NIPT or IPD/intermediate risk (1:101‐1:2500) offered NIPT | 3698 |
NIPT: 16 IPD: 27 |
12 |
NIPT: 7 IPD: 25 |
NIPT: 0 IPD: 0 |
NIPT: 9 IPD: 2 |
NIPT: 44% IPD: 93% Overall: 73% |
| Gil et al38 | Spain | Retrospective audit at 1 centre (Jan 2015‐Jan 2016) | High risk (1 > 250) | 54 |
NIPT: 1 IPD: 4 |
1 |
NIPT: 1 IPD: 4 |
NIPT: 0 IPD: 0 |
NIPT: 0 IPD: 0 |
NIPT: 100% IPD: 100% Overall: 100% |
| Qiang et al40 | China | Retrospective audit at 1 centre (Mar 2012‐Mar 2015) | High riskc | 1901 | 15 | 14 | 13 | 1 | 0 (1 lost to follow‐upd) | 87% |
| Le Conte et al39 | France | Prospective study of tests performed at 1 laboratory (Nov 2013‐Aug 2015) | Twin pregnancies without ultrasound anomalies | 492 | 4 | 4 (includes 1 false positive) | 3 | 0 | 0 | 100% |
Termination rates were calculated as a proportion of all pregnancies that had a high‐risk result for DS from NIPT (false positives and negatives excluded). This may include cases confirmed with IPD and those confirmed at birth who did not undergo confirmatory IPD. Where additional data were available, termination rates were calculated for “NIPT”—those undergoing NIPT (including cases confirmed with IPD and those confirmed at birth without confirmatory IPD), “IPD”—those going directly to IPD without NIPT, and “Overall”—the termination rate for all cases of DS diagnosed prenatally.
Definition of “high risk” includes; advanced maternal age, fetal ultrasound finding suggestive of aneuploidy, family history of aneuploidy, or positive maternal serum screening result. Aligned with American College of Obstetricians and Gynecologist (ACOG) Committee Opinion.
NIPT was offered to women with a risk of one or more anomaly. All women had DS screening prior to NIPT.
The case lost to follow‐up declined confirmatory invasive testing.
Abbreviations: DS, Down syndrome; IPD, invasive prenatal diagnosis; NIPT, noninvasive prenatal testing.
3.2. Audit of pregnancy outcomes following NIPT as a clinical service in Singapore
Between April 1, 2014, and January 31, 2017, there were 20 701 new bookings at National University Hospital Singapore; 700 women had NIPT (3.4%). For 6 women NIPT indicated that the baby had a high risk of being affected with DS. Five chose to confirm their results by amniocentesis, and the sixth declined further testing. There were no false positives. Of the 5 women with a confirmed pregnancy affected with DS, 4 opted for a termination (80%), and 1 continued the pregnancy. The woman who declined further testing following NIPT also continued the pregnancy. The termination rate for these women with high‐risk NIPT results for DS was 67%. Nine women were lost to follow‐up, and it was not possible to issue a result in 4 cases, because of high variance or low fetal fraction. Over the same period, 706 women (3.4%) opted for an invasive test without prior NIPT, and 39 had a positive result for DS. Twelve cases were lost for follow‐up; of the remaining, 27 women, 25 women chose to terminate their affected pregnancy (92.6%), including 1 woman who had a selective feticide for a single affected twin. There was 1 intrauterine death, and 1 continued pregnancy. Overall, from the 33 cases with confirmed DS following invasive testing or high risk NIPT where outcomes were known, 29 terminated (87.9%) and 4 (12.1%) continued with 1 being stillborn. A summary of the audit findings is presented in Figure 1.
Figure 1.
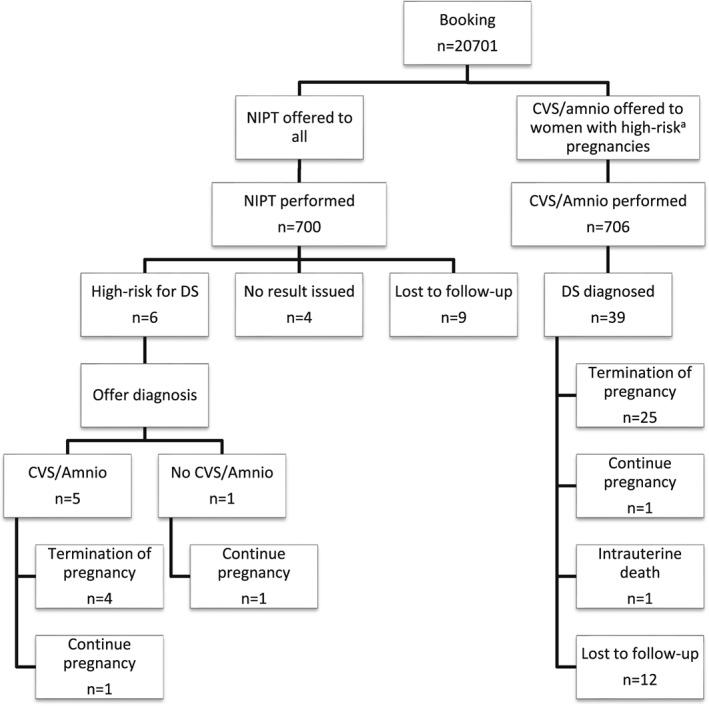
Flowchart showing numbers of women and outcomes for the Singapore audit
3.3. Audit of pregnancy outcomes following NIPT offered as a clinical service in the United Kingdom
Nine hundred and sixty seven women with a high‐risk DS screening result in the North Thames Region had NIPT and 46 were found to be highly likely to have DS. There were no false positive DS results; however, 3 women in this group were lost to follow‐up. Of the 46 women found to be highly likely to have DS by NIPT, outcomes were available for 43. There were 27 (62.7%) women who chose to terminate the pregnancy, 1 had a still birth (2.3%), and 15 (34.9%) continued the pregnancy. Out of 15 parents who chose to continue the pregnancy, 12 did not have their NIPT result confirmed by invasive testing, 2 had invasive testing, and 1 decision was unknown. Twenty one of the 27 parents who chose to have a termination of pregnancy had invasive testing to confirm the NIPT result, 1 declined and the decision was not reported for 5. A summary of the audit findings is presented in Figure 2.
Figure 2.
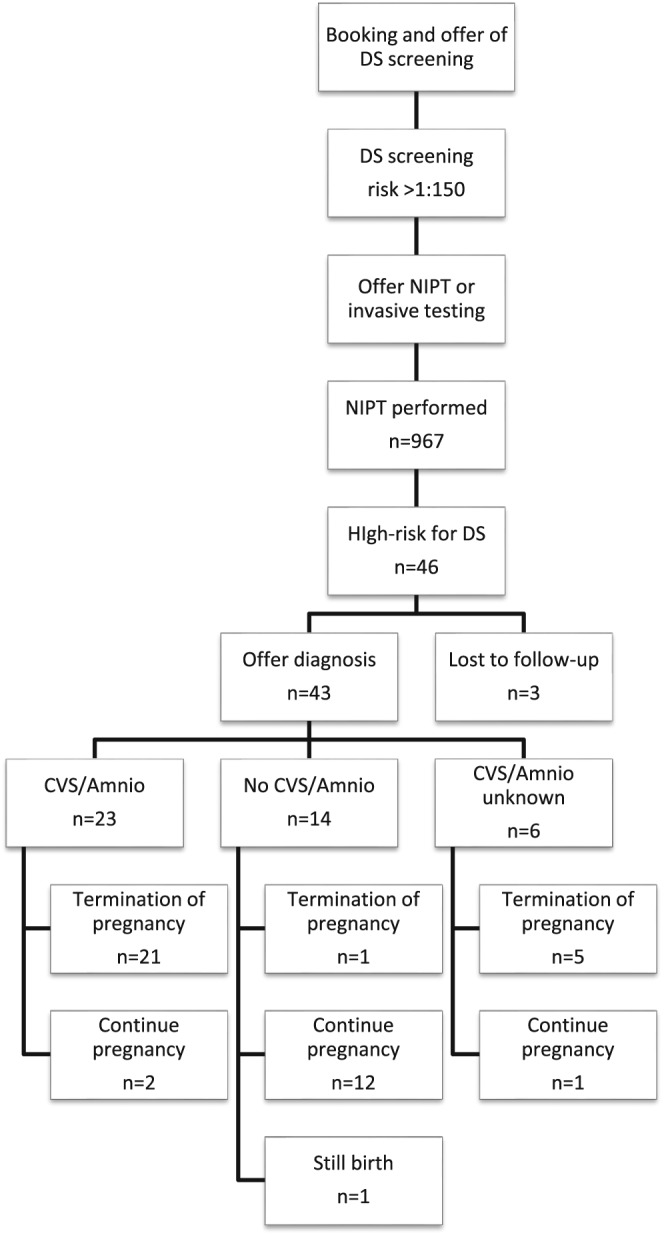
Flowchart showing numbers of women and outcomes for the UK audit
4. DISCUSSION
Our literature review and audits show that many women opting for NIPT who had a result indicating they were highly likely to have a baby with DS chose to continue their pregnancy. Although numbers are relatively small, when we look at the data as a whole our findings suggest that the high uptake of NIPT worldwide includes many women who would like additional information about their baby that will not necessarily be used for decision making about termination of pregnancy. In addition, several studies in the review reported that some women declined an invasive test to confirm the NIPT result and continued their pregnancy, further emphasising that many women choosing to have NIPT want information about the health of their baby, but would not risk miscarriage with an invasive test. Differences in the number of live births were seen between countries as women chose to continue pregnancies highly likely to be affected with DS in the United States, the United Kingdom, the Netherlands, and Singapore, but there were no confirmed live births reported in the studies from China, Hong Kong, Taiwan, France, and Spain.
Termination rates for DS prior to the introduction of NIPT vary quite markedly throughout the world. In the United States, a systematic review of termination rates in studies published between 1995 and 2011 found that 67% of women opted for termination of pregnancy following prenatal diagnosis of DS.41 Termination rates of over 90% have been reported in the United Kingdom,21 Australia,42, 43 China,24 and the Netherlands.44 In Taiwan, a termination rate of 67.5% was reported for 200145; however, prenatal screening and diagnosis has since been more widely introduced and the live birth rate for children with DS decreased from 48.7% in 2001 to 6% in 2010.23 Comparison between termination rates reported prior to the introduction of NIPT with termination rates seen in the reviewed studies and audits suggests a general trend towards a decrease in termination rates compared with pre‐NIPT rates (Table 2). Data from the UK audit and research studies35, 36, 37 indicate that termination rates for women having NIPT are considerably lower than the pre‐NIPT termination rate of 92%.21 For the US8, 28, 29, 31, 33 and Dutch34 studies, decreases in the termination rate compared to pre‐NIPT rates were also seen. Notably in the United States, termination rates following a prenatal diagnosis of DS are already low (67%) compared to other countries (>90%). In some countries termination rates were unchanged, for example, in Hong Kong, in the studies reported by Lau et al,26, 27 a termination rate of 100% is in line with reported termination rates above 90% for mainland China.24
Table 2.
Termination rates determined from reviewed studies and the audits reported here compared to pre‐NIPT termination rates
| Citation | Termination Ratea | Country | Pre‐NIPT Termination Rate for DSb (year(s) obtained) |
|---|---|---|---|
| Lau et al26, 27 | 100%c | Hong Kong | Not found |
| Pettit et al28 | 63% | United States | 67% (1995‐2011)41 |
| Vahanian et al29 | 0% | United States | 67% (1995‐2011)41 |
| Shaw et al30 | 100%c | Taiwan | 68% (2001)45 |
| Beamon et al31 | 60% | United States | 67% (1995‐2011)41 |
| Tiller et al8 | 40% | United States | 67% (1995‐2011)41 |
| Song et al32 | 67% | China | 94% (2003‐2011)24 |
| Dobson et al33 |
Singletons: 64% Twins: 40% |
United States | 67% (1995‐2011)41 |
| Oepkes et al34 | 86%c | The Netherlands | 93% (2010)44 |
| Chitty et al35 |
NIPT: 70% IPD: 93% Overall: 79% |
United Kingdom | 92% (2007‐2008)21 |
| Gil et al36, 37 |
NIPT:44% IPD: 93% Overall: 73% |
United Kingdom | 92% (2007‐2008)21 |
| Gil et al38 | 100%c | Spain | 96%d (2002‐2004)46 |
| Qiang et al40 | 87% | China | 94% (2003‐2011)24 |
| Le Conte et al39 | 100%c | France | 96%e (2002‐2004)46 |
| Reported here |
NIPT: 67% IPD: 93% Overall: 88% |
Singapore | Not found |
| Reported here | 62.7% | United Kingdom | 92% (2007‐2008)21 |
Termination rate calculated as a proportion of all pregnancies that had a high‐risk result for DS from NIPT (false positives and negatives excluded).
Termination rate calculated as a proportion of all pregnancies that had a definitive prenatal diagnosis of DS by IPD.
All high‐risk results from NIPT were confirmed by IPD.
Termination rate for EUROCAT (European Surveillance of Congenital Anomalies) registry regions of Spain (Barcelona, Basque, Asturias, and Madrid).
Termination rate for EUROCAT (European Surveillance of Congenital Anomalies) registry regions of France (Auvergne, Paris, Central East and Strasbourg).
Abbreviations: DS, Down syndrome; IPD, invasive prenatal diagnosis; NIPT, noninvasive prenatal testing.
It is important to be cautious when interpreting comparisons between the studies discussed here and historical controls, as numbers are small in the included studies and there may be differences between the populations used for determining the historical termination rates and those of the included studies, for example, in the systematic review of termination rates from the United States, Natoli et al41 noted that the summary termination rate that the authors calculated may not be applicable to the entire US population. In addition, the termination rates from the historical controls only include women with a confirmed prenatal diagnosis of DS. To get a complete picture of the impact of NIPT we have calculated termination rates for all women with a high‐risk NIPT result, not all of whom chose to have confirmatory invasive testing. For some studies included in the review there are no differences in how termination rates were calculated compared to historical controls, as all women in the study had their high‐risk NIPT result confirmed.26, 27, 30, 34, 38, 39 To look at termination rates across subgroups in the remaining studies, we could calculate the termination rates for women with and without a confirmed prenatal diagnosis of DS separately. However, this is not possible for all studies as the required information was either not reported or participant numbers were too low to allow a meaningful breakdown. In Chitty et al35 the termination rates for women with and without a confirmed diagnosis after a high‐risk NIPT result were 83% (29/35) and 12.5% (1/8), respectively, and in the UK audit 88% (21/24) and 8% (1/13), respectively. In the US study reported by Dobson et al,33 the termination rates for women with and without a confirmed diagnosis were 80% (28/35) and 27% (4/15), respectively. Notably, Dobson et al33 concluded, as we did, that the overall termination rate was not higher than historical controls and stated that their findings argue against the concern that cfDNA screening would increase rates of pregnancy termination.
As one of the common reasons women have declined screening in the past is the miscarriage risk associated with invasive testing,47, 48 it is not surprising that NIPT is being adopted widely around the world.1 However, our findings suggest that this increase may not impact greatly on the number of babies born with DS as many parents will use NIPT for information and not for decisions about termination of pregnancy. The decrease in termination rates compared with pre‐NIPT rates observed in the reviewed studies, presumably reflects, at least in part, the uptake of NIPT by women seeking information who would not have had prenatal testing in the past as they would not put their pregnancies at risk with invasive testing. We also found evidence that the termination rate for women opting for NIPT was lower when compared to women who chose to go directly to invasive testing following a high‐risk screening result. In the Singapore audit the number of women opting for termination following NIPT (4/6—66.7%) was lower than those who chose invasive testing (25/27—92.6%). Similarly, in the United Kingdom, in 2 prospective studies where NIPT was offered to both high and intermediate risk women the number of live births of children with DS were significantly higher amongst women opting for NIPT compared to women who chose to go straight to invasive testing.35, 36, 37 This difference is most likely due to the variances in motivation for women choosing NIPT versus invasive testing, with the latter group perhaps being more likely to want diagnostic information to make decisions about termination.49
We know from the literature that the uptake of NIPT is high,34, 35 making it likely that detection of DS will increase, but the lower termination rates following NIPT in some countries suggest that live birth rates may remain largely unchanged compared to termination rates prior to the introduction of NIPT. Termination rates did not, however, fall in all studies reviewed here and in settings where NIPT uptake is high and termination rates remain unchanged there will be an overall increase in numbers of terminations of pregnancy and a corresponding decrease in the live birth rate.
Ultimately, however, the research included here describes relatively small numbers of women and can only give insights into pregnancy outcomes following prenatal testing and we do not know how many children with DS were born to parents who chose not to have prenatal testing. Population‐based studies of live birth rates are therefore essential to allow us to see the overall impact of NIPT. The importance of the population‐based studies is highlighted by reports from countries such as the Netherlands where the termination rate following a prenatal diagnosis of DS is high, but the overall live birth rate has increased over time as many women opt not to have DS screening.20 Uptake of DS screening is low in the Netherlands compared to other European countries. This may reflect the fact that parents must make a financial contribution to screening, also that the offer of DS screening is not presented as a routine test and is discussed in a way that emphasises the right not to know.50 Moreover, several studies looking at hypothetical choices have shown that even with NIPT as an option many women will still choose not to have any prenatal testing. In a survey of 2666 women from 9 countries, there was a sizable proportion of women who said they would not have any prenatal testing for DS, including more than one third of women in the Netherlands and Israel.51 Similarly, studies from the United States and the Netherlands found that around one third of people surveyed reported not wanting any tests for DS.52, 53
Research looking at women's hypothetical choices regarding how they would respond to an NIPT result that suggests DS is highly likely support our findings that many women will choose to continue their pregnancies.18, 49, 52, 54 While the data on hypothetical choices needs to be interpreted with caution, as parents may make different choices when faced with real‐life situations, these studies indicate that a significant number of parents would use NIPT for information only, so that they could plan and prepare for the birth of an affected child rather than using the information to make decisions about termination of pregnancy.18, 52, 55, 56 The anticipated high uptake of NIPT suggested in these studies indicates that parents value having screening tests available, regardless of their intention to either terminate or continue an affected pregnancy. Recent research in the United States that surveyed 217 individuals, representative the US population by gender, income, and education, found that the majority (65%) saw the value of having reliable information when making health care decisions and therefore supported having NIPT and other prenatal tests available.57
Decision making about next steps following a diagnosis of DS is complex. Every woman must have access to the support they need to make informed decisions that take into consideration their own circumstances, experiences, the needs of their family and are in keeping with their personal beliefs and values.58, 59 Recent research has highlighted best care practices following a prenatal diagnosis of aneuploidy that include the provision of clear, accurate, and respectful communication about the testing process and results; empathic, nonjudgemental professional support; timely access to services; health professional acknowledgement of the enormity of the decision; and opportunities to discuss the diverse range of feelings that accompany prenatal diagnosis.59 As many parents will have prenatal testing with NIPT for information only, there will be more parents continuing pregnancies knowing that the baby has DS. As many of these women receive the diagnosis in early pregnancy it is important that their ongoing needs for emotional and clinical support are met. Furthermore, knowledge of fetal DS status may allow increased surveillance to prevent intrauterine death as highlighted by recent research from the United States, which found elevated rates of growth restriction, early delivery due to nonreassuring fetal status, and placental insufficiency in a cohort of 64 women continuing the pregnancy with a diagnosis of DS.60 Provision of practical and psychosocial support for individuals with DS and their families will also continue to be needed.
5. CONCLUSIONS
Parents value having the option of NIPT so that they can obtain information about DS early in pregnancy without putting their pregnancy at risk of miscarriage. The data presented here suggest that parents choose NIPT for different reasons, and a significant number of parents will use the results for information so that they can prepare for the birth of a child with DS. Comparison of termination rates reported in the studies reviewed here with termination rates reported prior to the introduction of NIPT suggest that in many settings the implementation of NIPT may not alter the overall numbers of children born with DS. Long‐term population‐based studies are needed to accurately determine the impact of NIPT on the number of children born with DS. Future research should also consider the implications for cost‐effectiveness and service provision of NIPT being used for information and planning as well as to direct decisions about termination of pregnancy.
CONFLICTS OF INTEREST
J.F. is used by the charity Antenatal Results and Choices (ARC), which in the past has received small amounts of funding from some commercial companies marketing NIPT. All other authors declare no conflict of interest.
ACKNOWLEDGEMENTS
We thank Cecille Laureano Asibal, Maylene Tan Zipagan, and Cynthia Zapata Tagarino for assistance in collecting the data for the Singapore audit. We thank Emilia Gnat and Samantha Edwards at Great Ormond Street Hospital NHS Foundation Trust and the clinical and research teams at each of the participating units for their assistance in collecting the data for the UK audit. Thank you also to Dr Sevan Vahanian for providing additional detail on the pregnancy outcomes included in her 2014 report. This manuscript presents independent research partially funded by the National Institute for Health Research (NIHR) under the Programme Grants for Applied Research programme (RP‐PG‐0707‐10107) (the “RAPID” project). L.S.C. is partially funded by the NIHR Biomedical Research Centre at Great Ormond Street Hospital. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Hill M, Barrett A, Choolani M, Lewis C, Fisher J, Chitty LS. Has noninvasive prenatal testing impacted termination of pregnancy and live birth rates of infants with Down syndrome? Prenatal Diagnosis. 2017;37:1281–1290. https://doi.org/10.1002/pd.5182
REFERENCES
- 1. Minear MA, Alessi S, Allyse M, Michie M, Chandrasekharan S. Noninvasive prenatal genetic testing: Current and emerging ethical, legal, and social issues. Annu Rev Genomics Hum Genet. 2015;16:369‐398. [DOI] [PubMed] [Google Scholar]
- 2. Gil MM, Quezada MS, Revello R, Akolekar R, Nicolaides KH. Analysis of cell‐free DNA in maternal blood in screening for fetal aneuploidies: Updated meta‐analysis. Ultrasound Obstet Gynecol. 2015;45:249‐266. [DOI] [PubMed] [Google Scholar]
- 3. American College of Obstetricians and Gynecologists . Committee opinion no. 640: cell‐free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126:e31‐e37. [DOI] [PubMed] [Google Scholar]
- 4. Norton ME, Jacobsson B, Swamy GK, et al. Cell‐free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372:1589‐1597. [DOI] [PubMed] [Google Scholar]
- 5. Dondorp W, de Wert G, Bombard Y, et al. Non‐invasive prenatal testing for aneuploidy and beyond: Challenges of responsible innovation in prenatal screening. Eur J Hum Genet. 2015;23:1438‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Larion S, Warsof SL, Romary L, Mlynarczyk M, Peleg D, Abuhamad AZ. Uptake of noninvasive prenatal testing at a large academic referral center. Am J Obstet Gynecol. 2014;211:651, e651‐7 [DOI] [PubMed] [Google Scholar]
- 7. Warsof SL, Larion S, Abuhamad AZ. Overview of the impact of noninvasive prenatal testing on diagnostic procedures. Prenat Diagn. 2015;35:972‐979. [DOI] [PubMed] [Google Scholar]
- 8. Tiller GE, Kershberg HB, Goff J, Coffeen C, Liao W, Sehnert AJ. Women's views and the impact of noninvasive prenatal testing on procedures in a managed care setting. Prenat Diagn. 2015;35:428‐433. [DOI] [PubMed] [Google Scholar]
- 9. Robson SJ, Hui L. National decline in invasive prenatal diagnostic procedures in association with uptake of combined first trimester and cell‐free DNA aneuploidy screening. Aust New Zeal J Obstet Gynaecol. 2015;55:507‐510. [DOI] [PubMed] [Google Scholar]
- 10. Li DZ, Zhen L, Pan M, Han J, Yang X, Ou YM. Non‐invasive prenatal testing: Impact on invasive prenatal diagnosis at a mainland Chinese tertiary medical center. J Matern Fetal Neonatal Med. 2016;29:3539‐3541. [DOI] [PubMed] [Google Scholar]
- 11. Lewis C, Silcock C, Chitty LS. Non‐invasive prenatal testing for Down's syndrome: Pregnant women's views and likely uptake. Public Health Genomics. 2013;16:223‐232. [DOI] [PubMed] [Google Scholar]
- 12. van Schendel RV, Kleinveld JH, Dondorp WJ, et al. Attitudes of pregnant women and male partners towards non‐invasive prenatal testing and widening the scope of prenatal screening. Eur J Hum Genet. 2014;22:1345‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Farrell R, Agatisa PK, Mercer B, Smith M, Philipson E. Balancing risks: The core of women's decisions about noninvasive prenatal testing. AJOB Empirical Bioethics. 2015;6:42‐53. [Google Scholar]
- 14. Alexander E, Kelly S, Kerzin‐Storrar L. Non‐invasive prenatal testing: UK genetic counselors' experiences and perspectives. J Genet Couns. 2015;24:300‐311. [DOI] [PubMed] [Google Scholar]
- 15. Skotko BG. With new prenatal testing, will babies with Down syndrome slowly disappear? Arch Dis Child. 2009;94:823‐826. [DOI] [PubMed] [Google Scholar]
- 16. Kellogg G, Slattery L, Hudgins L, Ormond K. Attitudes of mothers of children with Down syndrome towards noninvasive prenatal testing. J Genet Couns. 2014;23:805‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Schendel RV, Kater‐Kuipers A, van Vliet‐Lachotzki EH, Dondorp WJ, Cornel MC, Henneman L. What do parents of children with Down yndrome think about non‐invasive prenatal testing (NIPT)? J Genet Couns. 2017;26:529‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lewis C, Hill M, Silcock C, Daley R, Chitty L. Non‐invasive prenatal testing for trisomy 21: A cross‐sectional survey of service users' views and likely uptake. BJOG. 2014;121:582‐594. [DOI] [PubMed] [Google Scholar]
- 19. de Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am J Med Genet A. 2015;167a:756‐767. [DOI] [PubMed] [Google Scholar]
- 20. de Graaf G, Engelen JJ, Gijsbers AC, et al. Estimates of live birth prevalence of children with Down syndrome in the period 1991‐2015 in the Netherlands. J Intellect Disabil Res. 2017;61:461‐470. [DOI] [PubMed] [Google Scholar]
- 21. Morris JK, Alberman E. Trends in Down's syndrome live births and antenatal diagnoses in England and Wales from 1989 to 2008: Analysis of data from the National Down Syndrome Cytogenetic Register. BMJ. 2009;339:b3794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bittles AH, Bower C, Hussain R, Glasson EJ. The four ages of Down syndrome. Eur J Public Health. 2007;17:221‐225. [DOI] [PubMed] [Google Scholar]
- 23. Lin SY, Hsieh CJ, Chen YL, et al. The impact of Down syndrome screening on Taiwanese Down syndrome births: A nationwide retrospective study and a screening result from a single medical centre. PLoS One. 2013;8:e75428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Deng C, Yi L, Mu Y, et al. Recent trends in the birth prevalence of Down syndrome in China: Impact of prenatal diagnosis and subsequent terminations. Prenat Diagn. 2015;35:311‐318. [DOI] [PubMed] [Google Scholar]
- 25. Loane M, Morris JK, Addor MC, et al. Twenty‐year trends in the prevalence of Down syndrome and other trisomies in Europe: Impact of maternal age and prenatal screening. Eur J Hum Genet. 2013;21:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lau TK, Chan MK, Lo PS, et al. Clinical utility of noninvasive fetal trisomy (NIFTY) test—early experience. J Matern Fetal Neonatal Med. 2012;25:1856‐1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau TK, Cheung SW, Lo PS, et al. Non‐invasive prenatal testing for fetal chromosomal abnormalities by low‐coverage whole‐genome sequencing of maternal plasma DNA: Review of 1982 consecutive cases in a single center. Ultrasound Obstet Gynecol. 2014;43:254‐264. [DOI] [PubMed] [Google Scholar]
- 28. Pettit KE, Hull AD, Korty L, Jones MC, Pretorius DH. The utilization of circulating cell‐free fetal DNA testing and decrease in invasive diagnostic procedures: An institutional experience. J Perinatol. 2014;34:750‐753. [DOI] [PubMed] [Google Scholar]
- 29. Vahanian SA, Baraa Allaf M, Yeh C, Chavez MR, Kinzler WL, Vintzileos AM. Patient acceptance of non‐invasive testing for fetal aneuploidy via cell‐free fetal DNA. J Matern Fetal Neonatal Med. 2014;27:106‐109. [DOI] [PubMed] [Google Scholar]
- 30. Shaw SW, Hsiao CH, Chen CY, et al. Noninvasive prenatal testing for whole fetal chromosomal aneuploidies: A multicenter prospective cohort trial in Taiwan. Fetal Diagn Ther. 2014;35:13‐17. [DOI] [PubMed] [Google Scholar]
- 31. Beamon CJ, Hardisty EE, Harris SC, Vora NL. A single center's experience with noninvasive prenatal testing. Genet Med. 2014;16:681‐687. [DOI] [PubMed] [Google Scholar]
- 32. Song Y, Huang S, Zhou X, et al. Non‐invasive prenatal testing for fetal aneuploidies in the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2015;45:55‐60. [DOI] [PubMed] [Google Scholar]
- 33. Dobson LJ, Reiff ES, Little SE, Wilkins‐Haug L, Bromley B. Patient choice and clinical outcomes following positive noninvasive prenatal screening for aneuploidy with cell‐free DNA (cfDNA). Prenat Diagn. 2016;36:456‐462. [DOI] [PubMed] [Google Scholar]
- 34. Oepkes D, Page‐Christiaens GC, Bax CJ, et al. Trial by Dutch laboratories for evaluation of non‐invasive prenatal testing. Part I‐clinical impact Prenat Diagn. 2016;36:1083‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chitty LS, Wright D, Hill M, et al. Uptake, outcomes, and costs of implementing non‐invasive prenatal testing for Down's syndrome into NHS maternity care: Prospective cohort study in eight diverse maternity units. BMJ. 2016;354:i3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gil MM, Giunta G, Macalli EA, Poon LC, Nicolaides KH. UK NHS pilot study on cell‐free DNA testing in screening for fetal trisomies: Factors affecting uptake. Ultrasound Obstet Gynecol. 2015;45:67‐73. [DOI] [PubMed] [Google Scholar]
- 37. Gil MM, Revello R, Poon LC, Akolekar R, Nicolaides KH. Clinical implementation of routine screening for fetal trisomies in the UK NHS: Cell‐free DNA test contingent on results from first‐trimester combined test. Ultrasound Obstet Gynecol. 2016;47:45‐52. [DOI] [PubMed] [Google Scholar]
- 38. Gil MM, Brik M, Casanova C, et al. Screening for trisomies 21 and 18 in a Spanish public hospital: From the combined test to the cell‐free DNA test. J Matern Fetal Neonatal Med. 2017;30:2476‐2482. [DOI] [PubMed] [Google Scholar]
- 39. Le Conte G, Letourneau A, Jani J, et al. Cell‐free fetal DNA analysis in maternal plasma as a screening test for trisomy 21, 18 and 13 in twin pregnancies. Ultrasound Obstet Gynecol. 2017; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40. Qiang R, Cai N, Wang X, et al. Detection of trisomies 13, 18 and 21 using non‐invasive prenatal testing. Exp Ther Med. 2017;13:2304‐2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Natoli JL, Ackerman DL, McDermott S, Edwards JG. Prenatal diagnosis of Down syndrome: A systematic review of termination rates (1995‐2011). Prenat Diagn. 2012;32:142‐153. [DOI] [PubMed] [Google Scholar]
- 42. Collins VR, Muggli EE, Riley M, Palma S, Halliday JL. Is Down syndrome a disappearing birth defect? J Pediatr. 2008;152:20, 24.e21‐24. [DOI] [PubMed] [Google Scholar]
- 43. Maxwell S, Bower C, O'Leary P. Impact of prenatal screening and diagnostic testing on trends in Down syndrome births and terminations in Western Australia 1980 to 2013. Prenat Diagn. 2015;35:1324‐1330. [DOI] [PubMed] [Google Scholar]
- 44. Verweij EJ, Oepkes D, de Boer MA. Changing attitudes towards termination of pregnancy for trisomy 21 with non‐invasive prenatal trisomy testing: A population‐based study in Dutch pregnant women. Prenat Diagn. 2013;33:397‐399. [DOI] [PubMed] [Google Scholar]
- 45. Jou HJ, Kuo YS, Hsu JJ, Shyu MK, Hsieh TT, Hsieh FJ. The evolving national birth prevalence of Down syndrome in Taiwan. A study on the impact of second‐trimester maternal serum screening. Prenat Diagn. 2005;25:665‐670. [DOI] [PubMed] [Google Scholar]
- 46. Boyd PA, Devigan C, Khoshnood B, et al. Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down's syndrome. BJOG. 2008;115:689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gottfredsdottir H, Bjornsdottir K, Sandall J. How do prospective parents who decline prenatal screening account for their decision? A qualitative study. Soc Sci Med. 2009;69:274‐277. [DOI] [PubMed] [Google Scholar]
- 48. Markens S, Browner CH, Press N. ‘Because of the risks’: How US pregnant women account for refusing prenatal screening. Soc Sci Med. 1999;49:359‐369. [DOI] [PubMed] [Google Scholar]
- 49. Lewis C, Hill M, Chitty LS. Women's experiences and preferences for service delivery of non‐invasive prenatal testing for aneuploidy in a public health setting: a mixed methods study. PLoS One. 2016;11:e0153147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Crombag NM, Boeije H, Iedema‐Kuiper R, Schielen PC, Visser GH, Bensing JM. Reasons for accepting or declining Down syndrome screening in Dutch prospective mothers within the context of national policy and healthcare system characteristics: a qualitative study. BMC Pregnancy Childbirth. 2016;16:121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hill M, Johnson JA, Langlois S, et al. Preferences for prenatal tests for Down syndrome: An international comparison of the views of pregnant women and health professionals. Eur J Hum Genet. 2016;24:968‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Allyse M, Sayres LC, Goodspeed TA, Cho MK. Attitudes towards non‐invasive prenatal testing for aneuploidy among US adults of reproductive age. J Perinatol. 2014;34:429‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Schendel RV, Dondorp WJ, Timmermans DR, et al. NIPT‐based screening for Down syndrome and beyond: What do pregnant women think? Prenat Diagn. 2015;35:598‐604. [DOI] [PubMed] [Google Scholar]
- 54. van Schendel RV, Page‐Christiaens GC, Beulen L, et al. Trial by Dutch laboratories for evaluation of non‐invasive prenatal testing. Part II‐women's perspectives Prenat Diagn. 2016;36:1091‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sayres LC, Allyse M, Goodspeed TA, Cho MK. Demographic and experiential correlates of public attitudes towards cell‐free fetal DNA screening. J Genet Couns. 2014;23:957‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuppermann M, Norton ME, Thao K, et al. Preferences regarding contemporary prenatal genetic tests among women desiring testing: Implications for optimal testing strategies. Prenat Diagn. 2016;36:469‐475. [DOI] [PubMed] [Google Scholar]
- 57. Steinbach RJ, Allyse M, Michie M, Liu EY, Cho MK. “This lifetime commitment”: Public conceptions of disability and noninvasive prenatal genetic screening. Am J Med Genet A. 2016;170a:363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reed AR, Berrier KL. A qualitative study of factors influencing decision‐making after prenatal diagnosis of Down syndrome. J Genet Couns. 2017;26:814‐828. [DOI] [PubMed] [Google Scholar]
- 59. Hodgson J, Pitt P, Metcalfe S, et al. Experiences of prenatal diagnosis and decision‐making about termination of pregnancy: a qualitative study. Aust N Z J Obstet Gynaecol. 2016;56:605‐613. [DOI] [PubMed] [Google Scholar]
- 60. Guseh SH, Little SE, Bennett K, Silva V, Wilkins‐Haug LE. Antepartum management and obstetric outcomes among pregnancies with Down syndrome from diagnosis to delivery. Prenat Diagn. 2017;37:640‐646. [DOI] [PubMed] [Google Scholar]


