Abstract
Background:
Orexins are excitatory neuropeptides which stimulate the central regulatory pathways. Orexins increase the penicillin-induced epileptic activity in rats. Orexin-A increases in different types of seizures and its elevated level is the characteristic feature in the epileptic children during polysomnography. Recently, the orexin receptor blockage has been reported to increase seizure threshold in mice; however, effect of the selective orexin-A receptor antagonist (SB-334867) on 4-aminopyridine (4-AP)-induced seizures has not been investigated.
Materials and Methods:
We used the intraperitoneal injection of 4-AP to induce seizure in male rats. Under urethane anesthesia, SB-334867 (50 and 100 nmol) was injected stereotaxically into the ventral hippocampal commissure. Using video recording, the effects of SB-334867 on electroencephalogram and tonic–clonic convulsions were compared to those that received diazepam or dimethyl sulfoxide (DMSO).
Results:
SB-334867 significantly decreased the duration of spike trains compared to DMSO-treated rats (P < 0.001) and reduced the duration of convulsive seizures (P < 0.05). Seizure onset was increased significantly by SB-334867, 50 nmol, compared to DMSO (P < 0.05) and diazepam (P < 0.01) treated rats.
Conclusion:
Antagonism of orexin-A receptor by a low-dose SB-334867 showed protective effects in 4-AP-induced seizure-like activities in anesthetized rats.
Keywords: 4-aminopyridine, electroencephalogram, microinjection, orexin-A receptor antagonist
Introduction
Orexinergic (hypocretinergic) neurons were initially discovered in 1998.[1] They produce two excitatory neuropeptides, orexin-1 (A) and orexin-2 (B).[2] Orexin receptors have two subtypes (OXR-1and OXR-2) which exist in many brain regions including hippocampus. There are two hippocampal orexin receptor subtypes, OXR-1 (in CA2) and OXR-2 (in CA3).[1] Orexin-A stimulates both receptors, which leads to in vitro calcium mobilization, locomotor activity increment,[3] awake state induction,[4] sympathetic nervous system trigger,[5] and food intake stimulation in rats.[6]
There are controversial reports which point to orexin involvement in epilepsy. Orexins increased the penicillin-induced cortical epileptic activity,[7] and hippocampal expression of prepro-orexin mRNA in rats increased after pilocarpine-induced status epilepticus;[8] however, the cerebrospinal fluid orexin-A level is decreased after repetitive seizures in patients.[9] The orexin-A level has been reported low in epileptic children without seizures during polysomnography versus parasomnic children; however, it was high in those with seizures.[10]
Orexins affect central regulatory pathways, hippocampal synaptic plasticity,[9,11] and reduce gamma-aminobutyric acid (GABA) content;[10] therefore, blocking the hippocampal orexin receptors may have protective effects in epilepsy. Despite a lot of experiments about orexins, narcolepsy, and sleep waves, there are very few reports about the orexin receptors antagonists in epilepsy. For example, blocking orexin receptors (OXR-1 and OXR-2) in pentylenetetrazole (PTZ)-induced seizures decreased the severity of convulsions through modification of GABA and glutamate content.[12] Recently, the orexin receptor blockage by orexin-A receptor antagonist (SB-334867) has been reported to increase seizure threshold in mice in acute seizure tests such as intravenous PTZ seizure threshold test, maximal electroshock seizure test, and 6-Hz psychomotor seizure test.[13] Furthermore, it has been reported that using almorexant (a dual orexin receptor antagonist) in Kcna1-null mice (a genetic model of temporal lobe epilepsy) has improved sleep, decreased the occurrence of seizures, and reduced the frequency of severe convulsions.[14]
Altogether, there are very few reports about the SB-334867 in different epilepsy models, and antagonism of orexin receptors has not been studied in 4-aminopyridine (4-AP) seizures in vivo. 4-AP can cause tonic–clonic seizure-like events (SLEs) in acute seizure models (slices) and is used for inducing pharmacoresistant models of status epilepticus,[15] so it can offer a good model for investigating new drug targets. Therefore, we aimed to investigate the effect of SB-334867 microinjection in 4-AP-induced seizures in male rats.
Materials and Methods
All chemical compounds were analytical grade and obtained as follows: 4-AP and urethane (Sigma-Aldrich Inc., China), SB-334867 (Tocris Bioscience Co, UK), dimethyl sulfoxide (DMSO) (AppliChem GmbH, Germany), ketamine 10% and xylazine 2% (Alfasan Co., The Netherlands), and diazepam (Caspian Tamin pharmaceutical Co, Iran). Diazepam and 4-AP (freshly prepared) were diluted in a saline solution. SB-334867 was dissolved in DMSO at the final concentration of 4 mM; aliquot of SB-334867 was kept at −20°C.[11]
Experimental design
After approval by the Institutional Research Committee, the study was performed with consideration of the principles of care and use of laboratory animals (8th edition) and 3R rules (Basel declaration). Mature male Wistar rats were purchased from the animal house of School of Medicine and maintained in Plexiglas cages under 12-h light-dark cycle, light off at 6 pm, and 22°C–25°C. The adaptation period was 1 week with free access to food and water. All experiments were performed within 08:00–16:00 h to remove the circadian effect. The rats were randomly assigned to six experimental groups of seven animals each. 4-AP was used to induce convulsions along with SB-334867, 50 and 100 nmol/rat (SB1 and SB2). Control groups received DMSO and diazepam, and one group received SB 100 nmol along with normal saline (NS) intraperitoneal (IP) instead of 4-AP.
We injected 0.5 μl of diazepam (0.1 g/L),[16] DMSO, and SB-334867 (50 and 100 nmol);[12] after 20 min, 4-AP, 6 mg/kg IP or the same volume of NS was administered.
Surgery
Anesthesia was induced by IP injection of ketamine (120 mg/kg) and xylazine (10 mg/kg). Considering the antiseptic technique, the stereotaxic surgery (Stoelting Co, USA) was done with a hand driller to implant four hand-made guides (needles 22-gauge) (two for monopolar electroencephalogram [EEG] recording electrodes, one for reference electrode, and one guide for injection needle) in addition to two small anchoring screws in lateral sides. The epidural EEG electrodes located on parietal bones and the reference electrode on the cerebellum. This assembly was fixed together by dental acrylic cement. The injection guide was 4 mm in length while other guides inserted in 1 mm holes. A stylet was inserted into each guide to prevent the occlusion. The relevant coordinates according to the rat brain atlas[17] were as follows: the left ventral hippocampal commissure, AP: −1.56, L: +1.4, H: 4 mm. Animals were kept warm and closely observed during surgery; then, individually put in a Plexiglas cage for 2 days to regain the normal conditions. On the 3rd day, animals were anesthetized by urethane, 2 g/kg IP, the stylet was removed. Then, the injection needle (27-gauge dental needles) and monopolar electrodes (ADInstruments, Australia) were inserted in their place. The end of the injection needle was 1 mm beyond the tip of its guide to ensure easy access to brain tissue. After 2 min, DMSO, diazepam, or SB-334867 were administered, each 0.5 μl in 5 min, using a syringe pump. The injecting needle was left in place for at least 10 min to prevent the drug backflow. Initially, we recorded the EEG for 20–30 min until stabilization. After 20 min, we injected 4-AP, 6 mg/kg IP.[18,19] The EEG recording continued for 1.5 h. Finally, Evans blue, 0.5 μl, was injected for verification of the injection site, animals were rapidly decapitated, and the brain was removed and placed in formalin/saline 10%. The spike trains were measured as previously published data.[20] Briefly, the spike train was determined if it included the spikes and waves with an amplitude of at least 2 times the basal level, duration of at least 1 s, and frequency of 5–9 Hz.
Furthermore, an expert observer in a nonblinded manner recorded the behavioral manifestations at the beginning of the study; after injection of DMSO, diazepam, SB-334867, and 4-AP, obtaining 30–60 s videotapes every 15 min; at least six films for each rat. We used the films for data analysis to prevent the experiment's bias. Latency to initiate tonic–clonic SLEs and the total duration of such severe seizure-like behaviors in each animal were measured. We considered the onset of repeated generalized convulsions in whole body and the duration of such condition.
Data analysis
Normal distribution was examined by Kolmogorov–Smirnov test. Using the GraphPad Prism5, (GraphPad software Inc, San Diego, USA) Friedman test followed by Dunn's test (comparison of time points), Kruskal–Wallis test followed by Dunn's test (comparison of train duration in different groups), and one-way ANOVA followed by Bonferroni post hoc tests (comparison of onset and duration of seizure behaviors) were used to determine statistical significant differences. P < 0.05 was defined as significant. All the values were expressed as mean ± standard error mean.
Results
Administration of either SB-334867 + NS or DMSO + NS did not induce seizure activity. 4-AP injection induced SLEs with increased severity over time. The first behavioral demonstrations were symptoms such as hair-raising, increased respiratory rate, ear twitches, facial myoclonic jerks including vibrissae twitching, and eye clonus. In later stages, animals showed forelimb clonus, hindlimb clonus (at first unilateral then bilateral), and wet dog shakes. Ultimately, the generalized tonic–clonic SLEs and even running-like behavior of hind limbs (1 rat) were observed.
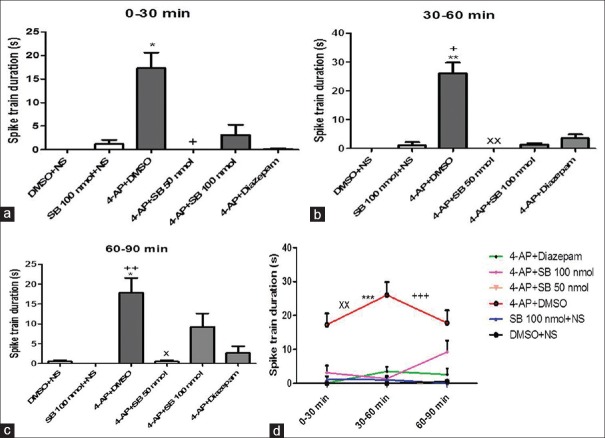
SB-334867 significantly decreased the duration of trains compared to DMSO-treated rats (P < 0.001) [Figure 1d]. The EEG traces 30 min after 4-AP injection showed slow waves in SB1 group; diazepam and SB2 rats, however, showed EEG irregularity [Figure 2]. Duration of severe seizures was significantly decreased by SB-334867 (both doses) compared to DMSO- and diazepam-treated rats (P < 0.05) [Figure 3], and seizure onset increased significantly by SB (50 nmol) compared to DMSO- and diazepam-treated rats (P < 0.05 and P < 0.01, respectively) [Figure 4]. Since no seizure-like behavior was demonstrated in control groups of DMSO + NS or SB-334867 + NS, so these groups were not depicted in Figures 3 and 4. The injection site was verified under light microscopy and those with unjustified site excluded from the study [Figure 5].
Figure 1.
Duration of spike trains in treated groups with DMSO + normal saline, 4-aminopyridine + DMSO, SB-334867 (50 and 100 nmol) +4-aminopyridine, SB-334867 (100 nmol) + normal saline, and 4-aminopyridine + diazepam after intraperitoneal 4-aminopyridine injection. (a) *P < 0.05 versus DMSO + normal saline, +P < 0.05 versus 4-aminopyridine + DMSO. (b) **P < 0.01 versus DMSO + normal saline, +P < 0.05 versus SB 100 nmol + normal saline, xxP < 0.01 versus 4-aminopyridine + DMSO. (c) *P < 0.05 versus DMSO + normal saline, ++P < 0.01 versus SB 100 nmol + normal saline, *P < 0.05 versus 4-aminopyridine + DMSO. Friedman test followed by Dunn's test. (d) ***P < 0.001 versus DMSO + normal saline, xxP < 0.01 versus SB 100 nmol + normal saline, and +++P < 0.001 versus 4-aminopyridine + SB 50 nmol. Kruskal–Wallis followed by Dunn's test. P <0.05 was considered as significant
Figure 2.
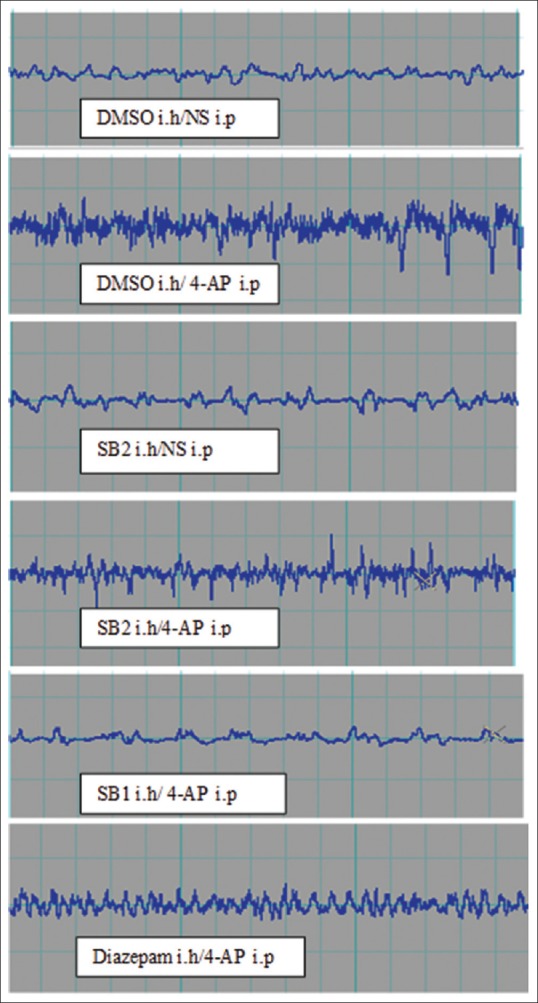
Electroencephalogram patterns in different treated groups with DMSO, SB-334867 50 nmol (SB1), 100 nmol (SB2), and diazepam 30 min after intraperitoneal 4-aminopyridine injection. Calibration is 1 s on X axis, 1 mv on Y axis on each little square. ih = Intrahippocampal commissure, ip = Intraperitoneal
Figure 3.
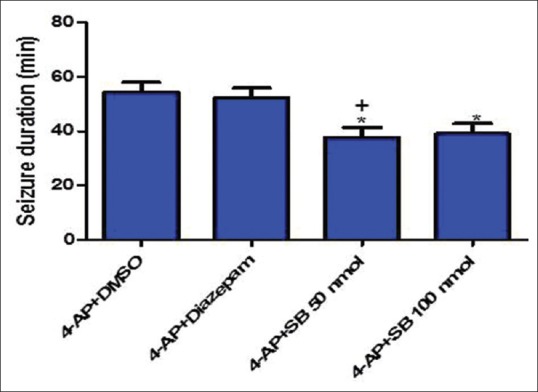
Duration of convulsive-like seizures in different treated groups with DMSO, SB-334867 (50 and 100 nmol), and diazepam after intraperitoneal 4-aminopyridine injection. One-way ANOVA followed by Bonferroni test. P < 0.05 was considered as significant. *P < 0.05: 4-aminopyridine + DMSO versus 4-aminopyridine + SB 50 and 100 nmol, +P < 0.05: 4-aminopyridine + diazepam versus 4-aminopyridine + SB 50 nmol. DMSO + normal saline and SB 100 nmol + normal saline did not induce seizure behaviors, so they were neglected in data analysis
Figure 4.
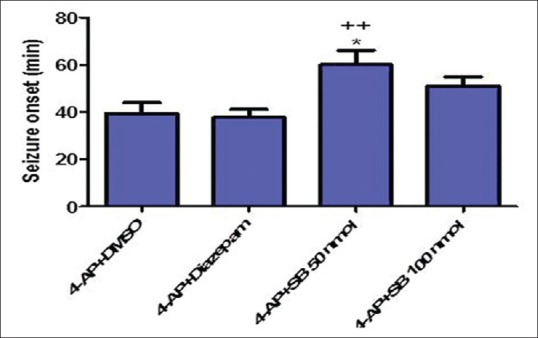
Seizure onset in different treated groups with DMSO, SB-334867 (50 and 100 nmol/rat), and diazepam after intraperitoneal 4-aminopyridine injection. One-way ANOVA followed by Bonferroni test. P < 0.05 was considered as significant. *P < 0.05: 4-aminopyridine + DMSO versus 4-aminopyridine + SB 50 nmol, ++P < 0.01: 4-aminopyridine + diazepam versus 4-aminopyridine + SB 50 nmol. DMSO + normal saline and SB 100 nmol + normal saline did not induce seizure behaviors, so they were neglected in data analysis
Figure 5.
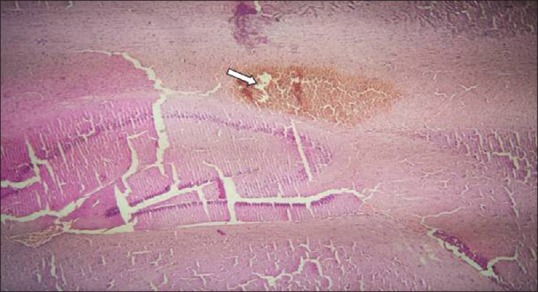
Injection needle tip site through ventral hippocampal commissure. White arrow represents the site of injection needle. Hematoxylin eosin staining, (×100))
Discussion
This is for the first time that 4-AP is used for investigating the protective effect of SB-334867 in EEG and seizure-like behaviors. Unilateral SB-334867 microinjection attenuated convulsive seizures, which supports recent findings in PTZ-induced seizure in freely moving rats that SB-334867 reduced the seizure severity and duration.[12] The duration of spike trains was also reduced by SB-334867. To our knowledge, there was not any report about EEG finding of SB-334867 in seizure to compare with current result.
We used 4-AP because it makes hippocampal slices epileptiform even after 4-AP wash out[21] and induces epileptic discharges even in Nembutal-anesthetized rats.[22] Furthermore, intrahippocampal injection of 4-AP has been reported to induce status epilepticus-like activities in urethane-anesthetized rats.[23]
We observed convulsive seizures using 4-AP and urethane anesthesia. It has been reported urethane anesthesia permitted 3–4 h acute phase of status epilepticus,[24] and limbic cortical seizures can be induced under urethane anesthesia.[25] However, the motor convulsion have not been observed in piriform cortex excitation,[26] kindled seizures, and EEG or PTZ convulsions under urethane-induced anesthesia.[27] This controversy may be due to using different convulsions inducing agents. Seizure behavior has been studied in different seizure types under urethane anesthesia.[24,26,28] In urethane anesthesia, animals can breathe on their own and different polysynaptic activities take place.[24,29] Thus, physiologic changes in response to pharmacological manipulation mimic those of wakeful animals.[29] It is also a well-established method in electrophysiological studies. Despite motor convulsions seen in this model, no lethality occurred until the end of the experiment (90 min after 4-AP injection), which may be accounted as the advantage of urethane anesthesia.
The lower SB-334867 dose was more effective in this study. This result supports the recent microdialysis findings; the lower SB-334867 dose has shown the highest glutamate depletion and GABA increment while the higher dose did not affect GABA and glutamate reduction was less pronounced than the lower dose.[12] It is speculated that SB-334867 exerts antagonistic effects in low dose compared to agonistic effects in high dose.[30] The antagonistic effect of low SB-334867 dose may be related to autoreceptor-mediated dynorphin responses.[30,31] Dynorphin which is co-released with orexin has shown immunoreactivity changes in the hippocampus in different types of seizures.[32]
It is well known that orexin-A depolarizes the rat brain cells and increases calcium concentration[4,33] which is a key element in seizure.[34] Different seizure types enhance orexin-A,[13] and SB-334867 inhibits the OXR-A-mediated calcium enhancement;[35] thus, its beneficial effect in seizure may be associated to the reduction of calcium.[13] However, this speculation remains to be elucidated in future investigations.
Duration of seizures can affect the responsiveness of anticonvulsant drugs; if convulsive seizures last >30 min, it will be more resistance to the effects of antiseizure drugs such as diazepam.[36] In the present study, the seizures lasted for more than 40 min, especially in DMSO + 4-AP and diazepam + 4-AP groups. In this study, diazepam did not prevent the induction or propagation of seizures perfectly which supports the findings of previous reports.[15,37] However, in a recent study, it has been shown that seizure onset delay was significantly increased by diazepam compared to magnesium sulfate in 4-AP-induced seizures in Swiss mice.[38] Furthermore, the status epilepticus following pilocarpine injection in the piriform cortex or hippocampus was halted by diazepam within 2 h after epilepsy induction.[39]
Conclusion
The findings of this study further support the beneficial effect of SB-334867 in EEG and convulsion-like behaviors in urethane-anesthetized 4-AP-induced seizures.
Financial support and sponsorship
The study was supported financially by Council of Research, Mashhad University of Medical Sciences (Grant no: 910781).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study is part of a Ph.D. dissertation and has been supported financially by Council of Research, Mashhad University of Medical Sciences (Grant no: 910781).
References
- 1.Tsujino N, Sakurai T. Orexin/hypocretin: A neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev. 2009;61:162–76. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 2.Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: Implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–65. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- 3.Samson WK, Bagley SL, Ferguson AV, White MM. Orexin receptor subtype activation and locomotor behaviour in the rat. Acta Physiol (Oxf) 2010;198:313–24. doi: 10.1111/j.1748-1716.2009.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: Role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol. 2007;292:R382–7. doi: 10.1152/ajpregu.00496.2006. [DOI] [PubMed] [Google Scholar]
- 6.Thorpe AJ, Doane DF, Sweet DC, Beverly JL, Kotz CM. Orexin A in the rostrolateral hypothalamic area induces feeding by modulating GABAergic transmission. Brain Res. 2006;1125:60–6. doi: 10.1016/j.brainres.2006.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kortunay S, Erken HA, Erken G, Genç O, Sahiner M, Turgut S, et al. Orexins increase penicillin-induced epileptic activity. Peptides. 2012;34:419–22. doi: 10.1016/j.peptides.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Morales A, Bonnet C, Bourgoin N, Touvier T, Nadam J, Laglaine A, et al. Unexpected expression of orexin-B in basal conditions and increased levels in the adult rat hippocampus during pilocarpine-induced epileptogenesis. Brain Res. 2006;1109:164–75. doi: 10.1016/j.brainres.2006.06.075. [DOI] [PubMed] [Google Scholar]
- 9.Rejdak K, Papuc E, Grieb P, Stelmasiak Z. Decreased cerebrospinal fluid hypocretin-1 (orexin A) in patients after repetitive generalized tonic-clonic seizures. Epilepsia. 2009;50:1641–4. doi: 10.1111/j.1528-1167.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- 10.Kacinski M, Budziszewska B, Lason W, Zajac A, Skowronek-Bala B, Leskiewicz M, et al. Level of S100B protein, neuron specific enolase, orexin A, adiponectin and insulin-like growth factor in serum of pediatric patients suffering from sleep disorders with or without epilepsy. Pharmacol Rep. 2012;64:1427–33. doi: 10.1016/s1734-1140(12)70940-4. [DOI] [PubMed] [Google Scholar]
- 11.Dias MB, Li A, Nattie EE. Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol. 2009;587(Pt 9):2059–67. doi: 10.1113/jphysiol.2008.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goudarzi E, Elahdadi Salmani M, Lashkarbolouki T, Goudarzi I. Hippocampal orexin receptors inactivation reduces PTZ induced seizures of male rats. Pharmacol Biochem Behav. 2015;130:77–83. doi: 10.1016/j.pbb.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Socala K, Szuster-Ciesielska A, Wlaz P. SB 334867, a selective orexin receptor type 1 antagonist, elevates seizure threshold in mice. Life Sci. 2016;150:81–8. doi: 10.1016/j.lfs.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 14.Roundtree HM, Simeone TA, Johnson C, Matthews SA, Samson KK, Simeone KA. Orexin receptor antagonism improves sleep and reduces seizures in Kcna1-null mice. Sleep. 2016;39:357–68. doi: 10.5665/sleep.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahab A, Albus K, Gabriel S, Heinemann U. In search of models of pharmacoresistant epilepsy. Epilepsia. 2010;51(Suppl 3):154–9. doi: 10.1111/j.1528-1167.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 16.Lu YJ, Zhou J, Zhang SM, Zhang HY, Zheng XX. Inhibitory effects of jujuboside A on EEG and hippocampal glutamate in hyperactive rat. J Zhejiang Univ Sci B. 2005;6:265–71. doi: 10.1631/jzus.2005.B0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C, editors. The Rat Brain in Stereotaxic Coordinates. 6th ed. London: Elsevier Academic Press; 2007. [Google Scholar]
- 18.Baracskay P, Kiglics V, Kékesi KA, Juhász G, Czurkó A. Status epilepticus affects the gigantocellular network of the pontine reticular formation. BMC Neurosci. 2009;10:133. doi: 10.1186/1471-2202-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu W, Smith AB, Pilitsis J, Shin DS. Isovaline attenuates epileptiform activity and seizure behavior in 4-aminopyridine treated rats. Epilepsy Res. 2014;108:331–5. doi: 10.1016/j.eplepsyres.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Sadeghnia HR, Cortez MA, Liu D, Hosseinzadeh H, Snead OC Antiabsence effects of safranal in acute experimental seizure models: EEG and autoradiography. J Pharm Pharm Sci. (3rd) 2008;11:1–14. doi: 10.18433/j38g6j. [DOI] [PubMed] [Google Scholar]
- 21.Salah A, Perkins KL. Persistent ictal-like activity in rat entorhinal/perirhinal cortex following washout of 4-aminopyridine. Epilepsy Res. 2011;94:163–76. doi: 10.1016/j.eplepsyres.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovács A, Mihály A, Komáromi A, Gyengési E, Szente M, Weiczner R, et al. Seizure, neurotransmitter release, and gene expression are closely related in the striatum of 4-aminopyridine-treated rats. Epilepsy Res. 2003;55:117–29. doi: 10.1016/s0920-1211(03)00113-x. [DOI] [PubMed] [Google Scholar]
- 23.Martín ED, Pozo MA. Valproate suppresses status epilepticus induced by 4-aminopyridine in CA1 hippocampus region. Epilepsia. 2003;44:1375–9. doi: 10.1046/j.1528-1157.2003.11603.x. [DOI] [PubMed] [Google Scholar]
- 24.Saito T, Sakamoto K, Koizumi K, Stewart M. Repeatable focal seizure suppression: A rat preparation to study consequences of seizure activity based on urethane anesthesia and reversible carotid artery occlusion. J Neurosci Methods. 2006;155:241–50. doi: 10.1016/j.jneumeth.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto K, Saito T, Orman R, Koizumi K, Lazar J, Salciccioli L, et al. Autonomic consequences of kainic acid-induced limbic cortical seizures in rats: Peripheral autonomic nerve activity, acute cardiovascular changes, and death. Epilepsia. 2008;49:982–96. doi: 10.1111/j.1528-1167.2008.01545.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang DX, Bertram EH. Suppressing limbic seizures by stimulating medial dorsal thalamic nucleus: Factors for efficacy. Epilepsia. 2015;56:479–88. doi: 10.1111/epi.12916. [DOI] [PubMed] [Google Scholar]
- 27.Cain DP, Raithby A, Corcoran ME. Urethane anesthesia blocks the development and expression of kindled seizures. Life Sci. 1989;44:1201–6. doi: 10.1016/0024-3205(89)90315-9. [DOI] [PubMed] [Google Scholar]
- 28.Stringer JL. Amygdala stimulation produces two types of hippocampal afterdischarges in the urethane-anesthetized rat. Brain Res. 1992;591:103–8. doi: 10.1016/0006-8993(92)90983-g. [DOI] [PubMed] [Google Scholar]
- 29.Hara K, Harris RA. The anesthetic mechanism of urethane: The effects on neurotransmitter-gated ion channels. Anesth Analg. 2002;94:313–8. doi: 10.1097/00000539-200202000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Kukkonen JP. Physiology of the orexinergic/hypocretinergic system: A revisit in 2012. Am J Physiol Cell Physiol. 2013;304:C2–32. doi: 10.1152/ajpcell.00227.2012. [DOI] [PubMed] [Google Scholar]
- 31.Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–47. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signalling cascades. Br J Pharmacol. 2014;171:314–31. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cain SM, Snutch TP. Voltage-gated calcium channels in epilepsy. In: Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Noebels J, Avoli M, Rogawski M, Olsen R, Delgado-Escueta A, editors. Vancouver: Oxford University Press; 2012. [Google Scholar]
- 35.Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, et al. SB-334867-A: The first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–82. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Löscher W. Single versus combinatorial therapies in status epilepticus: Novel data from preclinical models. Epilepsy Behav. 2015;49:20–5. doi: 10.1016/j.yebeh.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 37.Rajasekaran K, Zanelli SA, Goodkin HP. Lessons from the laboratory: The pathophysiology, and consequences of status epilepticus. Semin Pediatr Neurol. 2010;17:136–43. doi: 10.1016/j.spen.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kebe B, Obeten E, Mfem C, Usun O. Comparative studies of the effect of diazepam and magnetic sulphate on motor coordination and seizures in 4-aminopyridine induced seizures in swiss mice. Glob J Sci Front Res C Biol Sci. 2016;16:1–13. [Google Scholar]
- 39.Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: Pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–99. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]