Abstract
Context:
Human dentin powder (HD), bovine serum albumin (BSA) and endotoxin (LPS) may affect the antimicrobial activity of irrigating solutions.
Aim:
To evaluate the inhibitory effect of HD powder, BSA, and LPS on the antibacterial activity of 0.5%, 1%, 2.5%, and 5% sodium hypochlorite (NaOCl), 2% chlorhexidine (CHX) gel, BioPure mixture of tetracycline, citric acid, and detergent (MTAD), and QMix.
Methods:
The direct contact test against Enterococcus faecalis (ATCC 29212) for 2-min, 30-min and 6-h was used. Sterile pyrogen-free water was the negative control. After experimental periods, a neutralizing agent was used. Colony-forming units were determined by 10-fold serial dilutions and culture on agar plates. Data were analyzed by Kruskal–Wallis and Dunn's test (α = 5%).
Results:
In the absence of inhibitors, all irrigants eliminated E. faecalis. In contact with HD, all solutions eliminated E. faecalis within 2-min, with the exception of MTAD. In the presence of BSA, only 5% NaOCl killed E. faecalis within 2-min. LPS did not affect the antibacterial effect of any irrigant. At 30-min and at 6-h, all substances eliminated E. faecalis.
Conclusions:
In the presence of albumin, irrigants needed >2-min to eliminate E. faecalis, except for 5% NaOCl. The same was observed in the presence of dentin when E. faecalis was exposed to MTAD.
Keywords: Antibacterial agents, Enterococcus faecalis, root canal irrigants
INTRODUCTION
Microorganisms are the major etiologic agents of pulp and periapical disease. Endodontic therapy aims to eliminate, or at least significantly reduce, the bacterial load within canals to levels that are compatible with a favorable host response.[1] Mechanical instrumentation is essential for the removal of infected dental structures, but its action may be impaired by the complex canal morphology. For this reason, the use of chemical substances as irrigants is necessary to achieve proper disinfection.[2]
Despite technological and scientific advances, root canal treatment may fail, and the presence of resistant microbes and/or reinfection is the most common cause of this outcome.[3] Enterococcus faecalis is a Gram-positive and facultative bacterium, widely used in endodontic research due to its prevalence (24%–74%) in persistent and asymptomatic endodontic infections.[4] Although some recent studies have questioned the role of this microorganism in the persistence of periapical lesions,[5] it deserves attention due to its resistance to antimicrobial agents and capacity to survive in hostile environments.
Irrigants enhance bacterial elimination and facilitate removal of debris from canals. They prevent the apical packing of debris as well as their extrusion into the periapical area.[2] Sodium hypochlorite (NaOCl) is the most commonly used irrigant and can kill a wide range of microorganisms due to the effect of chlorine and oxygen released. It is also a nonspecific proteolytic agent, capable of dissolving pulp tissue.[6] Chlorhexidine gluconate (CHX) has been recommended as an auxiliary substance in endodontics due to its wide antimicrobial effect, substantivity, and prolonged action resulting from its binding to dentin.[7]
After using NaOCl or CHX, 17% ethylenediaminetetraacetic acid (EDTA) is recommended to remove the smear layer and provide a clean surface for canal filling.[2] As an alternative to EDTA, associations containing chelating substances in their composition have been developed.[8,9] BioPure mixture of tetracycline, citric acid, and detergent (MTAD) consists of doxycycline, citric acid, and a detergent.[9] Its use has been recommended after irrigation with 1.3% NaOCl and it has shown effectiveness against different strains of E. faecalis.[10]
With a similar purpose, QMix has been released in the USA. It contains CHX, EDTA, and a detergent (cetrimide), and was designed to remove the smear layer and disinfect the root canal at the same time.[8] It is indicated as a final irrigant and substitute for EDTA and causes less dentin erosion than the former.[11] The chemical design of QMix prevents the formation of para-chloroaniline, which is expected from the interaction between CHX and NaOCl.[12] As seen in BioPure MTAD, the addition of a detergent to the irrigating solution reduces surface tension and may promote increased penetration into the irregularities of the root canal system.[13]
Laboratory studies have shown that dentin and organic compounds present in canals may affect the antimicrobial action of irrigating solutions and intracanal medications.[14,15,16,17,18] The buffering effect of dentin may explain the inhibition of alkaline substances, such as calcium hydroxide-based medications and NaOCl.
Albumin is the main protein of human and bovine serum, and it also represents the major component of inflammatory exudates.[19] The exudate from periapical tissues may enter the canal via apical foramen in cases of purulent infection.[20] In this context, the presence of albumin may inhibit or delay the antimicrobial action of irrigants.[16,17,18] Finally, lipopolysaccharide (LPS) is another potential inhibitor found in the cell wall of Gram-negative bacteria, which can form electrostatic complexes with some disinfectants, thereby impairing their effects.[18]
Therefore, this study aimed to evaluate the effect of human dentin powder (HD), bovine serum albumin (BSA), and LPS on the antibacterial activity of irrigants used in canal preparation: NaOCl (0.5%, 1%, 2.5%, 5%), 2% CHX gel, Biopure MTAD and QMix, using a sterile pyrogen-free water as negative control, against a planktonic culture of E. faecalis.
METHODS
The study was approved by the research ethics committee of the Federal University of Rio Grande do Sul.
HD, BSA (18% BSA, Sigma Aldrich, St. Louis, MO, USA), and Escherichia coli LPS (1 μL/mL LPS; Sigma Aldrich, St. Louis, MO, USA)[18] were tested as potential inhibitors.
HD was prepared as previously described,[15,18] from five distal roots of mandibular molars and was suspended in distilled water, 28 mg in an aliquot of 50 μL, as recommended by Haapasalo et al.[14] The aliquots were placed in plastic test tubes, autoclaved, and maintained at 4°C until use.
BioPure MTAD (Dentsply Tulsa Dental Specialties, OK, USA), QMix (Dentsply Tulsa Dental Specialties, OK, USA), 0.5%, 1%, 2.5%, and 5% NaOCl solution (Asfer, São Caetano do Sul, SP, Brazil), and 2% chlorhexidine (CHX) gel (Essential Farma, Itapetininga, SP, Brazil) were the irrigants evaluated. The negative control was sterile pyrogen-free water.
E. faecalis (ATCC 29212) was grown overnight on brain heart infusion (BHI; Difco Laboratories, Detroit, MI, USA) agar and evaluated for purity by Gram staining and colony morphology. Afterwards, it was suspended in 5 mL of sterile BHI broth. The bacterial suspension was adjusted by spectrophotometry (FANEM LTDA, São Paulo, SP, Brazil) to a cell density of 1.5 × 108 CFU/mL.
The antibacterial activity was evaluated by the direct contact test.[17] After adjusting the bacterial suspension, using Falcon tubes (one for each irrigant with each inhibitor), aliquots of 450 μL of HD, BSA or LPS suspensions were thoroughly mixed with 450 μL of an irrigant and 450 μL of the bacterial suspension for a total volume of 1,350 μL. In the negative control, pyrogen-free water was added to keep the total volume. To control the influence of inhibitors on the antibacterial activity, pyrogen-free water, rather than inhibitors, was added, using plastic tubes (one for each irrigant).
After incubation for 2-min, 30-min and 6-h, aliquots of 100 μL were taken and submitted to 10-fold serial dilutions up to 10−4. When an irrigant was present in the suspension, the first two tubes in the dilution sequence contained an inactivating agent:[21] Three percent Tween 80 + 0.3% α-lecithin (Sigma-Aldrich, St. Louis, MO, USA) for MTAD, Qmix, and CHX, and 0.5% sodium thiosulfate (Mallickrodt Chemical Works, St. Louis, MO, USA) for NaOCl. Three aliquots of 20 μL from each dilution tube were plated onto BHI agar and incubated at 37°C for 24-h. Bacterial colonies were counted using a CFU count limit between 5 and 50; purity was assessed and the number of CFU/mL was calculated. All experiments were performed in triplicate.
Statistical analyses were performed using SPSS 16.0 software (SPSS Co. Chicago, IL, USA). The Kruskal–Wallis, followed by Dunn's test, compared the different irrigants and water in each experimental period, in contact with one of the inhibitors or in their absence, and the effect of the inhibitors in each experimental period was then compared, considering each irrigant and water. A 5% significance level was established.
RESULTS
Table 1 summarizes the results. All irrigants eliminated E. faecalis at 30-min and 6-h, even in the presence of HD, BSA or LPS, and were different from the respective control. The presence of inhibitors at all time points did not alter E. faecalis growth in the absence of irrigants.
Table 1.
Median, minimum and maximum values for the number of colony-forming unit (×105/mL) of Enterococcus faecalis when exposed to irrigants, with or without tested potential inhibitors, after different periods of time (2 min, 30 min or 6 h)
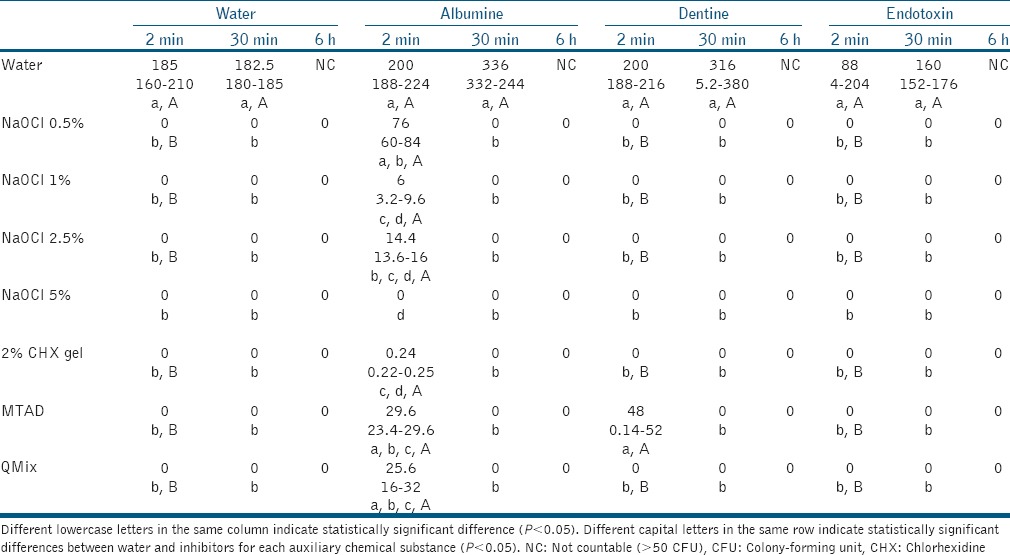
At 2-min, in the presence of endotoxin, all substances were significantly different from the negative control, and eventually killed E. faecalis. In contact with BSA, only 5% NaOCl was capable of eliminating E. faecalis. Other substances reduced bacterial counts, while 0.5% NaOCl, MTAD, and QMix were not different from the control. In contact with HD, all solutions eliminated E. faecalis within 2-min, with the exception of MTAD, which showed a similar number of colonies in comparison with the control.
DISCUSSION
This study investigated the influence of tissue components and bacterial endotoxin on the antibacterial activity of irrigants. The appropriate action of chemical substances against endodontic bacteria in vitro[22] can be impaired in vivo.[23] This fact may be explained by the presence of dentin and various other substances that can inhibit the antimicrobial activity of irrigating solutions used in endodontics.[14,17]
The results showed that only 5% NaOCl was not influenced by the inhibitors in all experimental periods, and was the most effective irrigant. This finding is consistent with previous studies that also demonstrate the superiority of this solution at high concentrations.[15,22] NaOCl is the most commonly used irrigant in endodontic, but there is no consensus regarding its optimal concentration.[2] Despite its advantage in terms of antimicrobial activity, the use of a highly concentrated NaOCl is controversial because it can cause severe damage in cases of accidental extrusion into periapical tissues.[6]
The presence of BSA caused a delay in the antimicrobial activity of irrigants, except for 5% NaOCl, as they were able to eliminate E. faecalis only after 30-min. According to Pappen et al.,[16] the reaction between albumin and NaOCl results in protein degradation and hypochlorite reduction. The presence of high concentrations of BSA consumes some of the NaOCl, which results in the late killing of bacteria. However, the antibacterial effect of NaOCl in the highest concentration was not affected, as previosly repported.[24] Moreover, Portenier et al.[17] found that the action of MTAD and CHX was delayed in the presence of albumin. In the same study, albumin also influenced the antimicrobial action of chlorhexidine combined with cetrimide. Because QMix contains such solutions, there was a delayed effect of the product on E. faecalis in the presence of albumin.
The dentin powder model proposed by Haapasalo et al.[14] has been used to investigate the effect of dentin on the antimicrobial activity of chemical substances.[15,17,18] As observed herein, in the study of Portenier et al.,[17] the antibacterial effect of MTAD was delayed by the presence of dentin. The other tested irrigants were not affected by this component, unlike previous findings,[14] in which the action of 1% NaOCl and 0.05% CHX was reduced in the presence of dentin powder. Likewise, Portenier et al.[17] found that dentin delayed the antibacterial activity of 0.02% CHX. Such controversial results may be attributable to the lower CHX concentrations used in those studies. When a 2% concentration was tested, the results were similar to those presented herein.[15]
Moreover, Morgental et al.[15] verified that the presence of dentin powder delayed the antibacterial action of QMix and of 1% and 6% NaOCl, unlike the findings of this research. These discrepancies may be explained by the short experimental periods (10-s and 1-min), not evaluated in this study, and by the fact that the authors used bovine dentin, whose chemical composition has been poorly investigated. This may influence the intensity of the dentin buffering effect, responsible for the inhibition of irrigants.
LPS showed no inhibitory effect on the irrigants. It is released during the growth, proliferation, and lysis of Gram-negative bactérias, being a common component in infected canals. Since it is able to form electrostatic components with some disinfectant solutions,[18] it was hypothesized that this component could negatively influence the antibacterial action of irrigants against E. faecalis, but the same was not observed in the present investigation. In infected canals, it has been shown that the concentration of LPS varies according to the presence or absence of symptoms.[25] Therefore, although this study used the average concentration of endotoxin found in necrotic root canals,[25] the effect of variations in its concentration should be investigated in future research.
Due to technological advances over the last decades, canal preparation has become an increasingly fast procedure. Some concerns exist about the time necessary for irrigants to act against the endodontic microbiota. The dataset obtained in this study provides additional information about the possible interactions of irrigants and components of canals in an in vitro model. The delay caused by albumin in the antibacterial activity of 0.5%, 1%, and 2.5% NaOCl, 2% CHX, MTAD, and QMix, and by dentin in the case of MTAD, evidenced the influence of these tissue elements on the action of irrigants. However, factors that are not present in this experimental model can play an important role in the effectiveness of these substances as well as in their mode of action in vivo. Thus, these findings should be complemented with in vivo investigations to better represent the clinical application of these irrigants.
CONCLUSIONS
Within the limitations of this study, it can be concluded that in a short period of time, dentin and endotoxin did not affect the antibacterial action of the tested irrigants against E. faecalis. In the presence of albumin, those substances needed a longer period of time to eliminate the microorganism, except for 5% NaOCl. The same was observed in the presence of dentin when E. faecalis was exposed to MTAD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Siqueira JF, Jr, Rôças IN. Clinical implications and microbiology of bacterial persistence after treatment procedures. J Endod. 2008;34:1291–301.e3. doi: 10.1016/j.joen.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Haapasalo M, Udnaes T, Endal U. Persistent, recurrent and acquired infection of the root canal system post-treatment. Endod Top. 2003;6:29–56. [Google Scholar]
- 4.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 5.Hong BY, Lee TK, Lim SM, Chang SW, Park J, Han SH, et al. Microbial analysis in primary and persistent endodontic infections by using pyrosequencing. J Endod. 2013;39:1136–40. doi: 10.1016/j.joen.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Mohammadi Z. Sodium hypochlorite in endodontics: An update review. Int Dent J. 2008;58:329–41. doi: 10.1111/j.1875-595x.2008.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 7.Gomes BP, Vianna ME, Zaia AA, Almeida JF, Souza-Filho FJ, Ferraz CC, et al. Chlorhexidine in endodontics. Braz Dent J. 2013;24:89–102. doi: 10.1590/0103-6440201302188. [DOI] [PubMed] [Google Scholar]
- 8.Stojicic S, Shen Y, Qian W, Johnson B, Haapasalo M. Antibacterial and smear layer removal ability of a novel irrigant, QMiX. Int Endod J. 2012;45:363–71. doi: 10.1111/j.1365-2591.2011.01985.x. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Khademi AA, Babagoli J, Cho Y, Johnson WB, Bozhilov K, et al. Anew solution for the removal of the smear layer. J Endod. 2003;29:170–5. doi: 10.1097/00004770-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Tong Z, Ling J, Lin Z, Li X, Mu Y. The effect of MTADN on 10 Enterococcus faecalis isolates and biofilm: An in vitro study. J Endod. 2013;39:674–8. doi: 10.1016/j.joen.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Aranda-Garcia AJ, Kuga MC, Chavéz-Andrade GM, Kalatzis-Sousa NG, Hungaro Duarte MA, Faria G, et al. Effect of final irrigation protocols on microhardness and erosion of root canal dentin. Microsc Res Tech. 2013;76:1079–83. doi: 10.1002/jemt.22268. [DOI] [PubMed] [Google Scholar]
- 12.Arslan H, Uygun AD, Keskin A, Karatas E, Seçkin F, Yıldırım A, et al. Evaluation of orange-brown precipitate formed in root canals after irrigation with chlorhexidine and QMix and spectroscopic analysis of precipitates produced by a mixture of chlorhexidine/NaOCl and QMix/NaOCl. Int Endod J. 2015;48:1199–203. doi: 10.1111/iej.12427. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Shen Y, Ma J, Haapasalo M. The effect of detergents on the antibacterial activity of disinfecting solutions in dentin. J Endod. 2012;38:948–53. doi: 10.1016/j.joen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Haapasalo HK, Sirén EK, Waltimo TM, Ørstavik D, Haapasalo MP. Inactivation of local root canal medicaments by dentine: An in vitro study. Int Endod J. 2000;33:126–31. doi: 10.1046/j.1365-2591.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 15.Morgental RD, Singh A, Sappal H, Kopper PM, Vier-Pelisser FV, Peters OA, et al. Dentin inhibits the antibacterial effect of new and conventional endodontic irrigants. J Endod. 2013;39:406–10. doi: 10.1016/j.joen.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Pappen FG, Qian W, Aleksejūniene J, Leonardo Rde T, Leonardo MR, Haapasalo M, et al. Inhibition of sodium hypochlorite antimicrobial activity in the presence of bovine serum albumin. J Endod. 2010;36:268–71. doi: 10.1016/j.joen.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Portenier I, Waltimo T, Ørstavik D, Haapasalo M. Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. J Endod. 2006;32:138–41. doi: 10.1016/j.joen.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha A, Kishen A. The effect of tissue inhibitors on the antibacterial activity of chitosan nanoparticles and photodynamic therapy. J Endod. 2012;38:1275–8. doi: 10.1016/j.joen.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Tögl-Leimüller A, Egger G, Porta S. Albumin as one-way transport vehicle into sites of inflammation. Exp Pathol. 1986;30:91–6. doi: 10.1016/s0232-1513(86)80066-4. [DOI] [PubMed] [Google Scholar]
- 20.Kishen A. Periapical biomechanics and the role of cyclic biting force in apical retrograde fluid movement. Int Endod J. 2005;38:597–603. doi: 10.1111/j.1365-2591.2005.00986.x. [DOI] [PubMed] [Google Scholar]
- 21.Pappen FG, Shen Y, Qian W, Leonardo MR, Giardino L, Haapasalo M, et al. In vitro antibacterial action of tetraclean, MTAD and five experimental irrigation solutions. Int Endod J. 2010;43:528–35. doi: 10.1111/j.1365-2591.2010.01712.x. [DOI] [PubMed] [Google Scholar]
- 22.Gomes BP, Ferraz CC, Vianna ME, Berber VB, Teixeira FB, Souza-Filho FJ, et al. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34:424–8. doi: 10.1046/j.1365-2591.2001.00410.x. [DOI] [PubMed] [Google Scholar]
- 23.Vianna ME, Horz HP, Gomes BP, Conrads G. In vivo evaluation of microbial reduction after chemo-mechanical preparation of human root canals containing necrotic pulp tissue. Int Endod J. 2006;39:484–92. doi: 10.1111/j.1365-2591.2006.01121.x. [DOI] [PubMed] [Google Scholar]
- 24.Khedmat S, Aligholi M, Sadeghi S. Influence of bovine serum albumin on the antibacterial activity of endodontic irrigants against Enterococcus faecalis. Iran Endod J. 2009;4:139–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Jacinto RC, Gomes BP, Shah HN, Ferraz CC, Zaia AA, Souza-Filho FJ, et al. Quantification of endotoxins in necrotic root canals from symptomatic and asymptomatic teeth. J Med Microbiol. 2005;54:777–83. doi: 10.1099/jmm.0.45976-0. [DOI] [PubMed] [Google Scholar]


