Abstract
AIM
To investigate whether laparoscopic surgery is as safe and feasible as open resection for patients with larger gastrointestinal stromal tumors (GISTs) (≥ 5 cm).
METHODS
A systematic search of PubMed, EMBASE, Web of Science and the Cochrane Library database was performed. Relevant studies of laparoscopic and open surgery for GISTs of > 5 cm published before December 2016 were identified from these databases. The quality of the studies was assessed by the Newcastle-Ottawa Quality Assessment Scale. The tumor size, operation time, blood loss, postoperative hospital stay, complication rate, and disease-free survival rate were assessed. The software Stata (version 12.0) was used for the meta-analysis.
RESULTS
Five clinical trials comprising 209 patients with GISTs of similar larger sizes were evaluated. The pooled analysis of 100 patients in the laparoscopic resection group and 109 patients in the open resection group demonstrated that laparoscopic surgery was significantly associated with a shorter postoperative hospital stay (P < 0.001) and less blood loss (P = 0.002). Moreover, there were no statistically significant differences in the operation time (P = 0.38), postoperative complication rate (P = 0.88), or disease-free survival rate (P = 0.20) between two groups.
CONCLUSION
Our findings revealed that for patients with large GISTs of comparable sizes, laparoscopic surgery did not significantly influence the operation factors or clinical outcomes compared with open surgery. This suggests that laparoscopic resection is as acceptable as open surgery for treatment of large gastric GISTs.
Keywords: Laparoscopic resection, Open resection, Gastrointestinal stromal tumor, Meta-analysis, Clinical outcome
Core tip: Whether laparoscopic resection is also effective and feasible for treatment of larger gastric gastrointestinal stromal tumors (GISTs) (> 5 cm) remains unknown. This meta-analysis collected up-to-date clinical data of comparison of laparoscopic and open resection for larger gastric GISTs (> 5 cm). Our results showed that laparoscopic resection is an upgraded minimal invasive technique with a shorter postoperative hospital stay and less intraoperative blood loss compared with open surgery in treating patients with larger GISTs.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common gastrointestinal sarcomas. They usually arise from the interstitial cells of Cajal and regulate gastrointestinal motility[1,2]. GISTs are often characterized by cellular markers such as CD117 (a receptor tyrosine kinase protein also known as tyrosine-protein kinase Kit). The stomach is the most prevalent location of GISTs, and the proximal stomach is involved in about two-thirds of suffering patients[3]. It is well accepted that the malignant potential of GISTs depends on the tumor size, cell mitotic rate, and tumor location[4].
Although substantial advances have been made in the targeted therapies for these tumors, surgical resection is still the most important component in the treatment of primary GISTs with no evidence of metastasis. Because wide margins (> 5 cm) and lymph node dissection are not necessary in the surgical management of GISTs[5], laparoscopic surgery seems to be more suitable for resection of these tumors. Various types of laparoscopic procedures for GISTs have been performed in a few specialized centers, including wedge resection of the stomach, intragastric tumor resection, and combined endoscopic–laparoscopic resection, etc. However, during laparoscopic surgery, these tumors must be handled with great care because rupture of their capsule confers a near 100% risk of recurrence.
Several studies and meta-analyses have shown that laparoscopic resection for gastric GISTs is as safe and efficacious as open surgery; additionally, laparoscopy is associated with less blood loss, less morbidity, and quicker recovery[6-8]. The long-term survival of patients with GISTs mainly depends on the tumor progression, and laparoscopic surgery does not increase the risk of tumor relapse and metastasis. The clinical practice guidelines for the management of GISTs released by the National Comprehensive Cancer Network and the Japanese Study Group on GIST note that laparoscopic surgical resection is the preferred therapy for relatively small GISTs with a diameter of < 5 cm[9].
However, most cohort studies have focused on laparoscopic surgery for relatively smaller tumors; few have been designed for evaluation of larger GISTs (> 5 cm)[10-14]. Although the size limit was not clearly stated, the practice guideline of the European Society for Medical Oncology recommends application of laparoscopic procedures in patients with large GISTs[15]. However, the complex surgical skills and long learning curve associated with laparoscopic surgery might prevent its application to larger GISTs to some extent[16]. Therefore, the feasibility and safety of laparoscopic surgery for GISTs of > 5 cm remains unclear. Additionally, whether 5 cm is the most appropriate cutoff for performance of minimally invasive procedures in patients with larger GISTs remains controversial. This meta-analysis was performed to assess the short- and long-term results of patients with larger gastric GISTs (> 5 cm) undergoing laparoscopic surgery.
MATERIALS AND METHODS
Literature search
Systematic electronic searches of PubMed, EMBASE, the Cochrane Library, the Clinical Trials Database, Web of Science, and Google Scholar were performed to identify relevant articles published up to 30 December 2016, utilizing the following search terms: “gastrointestinal stromal tumor,” “GIST,” “laparoscopic,” “laparoscopy,” “open resection,” “gastrectomy,” and “stomach”. Citations and references of identified studies were also reviewed for additional literature and trials. The language of the publications was limited to English.
Study selection
The inclusion criteria were as follows: (1) The studies involved patients with gastric GISTs larger than 5 cm; (2) The specific interventions were laparoscopic and open surgical resection; (3) The clinical outcomes were the operation time, intraoperative blood loss, conversion rate, length of hospital stay, adverse events, and long-term outcomes (overall survival, disease-specific survival, or recurrence rate); (4) Controlled studies (randomized controlled trials, cohort studies, and case-control studies) were included for the pooled analysis. However, case reports and case series were included for the systematic review; and (5) The informative data and full text of the articles were available.
The exclusion criteria were as follows: (1) The patients had GISTs that were located outside of the stomach or complicated with mixed disease; (2) Duplicate publications; (3) the size of the GIST was not specifically stated; (4) The article was a case report or review; and (5) The publication was in a language other than English.
Data extraction and management
Two reviewers independently screened the titles and abstracts of the publications. Once deemed acceptable, the whole manuscripts were obtained and screened. Controversial issues were resolved by discussion or referred to a third reviewer. Another two reviewers independently extracted the data using a unified form and resolved any discrepancies through discussion. The variables of interest included the author, study period, number of patients, tumor size, operation time, blood loss, length of postoperative hospital stay, complication rate, and long-term outcome (namely disease-free survival). In addition, if the original studies included the median, range, and size of a sample, we estimated the mean and variance using the methods described by Hozo et al[12].
The quality of the included papers was assessed using the Newcastle-Ottawa Quality Assessment Scale[17]. This scale ranges from 0 to 9 points; studies with a score of ≥ 6 were considered methodologically sound.
Statistical analysis
The meta-analysis was performed using weighted mean differences (WMDs) for continuous variables, odds ratios for dichotomous variables, and hazard ratios for time-to-event variables. Statistical heterogeneity was assessed by performing χ2 tests and calculating the Higgins I2 statistic, and a value of P < 0.10 or I2 > 50%, indicated statistical significance. A fixed-effects model was generally employed. If the heterogeneity was statistically significant, a random-effects model was adopted. Publication bias was evaluated by Begg’s test. A P value of < 0.05 was considered significant. Statistical analyses were performed using Stata software (version 12.0; StataCorp, College Station, TX, United States).
RESULTS
Enrolled studies and quality assessment
No eligible randomized controlled trials were identified, but 5 nonrandomized trials were analyzed (209 patients with GISTs of similar size). Overall, 100 patients underwent laparoscopic resection and 100 underwent open resection. A flow chart of the search strategy is illustrated in Figure 1. The main characteristics and quality assessment results of the included studies are shown in Tables 1 and 2, respectively.
Figure 1.
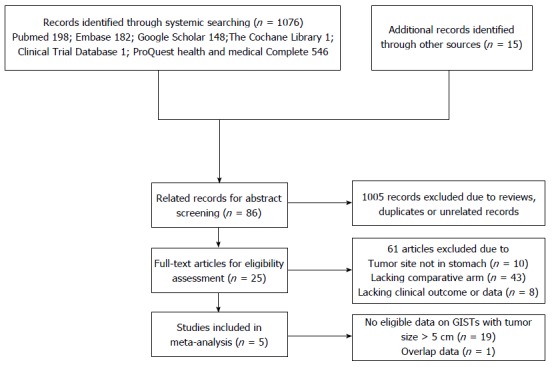
Flow chart of study selection process. GISTs: Gastrointestinal stromal tumors.
Table 1.
Main characteristics of enrolled trials
| Ref. | Region | Year | Study design | Study period |
Sample size |
Tumor size (cm) |
CS | Follow-up (mo) | ||
| LAP | Open | LAP | Open | |||||||
| Kim et al[10] | South Korea | 2012 | OCS (R) | 1998-2011 | 24 | 14 | 6.1 ± 1.3 | 7.2 ± 1.7 | 0 | 49.3 (8.4-164.4) |
| Lin et al[11] | China | 2014 | OCS (R) | 2007-2012 | 23 | 23 | 7.2 ± 1.6 | 7.3 ± 1.5 | 1 | 34.0 (6-78) |
| Hsiao et al[12] | Taiwan | 2015 | OCS (P) | 2002-2012 | 18 | 37 | 6.1 ± 1.0 | 6.0 ± 0.9 | 0 | 43.2 (16.8-133.2) |
| Takahashi et al[13] | Japan | 2015 | OCS (R) | 1995-2011 | 12 | 15 | 7.5 ± 1.9 | 5.5 ± 0.73 | 3 | 63 (7-154) |
| Khoo et al[14] | Japan | 2016 | OCS (R) | 2002-2015 | 23 | 36 | NA | NA | 1 | 45 |
OCS: Observational clinical study; R: Retrospective study; P: Prospective study; NA: Not available; CS: Convention surgery.
Table 2.
Newcastle-Ottawa Scale Assessment of enrolled studies
| Ref. |
Selection (0-4) |
Comparability |
Outcome (0-3) |
Total | ||||||
| REC | Snec | AE | OINP | SCB | SCA | AO | FU | AFC | ||
| Kim et al[10] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Lin et al[11] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| Hsiao et al[12] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Takahashi et al[13] | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 7 |
| Khoo et al[14] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
REC: Representativeness of the exposed cohort; SNEC: Selection of the no exposed cohort; AE: Ascertainment of exposure; OINP: Outcome of interest not presented in the start of study; SCB: Study controls for basic characteristics; SCA: Study controls for additional factor; AO: Assessment of outcome; FU: Follow-up; AFC: Adequacy of follow up.
Tumor size
Four studies reported no statistically significant differences in tumor size between the laparoscopy and open group, while Kim et al[10] reported that the tumor size in the open group was significantly larger than that in the laparoscopy group. Additionally, in the pooled data from a fixed-effects model with no significant heterogeneity (I2 = 53.3%, P = 0.073) (Table 3), no significant difference was identified in the total analysis [WMD = -0.038 cm, 95% confidence interval (95%CI): -0.699 to 0.362, P = 0.632] (Figure 2).
Table 3.
Summary results of meta-analysis of clinical outcomes
| Outcomes | No. of studies | Effect value | 95%CI of effect |
Heterogeneity |
|
| I2 (%) | P value | ||||
| Tumor size | 4 | WMD = -0.0.38 | -0.699 to 0.362 | 53.3 | 0.073 |
| Operation time | 5 | WMD = 7.17 min | -56.02 to 70.36 | 92.9 | 0.000 |
| Blood loss | 4 | WMD = -47.47 mL | -93.20 to -1.73 | 63.2 | 0.043 |
| Postoperative complications | 5 | OR = 0.93 | 0.34 to 2.50 | 0.0 | 0.858 |
| Postoperative stay | 5 | WMD = -2.81 d | -3.68 to -1.94 | 38.7 | 0.163 |
| Progression-free survival | 5 | HR = 0.64 | 0.35 to 1.19 | 0.0 | 0.553 |
WMD: Weighted mean differences.
Figure 2.
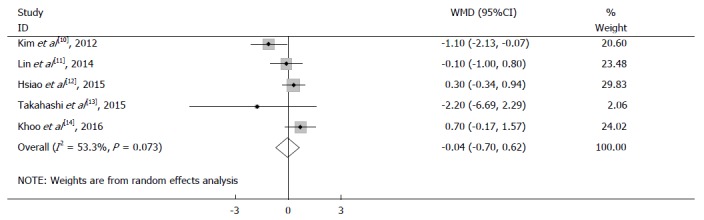
Meta-analysis of tumor size in laparoscopic surgery and open surgery groups.
Operative factors
All enrolled studies provided data for analysis of the operation time. The results showed no significant difference between the two groups (WMD = 7.17 min, 95%CI: -56.02 to 70.36, P = 0.824) (Figure 3A). Because obvious heterogeneity was detected (I2 = 92.9%, P = 0.000) (Table 3), a random-effects model was employed.
Figure 3.
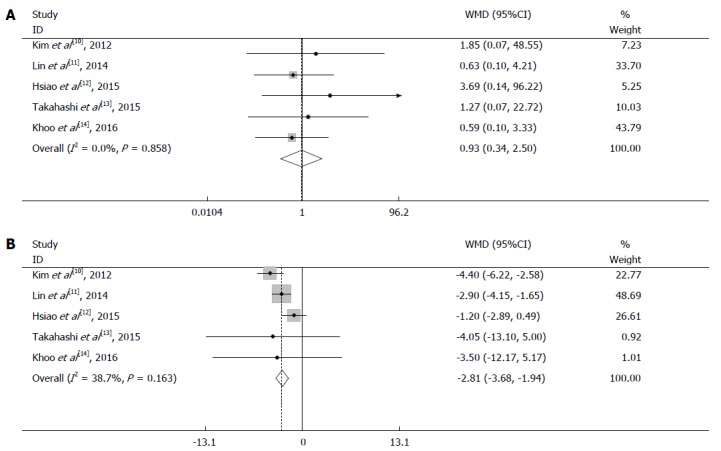
Meta-analysis of operative factors in laparoscopic surgery and open surgery group. A: Pooled analysis of operation time; B: Pooled analysis of blood loss.
Four studies reported data regarding intraoperative blood loss; Lin et al[11] reported that laparoscopic surgery was associated with less blood loss. The heterogeneity between the studies was significant (I2 = 63.2%, P = 0.043); therefore, the analysis was performed with a random-effects model. In the pooled data, a significant difference was found among these three groups (WMD = -47.47 mL, 95%CI: -93.20 to -1.73 mL, P = 0.042) (Figure 3B).
Among all enrolled studies, five patients in the laparoscopy group reportedly underwent conversion to open surgery. One conversion resulted from the surgeons’ initial learning curve for laparoscopy, one was due to dense adhesion to liver, and the other three occurred because of failure to secure the tumor in the visual field of the laparoscope.
Short-term outcomes
All five studies reported postoperative complications. The pooled data revealed no significant difference between the two groups (odds ratio = 0.93, 95%CI: 0.34 to 2.50, P = 0.88) (Figure 4A). A fixed-effects model was used because of the lack of significant heterogeneity (I2 = 0.0%, P = 0.858).
Figure 4.
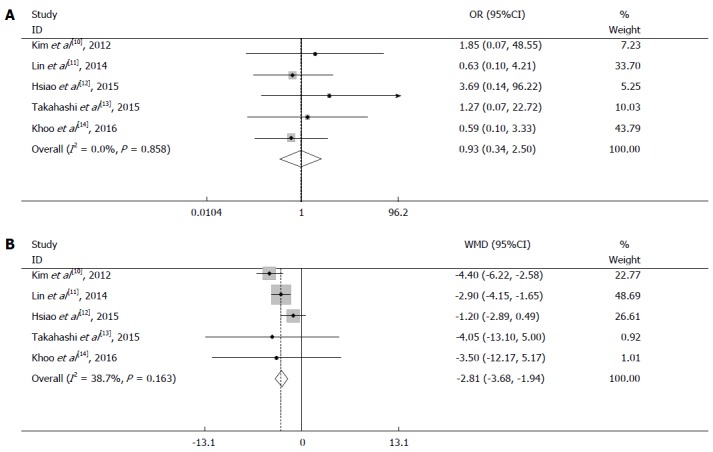
Meta-analysis of short-term outcomes in laparoscopic surgery and open surgery groups. A: Pooled analysis of postoperative complications; B: Pooled analysis of postoperative hospital stay.
Five studies reported data regarding the postoperative hospital stay. A fixed-effects model was employed because of insignificant heterogeneity (I2 = 38.7%, P = 0.163). The postoperative hospital stay was significantly shorter in the laparoscopy than open group (WMD = -2.81 d, 95%CI: -3.68 to -1.94, P < 0.001) (Figure 4B).
Long-term outcomes
All eligible studies reported the progression-free survival of patients. Figure 5 shows a forest plot of disease-free survival and the results of the meta-analysis. No significant difference was observed in patients with larger GISTs who underwent laparoscopic vs open surgery (hazard ratio = 0.64, 95%CI: 0.35 to 1.19, P = 0.157). No obvious heterogeneity was observed in this study; therefore, a fixed-effects model was applied in the survival meta-analysis (I2 = 0.0%, P = 0.553) (Figure 5).
Figure 5.
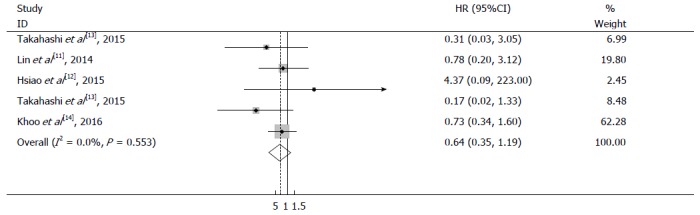
Meta-analysis of progression-free survival in laparoscopic surgery and open surgery groups.
Publication bias
Publication bias was evaluated based on the postoperative hospital stay using Begg’s and Egger’s tests. No publication bias was identified in the five studies (Begg’s test, P = 0.773; Egger’s test, P = 0.825) (Figure 6).
Figure 6.
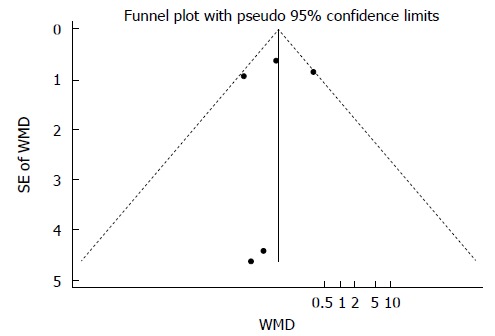
Funnel plot of postoperative hospital stay in laparoscopic surgery and open surgery groups. WMD: Weighted mean difference.
DISCUSSION
Recent studies have suggested that the prognosis of GISTs is mainly based on the tumor size and histological features rather than achievement of wide resection margins[18]. Therefore, laparoscopic resection is more frequently performed for treatment of patients with GISTs using the advances currently being made in surgical techniques.
Although randomized controlled trials are the first choice for high-quality meta-analyses, we failed to enroll any randomized controlled trials in this study. There are several obstacles to design and perform randomized controlled trials, such as ethical issues and organization difficulty[19]. Finally, five nonrandomized controlled studies (one prospective and four retrospective) were enrolled; all were assessed according to the Newcastle-Ottawa Quality Assessment Scale and scored > 6, ensuring their high quality.
Our pooled analysis demonstrated faster recovery and less blood loss in the laparoscopy than open surgery group. Less trauma caused by laparoscopic surgical intervention, only a mild acute inflammatory response, and earlier postoperative activities are considered to contribute to the shorter postoperative hospital stay. Although the blood loss volume might have varied according to the different methods used among the studies, the results of our work indicate that laparoscopic surgery might reduce patients’ surgical trauma to some extent. Furthermore, there was no difference in the postoperative complications between the two groups, adding to the safety of laparoscopic surgery in patients with larger GISTs.
Our review also indicated that laparoscopic resection for larger GISTs is feasible with a conversion rate of 5%, which is similar to other laparoscopic procedures such as laparoscopic gastrectomy[20,21]. The oncological outcome is one of the most concerning problems that prevents application of laparoscopy to the surgical treatment of larger GISTs[22]. Our results showed no difference in the disease-free survival of patients with larger GISTs who underwent laparoscopy vs open surgery (hazard ratio = 0.643, 95%CI: 0.349 to 1.185, P = 0.157), suggesting that the performance of a laparoscopic procedure does not profoundly influence the oncological outcome compared with open surgery.
Several limitations in our study should be addressed. First, the limited number of patients might affect the reliability of the results (209 patients across 5 studies). Second, most of the patients’ tumor sizes ranged from 5 to 10 cm; therefore, the results might not be suitable for patients with GISTs of > 10 cm. Third, treatment of larger GISTs in laparoscopic surgery requires greater surgical skill to prevent tumor rupture and gain adequate resection margins. Therefore, the inclusion of single-center studies with various levels of surgical techniques might have contributed to the bias of our meta-analysis. Finally, the use of different risk classifications and drug therapies within the groups might have also contributed to the bias of recurrence or progression-free survival[23].
In conclusion, this meta-analysis has demonstrated that laparoscopic surgery is as safe and feasible as open surgery for resection of larger GISTs (> 5 cm, mainly 5-10 cm). Moreover, laparoscopic surgery might offer the advantage of faster recovery and less trauma over open surgery in patients with GISTs. More multicenter randomized controlled clinical trials are needed to clarify and confirm the role of laparoscopic surgery in patients with larger GISTs.
ARTICLE HIGHLIGHTS
Research background
Laparoscopic resection of relatively small gastric gastrointestinal stromal tumors (GISTs) is currently well-accepted and has been proven as safe and feasible as traditional open surgery. However, whether laparoscopic resection is also effective and feasible for treatment of larger gastric GISTs (> 5 cm) remains unknown.
Research motivation
The authors aimed to explore whether laparoscopic resection is also effective and feasible for treatment of larger gastric GISTs (> 5 cm), just as the same situation in smaller GISTs.
Research objectives
Laparoscopic resection for small GISTs is now well-accepted. However, whether laparoscopic surgery is as safe and feasible as open resection for patients with larger GISTs (≥ 5 cm) remains controversial.
Research methods
A systematic search of PubMed, EMBASE, Web of Science and the Cochrane Library database was performed. Relevant studies of laparoscopic and open surgery for GISTs of > 5 cm published before December 2016 were identified from these databases. The meta-analysis was performed using Stata (version 12.0) applying weighted mean differences for continuous variables, odds ratios for dichotomous variables, and hazard ratios for time-to-event variables.
Research results
In terms of operative and oncological factors, our research demonstrated that laparoscopic surgery was significantly associated with a shorter postoperative hospital stay (P < 0.001) and less blood loss (P = 0.002) in resecting larger GISTs. Moreover, there were no statistically significant differences in the operation time (P = 0.38), postoperative complication rate (P = 0.88), or disease-free survival rate (P = 0.20) between two groups.
Research conclusion
This research stands as the first meta-analysis focusing on this specific type of GISTs. The meta-analysis has demonstrated that laparoscopic surgery is as safe and feasible as open surgery for resection of larger GISTs (> 5 cm, mainly 5-10 cm). Moreover, laparoscopic surgery might offer the advantage of faster recovery and less trauma over open surgery in patients with GISTs.
Research perspectives
Laparoscopic resection is as acceptable as open surgery for treatment of large gastric GISTs.
Footnotes
Supported by National Program on Key Basic Research Project of China, No. 2014CBA02002; National Key Research and Development Plan, No. 2016YFC0905302; National Natural Science Foundation of China, Nos. 81672319 and 81602507; and Beijing Municipal Science and Technology Project, No. D131100005313010.
Conflict-of-interest statement: This work was presented as an e-poster at the 12th International Gastric Cancer Congress (IGCC), April 20-23, 2017. We have no financial relationship to disclose.
Data sharing statement: No additional data are available.
Peer-review started: October 24, 2017
First decision: November 23, 2017
Article in press: December 6, 2017
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tanabe S, Yuan Y S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
Contributor Information
Jian-Xin Cui, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Yun-He Gao, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Hong-Qing Xi, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Ai-Zhen Cai, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Ke-Cheng Zhang, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Ji-Yang Li, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Bo Wei, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
Lin Chen, Department of General Surgery, Chinese PLA General Hospital, Beijing 100853, China.
References
- 1.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 3.Blanke CD, Corless CL. State-of-the art therapy for gastrointestinal stromal tumors. Cancer Invest. 2005;23:274–280. doi: 10.1081/cnv-200055972. [DOI] [PubMed] [Google Scholar]
- 4.Lai IR, Lee WJ, Yu SC. Minimally invasive surgery for gastric stromal cell tumors: intermediate follow-up results. J Gastrointest Surg. 2006;10:563–566. doi: 10.1016/j.gassur.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Goh BK, Chow PK, Yap WM, Kesavan SM, Song IC, Paul PG, Ooi BS, Chung YF, Wong WK. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol. 2008;15:2153–2163. doi: 10.1245/s10434-008-9969-z. [DOI] [PubMed] [Google Scholar]
- 6.Goh BK, Goh YC, Eng AK, Chan WH, Chow PK, Chung YF, Ong HS, Wong WK. Outcome after laparoscopic versus open wedge resection for suspected gastric gastrointestinal stromal tumors: A matched-pair case-control study. Eur J Surg Oncol. 2015;41:905–910. doi: 10.1016/j.ejso.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Goh BK, Chow PK, Chok AY, Chan WH, Chung YF, Ong HS, Wong WK. Impact of the introduction of laparoscopic wedge resection as a surgical option for suspected small/medium-sized gastrointestinal stromal tumors of the stomach on perioperative and oncologic outcomes. World J Surg. 2010;34:1847–1852. doi: 10.1007/s00268-010-0590-5. [DOI] [PubMed] [Google Scholar]
- 8.Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007;33:444–447. doi: 10.1016/j.ejso.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Demetri GD, Benjamin RS, Blanke CD, Blay JY, Casali P, Choi H, Corless CL, Debiec-Rychter M, DeMatteo RP, Ettinger DS, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5 Suppl 2:S1–S29; quiz S30. [PubMed] [Google Scholar]
- 10.Kim KH, Kim MC, Jung GJ, Kim SJ, Jang JS, Kwon HC. Long term survival results for gastric GIST: is laparoscopic surgery for large gastric GIST feasible? World J Surg Oncol. 2012;10:230. doi: 10.1186/1477-7819-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J, Huang C, Zheng C, Li P, Xie J, Wang J, Lu J. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): a size-matched comparison. Surg Endosc. 2014;28:2577–2583. doi: 10.1007/s00464-014-3506-x. [DOI] [PubMed] [Google Scholar]
- 12.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi T, Nakajima K, Miyazaki Y, Miyazaki Y, Kurokawa Y, Yamasaki M, Miyata H, Takiguchi S, Nishida T, Mori M, et al. Surgical strategy for the gastric gastrointestinal stromal tumors (GISTs) larger than 5 cm: laparoscopic surgery is feasible, safe, and oncologically acceptable. Surg Laparosc Endosc Percutan Tech. 2015;25:114–118. doi: 10.1097/SLE.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 14.Khoo CY, Goh BKP, Eng AKH, Chan WH, Teo MCC, Chung AYF, Ong HS, Wong WK. Laparoscopic wedge resection for suspected large (≥5 cm) gastric gastrointestinal stromal tumors. Surg Endosc. 2017;31:2271–2279. doi: 10.1007/s00464-016-5229-7. [DOI] [PubMed] [Google Scholar]
- 15.ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49–vii55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- 16.Fox AM, Pitzul K, Bhojani F, Kaplan M, Moulton CA, Wei AC, McGilvray I, Cleary S, Okrainec A. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single center. Surg Endosc. 2012;26:1220–1230. doi: 10.1007/s00464-011-2061-y. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33:478–483. doi: 10.1053/hupa.2002.124123. [DOI] [PubMed] [Google Scholar]
- 19.Chen QL, Pan Y, Cai JQ, Wu D, Chen K, Mou YP. Laparoscopic versus open resection for gastric gastrointestinal stromal tumors: an updated systematic review and meta-analysis. World J Surg Oncol. 2014;12:206. doi: 10.1186/1477-7819-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moisan F, Norero E, Slako M, Varas J, Palominos G, Crovari F, Ibañez L, Pérez G, Pimentel F, Guzmán S, et al. Completely laparoscopic versus open gastrectomy for early and advanced gastric cancer: a matched cohort study. Surg Endosc. 2012;26:661–672. doi: 10.1007/s00464-011-1933-5. [DOI] [PubMed] [Google Scholar]
- 21.Sica GS, Iaculli E, Biancone L, Di Carlo S, Scaramuzzo R, Fiorani C, Gentileschi P, Gaspari AL. Comparative study of laparoscopic vs open gastrectomy in gastric cancer management. World J Gastroenterol. 2011;17:4602–4606. doi: 10.3748/wjg.v17.i41.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen YH, Liu KH, Yeh CN, Hsu JT, Liu YY, Tsai CY, Chiu CT, Jan YY, Yeh TS. Laparoscopic resection of gastrointestinal stromal tumors: safe, efficient, and comparable oncologic outcomes. J Laparoendosc Adv Surg Tech A. 2012;22:758–763. doi: 10.1089/lap.2012.0115. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt NR, Collins D, Crotty P, Ridgway PF. Prognosis and management of adult wild type gastrointestinal stromal tumours (GISTs): A pooled analysis and review of literature. Surg Oncol. 2016;25:152–157. doi: 10.1016/j.suronc.2016.05.003. [DOI] [PubMed] [Google Scholar]


