Abstract
A deepened understanding of the cellular and molecular processes in the tumor microenvironment is necessary for the development of precision immunotherapy (IT). We simultaneously investigated CD3, PDL1, and IDO by immunohistochemistry in paired biopsies from various organs of 43 metastatic melanoma patients treated with IT and targeted therapy (TT). Intraindividual biopsies taken after a period of weeks to months demonstrate discordant results in 30% of the cases. Overlap of IDO and PDL1 increased after therapy. IT only marginally impacted PDL1 expression over time in contrast to TT. Standardized repeated assessments of multiple immune markers in repeated biopsies will generate detailed insights in melanoma's immune evolution and adaption during therapies and might be used to support treatment decisions.
Introduction
Immunotherapy (IT) has revolutionized the therapeutic landscape for advanced melanoma [1], [2], [3] and other tumor types. Multiple antibodies and other medications are under development that will widen the treatment options. In order to optimize IT, a deepened understanding of the cellular and molecular processes in the tumor microenvironment is mandatory that will allow a rational selection of the therapeutic tools resulting in precision IT [4].
Nivolumab and pembrolizumab bind to PD-1 and interrupt the interaction with its ligands PDL1 and PDL2 that inhibit antitumor T cell functions. Clinical benefit correlates with the expression levels of PDL1 in melanoma and other tumors such as lung carcinomas [5]. PDL1 expression on tumor cells helps to identify metastatic melanoma patients that achieve a similar benefit with nivolumab monotherapy than with the Nivo/Ipi combination therapy. Therefore, PDL1 may be a biomarker of clinical relevance [6].
Multiple, genetic, and epigenetic mechanisms regulate PDL1 expression levels on tumor cells [7]. In melanoma, PDL1 expression is enhanced by the presence of interferon-gamma–secreting lymphocytes in the tumor microenvironment in most cases, rendering its expression a surrogate for the intratumoral accumulation of T cells [8].
Interferon-gamma is also a strong inducer of indoleamin-2, 3-dioxygenase 1 (IDO). This cytosolic heme enzyme is expressed in various tissues including placenta where it is essential to promote immune privilege. IDO catabolizes tryptophan to kynurenine and other metabolites, resulting in tryptophan depletion suppressing T-cell functions [9].
In melanoma, the presence of IDO has negative prognostic implications as shown for IDO expression in peritumoral endothelium cells [10], in the sentinel lymph node [11], in nodal metastases, and for the kynurenine/tryptophan ratio in the serum [12]. Both PDL1 and IDO are important drug targets of monoclonal antibodies such as avelumab (MERCK) or the orally available epacadostat (INCB024360, Incyte). The combination of IDO inhibitors and an anti-PD1 antibody has demonstrated promising efficacy in early clinical trials and is currently investigated in larger phase III trials [13], [14].
There is limited information about the coexpression of IDO1 and the presence of tumor-infiltrating lymphocytes (TILs) such as T-cells and the consistency of these features during targeted and/or IT along with PDL1 expression in human melanoma biopsies. We here report results on these immune markers in a set of melanoma patients with multiple metastases and an expanded in silico investigation on these and other immune markers in the public database “The Cancer Genome Atlas (TCGA) Melanoma” [15], [16].
Patients and Methods
An extensive search in the internal dermatopathology database (Dermapro®) of the department of dermatology at the University Hospital of Zurich was performed. Keyword “melanoma” was used in full text search to identify patients with metastatic melanoma, from which serial tumor material is available. Clinical data were obtained from the KISIM electronic patient record of the University Hospital Zurich.
Based on this information, patients with serial melanoma biopsies before and after immuno- or targeted therapy were included. Treatments included immunotherapies including both anti–CTLA-4 and anti–PD-1 molecules and targeted therapies including BRAF- and MEK-inhibitors as well as chemotherapy and a tyrosine kinase inhibitor.
Exclusion criteria included insufficient or unavailable tumor material for staining, insufficient clinical follow-up data, and explicit refusal of patient data-based research at hospital admission.
Permission for this noninterventional research project was granted by the relevant authority (ethics committee of the Canton of Zurich, Switzerland, KEK-ZH-Nr. 2014-0193).
Immunohistochemistry
Formalin-fixed, paraffin-embedded samples from our histopathology archive were stained for CD3 in our research laboratory.
Standard automated protocols using a DAKO Autostainer with Real Detection System Alkaline Phosphatase was used. CD3 staining was performed using a monoclonal mouse antibody (DAKO, M7254, clone F7.2.38); heat-mediated antigen retrieval with EDTA buffer was performed. TRS high buffer was used for heat-mediated antigene retrieval. Normal human tonsil served as a positive control.
Stainings for IDO expression as described [10] were performed by Ines Chevolet (I.C.) in the laboratory of Liève Brochez (L.B.). A monoclonal mouse antibody (Millipore, clone 10.1) was used in a standard avidin-biotin-peroxidase protocol (Dako).
PDL1 immunoreactivity was assessed by an immunohistochemical assay for formalin-fixed, paraffin-embedded tumor specimens that incorporated a rabbit monoclonal anti-human PDL1 antibody (clone 28-8) and an automated staining procedure developed by DAKO. Percentage PDL1 expression was scored manually by a qualified pathologist where positive PDL1 staining was defined as complete circumferential or partial linear plasma membrane staining of tumor cells [17], [18]. Both staining and analysis for PDL1 expression were performed by Mosaic Laboratories, Lake Forest, CA, on behalf of Bristol Myers Squibb.
Scoring
Dermatopathological analysis was performed by a board-certified dermatopathologist (K.K.). H&E staining was used for orientation within the sample. With CD3, both peritumoral and intratumoral lymphocytes (infiltration) were graded using a semiquantitative approach defining both intra- and peritumoral lymphocytes as absent/mild, moderate, or severe. Special emphasis was on dynamics in tumor infiltration, especially on increasing lymphocyte infiltration after systemic treatments. CD3-positive tumor-infiltrating lymphocytes were used as a surrogate for TIL. IDO expression on high endothelial venules was assessed by I.C. as absent, focally positive, or positive. PDL1 expression on tumor cells was quantified by Mosaic Laboratories, Lake Forest, CA (on behalf of BMS). A cutoff of 1% positive tumor cells was used [19].
Expression of T-Cell–Associated Genes from TCGA
To confirm the prognostic relevance of T-cell–associated genes in melanoma, analysis of RNAseq data from the TCGA melanoma data set was used using an online tool [15]. T-cell marker expression including immune checkpoints, melanoma, and fibroblast specific genes were assessed. A comparison of overall survival comparing the top 10% to the bottom 10%, as well as the top 25% and bottom 25% expression groups was performed. The log-rank test was used to test for significant survival difference; a P value less than .05 was considered as statistically significant.
Results
Biopsy Locations
In total, paired tissue samples of metastases of 43 patients (for detailed description of the patient population, see Table 1) were available for definitive histological and clinical evaluation. Most pretreatment metastases were subcutaneous (n = 29) or nodal (n = 8) along with lung (n = 2), soft tissue (n = 2), liver (n = 1), and one metastasis of unknown origin (n = 1). Posttreatment metastases originated from similar locations (subcutaneous (n = 31), followed by nodal (n = 5), soft tissue (n = 4), bone (n = 1), ileum (n = 1), and cerebral (n = 1) (Table 1).
Table 1.
Patients' Characteristics
| Age (Median) | 56 (24-83) |
|---|---|
| Gender | |
| M | 23 |
| F | 20 |
| Melanoma subtypes | |
| SSM | 10 |
| NMM | 14 |
| ALM | 4 |
| Amel | 3 |
| Nev | 1 |
| Polyploid | 1 |
| UKT | 8 |
| UKO | 2 |
| Localization before | |
| Subcutaneous | 29 |
| Nodal | 8 |
| Lung | 2 |
| Soft tissue | 2 |
| Hepatic | 1 |
| Unknown | 1 |
| Localization after | |
| Subcutaneous | 31 |
| Nodal | 5 |
| Soft tissue | 4 |
| Bone | 1 |
| Ileum | 1 |
| Cerebral | 1 |
Histological Subtype
Types of the primary cutaneous melanoma included nodular (n = 15), superficial spreading (n = 10), unknown type (n = 8), acral-lentiginous (n = 4), amelanotic (n = 3), and nevoid (n = 1). In two patients, metastatic melanoma of unknown origin was diagnosed (n = 2) (Table 1).
Treatments
During the time between the biopsies, 10 patients underwent immunotherapies, including ipilimumab (n = 8) and pembrolizumab (n = 2); 18 patients underwent targeted therapy, including BRAF inhibitors (vemurafenib) (n = 12), MEK inhibitors (binimetinib, selumetinib, n = 5), and a combination of both (n = 1); 6 patients received sequential combinations of targeted and immunotherapy; 2 had sequential combinations of immunotherapy and other therapies (including chemotherapy); and 1 had all three treatment types (Table 1).
IDO Immunoreactivity on Peritumoral Endothelium Cells
Pretreatment samples presented IDO immunoreactivity in 17 out of 43 patients (39.53%) versus 25 (58.14%) IDO-negative with IDO status not evaluable due to technical reasons in one sample. After treatments, IDO-positive samples decreased slightly to 14 (32.56%), whereas IDO-negative samples increased to 28 (65.12%), with one sample not being evaluable (Table 2).
Table 2.
Evaluation of TIL, PD-L1, and IDO1 before and after Therapy
| TIL Infiltration Before | TIL Infiltration After | ||
|---|---|---|---|
| Moderate or severe | 15 | Moderate or severe | 16 |
| Less | 25 | Less | 24 |
| NA | 3 | NA | 3 |
| IDO1 before | IDO1 after | ||
| Yes | 17 | Yes | 14 |
| No | 25 | No | 28 |
| NA | 1 | NA | 1 |
| PDL1 (>1%) before | PDL1 (>1%) after | ||
| Yes | 7 | Yes | 15 |
| No | 32 | No | 25 |
| NA | 4 | NA | 3 |
| IDO1 and PDL1 positivity before | IDO1 and PDL1 positivity after | ||
| Double negative | 19 | Double negative | 17 |
| IDO1 positive | 13 | IDO1 positive | 7 |
| PDL1 positive | 3 | PDL1 positive | 8 |
| Double positive | 4 | Double positive | 7 |
Of the patients with only targeted therapy, 12 out of 18 (66.67%) showed consistent expression levels during treatment, 2 (11.11%) increased (Figure 1), and 4 (22.22%) decreased. With immunotherapy only, a decrease was observed in 2 out of 10 (25%), whereas 8 (75%) remained stable. Patients treated with both drugs (n = 6) remained stable (n = 5, 83.33%), and one patient (16.67%) showed increased IDO expression. In a subset of patients treated with other treatment types (n = 6), 1 (16.67%) increased, 2 (33.33%) decreased, and 3 (50%) stable cases were seen. Furthermore, one patient who received all treatment types remained stable.
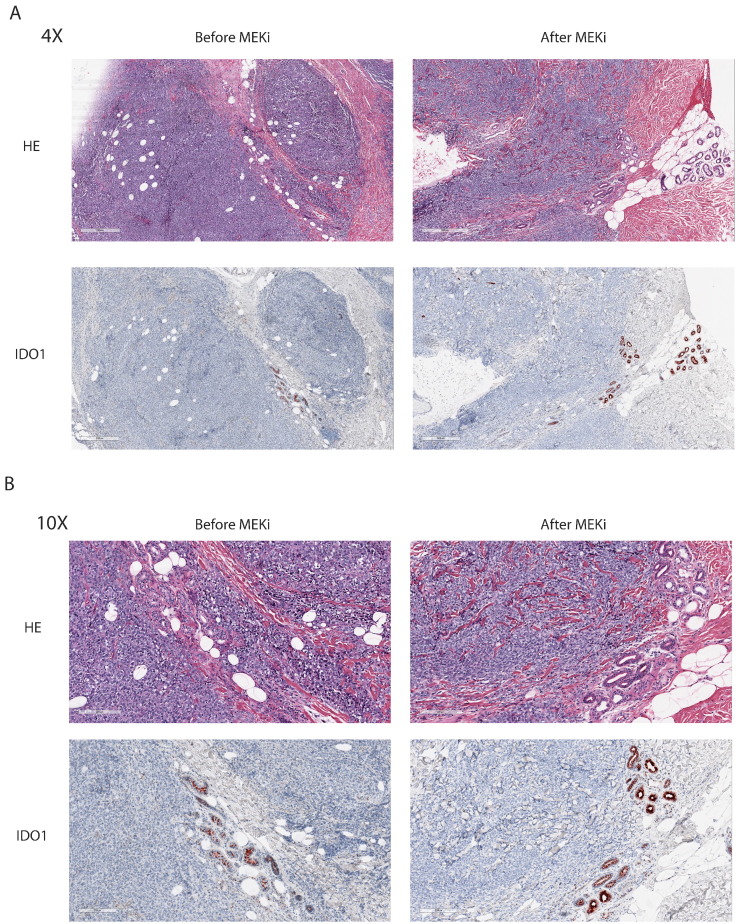
Figure 1.
Example of IDO1 expression increasing after targeted therapy.
(A) Histology of HE and IDO1 staining at 4× magnification of a patient before and after MEK inhibitor treatment. IDO1 staining was evaluated in the endothelial cells.
(B) Histology of HE and IDO1 staining at 10× magnification of a patient before and after MEK inhibitor treatment. IDO1 staining was evaluated in the endothelial cells.
Of 43 patients, we observed a decrease in IDO positivity under treatment in 8 (18.60%) patients, whereas we observed an increase in 5 (11.63%) of patients, while 30 (69.77%) patients displayed constant IDO status.
TILs
Out of 18 patients treated with targeted therapy only, 7 (38.89%) patients display increasing TILs.
No increase in TILs was observed in 9 (50%), and 2 (11.11%) were not amenable to complete evaluation. Ten patients were treated with only immunotherapies, and increasing TILs are seen in 4 (40%) patients versus 5 (50%) patients with stable infiltration, and 1 patient that could not be completely evaluated. In 7 patients with different regimens including other treatment types than targeted and immunotherapy, 3 (42.86%) patients display an increase in staining. Increasing tumor infiltrating lymphocytes are observed in 1 (16.67%) out of 6 patients treated with both immunotherapy and targeted therapy (Table 2).
PDL1
At baseline, PDL1 is expressed on more than 1% of tumor cells in 7 (16.28%) versus 15 (34.88%) samples. Four samples before and 3 samples after treatment were not evaluable; 32 (74.42%) pretreatment and 25 (58.14%) posttreatment display less than 1% of tumor cells expressing PDL1.
Patients treated with only immunotherapy (n = 10) remained mostly stable (70%), with 1 (10%) increase and 2 patients not amenable to complete evaluation (Figure 2A). Out of 18 patients with only targeted therapy, 10 (55.56%) displayed stable expression, 6 (33.33%) increased, and 2 were not amenable to complete evaluation (Figures 2B and 3).
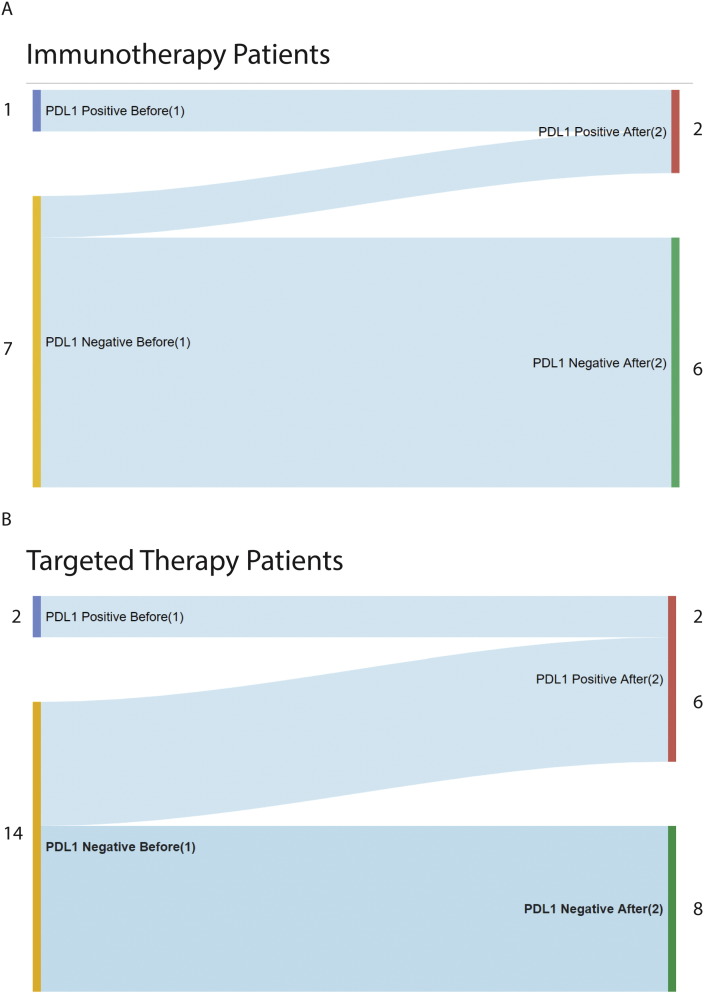
Figure 2.
The evolution of PDL1 immunoreactivity in IT (A) versus TT (B) patients demonstrating more increases in the TT population.
(A) Sankey plot showing changes of PDL1 expression levels in patients treated with immunotherapy.
(B) Sankey plot showing changes of PDL1 expression levels in patients treated with targeted therapy.
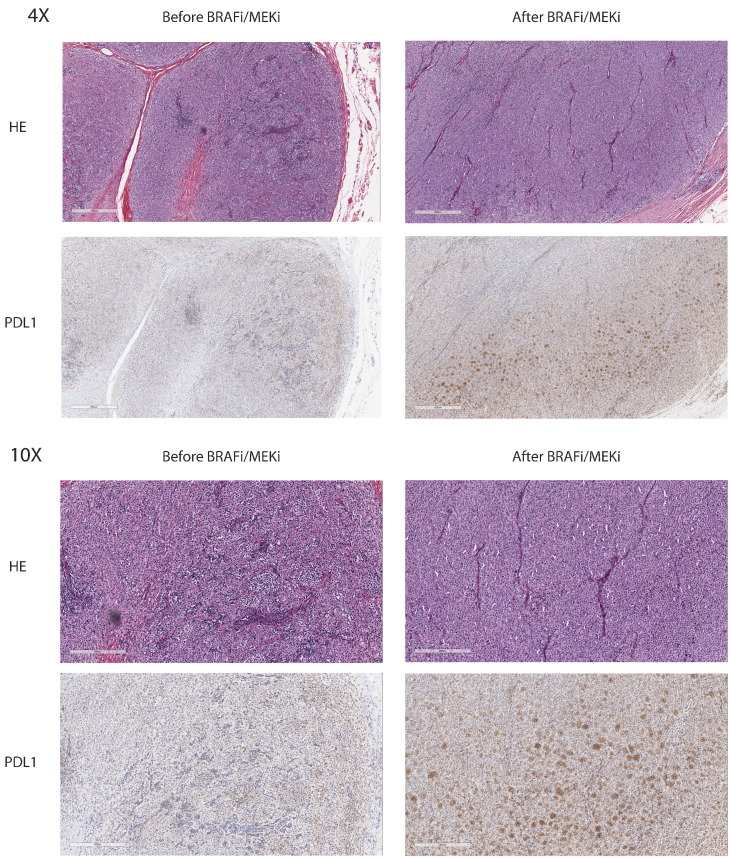
Figure 3.
Example of PDL1 expression increasing after targeted therapy.
(A) Histology of HE and PDL1 staining at 4× magnification of a patient before and after combination BRAF and MEK inhibitor treatment. PDL1 staining was evaluated in the tumor cells.
(B) Histology of HE and PDL1 staining at 10× magnification of a patient before and after combination BRAF and MEK inhibitor treatment. PDL1 staining was evaluated in the tumor cells.
After both targeted and immunotherapy (n = 6), an increase was observed in 1 (16.66%) case, 4 (66.66%) remained stable, and 1 (16.66%) was not amenable to evaluation. In 9 patients treated also with other treatment types, an increase was seen in 1 (11.11%) patient, decreases were seen in 2 (22.22%), 5 (55.56%) remained stable, and 1 (11.11%) patient was not amenable to complete evaluation (Table 2).
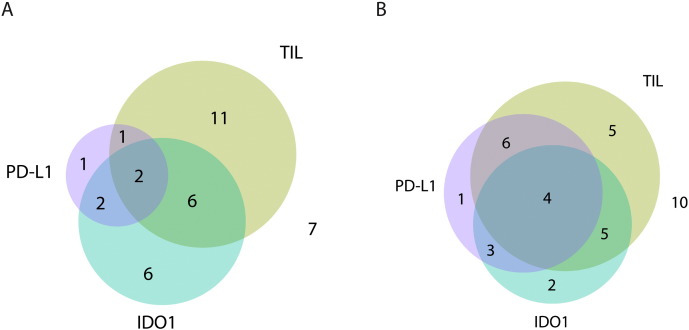
Coexpression of IDO1, PDL1, and TILs
Investigating IDO, PDL1, and TIL coexpression before treatment, 2 (5.56%) were triple positive, 2 (5.56%) were IDO1 and PDL1 double positive, 1 (2.78%) was PD-L1 and CD3 double positive, 6 (16.67%) were CD3 and IDO1 double positive, 1 (2.78%) was only PDL1 positive, 6 (16.67%) were only IDO1 positive, 11 (30.56%) were only CD3 positive, and 7 (19.44%) were negative for all markers (Figure 4A). After treatment, 4 (11.11%) were triple positive, 3 (8.33%) were IDO1 and PDL1 double positive, 6 (16.67%) were PD-L1 and CD3 double positive, 5 (13.89%) were CD3 and IDO1 double positive, 1 (2.78%) was only PDL1 positive, 2 (5.56%) were only IDO1 positive, 5 (13.89%) were only CD3 positive, and 10 (27.78%) were negative for all markers (Figure 4B).
Figure 4.
The relationship of TIL infiltration and PDL1 and IDO immunoreactivity before (A) and after therapy (B) demonstrating more overlap after therapy.
(A) Venn diagram showing the overlap in positive staining for CD3, PDL1, and IDO1 before treatment.
(B) Venn diagram showing the overlap in positive staining for CD3, PDL1, and IDO1 after treatment.
In Silico Analysis of the TCGA Data Set
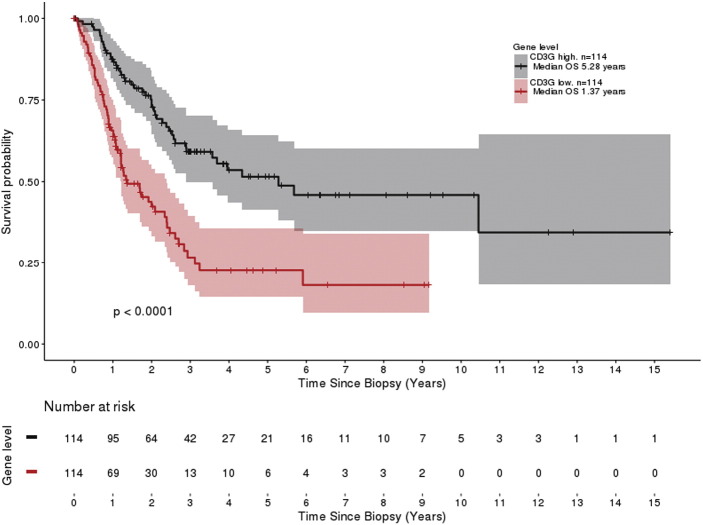
As our patient cohort was quite small, we used the TCGA to determine if significant differences in the overall survival of patients were associated with the expression of every T-cell specific gene assessed. At 25% expression levels which include 114 patients in each cohort, these include CD3G (5.28 vs 1.37 years, P < .0001) (Figure 5), CD8A (5.54 vs 1.34 years, P < .0001), CTLA4 (5.68 vs 1.7 years, P > .0001), CD274 (10.45 vs 1.29 years, P < .0001), PDCD1 (5.68 vs 1.28 years, P < .0001), and even IDO1 (5.54 vs 1.69 years, P < .0001).
Figure 5.
Example of survival curve from the TCGA database: CD3G (TIL).
Kaplan-Meier plot showing the overall survival difference between patients in the upper quartile versus the lower quartile in CD3G expression.
In the melanoma control groups, we tested various genes that are commonly overexpressed in melanoma cells including MLANA, CDKN2B, BRAF, NRAS, and MITF, all of which did not demonstrate any significant difference in median survival. However, for S100B, a significant (P = .014) difference of median survival of 3.19 years in the top 25% expression versus 1.87 years in the bottom 25% expression group was found.
When looking at PDGFRA and VIM, which are commonly expressed in fibroblasts, no survival difference was observed.
Discussion
Immunotherapeutic strategies are evolving rapidly. Within the next years, several treatment options including anti-CTLA4, PD1, PDL1, Lag-3, IDO inhibitors, and others may be available. Anti-PD1 therapy today is the backbone of IT. Our data for the first time demonstrate a clinical relevance of an immune marker assessed by immunohistochemistry. Based on ongoing translational research, other biomarkers appear promising such as LAG 3 expression [20], IDO expression, or genetic information (eg, mutational burden).
In the near future, it will be challenging to rationally establish treatment sequences of combinations of drugs. Biomarkers should be able to support clinical decision making and ideally should be the basis for precision immunotherapy. First steps in this direction include the immunoscore approach, PDL1 immunoreactivity, and immune signatures [21].
However, there is still limited information concerning the longitudinal consistency of immune markers such as PDL1 and the correlation with other immunological features such as the intratumoral presence of T-cells and the expression of IDO1.
Here we present the results of a comprehensive investigation of immunorelevant tissue markers that might be important for immunotherapy of metastatic melanoma. We have focused on patients with multiple biopsies and, in addition, used expression data of TCGA to describe the relevance of several immune markers in an independent data set.
The analyses of the TCGA have shown that all T-cell specific genes such as CD3, CD8, CTLA4, CD274, PD-1, and IDO1 are expressed at higher levels in patients with a prolonged overall survival. This means that any molecule analyzed that is associated with the presence of T-cells implies a better outcome, which is consistent with the published literature about the association of TILs with good prognosis.
We investigated metastatic tissues of 43 melanoma patients, which allowed us to compare both pre- and posttreatment lesions.
We were interested in staining for IDO1, the presence of T-cells infiltrates, and PDL1. Concerning TILs, we were especially interested in the CD3+, CD8+ T-cell population. This population may be the most important effector population during regression of melanoma metastasis [22].
IDO expression was analyzed on endothelial cells because expression at this site was reported to be of prognostic relevance in melanoma [10].
Our investigation revealed a number of interesting and highly relevant results. First, there is considerable inconsistency in the intraindividual and interindividual expression levels of the immune markers investigated with a limited overlap before therapy that evolves into high overlap after treatment. The type of treatment does not uniformly govern the immune marker expression. However, targeted therapy seems to upregulate PDL-1 more consistently than IT, which might favor sequential TT-IT or concomitant therapies.
Since IDO1 und PDL1 are both regulated by interferon gamma, we were especially interested in the frequency of biopsies that showed divergent expression. Actually, we were able to show that a number of biopsies show expression of a single marker only. One might speculate that the simultaneous presence of both markers might be predictive for a high response rate using IDO inhibitors and anti-PD1 combo treatments.
In conclusion, we demonstrate that simultaneous immunohistochemistry for IDO1 and PDL1 and the presence of TILs identify patients with various immune marker combinations. Theoretically, this investigation might help to select patients for specific IT approaches. The repeated use of these immune parameters together with others, for example, LAG-3 expression, will be helpful to develop precision immunotherapy in the near future [23]. Since IDO is the target for several new medications in clinical trials today, IDO expression on endothelial cells must be studied as a biomarker in the context of these clinical trials.
Conflicts of Interest
R. D. has intermittent, project-focused consulting and/or advisory relationships with Novartis, Merck Sharp & Dhome (MSD), Bristol-Myers Squibb (BMS), Roche, Amgen, Takeda, and Pierre Fabre outside the submitted work.
J. M. has temporary consultant or advisory relationships (Merck/Pfizer) and receives travel support from Merck Sharp & Dohme.
S. M. G. has intermittent advisory board relationships and travel grant support from BMS, MSD, Roche, and Novartis.
C. H. is an employee and owns stock with BMS.
L. K., K. K., I. C., L. B., M. L., and P. F. C. have no conflicts of interest to declare.
Acknowledgements
We thank Ines Kleiber and the staff of Mosaic Laboratories for their support with preparing and staining of histology slides.
Footnotes
This project was partially supported by the URPP and a research grant of BMS (Nos. CA209-109 BMS018 and CA184-496).
Contributor Information
Reinhard Dummer, Email: reinhard.dummer@usz.ch.
Phil F Cheng, Email: phil.cheng@usz.ch.
References
- 1.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1271. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dummer R, Hauschild A, Guggenheim M, Jost L, Pentheroudakis G. Group EGW, Melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl. 5):v194–7. doi: 10.1093/annonc/mdq188. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 4.Hoos A. Development of immuno-oncology drugs [mdash] from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2015;26:v126–v132. doi: 10.1093/annonc/mds229. [DOI] [PubMed] [Google Scholar]
- 7.Atsaves V, Tsesmetzis N, Chioureas D, Kis L, Leventaki V, Drakos E, Panaretakis T., Grander D., Medeiros L.J., Young K.H., Rassidakis G.Z. PD-L1 is commonly expressed and transcriptionally regulated by STAT3 and MYC in ALK-negative anaplastic large-cell lymphoma. Leukemia. 2017;31:1633–1637. doi: 10.1038/leu.2017.103. [DOI] [PubMed] [Google Scholar]
- 8.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T<sub>regs</sub> in the melanoma tumor microenvironment is driven by CD8<sup>+</sup> T Cells. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006504. [200ra116-200ra116] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer. 2017;76:167–182. doi: 10.1016/j.ejca.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Chevolet I, Speeckaert R, Haspeslagh M, Neyns B, Krüse V, Schreuer M, Van Gele M., Van Geel N., Brochez L. Peri-tumoral indoleamine 2,3-dioxygenase expression in melanoma: an early marker of resistance to immune control? Br J Dermatol. 2014;171(5):987–995. doi: 10.1111/bjd.13100. [DOI] [PubMed] [Google Scholar]
- 11.Speeckaert R, Vermaelen K, van Geel N, Autier P, Lambert J, Haspeslagh M, van Gele M., Thielemans K., Neyns B., Roche N. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. Eur J Cancer. 2012;48:2004–2011. doi: 10.1016/j.ejca.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. 2007;214:8–14. doi: 10.1159/000096906. [DOI] [PubMed] [Google Scholar]
- 13.Gangadhar TC, Schneider BJ, Bauer TM, Wasser JS, Spira AI, Patel SP, Balmanoukian AS, Bauml J, Schmidt EV, Zhao Y. American Society of Clinical Oncology; 2017. Efficacy and safety of epacadostat plus pembrolizumab treatment of NSCLC: preliminary phase I/II results of ECHO-202/KEYNOTE-037. [Google Scholar]
- 14.Hamid O, Bauer TM, Spira AI, Smith DC, Olszanski AJ, Tarhini AA, Lara P, Gajewski T, Wasser JS, Patel SP. Epacadostat plus pemborlizumab in patients with advanced melanoma: phase 1 and 2 efficacy and safety results from ECHO-202/KEYNOTE-037. ESMO. Madrid. Ann Oncol. 2017;28(5):v428–v448. [Google Scholar]
- 15.Cheng PF, Dummer R, Levesque MP. Data mining The Cancer Genome Atlas in the era of precision cancer medicine. Swiss Med Wkly. 2015;145:w14183. doi: 10.4414/smw.2015.14183. [DOI] [PubMed] [Google Scholar]
- 16.Genomic classification of cutaneous melanomaCell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips T, Simmons P, Inzunza HD, Cogswell J, Novotny J, Jr., Taylor C, Zhang X. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non–small cell lung cancer. Appl Immunohistochem Mol Morphol. 2015;23:541–549. doi: 10.1097/PAI.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richard K, Antoni R, Hamid O, Caroline R, Adil D, Wolchok JD, Joshua AM, Hodi FS, Gangadhar TC, Hersey P. Clinical efficacy and correlation with tumor PD-L1 expression in patients (pts) with melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol. 2014;32:3005. [Google Scholar]
- 20.Ascierto PA, Bono P, Bhatia S, Melero I, Nyakas MS, Svane I, Larkin J, Gomez-Roca C, Schadendorf D, Dummer R. ESMO; Madrid: 2017. Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma. [Google Scholar]
- 21.Ascierto P, Capone M, Urba W, Bifulco C, Botti G, Lugli A, Marincola F, Ciliberto G, Galon J, Fox B. The additional facet of immunoscore: immunoprofiling as a possible predictive tool for cancer treatment. J Transl Med. 2013;11:54. doi: 10.1186/1479-5876-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, Rosenblum M, Harview CL, Taube JM, Handley N. Liver metastasis and treatment outcome with anti–PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blank CU, Haanen JB, Ribas A, Schumacher TN. The “cancer immunogram”. Science. 2016;352:658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]