Abstract
Background:
Chronic stress adversely influences brain functions while crocin, as an effective component of saffron, exhibits positive effects on memory processes. This study investigated the effects of different doses of crocin on the improvement of learning and memory as well as corticosterone (CORT) levels in the hippocampus and frontal cortex of rats subjected to chronic stress.
Materials and Methods:
Forty male rats were randomly allocated to five different groups (n = 8): Control, sham; stress (6 h/day for 21 days) groups, and two groups receiving daily intraperitoneal injections of one of two doses (30 and 60 mg/kg) of crocin accompanied by 21 days of restraint stress. Latency was evaluated as a brain function using the passive avoidance test before and one-day after a foot shock. CORT levels were measured in the homogenized hippocampus and frontal cortex.
Results:
Results revealed that chronic stress had a significantly (P < 0.01) negative effect on memory. Crocin (30 and 60 mg/kg), however, gave increase to significantly (P < 0.01 and P < 0.05; respectively) improved memory functions in the stressed rats. Furthermore, the CORT levels in the hippocampus and frontal cortex declined significantly (P < 0.05) in the stress group compared to the control. Only a crocin dose of 30 mg/kg was observed modulate significantly (P < 0.05) the CORT levels in the hippocampus and frontal cortex in the stressed group.
Conclusions:
It was found that the lower crocin dose (30 mg/kg) had more beneficial effects than its higher (60 mg/kg) dose on learning and memory under chronic stress conditions. Moreover, it was speculated that different doses of crocin act on different neurotransmitters and biochemical factors in the brain.
Keywords: Corticosterone, crocin, hippocampus, memory, stress
Introduction
Stress is known as a state of psychological response to actual or potential stressors that tend to disturb the homeostasis of the body, especially the brain functions.[1] Stress alters the activity of the hypothalamic-pituitary-adrenal (HPA) axis and affects the secretion of glucocorticoids such as corticosterone (CORT) in rodents.[2] Previous study has shown alterations due to chronic stress in many factors including levels of hormones as well as many biochemical and neurochemical substances in both the serum and the brain.[1,2,3] Hence, stress can impair biological, behavioral, and electrophysiological activities in subjects.[4,5,6] Moreover, memory processes can be impaired by changes in the functions of the hippocampus and probably the frontal cortex under stressful conditions.[7,8,9] These structures are the most important regions involved in memory, moods, cognition, and neuroendocrine stress reactivity.[7,10,11,12]
As an immediate remedial strategy, plant drugs have been proposed to enhance brain functions and public attention has been increasingly drawn to herbal medication as supplementary medicines due to their availability and supposedly lower side effects.[1]
Cultivated in many parts of the world, Crocus sativus L. (saffron) is especially grown in Iran, Italy, Greece, Spain, and China.[13] One of the most important active components of saffron is crocin (carotenoid pigment), which is responsible for its characteristic color[14] and is a glucosyl ester of crocetin and water-soluble carotenoids.[15] Crocin as an anti-oxidant has various pharmaceutical advantages such protection against oxidative stress[16,17,18,19] and improvement of memory deficits.[13,20] It is seam that daily consumption of saffron can be useful for ameliorating the destructive effects of stress. Hypothesizing that different doses of crocin might induce changes in chronic emotional stress, we designed and conducted this study to explore the effects of different doses of crocin on learning, memory, and both hippocampal and frontal cortical CORT levels to verify the protection it might offer against memory deficit in rats subjected to chronic restraint stress.
Materials and Methods
Experimental animals
Forty male Wistar rats with initial weights of 250-300 g were obtained from Pasteur Institute, Tehran, Iran. All the experimental protocols were approved by the Committee of Ethics of Isfahan University of Medical Sciences (Isfahan, Iran) and implemented in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23, revised in 1996). The rats were housed under controlled humidity (50% ± 5%) and light conditions (12 h light/dark cycle; lights on 07:00 am to 19:00 pm). Room temperature was set to 23 ± 2°C and food and water were made available ad libitum, except during stress sessions. Cage size and colony grouping were completely similar across the groups. All the behavioral experiments were carried out at 15:00-16:00 pm. A 2-week period was allowed for adaptation before the animals were randomly divided into the following five groups (n = 8 in each):
Control group (Co): Rats were maintained in the laboratory room receiving no special treatment
Sham group (Sh): Rats received equal volumes of saline (drug solvent) for 21 days
Restraint stress group (St.R): Rats were under restraint stress for 6 h/day over the 21-day experimental period
Restraint stress-crocin 30 group (St.R-C30): Rats were under restraint stress for 6 h/day over the 21-day experimental period and received daily injection of 30 mg/kg crocin
Restraint stress-crocin 60 group (St.R-C60): Rats received the same treatment as the St.R-C30 group except that their daily injections contained 60 mg/kg of crocin.
Experimental procedures
Drugs
Depending on their experimental group, the stressed rats received intraperitoneal (i.p) injections of saline-dissolved 30 or 60 mg/kg of crocin (Sigma-Aldrich Co. USA) for 21 days.
Stress Paradigms
In the chronic stress model, the rats would be placed in tightly fit restrainers for 6 h/day over the whole 21 days[7,21] of the experiment. Hence, restraint was a powerful emotional stress.[22] The restraint stress was induced throughout the experimental period from 8:00 am to 14:00 pm each day.[21,23]
Behavioral Paradigms
The passive avoidance test, which is a hippocampus-dependent memory task involving the cognitive memory,[2,24,25] was used in this study with the passive avoidance apparatus (Shuttle box 64 cm × 25 cm × 35 cm) divided into two rooms of identical size (32 cm × 25 cm × 35 cm) with sliding guillotine doors and grid floors. On day 19 of the experiment, each rat was placed in the apparatus for 300 s for habituation. On the following day (day 20), a single learning trial was performed. On day 21, the memory trial of the passive avoidance test was evaluated. Both habituation and memory trial were accomplished without any foot shocks. In the learning trial, the rats were placed individually in the light room for 60 s before the guillotine door was raised. When the rat entered the dark room, the door would be closed and a single electrical shock (0.5 mA, 2 s; once) would be delivered to the animal's foot through the grid floor using an isolated stimulator.[26] The initial latency (IL) of entrance into the dark room was recorded before inducing the electrical shock. In the memory trial, the step-through latency (STL), defined as the delay of entrance into the dark room from the light room (up to a maximum of 300 s), was measured. Finally, the difference between the initial and the step-through latencies was interpreted as the occurrence of learning in the animal experimental research.[27]
Assessment of corticosterone levels in the hippocampus and frontal cortex
At the end of the experiments, the animals were anesthetized with an i.p injection of urethane (1.5 g/kg, Sigma-Aldrich Co., USA) and sacrificed at 16:00–18:00 by decapitation on day 22. Following decapitation, the brain of each animal was immediately removed from the skull and the hemi-hippocampus and hemi-frontal cortex were instantly dissected to be kept on dry ice, which were subsequently immersed in Problock™-50, EDTA-free (Gold Bio Co., USA) and in a phosphate buffer solution (PBS buffer, 0.01 M, pH 7.4), separately. Indeed, this solution contained a complete protease inhibitor cocktail.[28] The hippocampus and frontal cortex were homogenized and centrifuged in a cooled centrifuge (4°C, 10,000 g) for 20 min. The supernatant was collected and stored at − 80°C until assessment. The commercial Enzyme-Linked Immuno Sorbent Assay (ELISA) kit (Zellbio Co., Marburg, Germany) was used to assess the CORT levels in the hippocampus and frontal cortex. The amount of CORT was determined in a given volume of the supernatant.[2,28,29]
Statistical analysis
The IL and STL of the passive avoidance test, CORT levels, body weight, and daily BWDs were analyzed by ANOVA followed by Tukey's post hoc test for multiple groups. Comparisons of IL and STL (within groups) were analyzed using the paired sample t-test. Furthermore, body weight trends were compared using repeated measures ANOVA followed by Tukey's post hoc test. Pearson's correlation analysis was performed to find the correlation between and among the variables. All the data were reported as means ± standard error of mean. The value of P < 0.05 was considered statistically significant.
Results
Not all the data showed significant differences between the control (Co) and the sham (Sh), indicating that the injection had no significant effects on these parameters.
Step-through latency
Figures 1 and 2, respectively, show the initial and the stepthrough (IL and STL, respectively) latency results in the passive avoidance test after 1 day for all the groups. Based on the one-way ANOVA, no significant differences were observed in IL values across the groups [Figure 1].
Figure 1.
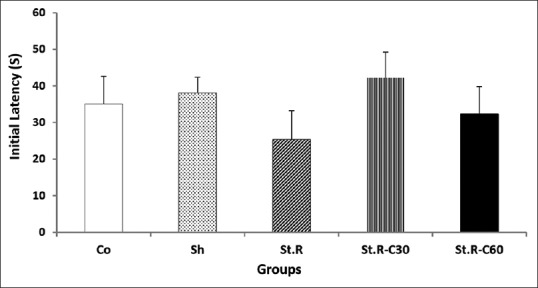
Initial latency to enter the dark room of the passive avoidance apparatus for all the groups before receiving a foot shock (n = 8). Results are expressed as means ± standard error of mean (one-way of ANOVA followed by Tukey's post hoc test). No significant differences were observed among the groups. Co: Control group, Sh: Sham group, St.R: Restrain stress group, St.R-C30: Restrain stress-Crocin 30 group, St.R-C60: Restrain stress-Crocin 60 group
Figure 2.
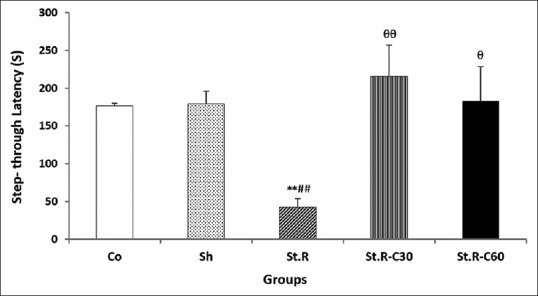
Step-through latency to enter the dark room of the passive avoidance apparatus for all the groups 1 day after receiving the foot shock (n = 8). Results are expressed as means ± standard error of mean (one-way of ANOVA followed by Tukey's post hoc test). **P < 0.01 compared to the control, ##P < 0.01 compared to the Sham, θP < 0.05 and θθP < 0.01 compared to the Stress group. Co: Control group, Sh: Sham group, St.R: Restrain stress group, St.R-C30: Restrain stress-Crocin 30 group, St.R-C60: Restrain stress-Crocin 60 group
In the stress (St.R) group, the STL values were significantly (P < 0.01) lower than those recorded for the control and sham groups, indicating the reduced passive avoidance memory as a result of the restraint stress [Figure 2].
As shown in Figure 2, the values for STL showed significant enhancements (P < 0.01 and P < 0.05, respectively) in the stress-crocin 30 (St.R-C30) and stress-crocin 60 (St.R-C60) groups when compared with the St.R group, indicating that although chronic crocin treatment was able to produce a significant increase in memory after 1 day, daily administration of 30 mg/kg of crocin had more beneficial effects than did the 60 mg/kg dose on cognitive memory in the emotional stress model.
The results of initial and stepthrough latencies (IL and STL, respectively) were analyzed using the paired sample t-test to evaluate within group latency changes. As shown in Figure 3, significant differences were detected between IL and STL in all the groups, except between all the groups and the St.R one (P < 0.001 in Co and Sh groups, P < 0.01 in St.R-C30, and P < 0.05 in St.R-C60 groups).
Figure 3.
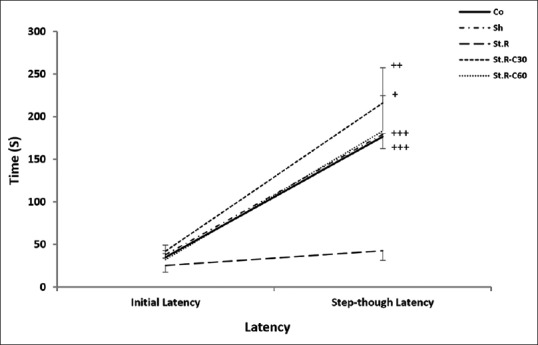
Initial latency and step-through latency after 1 day to enter the dark room of the passive avoidance apparatus before and after the foot shock (within groups) (n = 8). Results are expressed as means ± standard error of mean (paired sample t-test). +P < 0.05, ++P < 0.01 and +++P < 0.001 initial latency relative to the step-through latency. Co: Control group, Sh: Sham group, St.R: Restrain stress group, St.R-C30: Restrain stress-Crocin 30 group, St.R-C60: Restrain stress-Crocin 60 group
Assessment of corticosterone levels in the hippocampus and frontal cortex
As shown in Figures 4 and 5, significant enhancements (P < 0.05) were observed in the hippocampal and frontal cortical CORT levels in the St.R group when compared with the control [Figures 2 and 3]. CORT levels decreased in the St.R C30 and St.R C60 groups relative to those in the St.R group. However, the decline was significant only in the St.R-C30 group rather than in the St.R [Figures 4 and 5].
Figure 4.
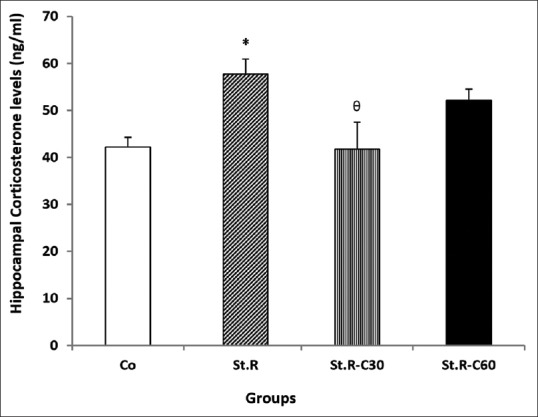
Effects of chronic restraint stress and different doses of crocin on hippocampal corticosterone levels (ng/ml) in the different groups (n = 8). Results are expressed as mean ± standard error of mean (ANOVA test Tukey's post hoc test). *P < 0.05 compared to the control, θP < 0.05 compares to the Stress group. Co: Control group, Sh: Sham group, St.R: Restrain stress group, St.R-C30: Restrain stress-Crocin 30 group, St.R-C60: Restrain stress-Crocin 60 group
Figure 5.
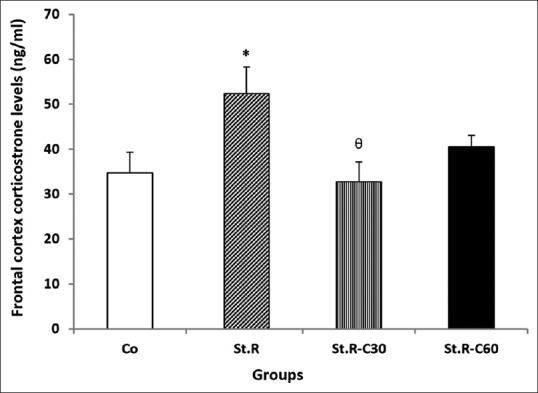
Effects of chronic restraint stress and different doses of crocin on the frontal cortex corticosterone levels (ng/ml) in the different groups (n = 8). Results are expressed as mean ± standard error of mean (ANOVA test Tukey's post hoc test). *P < 0.05 compared to the control, θP < 0.05 compared to the Stress group. Co: Control group, Sh: Sham group, St.R: Restrain stress group, St.R-C30: Restrain stress-Crocin 30 group, St.R-C60: Restrain stress-Crocin 60 group
Correlation between hippocampal and frontal cortical corticosterone levels
A positive correlation was established between the hippocampus and the frontal cortex with respect to their CORT levels across the groups ([P < 0.01 in St.R-C30 and P < 0.05 in others]; Pearson's correlations; r = 0.895 in Co, r = 0.883 in St.R, r = 0.967 in St.R-C30, and r = 0.880 in St.R-C60) [Figure 6]. These findings revealed a direct relationship between the CORT levels in the hippocampus and the frontal cortex.
Figure 6.
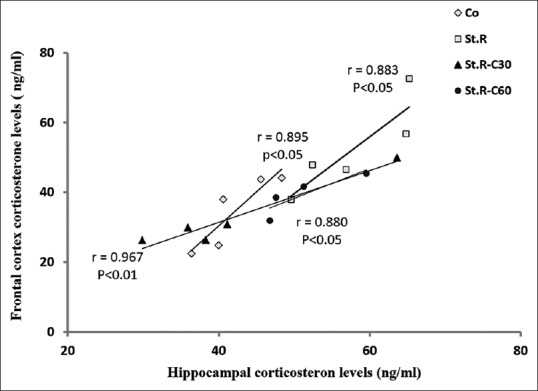
Correlation analysis of the frontal cortex and hippocampal corticosterone levels in the different groups (n = 8). Results are expressed as means ± standard error of mean (Pearson's correlation t-test). A significant (P < 0.05) and positive correlation was established between the frontal cortex corticosterone levels and those of the hippocampus in all the groups examined. Co: Control group, Sh: Sham group, St.R: Restrain stress group, St.R-C30: Restrain stress-Crocin 30 group, St.R-C60: Restrain stress-Crocin 60 group
Discussion
The effects of different doses (30 and 60 mg/kg) of crocin were investigated on learning, memory, and the hippocampal and frontal cortical CORT levels in rats subjected to chronic restraint stress as a chronic emotional stress model.
The findings confirm the hypothesis that chronic stress is accompanied by a disturbance in STL as memory. It has been shown that chronic restraint stress impairs brain functions and memory processing in rats,[2,30,31,32,33] whereas it also alters such neuronal properties as suppression of neurogenesis in the dentate gyrus of the hippocampus[34,35,36] and changes the emotional and stress-related responses in animals.[2,30] Various mechanisms are involved in the stress responses; these include changes in stress biomarkers, hormones, and neurotransmitters as well as free radical generation and anti-oxidant enzyme activities.[2,37,38] In contrast to the present results, one study reported on the habituation of animals to chronic stress conditions.[36] The discrepancy observed between the two results may be attributed to differences in the types and durations of stress induced and/or types of task behavior designed.[31]
Another aspect of the present study involved crocin dosage which revealed that daily administration of crocin (30 and 60 mg/kg) led to beneficial effects on learning in stressed rats. Moreover, crocin improved the memory deficit induced by stress although a 30 mg/kg dose was found to be more effective than the 60 mg/kg one on the cognitive memory deficit as measured by the passive avoidance test under restraint stress conditions (as an emotional stress model) [Figures 2 and 3]. Using the Morris water maze task, Ghadrdoost et al. observed that crocin prevented spatial memory impairment induced by chronic stress.[39] In agreement with this finding, previous studies had also shown that administration of crocin (30 mg/kg) led to a significantly reduced memory deficit induced by scopolamine.[13,39] In general, it seems that crocin has beneficial effects on different kinds of memory and that the harmful effects of chronic stress on brain functions can be prevented or remedied by crocin. In contrast to the results of the current results, low doses (15 and 30 mg/kg) of crocin were reported to have no influence on animal behavior.[13] Based on these observations, it might be claimed that lower doses of crocin probably reverse memory impairment through a variety of mechanisms involving different biochemical factors.[40,41] In addition, it is important to note that the differences observed in the effects of crocin among different studies might be related to differences in the behavioral tasks used in different experimental protocols.[42]
Chronic stress in the current study was observed to give rise to a significant increase in the hippocampal and frontal cortical CORT levels. Furthermore, the hippocampus and frontal cortex were found to be positively correlated with respect to their CORT levels. It has been demonstrated that stress disrupts brain cognitive functions by causing impairments in the prefrontal cortex.[43] In the light of what went above, this can now be attributed to changes in CORT levels. In addition, previous studies have shown that enhanced CORT levels might have implications for post-stress brain plasticity and behavioral changes in rat's brain and hippocampus.[2,7] Other studies demonstrated that the aqueous extract of saffron gave rise to increased release of such important neurotransmitters as dopamine and glutamate in rats’ brains, as a result of which depression symptoms reduced. Crocin has also been shown to inhibit N-methyl-D-aspartate (NMDA) glutamate receptors and sigma opioid receptors isolated from rat spinal cord.[44]
Our current findings revealed no significant correlations in the stressed groups between their memory functions and their CORT levels in the hippocampus, frontal cortex, or both. It may, therefore, be suggested that a wide variety of mechanisms and multiple factors such as changes in neurotransmitters’ release, neurotrophic factors, leptin, and oxidative stress affecting CORT level might be involved in induced memory deficit as a result of stress.[45,46,47]
Administration of crocin decreased CORT levels in both the hippocampus and the frontal cortex of stressed rats although the effects were significant only in the St.R-C30 group. Compared to the high dose of 60 mg/kg, the low crocin dose of 30 mg/kg administered in the present study led to improved CORT levels. This is somehow in agreement with Ghadrdoost and et al. who reported more beneficial effects of a crocin dose of 30 mg/kg than one of 15 mg/kg of crocin.[39] It, therefore, seems that the golden crocin dose of 30 mg/kg, no less and no more, may not only play a crucial role in the development and maintenance of memory but also decrease CORT levels in the hippocampus and frontal cortex in emotionally stressed subjects. Another conclusion to be drawn is that the beneficial effects of crocin are dose-dependent. Finally, a study has suggested that crocin might have free radical scavenging and antioxidant activities.[48] If so, it seems that crocin protects the hippocampus and the frontal cortex against the oxidative damage of chronic stress.
Based on the above observations, the central mechanisms such as interactions with the HPA axis and other brain areas or the involvement of peripheral structures might be similar to other memory mechanisms.[14] However, the hippocampus and the fontal cortex are more similar in their functions such as regulation of the HPA axis.[10,49] and expression of glucocorticoid receptors.[10,50] Some scholars have, however, shown that the hippocampus contains higher densities of the glucocorticoid receptors than does the prefrontal cortex.[10] It, thus, be concluded that the former is more responsive to corticosteroids than the latter is.[51]
Conclusions
The low dose (30 mg/kg of crocin) was found capable of reversing the harmful effects of chronic restraint stress on memory and also able to lower the CORT levels in both the hippocampus and the frontal cortex. Based on the results obtained from the present and previous studies, it may be suggested that the potential effects of crocin on emotional chronic stress are dose-dependent. Further research is, however, required to shed more light on the possible mechanism(s) involved. Investigation of receptor regulation, assessment of other hormones, and identification of biochemical factors through which crocin might affect brain functions might be suggested for future study. In addition, the molecular pathway of glucocorticoid receptors in response to crocin may be a more appropriate topic of research to determine the mechanism through which crocin affects learning and memory in animals under stress.
Financial support and sponsorship
This study was funded by Isfahan University of Medical Sciences rant number 394934.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to express their gratitude to Isfahan University of Medical Sciences, Isfahan, Iran, without whose support.
References
- 1.Bandegi AR, Rashidy-Pour A, Vafaei AA, Ghadrdoost B. Protective effects of Crocus sativus L.extract and crocin against chronic-stress induced oxidative damage of brain, liver and kidneys in rats. Adv Pharm Bull. 2014;4(Suppl 2):493–9. doi: 10.5681/apb.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. Effects of different timing of stress on corticosterone, BDNF and memory in male rats. Physiol Behav. 2015;139:459–67. doi: 10.1016/j.physbeh.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Sahin E, Gümüslü S. Immobilization stress in rat tissues: Alterations in protein oxidation, lipid peroxidation and antioxidant defense system. Comp Biochem Physiol C Toxicol Pharmacol. 2007;144:342–7. doi: 10.1016/j.cbpc.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Moberg GP. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. CABI. 2000:1–21. [Google Scholar]
- 5.Conrad CD. A critical review of chronic stress effects on spatial learning and memory. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:742–55. doi: 10.1016/j.pnpbp.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Azadbakht AA, Eidelkhani N, Kazemi M, Radahmadi M, Reisi P. Doxepin improves stress-impaired long-term potentiation and gene expression of BDNF in the rat hippocampus. Physiol Pharmacol. 2016;20:231–8. [Google Scholar]
- 7.Radahmadi M, Hosseini N, Nasimi A. Effect of chronic stress on short and long-term plasticity in dentate gyrus; study of recovery and adaptation. Neuroscience. 2014;280:121–9. doi: 10.1016/j.neuroscience.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holtzer R, Schoen C, Demetriou E, Mahoney JR, Izzetoglu M, Wang C, et al. Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions. Eur J Neurosci. 2017;45:660–70. doi: 10.1111/ejn.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerqueira JJ, Pêgo JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto T, Yoshida M, Ono R, Murata S, Saji N, Niida S, et al. Frontal lobe function correlates with one-year incidence of urinary incontinence in elderly with Alzheimer disease. J Alzheimers Dis. 2017;56:567–74. doi: 10.3233/JAD-160923. [DOI] [PubMed] [Google Scholar]
- 12.Kim TK, Han PL. Functional connectivity of basolateral amygdala neurons carrying orexin receptors and melanin-concentrating hormone receptors in regulating sociability and mood-related behaviors. Exp Neurobiol. 2016;25:307–17. doi: 10.5607/en.2016.25.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitsikas N, Zisopoulou S, Tarantilis PA, Kanakis CD, Polissiou MG, Sakellaridis N. Effects of the active constituents of Crocus sativus L.crocins on recognition and spatial rats’ memory. Behav Brain Res. 2007;183:141–6. doi: 10.1016/j.bbr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Mobarakeh JI, Fakhraei N, Sadr ZA, Hamidipour A, Mostafavi E, Hosseini RH, et al. Effect of aqueous, ethanolic and acetonitrile Crocus sativus L.extracts on stress biomarkers in male rats. J Med Plants Res. 2013;7:3269–79. [Google Scholar]
- 15.Naghizadeh B, Mansouri MT, Ghorbanzadeh B, Farbood Y, Sarkaki A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013;20:537–42. doi: 10.1016/j.phymed.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Asdaq SM, Inamdar MN. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol. 2010;162:358–72. doi: 10.1007/s12010-009-8740-7. [DOI] [PubMed] [Google Scholar]
- 17.Ochiai T, Ohno S, Soeda S, Tanaka H, Shoyama Y, Shimeno H. Crocin prevents the death of rat pheochromyctoma (PC-12) cells by its antioxidant effects stronger than those of alpha-tocopherol. Neurosci Lett. 2004;362:61–4. doi: 10.1016/j.neulet.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 18.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54:8762–8. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 19.Naghizadeh B, Boroushaki MT, Vahdati Mashhadian N, Mansouri MT. Protective effects of crocin against cisplatin-induced acute renal failure and oxidative stress in rats. Iran Biomed J. 2008;12:93–100. [PubMed] [Google Scholar]
- 20.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res. 2000;14:149–52. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. Preventive and therapeutic effect of treadmill running on chronic stress-induced memory deficit in rats. J Bodyw Mov Ther. 2015;19:238–45. doi: 10.1016/j.jbmt.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Avishai-Eliner S, Eghbal-Ahmadi M, Tabachnik E, Brunson KL, Baram TZ. Down-regulation of hypothalamic corticotropin-releasing hormone messenger ribonucleic acid (mRNA) precedes early-life experience-induced changes in hippocampal glucocorticoid receptor mRNA. Endocrinology. 2001;142:89–97. doi: 10.1210/endo.142.1.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radahmadi M, Hosseini N, Alaei H, Sharifi MR. The effect of preventive, therapeutic and protective exercises on hippocampal memory mediators in stressed rats. Malays J Med Sci. 2016;23:29–37. doi: 10.21315/mjms2016.23.5.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93:487–94. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Reiriz AB, Reolon GK, Preissler T, Rosado JO, Henriques JA, Roesler R, et al. Cancer chemotherapy and cognitive function in rodent models: Memory impairment induced by cyclophosphamide in mice. Clin Cancer Res. 2006;12:5000. doi: 10.1158/1078-0432.CCR-06-0138. [DOI] [PubMed] [Google Scholar]
- 26.Huang AC, Shyu BC, Hsiao S, Chen TC, He AB. Neural substrates of fear conditioning, extinction, and spontaneous recovery in passive avoidance learning: A c-fos study in rats. Behav Brain Res. 2013;237:23–31. doi: 10.1016/j.bbr.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. The effect of synchronized running activity with chronic stress on passive avoidance learning and body weight in rats. Int J Prev Med. 2013;4:430–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. Effect of forced exercise and exercise withdrawal on memory, serum and hippocampal corticosterone levels in rats. Exp Brain Res. 2015;233:2789–99. doi: 10.1007/s00221-015-4349-y. [DOI] [PubMed] [Google Scholar]
- 29.Wolf SA, Steiner B, Wengner A, Lipp M, Kammertoens T, Kempermann G. Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus. FASEB J. 2009;23:3121–8. doi: 10.1096/fj.08-113944. [DOI] [PubMed] [Google Scholar]
- 30.Raju T. Chronic restraint stress impairs acquisition and retention of spatial memory task in rats. Curr Sci. 2000;79:1581–5. [Google Scholar]
- 31.Ranjbar H, Radahmadi M, Alaei H, Reisi P, Karimi S. The effect of basolateral amygdala nucleus lesion on memory under acute, mid and chronic stress in male rats. Turk J Med Sci. 2016;46:1915–25. doi: 10.3906/sag-1507-7. [DOI] [PubMed] [Google Scholar]
- 32.Ranjbar H, Radahmadi M, Reisi P, Alaei H. Effects of electrical lesion of basolateral amygdala nucleus on rat anxiety-like behaviour under acute, sub-chronic, and chronic stresses. Clin Exp Pharmacol Physiol. 2017;44:470–9. doi: 10.1111/1440-1681.12727. [DOI] [PubMed] [Google Scholar]
- 33.Ranjbar H, Radahmadi M, Alaei H, Reisi P. Effect of different durations of stress on spatial and cognitive memory in male rats. J Isfahan Med Sch. 2015;32:1933–43. [Google Scholar]
- 34.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 35.Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T. Chronic stress induces impairment of spatial working memory because of prefrontal dopaminergic dysfunction. J Neurosci. 2000;20:1568–74. doi: 10.1523/JNEUROSCI.20-04-01568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS. Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–77. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 37.Radahmadi M, Alaei H, Sharifi MR, Hosseini N. Stress biomarker responses to different protocols of forced exercise in chronically stressed rats. J Bodyw Mov Ther. 2017;21:63–8. doi: 10.1016/j.jbmt.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Gümüslü S, Sarikçioglu SB, Sahin E, Yargiçoglu P, Agar A. Influences of different stress models on the antioxidant status and lipid peroxidation in rat erythrocytes. Free Radic Res. 2002;36:1277–82. doi: 10.1080/1071576021000016508. [DOI] [PubMed] [Google Scholar]
- 39.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–9. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26:381–6. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 41.Pitsikas N, Tarantilis PA. Crocins, the active constituents of Crocus sativus L.counteracted apomorphine-induced performance deficits in the novel object recognition task, but not novel object location task, in rats. Neurosci Lett. 2017;644:37–42. doi: 10.1016/j.neulet.2017.02.042. [DOI] [PubMed] [Google Scholar]
- 42.Li S, Wang C, Wang W, Dong H, Hou P, Tang Y. Chronic mild stress impairs cognition in mice: From brain homeostasis to behavior. Life Sci. 2008;82:934–42. doi: 10.1016/j.lfs.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: Role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog Brain Res. 2000;126:183–92. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- 44.Ettehadi H, Mojabi SN, Ranjbaran M, Shams J, Sahraei H, Hedayati M, et al. Aqueous extract of saffron (Crocus sativus) increases brain dopamine and glutamate concentrations in rats. J Behav Brain Sci. 2013;3:315–19. [Google Scholar]
- 45.Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA. Chronic psychosocial stress-induced impairment of hippocampal LTP: Possible role of BDNF. Neurobiol Dis. 2006;22:453–62. doi: 10.1016/j.nbd.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Chen JX, Li W, Zhao X, Yang JX. Effects of the Chinese traditional prescription Xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and brain BDNF, TrkB, and NT-3 in rats. Cell Mol Neurobiol. 2008;28:745–55. doi: 10.1007/s10571-007-9169-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris RB, Mitchell TD, Simpson J, Redmann SM, Jr, Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol. 2002;282:R77–88. doi: 10.1152/ajpregu.2002.282.1.R77. [DOI] [PubMed] [Google Scholar]
- 48.Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of Crocus sativus stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol. 2008;27:657–64. doi: 10.1089/dna.2008.0767. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 50.Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J Psychiatr Res. 2000;34:383–92. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- 51.Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;19:1837–46. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]


