Abstract
In present study 30 groundwater samples were collected from Sarpol-e Zahab area, Kermanshah province of Iran in order to assess the quality of groundwater in subjected area and determining its suitability for drinking and agricultural purposes. Also the variations in the quality levels of groundwater were compared over the years of 2015 and 2016. Statistical analyses including Spearman correlation coefficients and factor analysis display good correlation between physicochemical parameters (EC, TDS and TH) and Na+, Mg2+, Ca2+, Cl− and ionic constituents. Also in order to assess water quality for irrigation we used the United States Department of Agriculture (USDA) classification which is based on SAR for irrigation suitability assessment. In addition, the residual sodium carbonate (RSC), %Na, PI, KR, SSP, MH, EC characteristics were calculated for all samples and used for assessment of irrigation suitability. Based on these indicators, for every two years, the quality of water for agriculture is in good and excellent category. The Piper classification for hydro geochemical facies indicates that the water in the study area is of Ca-HCO3− type. However, the study of water hardness shows that more than 80% of samples are in hard and very hard water class. Therefore, there is a need for decisions to refine and soften the water.
Keywords: Groundwater quality index, Rural area, Sarpol-e Zahab, Iran
Specifications Table
| Subject area | Chemistry |
| More specific subject area | Describe narrower subject area |
| Type of data | Tables and figures |
| How data was acquired | Experiments have been done in two total categories of system tests and titrimetric tests including temporary and permanent hardness, calcium, magnesium and chloride. Also system tests including pH and electrical conductivity (EC) measured by pH meter device (pHwtw model) and Esi meter (wbw), respectively. The analysis of anions and cations of sulfate was also done by spectrophotometer Hatch (DR 5000 model) in water and wastewater laboratory of Kermanshah. Total hardness was determined by EDTA titrimetric method and TDS was measured gravimetrically. |
| Data format | Raw, Analyzed |
| Experimental factors | All water samples in polyethylene bottles were stored in a dark place at room temperature until the metals analysis |
| Experimental features | The mentioned parameters above, in abstract section, were analyzed according to the standards for water and wastewater treatment handbook. |
| Data source location | Sarpol-e Zahab, Kermanshah province, Iran |
| Data accessibility | Data are included in this article and supplement file excel |
Value of the data
-
•
Determination of the physical and chemical parameter including EC, pH, TDS, TH, Ca, Mg, CO3, HCO3, Na, K, Cl and SO4 in ground water was investigated in rural area, Sarpol-e Zahab city, Iran.
-
•
Due to limited studies in the study area, the data of this study can help to better understand the quality of groundwater in the area and provide further studies.
-
•
The result of data analysis shows that water in this area is suitable for agricultural according to calculated indices.
1. Data
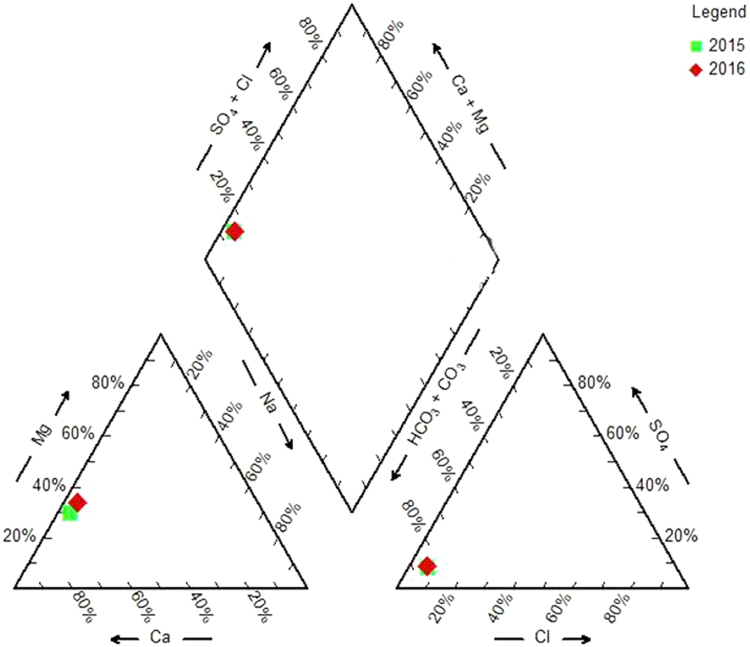
The data presented here deal with monitoring of physical and chemical characteristics of groundwater including pH, EC, TDS, HCO3, CO3, SO4, Cl, Ca, Mg, and Na as well as in Sarpol-e Zahab city, Kermanshah, Iran. The study area and the sampling points are shown in Fig. 1. Also a summary of water quality characteristics are presented in Table 1, Table 2. Results of quality assessment of groundwater samples from rural area in city for drinking purpose (BIS standard) are presented in Table 3, Table 4 [1]. Also classification of groundwater samples for irrigation use on the basis of EC, SAR, RSC, KR, SSP, PI, MH, Na%, T.H are presented in Table 5. Finally, the Piper diagram indicates that the Hydrochemical type of water is of Ca-HCO3 type (Fig. 2) (Table 6, Table 7).
Fig. 1.
The map and location of sampling villages.
Table 1.
Water level and physico-chemical analyses of groundwater samples of study area collected during 2015 year.
| Well | pH | Na | Mg | Ca | Cl | CO3 | HCO3 | SO4 | TDS | EC | T.H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| no | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/l) | (μmhos/cm) | (mg/l) | |
| P1 | 7.33 | 5.75 | 21.78 | 90 | 17.75 | 0 | 335.5 | 25.44 | 430 | 672 | 315 |
| P2 | 7.47 | 4.6 | 20.57 | 78 | 14.2 | 0 | 311.1 | 13.44 | 376 | 587 | 280 |
| P3 | 7.48 | 4.6 | 16.94 | 76 | 10.65 | 0 | 292.8 | 13.44 | 354 | 553 | 260 |
| P4 | 8.07 | 5.75 | 12.1 | 64 | 10.65 | 0 | 189.1 | 49.44 | 292 | 457 | 210 |
| P5 | 7.19 | 25.07 | 18.15 | 90 | 28.4 | 0 | 335.5 | 36.96 | 465 | 715 | 300 |
| P6 | 7.38 | 20.01 | 14.52 | 80 | 24.85 | 0 | 305 | 16.8 | 395 | 617 | 260 |
| P7 | 8.03 | 4.6 | 20.57 | 58 | 14.2 | 0 | 244 | 18.24 | 316 | 493 | 230 |
| P8 | 8.15 | 6.44 | 29.04 | 58 | 17.75 | 0 | 262.3 | 36.48 | 365 | 570 | 265 |
| P9 | 7.7 | 7.36 | 18.15 | 90 | 17.75 | 0 | 305 | 38.4 | 412 | 644 | 300 |
| P10 | 7.71 | 2.76 | 16.94 | 56 | 10.65 | 0 | 225.7 | 14.4 | 272 | 425 | 210 |
| P11 | 7.55 | 8.97 | 33.88 | 92 | 17.75 | 0 | 408.7 | 27.36 | 519 | 798 | 370 |
| P12 | 8.28 | 3.68 | 16.94 | 62 | 10.65 | 0 | 244 | 16.32 | 306 | 478 | 225 |
| P13 | 7.62 | 2.76 | 18.15 | 54 | 10.65 | 0 | 225.7 | 14.4 | 283 | 442 | 210 |
| P14 | 7.81 | 4.6 | 16.94 | 76 | 10.65 | 0 | 280.6 | 23.04 | 351 | 548 | 260 |
| P15 | 8.04 | 3.68 | 19.36 | 54 | 10.65 | 0 | 231.8 | 16.32 | 295 | 461 | 215 |
| P16 | 8.06 | 6.44 | 12.1 | 58 | 10.65 | 0 | 219.6 | 12.48 | 274 | 428 | 195 |
| P17 | 7.71 | 1.38 | 15.73 | 52 | 7.1 | 0 | 213.5 | 11.52 | 265 | 414 | 195 |
| P18 | 7.45 | 5.75 | 21.78 | 80 | 14.2 | 0 | 305 | 30.24 | 393 | 614 | 290 |
| P19 | 7.68 | 4.6 | 16.94 | 72 | 10.65 | 0 | 280.6 | 13.44 | 342 | 534 | 250 |
| P20 | 7.65 | 4.6 | 16.94 | 80 | 14.2 | 0 | 298.9 | 13.44 | 367 | 573 | 270 |
| P21 | 7.97 | 5.75 | 25.41 | 76 | 14.2 | 0 | 305 | 35.04 | 401 | 626 | 295 |
| P22 | 7.71 | 4.6 | 19.36 | 70 | 10.65 | 0 | 262.3 | 32.64 | 346 | 540 | 255 |
| P23 | 7.35 | 11.73 | 33.88 | 100 | 24.85 | 0 | 408.7 | 42.72 | 550 | 846 | 390 |
| P24 | 7.46 | 3.68 | 16.94 | 60 | 10.65 | 0 | 244 | 11.52 | 302 | 472 | 220 |
| P25 | 7.66 | 2.76 | 18.15 | 50 | 7.1 | 0 | 225.7 | 9.6 | 269 | 420 | 200 |
| P26 | 7.28 | 9.89 | 25.41 | 84 | 21.3 | 0 | 347.7 | 19.68 | 438 | 685 | 315 |
| P27 | 8.18 | 2.07 | 10.89 | 66 | 10.65 | 0 | 225.7 | 12.96 | 284 | 444 | 210 |
| P28 | 7.73 | 4.6 | 13.31 | 90 | 14.2 | 0 | 305 | 18.24 | 381 | 596 | 280 |
| P29 | 7.51 | 3.68 | 15.73 | 68 | 7.1 | 0 | 268.4 | 11.52 | 319 | 499 | 235 |
| P30 | 8 | 5.06 | 26.62 | 70 | 14.2 | 0 | 317.2 | 14.4 | 384 | 600 | 285 |
| Min | 7.2 | 1.4 | 10.9 | 50.0 | 7.1 | 0.0 | 189.1 | 9.6 | 265.0 | 414.0 | 195.0 |
| Max | 8.3 | 25.1 | 33.9 | 100.0 | 28.4 | 0.0 | 408.7 | 49.4 | 550.0 | 846.0 | 390.0 |
| Ave | 7.7 | 6.2 | 19.4 | 71.8 | 14.0 | 0.0 | 280.8 | 21.7 | 358.2 | 558.4 | 259.8 |
| SD | 0.30 | 5.00 | 5.77 | 13.95 | 5.35 | 0.00 | 54.12 | 11.02 | 73.08 | 111.47 | 49.30 |
Table 2.
Water level and physico-chemical analyses of groundwater samples of study area collected during 2016 year.
| Well | pH | Na | Mg | Ca | Cl | CO3 | HCO3 | SO4 | TDS | EC | T.H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| no | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/l) | (μ mhos/cm) | (mg/l) | |
| P1 | 7.3 | 14.95 | 42.35 | 104 | 31.95 | 0 | 378.2 | 107.04 | 614 | 944 | 435 |
| P2 | 7.58 | 3.68 | 24.2 | 60 | 10.65 | 0 | 274.5 | 16.32 | 336 | 525 | 250 |
| P3 | 7.63 | 5.75 | 12.1 | 60 | 10.65 | 0 | 219.6 | 15.84 | 281 | 439 | 200 |
| P4 | 7.74 | 2.76 | 16.94 | 54 | 10.65 | 0 | 219.6 | 14.4 | 276 | 432 | 205 |
| P5 | 7.27 | 25.07 | 22.99 | 80 | 24.85 | 0 | 359.9 | 17.76 | 463 | 712 | 295 |
| P6 | 7.54 | 3.68 | 24.2 | 56 | 10.65 | 0 | 262.3 | 16.32 | 323 | 505 | 240 |
| P7 | 7.54 | 4.6 | 25.41 | 74 | 10.65 | 0 | 323.3 | 18.24 | 388 | 607 | 290 |
| P8 | 7.56 | 7.36 | 25.41 | 74 | 14.2 | 0 | 329.4 | 14.4 | 401 | 627 | 290 |
| P9 | 7.83 | 2.76 | 15.73 | 54 | 10.65 | 0 | 213.5 | 14.4 | 268 | 418 | 200 |
| P10 | 7.29 | 7.36 | 24.2 | 82 | 17.75 | 0 | 341.6 | 14.4 | 420 | 656 | 305 |
| P11 | 7.84 | 7.36 | 10.89 | 60 | 10.65 | 0 | 219.6 | 14.4 | 275 | 430 | 195 |
| P12 | 7.74 | 8.97 | 20.57 | 76 | 10.65 | 0 | 305 | 27.36 | 383 | 598 | 275 |
| P13 | 7.42 | 5.75 | 25.41 | 74 | 17.75 | 0 | 317.2 | 15.84 | 392 | 612 | 290 |
| P14 | 7.63 | 4.6 | 22.99 | 70 | 10.65 | 0 | 298.9 | 18.24 | 364 | 569 | 270 |
| P15 | 7.46 | 5.75 | 24.2 | 68 | 17.75 | 0 | 292.8 | 15.84 | 372 | 581 | 270 |
| P16 | 7.58 | 4.6 | 22.99 | 80 | 10.65 | 0 | 329.4 | 18.24 | 397 | 620 | 295 |
| P17 | 7.56 | 7.36 | 24.2 | 78 | 14.2 | 0 | 335.5 | 14.4 | 405 | 633 | 295 |
| P18 | 7.53 | 8.97 | 18.15 | 84 | 17.75 | 0 | 317.2 | 17.76 | 398 | 622 | 285 |
| P19 | 7.34 | 3.68 | 21.78 | 60 | 10.65 | 0 | 262.3 | 16.32 | 324 | 506 | 240 |
| P20 | 7.87 | 2.76 | 24.2 | 48 | 10.65 | 0 | 237.9 | 14.4 | 274 | 428 | 220 |
| P21 | 7.34 | 4.6 | 19.36 | 70 | 10.65 | 0 | 280.6 | 18.24 | 344 | 538 | 255 |
| P22 | 7.62 | 7.36 | 18.15 | 48 | 10.65 | 0 | 219.6 | 14.4 | 279 | 436 | 195 |
| P23 | 7.59 | 5.75 | 15.73 | 58 | 10.65 | 0 | 231.8 | 15.84 | 292 | 457 | 210 |
| P24 | 7.57 | 14.95 | 38.72 | 110 | 31.95 | 0 | 396.5 | 92.64 | 616 | 497 | 435 |
| P25 | 7.06 | 6.44 | 27.83 | 76 | 10.65 | 0 | 335.5 | 26.88 | 414 | 647 | 305 |
| P26 | 7.52 | 2.76 | 20.57 | 48 | 10.65 | 0 | 219.6 | 14.4 | 278 | 435 | 205 |
| P27 | 7.33 | 4.6 | 25.41 | 50 | 10.65 | 0 | 244 | 23.04 | 312 | 487 | 230 |
| P28 | 7.23 | 9.89 | 36.3 | 96 | 17.75 | 0 | 451.4 | 14.88 | 541 | 833 | 390 |
| P29 | 7.26 | 8.05 | 29.04 | 88 | 21.3 | 0 | 305 | 59.04 | 470 | 723 | 340 |
| P30 | 7.21 | 5.75 | 29.04 | 80 | 10.65 | 0 | 353.8 | 25.44 | 431 | 673 | 320 |
| Min | 7.1 | 2.8 | 10.9 | 48.0 | 10.7 | 0.0 | 213.5 | 14.4 | 268.0 | 418.0 | 195.0 |
| Max | 7.9 | 25.1 | 42.35 | 110.0 | 32.0 | 0.0 | 451.4 | 107.0 | 616.0 | 944.0 | 435.0 |
| Ave | 7.5 | 6.9 | 23.6 | 70.7 | 14.3 | 0.0 | 295.9 | 24.2 | 377.7 | 573.0 | 274.3 |
| SD | 0.20 | 4.60 | 6.94 | 16.27 | 6.15 | 0.00 | 60.70 | 22.30 | 94.10 | 126.54 | 64.62 |
Table 3.
Calculation of RSC, PI, KR, MH, Na%, SAR and SSP of groundwater for 2015and 2016 years.
| Well |
2015 Year |
2016 Year |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | RSC | PI | KR | MH | Na% | SAR | SSP | RSC | PI | KR | MH | Na% | SAR | SSP |
| P1 | − 0.80 | 39.62 | 0.04 | 28.57 | 3.82 | 0.14 | 3.82 | − 2.5 | 33.58 | 0.07 | 40.23 | 6.95 | 0.31 | 6.95 |
| P2 | − 0.50 | 42.38 | 0.04 | 30.36 | 3.45 | 0.12 | 3.45 | − 0.5 | 44.21 | 0.03 | 40.00 | 3.10 | 0.10 | 3.10 |
| P3 | − 0.40 | 44.28 | 0.04 | 26.92 | 3.70 | 0.12 | 3.70 | − 0.4 | 50.53 | 0.06 | 25.00 | 5.88 | 0.18 | 5.88 |
| P4 | − 1.10 | 45.18 | 0.06 | 23.81 | 5.62 | 0.17 | 5.62 | − 0.5 | 47.80 | 0.03 | 34.15 | 2.84 | 0.08 | 2.84 |
| P5 | − 0.50 | 48.45 | 0.18 | 25.00 | 15.37 | 0.63 | 15.37 | 0 | 50.34 | 0.18 | 32.20 | 15.59 | 0.63 | 15.59 |
| P6 | − 0.20 | 51.17 | 0.17 | 23.08 | 14.33 | 0.54 | 14.33 | − 0.5 | 45.03 | 0.03 | 41.67 | 3.23 | 0.10 | 3.23 |
| P7 | − 0.60 | 45.83 | 0.04 | 36.96 | 4.17 | 0.13 | 4.17 | − 0.5 | 41.70 | 0.03 | 36.21 | 3.33 | 0.12 | 3.33 |
| P8 | − 1.00 | 42.18 | 0.05 | 45.28 | 5.02 | 0.17 | 5.02 | − 0.4 | 43.20 | 0.06 | 36.21 | 5.23 | 0.19 | 5.23 |
| P9 | − 1.00 | 40.44 | 0.05 | 25.00 | 5.06 | 0.18 | 5.06 | − 0.5 | 48.32 | 0.03 | 32.50 | 2.91 | 0.08 | 2.91 |
| P10 | − 0.50 | 47.30 | 0.03 | 33.33 | 2.78 | 0.08 | 2.78 | − 0.5 | 41.84 | 0.05 | 32.79 | 4.98 | 0.18 | 4.98 |
| P11 | − 0.70 | 38.23 | 0.05 | 37.84 | 5.01 | 0.20 | 5.01 | − 0.3 | 52.54 | 0.08 | 23.08 | 7.58 | 0.23 | 7.58 |
| P12 | − 0.50 | 46.35 | 0.04 | 31.11 | 3.43 | 0.11 | 3.43 | − 0.5 | 44.59 | 0.07 | 30.91 | 6.62 | 0.24 | 6.62 |
| P13 | − 0.50 | 47.30 | 0.03 | 35.71 | 2.78 | 0.08 | 2.78 | − 0.6 | 41.82 | 0.04 | 36.21 | 4.13 | 0.15 | 4.13 |
| P14 | − 0.60 | 43.42 | 0.04 | 26.92 | 3.70 | 0.12 | 3.70 | − 0.5 | 43.10 | 0.04 | 35.19 | 3.57 | 0.12 | 3.57 |
| P15 | − 0.50 | 47.30 | 0.04 | 37.21 | 3.59 | 0.11 | 3.59 | − 0.6 | 43.20 | 0.05 | 37.04 | 4.42 | 0.15 | 4.42 |
| P16 | − 0.30 | 52.09 | 0.07 | 25.64 | 6.70 | 0.20 | 6.70 | − 0.5 | 41.37 | 0.03 | 32.20 | 3.28 | 0.12 | 3.28 |
| P17 | − 0.40 | 48.76 | 0.02 | 33.33 | 1.52 | 0.04 | 1.52 | − 0.4 | 42.85 | 0.05 | 33.90 | 5.14 | 0.19 | 5.14 |
| P18 | − 0.80 | 41.09 | 0.04 | 31.03 | 4.13 | 0.15 | 4.13 | − 0.5 | 43.85 | 0.07 | 26.32 | 6.40 | 0.23 | 6.40 |
| P19 | − 0.40 | 45.09 | 0.04 | 28.00 | 3.85 | 0.13 | 3.85 | − 0.5 | 45.03 | 0.03 | 37.50 | 3.23 | 0.10 | 3.23 |
| P20 | − 0.50 | 43.10 | 0.04 | 25.93 | 3.57 | 0.12 | 3.57 | − 0.5 | 46.35 | 0.03 | 45.45 | 2.65 | 0.08 | 2.65 |
| P21 | − 0.90 | 40.42 | 0.04 | 35.59 | 4.07 | 0.15 | 4.07 | − 0.5 | 44.24 | 0.04 | 31.37 | 3.77 | 0.13 | 3.77 |
| P22 | − 0.80 | 42.90 | 0.04 | 31.37 | 3.77 | 0.13 | 3.77 | − 0.3 | 52.54 | 0.08 | 38.46 | 7.58 | 0.23 | 7.58 |
| P23 | − 1.10 | 37.29 | 0.07 | 35.90 | 6.14 | 0.26 | 6.14 | − 0.4 | 49.42 | 0.06 | 30.95 | 5.62 | 0.17 | 5.62 |
| P24 | − 0.40 | 47.37 | 0.04 | 31.82 | 3.51 | 0.11 | 3.51 | − 2.2 | 34.22 | 0.07 | 36.78 | 6.95 | 0.31 | 6.95 |
| P25 | − 0.30 | 49.60 | 0.03 | 37.50 | 2.91 | 0.08 | 2.91 | − 0.6 | 41.15 | 0.05 | 37.70 | 4.39 | 0.16 | 4.39 |
| P26 | − 0.60 | 41.86 | 0.07 | 33.33 | 6.39 | 0.24 | 6.39 | − 0.5 | 47.80 | 0.03 | 41.46 | 2.84 | 0.08 | 2.84 |
| P27 | − 0.50 | 46.94 | 0.02 | 21.43 | 2.10 | 0.06 | 2.10 | − 0.6 | 45.83 | 0.04 | 45.65 | 4.17 | 0.13 | 4.17 |
| P28 | − 0.60 | 42.00 | 0.04 | 19.64 | 3.45 | 0.12 | 3.45 | − 0.4 | 38.28 | 0.06 | 38.46 | 5.22 | 0.22 | 5.22 |
| P29 | − 0.30 | 46.45 | 0.03 | 27.66 | 3.29 | 0.10 | 3.29 | − 1.8 | 36.17 | 0.05 | 35.29 | 4.90 | 0.19 | 4.90 |
| P30 | − 0.50 | 42.24 | 0.04 | 38.60 | 3.72 | 0.13 | 3.72 | − 0.6 | 39.97 | 0.04 | 37.50 | 3.76 | 0.14 | 3.76 |
| Min | − 1.10 | 37.29 | 0.02 | 19.64 | 1.52 | 0.04 | 1.52 | − 2.50 | 33.58 | 0.03 | 23.08 | 2.65 | 0.08 | 2.65 |
| Max | − 0.20 | 52.09 | 0.18 | 45.28 | 15.37 | 0.63 | 15.37 | 0.00 | 52.54 | 0.18 | 45.65 | 15.59 | 0.63 | 15.59 |
| Ave | − 0.59 | 44.56 | 0.05 | 30.80 | 4.70 | 0.16 | 4.70 | − 0.64 | 44.03 | 0.05 | 35.41 | 5.01 | 0.18 | 5.01 |
| SD | 0.24 | 3.73 | 0.04 | 5.96 | 3.00 | 0.12 | 3.00 | 0.54 | 4.79 | 0.03 | 5.27 | 2.50 | 0.11 | 2.50 |
Table 4.
Quality of ground water sample samples from rural area in Sarpol-e Zahab city for drinking purpose (BIS standard) [2].
| Parameter | Desirable limit |
2015 Year samples (%) |
2016 Year samples (%) |
||
|---|---|---|---|---|---|
| Within limits | Exceed limits | Within limits | Exceed limits | ||
| pH | 6.5–8.5 | 100 | 0 | 100 | 0 |
| EC | 300 (μmhos/cm) | 0 | 100 | 0 | 100 |
| TDS | 500 (mg/L) | 93.3 | 6.7 | 90 | 10 |
| Total hardness | 200 (mg/L) | 13.4 | 86.6 | 20 | 80 |
| SO4 | 200 (mg/L) | 100 | 0 | 100 | 0 |
| Cl | 250 (mg/L) | 100 | 0 | 100 | 0 |
| Ca | 75 (mg/L) | 53.3 | 46.7 | 60 | 40 |
| Mg | 30 (mg/L) | 93.3 | 6.7 | 90 | 10 |
| Na | 200 (mg/L) | 100 | 0 | 100 | 0 |
Table 5.
Classification of groundwater sample for irrigation use on the basic of EC, SAR, RSC, KR, SSP, PI, MH, Na%, T.H [2].
| Parameters | Range | Water class |
Samples(%) |
|
|---|---|---|---|---|
| 2015 Year | 2016 Year | |||
| EC | < 250 | Excellent | Nil | Nil |
| 250–750 | Good | 93.3 | 93.3 | |
| 750–2250 | Permissible | 6.7 | 6.7 | |
| >2250 | Doubtful | Nil | Nil | |
| SAR | 0–10 | Excellent | 100 | 100 |
| 10–18 | Good | Nil | Nil | |
| 18–26 | Doubtful | Nil | Nil | |
| > 26 | Unsuitable | Nil | Nil | |
| RSC | < 1.25 | Good | 100 | 100 |
| 1.25–2.5 | Doubtful | Nil | Nil | |
| > 2.5 | Unsuitable | Nil | Nil | |
| KR | < 1 | suitable | 100 | 100 |
| 1–2 | Marginal suitable | Nil | Nil | |
| > 2 | Unsuitable | Nil | Nil | |
| SSP | < 50 | Good | 100 | 100 |
| > 50 | Unsuitable | Nil | Nil | |
| PI | > 75 | Class-I | Nil | Nil |
| 25–75 | Class-II | 100 | 100 | |
| < 25 | Class-III | Nil | Nil | |
| MH | < 50 | Suitable | 100 | 100 |
| > 50 | Harmful &Unsuitable | Nil | Nil | |
| Na% | < 20 | Excellent | 100 | 100 |
| 20–40 | Good | Nil | Nil | |
| 40–60 | Permissible | Nil | Nil | |
| 60–80 | Doubtful | Nil | Nil | |
| > 80 | Unsuitable | Nil | Nil | |
| T.H | < 75 | Soft | Nil | Nil |
| 75–150 | Moderately hard | Nil | Nil | |
| 150–300 | Hard | 86.7 | 76.7 | |
| > 300 | Very hard | 13.3 | 23.3 | |
Fig. 2.
The Piper diagram indicates that the hydrochemical type of water.
Table 6.
Summary of water quality indices in present study.
| Indices | Formula |
|---|---|
| Residual sodium carbonate (RSC) | |
| Permeability index (PI) | |
| Kelly's ratio (KR) | |
| Magnesium hazard(MH) | |
| Sodium percentage (Na %) | |
| Sodium adsorption ratio (SAR) | |
| Soluble sodium percentage (SSP) |
Table 7.
Pearson's correlation coefficient.
| pH | Na | Mg | Ca | HCO3 | CL | SO4 | TDS | EC | TH | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | |||||||||
| Na | − 0.416** | 1 | ||||||||
| Mg | − 0.424** | 0.30* | 1.00 | |||||||
| Ca | − 0.451** | 0.578** | 0.544** | 1.00 | ||||||
| HCO3 | − 0.569** | 0.551** | 0.753** | 0.884** | 1 | |||||
| CL | − 0.384** | 0.820** | 0.572** | 0.749** | 0.672** | 1 | ||||
| SO4 | − 0.148 | 0.425** | 0.591** | 0.581** | 0.389** | 0.678** | 1 | |||
| TDS | − 0.516** | 0.641** | 0.799** | 0.924** | 0.938** | 0.829** | 0.671** | 1 | ||
| EC | − 0.551** | 0.573** | 0.695** | 0.835** | 0.895** | 0.690** | 0.462** | 0.890** | 1 | |
| TH | − 0.499** | 0.523** | 0.836** | 0.915** | 0.940** | 0.764** | 0.663** | 0.988** | 0.880** | 1 |
Correlation is significant at the 0.01 level (2-tailed).
Correlation is significant at the 0.05 level (2-tailed).
2. Experimental design, materials and methods
2.1. Description of study area
Sarpol-e Zahab city in Kermanshah province are located in west of Iran between the latitudes 34.4514 ° N and longitudes 45.8612 °E, encompassing an area of about 935.2 km2. Also the SarPol-e Zahab city has a cold and dry climate and the average altitude of the city is 550 m above sea level. It is worth noting that the average rainfall is 111 mm, with the minimum and maximum temperature of 1/1 ° C and 11.3 ° C, respectively.
2.2. Materials and methods
In order to assess the physico-chemical parameters, a total of 30 groundwater samples were collected from Sarpol-e Zahab city between years the of 2015 and 2016 (Fig. 1). Sampling was conducted with one‑liter polyethylene bottles which were immersed in nitric acid for 24 h then washed with 10% HCL and finally washed with distilled water. Before the samples were taken, sampling containers had been rinsed at least three times with water. Experiments have been done in two total categories of system tests and titrimetric tests including temporary and permanent hardness, calcium, magnesium and chloride. Also system tests including PH and electrical conductivity (EC) measured by PH meter device (pHwtw model) and Esi meter (wbw), respectively. The analysis of anions and cations of sulfate was also done by spectrophotometer Hatch (DR 5000 model) in water and wastewater laboratory of Kermanshah. Total hardness was determined by EDTA titrimetric method and TDS was measured gravimetrically [2], [3], [4], [5], [6], [7], [8], [9], [10].
Statistical analyses including Spearman correlation coefficients and factor analysis display good correlation between physicochemical parameters (EC, TDS and TH) and Na+, Mg2+, Ca2+, Cl− as well as ionic constituents of groundwater with SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp).
Finally, in order to understand chemical character of the groundwater and relationships between the dissolved ionic constituents, the hydrochemical data has been plotted on Piper diagram (Piper 1944) using AqQA software (Fig. 2).
Acknowledgements
The authors want to thank authorities of Tehran University of Medical Sciences for their comprehensives support for this study.
Footnotes
Transparency document associated with this article can be found in the online version at 10.1016/j.dib.2017.12.061.
Transparency document. Supplementary material
Transparency document
References
- 1.APHA, Standard Methods for the Examination of Water and Waste Water (APHA), 1995. [DOI] [PMC free article] [PubMed]
- 2.Yousefi M., Najafi Saleh H., Mohammad A.A., Mahvi A.H., Ghadrpoori M., Suleimani H. Data on water quality index for the groundwater in rural area Neyshabur County, Razavi province, Iran. Data Brief. 2017;15:901–907. doi: 10.1016/j.dib.2017.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asghari F.B., Mohammadi A.A., Aboosaedi Z., Yaseri M., Yousefi M. Data on fluoride concentration levels in cold and warm season in rural area of Shout (West Azerbaijan, Iran) Data Brief. 2017;15:528–531. doi: 10.1016/j.dib.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabizadeh Nodehi R., Mesdaghinia A., Nasseri S., Hadi M., Soleimani H., Bahmani P. Analysis of water corrosion tendency in water supply system using qualitative indices and calcium carbonate precipitation potential index. Iran. J. Health Environ. 2017;9:457–470. [Google Scholar]
- 5.Amouei A.I., Mahvi A.H., Mohammadi A.A., Asgharnia H.A., Fallah S.H., Khafajeh A.A. Physical and chemical quality assessment of potable groundwater in rural areas of Khaf, Iran. World Appl. Sci. J. 2012;18:693. [PubMed] [Google Scholar]
- 6.Mohammadi A.A., Najafi Saleh H., Mahv A.H., Alimohammadi M., Nabizadeh R., Yousefi M. Data on corrosion and scaling potential of drinking water resources using stability indices in Jolfa, East Azerbaijan, Iran. Data Breif. 2018 doi: 10.1016/j.dib.2017.11.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohammadi A.A., Yousefi M., Mahvi A.H. Fluoride concentration level in rural area in Poldasht city and daily fluoride intake based on drinking water consumption with temperature. Data Brief. 2017;13:312–315. doi: 10.1016/j.dib.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammadi A.A., Yaghmaeian K., Faraji H., Nabizadeh R., Dehghani M.H., Khaili J.K., Mahvi A.H. Temporal and spatial variation of chemical parameter concentration in drinking water resources of Bandar-e Gaz City using geographic information system. Desalin. Water Treat. 2017;68:170–176. [Google Scholar]
- 9.Abbasnia A., Alimohammadi M., Mahvi A.H., Nabizadeh R., Yousefi M., Mohammadi A.A., Pasalari H., Mirzabeigi M. Assessment of groundwater quality and evaluation of scaling and corrosiveness potential of drinking water samples in villages of Chabahr city, Sistan and Baluchistan province in Iran. Data Brief. 2018;16:182–192. doi: 10.1016/j.dib.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirzabeygi M., Yousefi N., Abbasnia A., Youzi H., Alikhani M., Mahvi A.H. Evaluation of groundwater quality and assessment of scaling potential and corrosiveness of water supply networks, Iran. J. Water Supply: Res. Technol.-Aqua. 2017:jws2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document