Abstract
Background:
The aim of this study was investigation of the effects of Nigella sativa (NS) seeds on hypothyroid pregnant rats and their progenies.
Materials and Methods:
Hypothyroidism was induced by propylthiouracil (PTU) 0.03% in drinking water. Female rats were divided into seven groups: control, PTU, PTU-NS (100, 200, and 400 mg/kg), and NS (100 and 400 mg/kg). All treatments were done 20 days before mating and during pregnancy. The weight of rat dams and progenies, number of progenies and serum T4, estradiol and prolactin (PRL) levels in rat dams were measured for all groups.
Results:
Serum T4 in all PTU-NS groups before mating was significantly increased versus PTU group. Body weight of rat dams before mating in all groups of PTU-NS was increased versus PTU group by P < 0.001, P < 0.05, and P < 0.001, respectively and in NS 100 and NS 400 was increased versus control group (P < 0.001). The number of offspring was significantly decreased in PTU and PTU-NS versus control group. The weight of progenies in NS 400 was higher than control group (P < 0.001) and was increased in PTU-NS 200 and PTU-NS 400 versus PTU group by P < 0.001 and P < 0.05, respectively. Serum PRL level in rat dams in control, PTU, and PTU-NS groups were not statistically different between groups but significantly increased in NS 400 group when compared to control group. Estradiol levels were not significantly different in rat dams at 5 days after delivery.
Conclusion:
These results demonstrated that feeding of rat dams with NS extract before mating has positive protective effects on progenies. These effects may be due to antioxidant properties of NS in reducing oxidative stress and thyroid damages induced by PTU.
Keywords: Hypothyroid, Nigella sativa, progeny, propylthiouracil, rat dams
Introduction
Thyroid dysfunctions are the second endocrine disorders that affect women of reproductive ages. Thyroid dysfunction during pregnancy is accompanied with complications such as: hypertension, miscarriage and premature birth, low birth weight of newborns, placental abruption, and fetal death.[1,2,3,4,5] The prevalence of hypothyroidism is 2%–4% in women during reproductive ages.[6] Hypothyroidism also can cause infertility, hypo/hypermenorrhea, amenorrhea, oligomenorrhea, bleeding, and recurrent pregnancy loss.[7] Several studies have reported that subclinical hypothyroidism is more common in unfertile women; premature ovarian failure, tube occlusion, and ovulation disorders are more common in subclinical hypothyroid women.[8,9]
According to the WHO, more than 1 billion people worldwide have iodine deficiency. Iodine deficiency is the common cause of hypothyroidism that even may affect pregnant women in areas with sufficient iodine. In many instances, the low iodine intake is not considered during pregnancy because thyroxin levels are in the normal range.[10] Several studies have reported that iodine intake lower than normal during pregnancy results in miscarriages and stillbirths and almost 1/5 of women with iodine deficiency had a history of abortion or stillbirth.[11]
Nigella sativa (NS) or black seeds as an herbal medicine have been used worldwide. Both grains and oil are commonly used as food and herbal medicine in different countries. In traditional medicine, NS has been used as an antihypertensive, diuretic, liver tonic, antidiarrhea, appetite stimulant, anti-inflammatory, antibacterial, and for treatment of skin disorders. Many studies have been conducted on different preparations of NS and its constituents, especially thymoquinone (TQ) which shows a range of pharmacological effects; these include antidiabetic, antihyperlipidemic and antiatherosclerotic, anticancer, immunomodulatory, anti-inflammatory, antibacterial, antispasmodic, bronchodilator, liver protection, and antinephrolithiasis, stomach protective, and antioxidant properties.[12,13,14,15,16] Active compounds of NS include 30%–40% fixed oil, 0.5%–1.5% essential oil, several sugars, and proteins; NS seeds also contain pharmacological active compounds including TQ, di thymoquinone, and nigellin. Based on Seghatoleslam's study, TQ is the main component of NS that the effects of NS can be attributed to it.[17] The amount of TQ in NS seeds oil from different origins is around 0.13%–0.17% w/v.[18] It seems that the seed extract and its components have a minimum level of toxicity.[19]
The effects of NS on thyroid disorders are less studied. In a recent study, it was reported that black seeds oil had antioxidant effects and reduced the oxidative stress and damage of thyroid follicles caused by propylthiouracil (PTU).[20] Another study has shown that NS oil increases levels of T3, T4, LH, and estrogen.[21] As thyroid dysfunctions can lead to fertility disorders, so its treatment is important in pregnancy and because of the low toxicity and less side effects of herbal remedies than chemical drugs, black seeds can be used. Hence, the aim of this study was an evaluation of the effects of the hydroalcoholic extract of NS on fertility disorders in hypothyroid rats.
Materials and Methods
Animals
Female Wistar rats (200 ± 10 g weight, 8 weeks of age) were obtained from the Animal House of medical school of Mashhad University of Medical Sciences. Throughout of the experiments, the animals were maintained under standard laboratory conditions (12 h light/dark cycle, controlled temperature of 21°C–25°C, and free access to food and water). The study protocol was approved by the Medical Ethics Committee of Mashhad University of Medical Sciences.
Experimental design
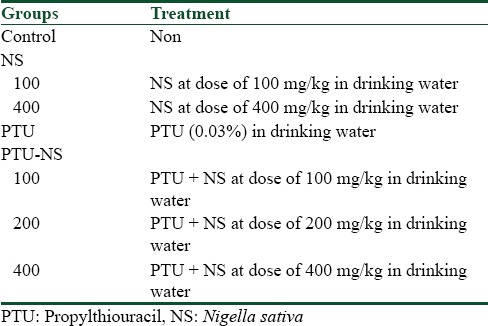
Seventy female rats were divided into seven groups of ten and treated as the following.
Control group
Rats in this group were left without any treatment.
Nigella sativa groups
In these groups, female rats were received drinking water containing NS extract at doses of 100 and 400 mg/kg body weight for 20 days before mating and during pregnancy. The dose of NS was selected based on a previous study.[17]
Propylthiouracil group
Female rats were received drinking water containing 0.03% of PTU (Sigma-Aldrich, China) for 20 days before mating and during pregnancy.
Propylthiouracil-Nigella sativa groups
In these groups, female rats were received drinking water containing 0.03% of PTU and NS extract at doses of 100, 200, and 400 mg/kg body weight for 20 days before mating and during pregnancy.
Rats in each group were placed in a cage with males (2:1) to become pregnant [Table 1].
Table 1.
Experimental groups and treatment
Preparation of hydroalcoholic extracts of Nigella sativa
The NS seeds were obtained from an herb store in Mashhad, Iran. NS seeds were powdered, and 100 g of powder was dissolved in sufficient amount of 70% ethanol and placed on a Soxhlet extractor. The obtained extract was concentrated under reduced pressure and kept in the refrigerator at 4°C until use.[22] The weight of dried extract was 30 g, so the extract is 30% w/w. The obtained extract was dissolved in distilled water to obtain the doses of 100, 200, and 400 mg/kg.
Mating procedure
The rats in each group were placed separately with male rats in a cage (female:male -2:1) for mating. The next morning each cage was visualized for vaginal plug. If the vaginal plug was seen that day was regarded as the day of mating (day 0) and the next day as the beginning of pregnancy. In the absence of vaginal plug, the same procedure was repeated for mating rats.
Measurement the body weight of animals
The body weight of progenies was measured on the 5th day after birth, and the body weight of female rat dams also was measured 1 day before mating for all groups.
Counting the number of offspring
Pregnant rats in each group were observed daily around gestation day, and the numbers of offspring of each rat dam were counted for all rats.
Hormones measurements
Blood samples were collected from orbital sinus before mating day for all groups and were centrifuged with 3000 rpm for 15 min; blood serum was separated and kept in -20°C until the measurement of hormones. Estradiol levels were assessed by Elisa kit (DiaMetra, Italy) under the protocols of kit manufactures. Prolactin (PRL) and T4 levels were assessed by a radioimmunoassay kit (RIAK, Korea) under the protocols of kit manufactures.
Statistical analysis
Data were prepared as mean ± standard error of mean and analyzed by SPSS 20 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp) using one-way ANOVA and Tukey's post hoc test to find out the different between groups. Differences were considered statistically significant with P < 0.05.
Results
In this study, reproductive and ovarian indicators including weight and number of progenies, maternal weight, and also the level of serum PRL and estradiol 5 days after delivery were evaluated in all studied groups to evaluate the effects of hydroalcoholic extract of NS on reproductive and ovarian parameters in hypothyroid rats.
Induction of hypothyroidism
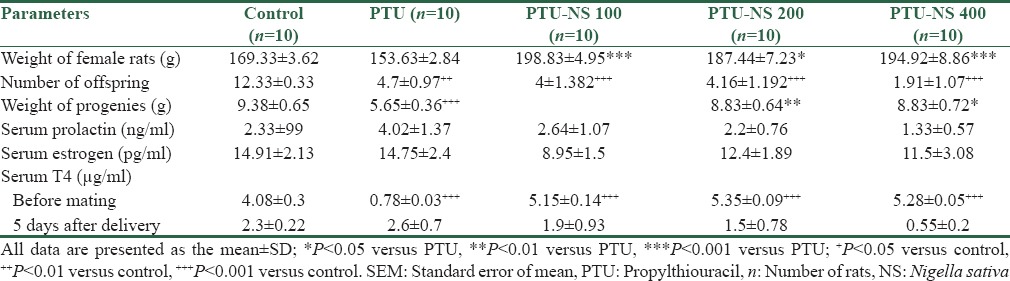
Administration of PTU for 3 weeks before mating significantly decreased serum T4 in PTU group when compared with control group (P < 0.001). A significant increase was observed in the serum T4 level of female rats in PTU-NS treated groups at the doses of 100, 200, and 400 in comparison with PTU group (P < 0.001). Serum T4 levels of female rats in PTU-NS treated groups with different dose of NS were not statistically different for all three groups (P > 0.05) [Table 2]. Serum T4 levels in all studied groups of rat dams at 5 days after parturition were not statistically different (P = 0.14) [Table 2].
Table 2.
Effects of Nigella sativa on measured parameters in treated groups with propylthiouracil
Results of treated groups with propylthiouracil and Nigella sativa
Weight of female rats
The weight of female rats before mating was significantly increased in treated groups with PTU-NS at doses of 100, 200, and 400 mg/kg of extract of NS when compared with PTU group by P < 0.001, P < 0.05, and P < 0.001, respectively [Table 2].
Number of offspring
The number of offspring born from each rat dam was significantly decreased in PTU group versus control group (P < 0.01). Treatment with doses of 100, 200, and 400 mg/kg of the extract was not able to increase the number of offspring in PTU-NS groups (P < 0.001 vs. control group) [Table 2].
Weight of progenies
The weights of progenies born from rat dams in control, PTU, and PTU-NS (200 and 400 mg/kg) groups are presented in Table 2. The weight of progenies born from rat dams in PTU-treated group was significantly decreased when compared with the control group (P < 0.001). The weight of progenies born from rat dams in PTU-NS 200 and PTU-NS 400 groups showed a significant increase when compared to PTU group, respectively by P < 0.01 and P < 0.05. The weight of progenies born from rat dams in PTU-NS groups at doses of 200 and 400 mg/kg were not different from control group (P > 0.05). No difference was observed between the weight of progenies born from mothers in PTU-NS 200 and PTU-NS 400 (P > 0.05) [Table 2].
Serum prolactin
Serum PRL level at 5 days after delivery in rat dams in control group, PTU group, and PTU-NS treated at the doses of 100, 200, and 400 mg/kg were not statistically different between groups (P > 0.05) [Table 2].
Serum estrogen
The results of serum estradiol in rat dams at 5 days after parturition were not statistically different between all studied groups (P = 0.33) [Table 2].
Results of treated groups with Nigella sativa
Weight of female rats
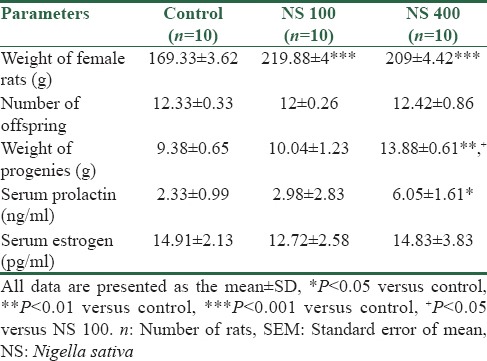
The weight of female rats treated with NS extract at dose of 100 and 400 mg/kg was significantly increased in comparison with control group (both P < 0.001). No significant differences were found between animals treated with 100 or 400 mg/kg of the extract (P > 0.05) [Table 3].
Table 3.
Effects of Nigella sativa on measured parameters in treated groups with Nigella sativa alone
Number of offspring
The number of offspring born from rat dams in both groups treated with doses of 100 and 400 mg/kg mg of extract was not different with control group (P > 0.05), and no significant difference was observed between the two treated groups with the extract (P > 0.05) [Table 3].
Weight of offspring
The weight of newborn from rat dams treated with NS extract at dose of 100 mg/kg was not different with control group (P > 0.05), but the weight of offspring born from rat dams treated with 400 mg/kg of extract was significantly increased when compared to the control group (P < 0.01). The weight of offspring in treated group at dose of 400 mg/kg showed a significant increase when compared with dose of 100 mg/kg (P < 0.05) [Table 3].
Prolactin levels
The results showed that the PRL levels significantly increased in rat dams treated with 400 mg/kg of NS extract when compared to control group (P < 0.05). However, serum PRL in rat dams treated at dose of 100 mg/kg of NS was not significantly different from control group (P > 0.05) [Table 3].
Serum estradiol
Estradiol levels in rat dams in control and treated groups with NS at doses of 100 and 400 mg/kg were compared with each other. No significant differences were evident between control and treated groups (P > 0.87) [Table 3].
Discussion
As seen in Table 3, administration of NS extracts in healthy female rats for 3 weeks before mating increased the weight of rat dams. NS also caused weight gain in hypothyroid rats, who received PTU and extract of NS for 3 weeks before mating and during pregnancy [Table 2]. Khalawi et al. studied the antioxidant effects of NS including thyroid effects and showed that black seeds oil improved body weight, food and water consumption in hypothyroid rats. They also showed that serum thyroid-stimulating hormone level decreased and triiodothyronine and thyroxine increased by treatment of black seeds oil. Histological studies by Khalawi showed an increase in follicular size and colloid content. They also showed that NS has antioxidant effects that reduced oxidative stress and also reduced the damaged thyroid follicles induced by PTU.[20]
The results of Khalawi's study about the effects of NS oil on weight gain in hypothyroid rats are consistent with our findings in the present study. It seems that the antioxidant compounds in black seeds may cause improvement of tissues and weight gain in hypothyroid rats.
Considering that more than 100 different types of compounds have been identified in black seeds and various properties for different constituents of black seeds such as antioxidant effects, the kidney protecting, and increasing insulin secretion from pancreatic beta cells has been reported in different studies. Therefore, it seems reasonable that the increase in body weight in healthy female rats and hypothyroid rats treated with extract of NS may be attributed to these effects, especially the increased insulin secretion.[23,24,25] A research work in our laboratory showed that TQ is the main component of NS that is responsible for most effects of NS seeds.[17]
Administration of NS at doses of 100 and 400 mg/kg as the low dose and high dose did not change the number of progenies which indicates that consumption of NS has no harmful effect on the number of offspring in normal animals. Keshri et al. treated rats with black seeds for 1–10 days after mating at a dose of 2 g/kg/day. They showed that NS prevented the pregnancy,[26] which is in contrast with our findings. This may be due to higher dose in Keshri's study or due to the different time of treatment in his study. Hypothyroidism induced by PTU decreased the number of progenies in our study [Table 2], and this finding has been also reported by other researches. NS extract could not increase the number of progenies in hypothyroid mothers. Our findings in this study are consistent with the findings of Keshri et al.[26]
The weight of progenies is one of the indicators that are affected by thyroid hormone disorders, especially hypothyroidism. In this study, the weight of progenies was measured on the 5th day after birth. The weight of progenies born from healthy mothers treated with low dose of NS extract (100 mg/kg) was not significantly different from the control group, but the high dose of NS extract (400 mg/kg) significantly increased the weight of progenies (P < 0.01) which indicated the improving of feeding of NS extract on body weight of newborn in this group [Table 3]. Treatment of hypothyroid rats with doses of 200 and 400 mg/kg of extracts significantly increased the weight of progenies [Table 2].
These findings show that NS extract effectively has increased the weight of progenies borne from hypothyroid rat dams. Farooq and collogues showed that administration of NS oil increased the litter size and weight of progenies, which is in favor of our findings.[27] Gaafar et al. also showed that treatment with NS oil increased litter size at birth in rabbits that confirms our findings.[13]
Hypothyroidism changes serum hormone levels and probably functions of other hormones. In this study, the serum PRL increased in healthy rats treated with a dose of 400 mg/kg of NS, but a dose of 100 mg/kg extract had no effect on serum PRL. It may be suggested that feeding animals with a medium dose of black seeds are accompanied by an increase in PRL which is the main hormone for milk production after delivery. This milk production is very important both in breast feeding human and animals. On the 5th day after delivery in PTU group, there was a slight increase in PRL levels and no significant changes in the serum PRL were observed in PTU-NS groups.
Hypothyroidism causes losing inhibitory feedback effects of thyroid hormones on pituitary and hypothalamus and brings overproduction of thyrotropin-releasing-hormone (TRH). TRH has a weak stimulatory effect on the lactotroph cells of the pituitary, so PRL levels are expected to increase in hypothyroidism. Decreased serum PRL in PTU-NS groups could be due to high levels of PRL during pregnancy and lactation such that PRL activates PRL receptors on dopamine neurons and also increased the activity of tyrosine hydroxylase a key enzyme in dopamine synthesis leading to increased dopamine synthesis and release which ultimately leads to PRL reduction.[28]
In this study, the estradiol levels did not show significant changes in treated rats at doses of 100 and 400 mg/kg of the extracts and were not different in hypothyroid and control groups. Estradiol levels after parturition in hypothyroid groups treated with either doses of the extract showed no significant difference [Table 3]. It seems that changes in serum estradiol during pregnancy is the result of entrance of high levels of placental estradiol in blood stream and this high levels of estradiol is not under the control of hypothalamus-pituitary-gonadal axis.
Serum T4 levels before mating in hypothyroid rats (treated 3 weeks with PTU) were reduced due to inhibitory effects of PTU on synthesis and secretion of thyroid hormones in the follicular cells.[28] Treatment with NS extract in hypothyroid rats caused a significant increase in the serum T4 levels. Khalawi et al. showed that black seeds oil increased T3 and T4 in hypothyroid rats treated with PTU, which is in favor of our study.[20] Sharif et al. studied the effects of ethanolic extracts of black seeds with a dose of 1 g/kg in diabetic and normal rats and reported an increase in T3 levels in diabetic rats and increase in T4 levels in healthy rats.[29] In the present study, serum T4 levels 5 days after parturition in rat dams had no significant difference with healthy control group, although a slight reduction was found in NS-treated group [Table 3]. This may be caused by the removal of the effect of PTU and increase the thyroid follicular cell activity which happened of the removal of PTU.
Conclusion
NS extracts in female rats before mating and during pregnancy increases the weight of female rat and weight of offspring. NS extract administration to healthy rats was without any detrimental effects on rat dams and their progenies. PTU administration caused hypothyroidism in rats and subsequently reduced the weight of dams and the number and weight of offspring. NS extract in particular at dose of 400 mg increased maternal weight and weight of offspring. It seems that the high doses of extract are more effective than the low doses. These effects may be due to antioxidant properties of NS in reducing oxidative stress and thyroid damages induced by PTU. The extract increased T4 levels in hypothyroid rats but PRL and estradiol levels were not much affected by the extracts. As the finding of this study shows NS, especially at high dose, is effective in hypothyroid states and improves some parameters related to both dams and progenies and outcome of pregnancy.
Financial support and sponsorship
This study was supported by a grant (931385) from the Council of Research, Mashhad University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge the Council of Research and Neurocognitive Research Center, Mashhad University of Medical Sciences for their support of this study.
References
- 1.Hirsch D, Levy S, Nadler V, Kopel V, Shainberg B, Toledano Y. Pregnancy outcomes in women with severe hypothyroidism. Eur J Endocrinol. 2013;169:313–20. doi: 10.1530/EJE-13-0228. [DOI] [PubMed] [Google Scholar]
- 2.Milanesi A, Brent GA. Management of hypothyroidism in pregnancy. Curr Opin Endocrinol Diabetes Obes. 2011;18:304–9. doi: 10.1097/MED.0b013e32834a91d1. [DOI] [PubMed] [Google Scholar]
- 3.Lahoti SK, Toppo L. Subclinical hypothyroidism and pregnancy outcomes. Annals of International Medical and Dental Research. 2012;1(3):324–6. [Google Scholar]
- 4.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–45. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 5.Haddow JE. Thyroid Study Group. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;106:198. doi: 10.1097/01.AOG.0000169596.53498.7a. [DOI] [PubMed] [Google Scholar]
- 6.Verma I, Sood R, Juneja S, Kaur S. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Int J Appl Basic Med Res. 2012;2:17–9. doi: 10.4103/2229-516X.96795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouzounian S, Bringer-Deutsch S, Jablonski C, Théron-Gérard L, Snaifer E, Cédrin-Durnerin I, et al. Hypothyroidism: From the desire for pregnancy to delivery. Gynecol Obstet Fertil. 2007;35:240–8. doi: 10.1016/j.gyobfe.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 8.Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, et al. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol Endocrinol. 2007;23:279–83. doi: 10.1080/09513590701259542. [DOI] [PubMed] [Google Scholar]
- 9.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–55. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 10.Berbel P, Obregón MJ, Bernal J, Escobar del Rey F, Morreale de Escobar G. Iodine supplementation during pregnancy: A public health challenge. Trends Endocrinol Metab. 2007;18:338–43. doi: 10.1016/j.tem.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Lakra K, Roy P. Pregnancy outcome of iodine deficiency: A study on tribal women in Orissa. Int J Res Dev Health. 2013;1:61–8. [Google Scholar]
- 12.Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, et al. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed. 2013;3:337–52. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaafar H, Ragab AA, El-Reidy K. Effect of diet supplemented with pumpkin (Cucurbita moschata) and black seed (Nigella sativa) oils on performance of rabbits: 2-Productive and reproductive performance of does and their offspring. Rep Opin. 2014;6:60–8. [Google Scholar]
- 14.Hadjzadeh MA, Mohammadian N, Rahmani Z, Rassouli FB. Effect of thymoquinone on ethylene glycol-induced kidney calculi in rats. Urol J. 2008;5:149–55. [PubMed] [Google Scholar]
- 15.Asgary S, Ghannadi A, Dashti G, Helalat A, Sahebkar A, Najafi S. Nigella sativa L. improves lipid profile and prevents atherosclerosis: Evidence from an experimental study on hypercholesterolemic rabbits. J Funct Foods. 2013;5:228–34. [Google Scholar]
- 16.Hayatdavoudi P, Khajavi Rad A, Rajaei Z, Hadjzadeh MA. Renal injury, nephrolithiasis and Nigella sativa: A mini review. Avicenna J Phytomed. 2016;6:1–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Seghatoleslam M, Alipour F, Shafieian R, Hassanzadeh Z, Edalatmanesh MA, Sadeghnia HR, et al. The effects of Nigella sativa on neural damage after pentylenetetrazole induced seizures in rats. J Tradit Complement Med. 2015;6:262–8. doi: 10.1016/j.jtcme.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houghton PJ, Zarka R, de las Heras B, Hoult JR. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–6. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 19.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 20.Khalawi AA, Al-Robai AA, Khoja SM, Shaker A. Can Nigella sativa oil (NSO) reverse hypothyroid status induced by PTU in rat?. Biochemical and histological studies. Life Sci J. 2013;10:802–811. [Google Scholar]
- 21.Jasim WK, Hassan MS, Keam GG. Study the effect of Nigella sativa on thyroid function and reproductive hormone of female rat. J Contemp Med Sci. 2016;2:67–9. [Google Scholar]
- 22.Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30:591–8. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farah N, Benghuzzi H, Tucci M, Cason Z. The effects of isolated antioxidants from black seed on the cellular metabolism of A549 cells. Biomed Sci Instrum. 2005;41:211–6. [PubMed] [Google Scholar]
- 24.Yaman I, Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62:183–90. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Gray JP, Burgos DZ, Yuan T, Seeram N, Rebar R, Follmer R, et al. Thymoquinone, a bioactive component of Nigella sativa, normalizes insulin secretion from pancreatic ß-cells under glucose overload via regulation of malonyl-CoA. Am J Physiol Endocrinol Metab. 2016;310:E394–404. doi: 10.1152/ajpendo.00250.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keshri G, Singh MM, Lakshmi V, Kamboj VP. Post-coital contraceptive efficacy of the seeds of Nigella sativa in rats. Indian J Physiol Pharmacol. 1995;39:59–62. [PubMed] [Google Scholar]
- 27.Juma FT, Hayfaa MA. The effects of Nigella sativa oil administration on some physiological and histological values of reproductive aspects of rats. Iraqi J Vet Med. 2011;35:52–60. [Google Scholar]
- 28.Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. Canada: Elsevier Health Sciences; 2015. [Google Scholar]
- 29.Sharif SH, Elmahdi BM, Mohammed AM, Mohammed AH. The effects of Nigella sativa L. ethanolic extract on thyroid function in normal and alloxan-induced diabetic rats. Thyroid Res Pract. 2012;9:48. [Google Scholar]


