Abstract
Risk stratification using molecular features could potentially help distinguish indolent from aggressive prostate cancer (PCa). Mutations in isocitrate dehydrogenase (IDH) acquire an abnormal enzymatic activity, resulting in the production of 2-hydroxyglutarate and alterations in cellular metabolism, histone modification, and DNA methylation. Mutant IDH1 has been identified in various human malignancies, and IDH1R132H constituted the vast majority of mutational events of IDH1. Most recent studies suggested that IDH1 mutations define a methylator subtype in PCa. However, the function of IDH1R132H in PCa development and progression is largely unknown. In this study, we showed that the prevalence of IDH1R132H in Chinese PCa patients is 0.6% (2/336). Of note, IDH1R132H-mutant PCa patients lacked other canonical genomic lesions (e.g., ERG rearrangement, PTEN deletion) that are common in most other PCa patients. The in vitro experiment suggested that IDH1R132H can promote proliferation of benign prostate epithelial cell RWPE-1 when under the situation of low cytokine. It could also promote migration capacity of RWPE-1 cells. Mechanistically, IDH1R132H was an important regulator of insulin-like growth factor 1receptor (IGF1R) by downregulating a set of microRNAs (miR-141-3p, miR-7-5p, miR-223-3p). These microRNAs were repressed by the alteration of epigenetic modification to decrease the enrichment of active marker H3K4me3 or to increase repressive marker H3K27me3 at their promoters. Collectively, we proposed a novel model for an IDH1R132H-microRNAs-IGF1R regulatory axis, which might provide insight into the function of IDH1R132H in PCa development.
Introduction
Prostate cancer (PCa) is the second leading malignancy in males and the fourth most common tumor type worldwide [1]. Currently, the established prognostic factors, Gleason score, pathological stage, and serum prostate-specific antigen (PSA), cannot precisely distinguish clinically aggressive PCas from clinically indolent ones [2], [3]. To meet this challenge, a better classification of the disease based on the underlying molecular features would be especially important in PCa. Several recent studies have explored the molecular basis of primary PCa and identified multiple recurrent genomic alterations, including mutations, DNA copy-number changes, rearrangements, and gene fusions [2].
Isocitrate dehydrogenases (IDHs) catalyze a redox reaction that converts isocitrate to α-ketoglutarate while reducing NADP to NADPH and liberating CO2. Mutations in IDHs have been identified in many human malignancies [4]. IDH1 mutations can cause alterations in cellular metabolism, histone modification, and DNA methylation [5]. Most recently, The Cancer Genome Atlas Research Network revealed a molecular taxonomy of PCa in which 74% of these tumors fell into one of seven subtypes defined by specific gene fusions (ERG, ETV1/4, and FLI1) or mutations (SPOP, FOXA1, and IDH1). Although the prevalence is low, IDH1 mutations may represent a methylator subtype in PCa. Interestingly, IDH1-mutant PCa patients seemed to possess fewer other common canonical genomic lesions in PCa [3]. To date, the exact biological role of IDH1 mutations has not been investigated in PCa so far.
Insulin-like growth factors 1 and 2 (IGFs) are proteins produced by the liver inducing cell proliferation, survival, and migration in many cell types [6]. IGF1R is the receptor of IGFs. The dysregulated expression of IGF1R has been described in many human malignancies [7]. IGF1R is often overexpressed in PCa, and it associates with carcinogenesis, proliferation, and migration of PCa [8], [9]. Targeting the IGF axis receptors showed promising antitumor effects in preclinical studies of PCa treatment [10].
MicroRNAs (miRNAs) are conserved small noncoding RNAs that act as posttranscriptional regulators of gene expression. Increasing evidence has shown that miRNAs play an important role in PCa progression [11]. Some studies suggested that IGF1R can be regulated by miRNAs [12], [13], [14]. Here we show that IDH1R132H mediates the suppression of miRNAs (miR-141-3p, miR-7-5p, miR-223-3p), leading to the upregulation of IGF1R which may promote malignant transformation of benign prostatic epithelium. This is the first time to systematically analyze the function of miRNAs in mutant IDH1 cells.
Material and Methods
Patients
A total of 336 paraffin-embedded tissues were retrieved from PCa patients with radical prostatectomy between 2001 and 2013 at Qilu Hospital of Shandong University (Jinan, China), Shandong Provincial Hospital (Jinan, China), General Hospital of Linyi (Linyi, China), and the Affiliated Hospital of Medical College Qingdao University (Qingdao, China). None of the patients received preoperative radiation or androgen deprivation therapy. A total of four tissue microarrays were constructed by incorporating two 1-mm cores from each representative tumor. The diagnosis was confirmed by three pathologists (B.H., M.Q., and J.H.). This study was approved by Institutional Review Board of Medical School of Shandong University (Jinan, China). Informed written consent was obtained from each patient.
Immunohistochemistry (IHC)
IHC was performed as previously described [15]. Briefly, the sections were incubated overnight with IDH1R132H MAb (DIA H09M) (Dianova; Hamburg, Germany), at a 1:400 dilution at 4°C and then evaluated blindly by two independent observers (B.H. and M.Q.). Cytoplasmic and nuclear immunostaining was scored into four grades (0, negative; 1-3, weak; 4-6, moderate; and 8-12, strong) based on its staining intensity (0, 1+, 2+, and 3+) and percentage of positive cells for each tumor [0 (0%), 1 (1%-25%), 2 (26%-50%), 3 (51%-75%), and 4 (76%-100%)] [16].
Mutational Analysis
IDH1R132H mutation was studied by polymerase chain reaction (PCR)–based sequencing. DNA was isolated from slices gained from formalin-fixed, paraffin-embedded (FFPE) PCa specimens using QIAamp DNA FFPE Tissue Kit (Qiagen, Germany) according to manufacturer's instructions. DNA from cell lines was extracted with the Puregene Cell and Tissue Kit (Qiagen, Germany). IDH1 mutation was analyzed by PCR using forward primer 5′-ACCAAATGGCACCATACGA-3′ and reverse primer 5′-TTCATACCTTGCTTAATGGGTGT-3′. The product was sequenced by standard technique using primer 5′-CGGTCTTCAGAGAAGCCATT-3′ [17].
Cell Culture and Reagents
RWPE-1, LNCaP, VCAP, and 22RV1 cell lines were kindly provided by Professor Huiqing Yuan (Department of Biochemistry, Shandong University). Cells were all maintained at 37°C with 5% CO2. LNCaP, VCAP, and 22RV1 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum. Nonneoplastic, immortalized human prostatic epithelial RWPE-1 cells were cultured in defined Keratinocyte-SFM (Gibco, 17005-042) supplemented with recombinant epidermal growth factor (EGF) and bovine pituitary extract (BPE) (Gibco). AGI-5198 (Xcessbio, San Diego, CA) was made by dissolving the compound in DMSO to 10 mM as stock. The cells were treated with TGF-β1 (10 ng/ml, RD Biosciences, CA, USA) for 72 hours. Medium was changed every 24 hours.
Plasmid Constructs and Lentivirus (LV)
Human wild-type IDH1 cDNA (accession# NM_005896) and mutant IDH1 cDNA [NM_005896 (R132H)] were subcloned into GV358 vector (Genechem, Shanghai, China). Lentiviral vectors encoding mutant IDH1R132H (LV-GFP-IDH1R132H), wild-type IDH1 (LV-GFP-IDH1WT), and an empty vector control (LV-GFP-VECTOR) were synthesized by Genechem. Following lentiviral infection, 2 μg/ml of puromycin was used for selecting stable expressed cell lines.
RNA Isolation and Quantitative Real-Time (qRT)-PCR Assay
Total RNA was isolated with TRIzol (Invitrogen, CA) and reverse-transcribed into cDNA by using ReverTra Ace qPCR RT kit (TOYOBO, Japan). qRT-PCR assay was carried out by using FastStart Universal SYBR Green Master (Roche, USA) on C1000TM RT-PCR system (BioRad, USA) according to manufacturer's instructions. GAPDH was used as an internal loading control for mRNA and pri-miRNA. Pre-U6 was used as an endogenous control for pre-miRNA. For detecting mature miRNA, qRT-PCR assay was performed using All-in-One miRNA qRT-PCR Detection Kit (GeneCopoeia, USA). RNU48 was used as an internal control. The primers of MiRNAs precursors were according to previous study [18]. The details of other primers were listed in Table S1.
Western Blot
Western blot analysis was performed as previously described [19]. Primary antibodies used were anti-IDH1R132H (DIA H09M, 1:800, Dianova), anti-IDH1 (#3997, 1:1000, CST), anti-IGF1R (ab39675, 1:1000, abcam), anti-AKT (1:1000; CST), anti-pAKT (Ser473, 1:1000; CST), anti-STAT3 (1:1000, CST), anti-pSTAT3 (Tyr705, 1:1000, CST), and anti-GAPDH (1:1000, Santa Cruz). Three independent experiments were performed.
Transient Transfection
The transfections of miRNA mimic, miRNA inhibitor, siRNA, and their corresponding control (GenePharma) were carried out with Hiperfect transfection reagent (Qiagen) following the manufacturer's instructions. The detail sequences were shown in Supplementary Table S2, Supplementary Table S3.
Immunofluorescence
Immunofluorescence was preformed according to the previous methods [20]. Cells grown on glass slides (NEST, China) were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS, and then incubated with the primary antibody anti-IGF1R (1:100, abcam) at 4°C overnight. After being washed in PBS and incubated for 1 hour at 37°C with fluorescence-labeled secondary antibody Alexa Fluor-594 (1:1000, Proteintech, Chicago, USA), the cells were then mounted with prolong gold antifade reagent with DAPI (Invitrogen) for 10 minutes.
MTS Assay
LNCaP and RWPE-1 cells (3000 and 5000 cells per well, respectively) were seeded in 96-well plates in 3 replicates, and proliferation was measured at 24-hour intervals by MTS assay (Promega, Madison, WI).
Transwell and Wound-Healing Assay
Wound-healing experiments were performed as previously described [21]. The average width of the remaining wounded gaps was measured every 24 hours. Transwell assay was performed in 24-well chemotaxis chambers (8-μm pores) (Costar/Corning, Lowell, MA). LNCaP (5 × 104 cells) or RWPE-1 cells (1 × 105 cells) were loaded into the top of each chamber in 200 μl of serum-free RPMI-1640 or Keratinocyte-SFM without EGF and BPE. The lower chamber was filled with 600 μl of RPMI-1640 containing 10% FBS for LNCaP, or Keratinocyte-SFM with EGF and BPE for RWPE-1 cells. After 48 hours, cells migrated through pores to the lower surface were fixed with 4% paraformaldehyde for 10 minutes and stained with crystal violet (Beyotime). Cells were counted in five randomly selected microscopic fields.
Dual Luciferase Assay
Dual luciferase assay was performed as previously described [22]. The normal and mutant potential recognizing regions of miR-141-3p, miR-7-5p, and miR-223-3pin 3′UTR region of IGF1R were, respectively, subcloned into pmirGLO vector (GenePharma, China). HEK293T cells were transiently transfected with reporter constructs together with miRNA mimics and the corresponding control using Tuberfect transfection reagent (Thermo). Cells were lysed at 48 hours after transfection. Dual luciferase assay reporter system was carried out according to the manufacturer's instructions (Promega).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was carried out according to previous description [19]. RWPE-1 cells expressing IDH1R132H or IDH1WT or VECTOR were fixed and immunoprecipitated using the EZ-Magna ChIP assay kit as recommended by the manufacturer (Millipore, Billerica, MD). Antibodies used to immunoprecipitate purified chromatin were validated antibody-Antitrimethyl-Histone H3 (Lys27) (07-622, Millipore) and Antitrimethyl-Histone H3 (Lys4) (07-614, Millipore). Primers used to amplify promoter region of miRNAs were shown in Table S1.
Tumor Xenografts
For the tumor xenograft assays, male athymic nude mice (nu/nu; 4 weeks old) were purchased from Weitonglihua Biotechnology (Beijing) and maintained in pathogen-free environment. A total of 2 × 106 LNCaP cells infected with IDH1R132H, IDH1WT, or empty vector were resuspended in 200 μl of serum-free RPMI-1640, mixed the same volumes of Matrigel (1:1) on ice bath for each mouse, and inoculated subcutaneously into two flanks of each nude mice (n = 6 per group). The tumor volume was calculated using the formula: length × (width)2 × 1/2. The experimental protocol was approved by the Shandong University Animal Care Committee.
mRNA Array Bioinformatics Analysis
MRNA expression of RWPE-1 cells expressing IDH1R132H and VECTOR was evaluated using Agilent Human 4x44K Gene Expression Microarrays v2 (KangCheng, Shanghai, China). MiRNA target analysis was carried out by TargetScan software (http://www.targetscan.org).
Gene Set Enrichment Analysis (GSEA)
GSEA was carried out according to standard procedure. Signatures for IGF1R upregulated were derived from GSE5225 (genes with P < .05, fold change > 2) and further analyzed by GSEA in the data set of differentially expressed mRNA of IDH1R132H and VECTOR in RWPE-1 cells.
Statistics
Statistical analysis was carried out using SPSS 20.0 software. Two-sided Student's t test was used for two groups; one-way analysis of variance is used for statistical comparisons of three or more groups. P < .05 was considered statistically significant.
Results
Mutational Analysis of IDH1R132H in PCa Patients
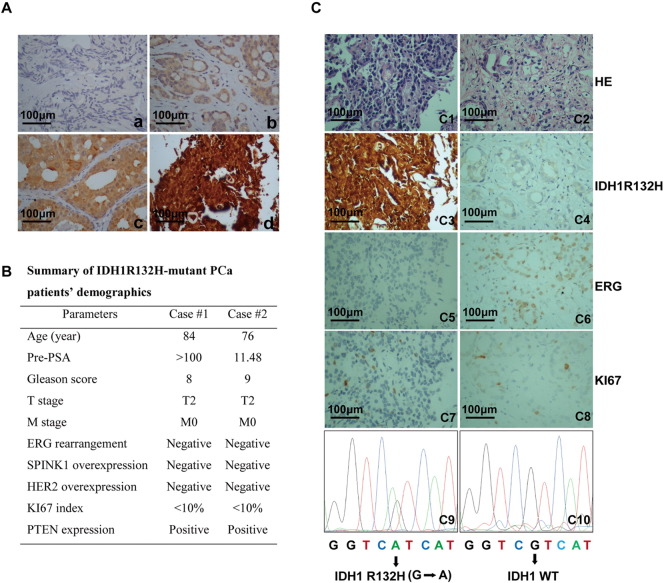
In addition to tumors of the central nervous system and leukemia, IDH1 mutations have been identified at low incidence rate in other solid malignancies [23]. In the current study, we first performed immunohistochemical staining of IDH1R132H on PCa patients. A total of four tissue microarrays including 336 PCa cases were constructed according to the protocols and quantification methods reported before [16], [24]. In all, 15 PCa patients were immunopositive for IDH1R132H. Among them, 11 showed weak scale, and 4 were moderate or strong scale for IDH1R132H. Representative IHC images of IDH1R132H staining intensity were shown in Figure 1A. To further characterize the IDH1 gene status, IDH1R132H IHC-positive PCa samples were further examined by PCR-based sequencing. In total, 2/336(0.6%) PCa samples were confirmed IDH1R132H (G to A) positive (Figure 1C). To avoid false-negative rate of this antibody, we also sequenced 90 IHC-negative patients for IDH1 mutations. No IDH1R132H mutation was found in these IHC-negative patients. Of note, for IDH1R132H mutant–positive cases, no ERG rearrangement or SPINK1 overexpression was identified. Both HER2 overexpression and PTEN deletion were negative, and KI67 index was less than 10% (Figure 1B). Representative IHC images of IDH1R132H-positive and -negative patients were shown in Figure 1C. Besides, the mutations of PCa cell lines RWPE-1, LNCaP, VCAP, and 22RV1 were also tested. No IDH1 mutations were found in these cell lines.
Figure 1.
Mutational analysis of IDH1R132H in PCa patients. (A) Representative IHC images were shown in a-d. Sections were stained with antibody against IDH1R132H in PCa patients (n = 336). Staining intensity was classified into four scales [a: negative staining (0+); b: weak staining (1+); c: moderate staining (2+); d: strong staining(3+)]. (B) Clinicopathological characteristic of IDH1R132H-positive PCa patients. The status of ERG rearrangement, SPINK1 overexpression, HER2 overexpression, PTEN deletion, and KI67 index were performed in our early studies. (C) Representative images of molecular pathological features of IDH1R132H-positive and -negative cases. For IHC, the slides were incubated with IDH1R132H antibody (c3, c4). The IHC of ERG and KI67 (c5-c8) and hematoxylin-eosin (HE) staining (c1, c2) were performed in our previous research. PCR-based gene sequencing of PCa patients. G to A mutation confirmed IDH1R132H mutation (c9, c10). Scale bar, 100 μm.
The Promotion of Malignant Transformation of Benign Prostatic Epithelium by IDH1R132H
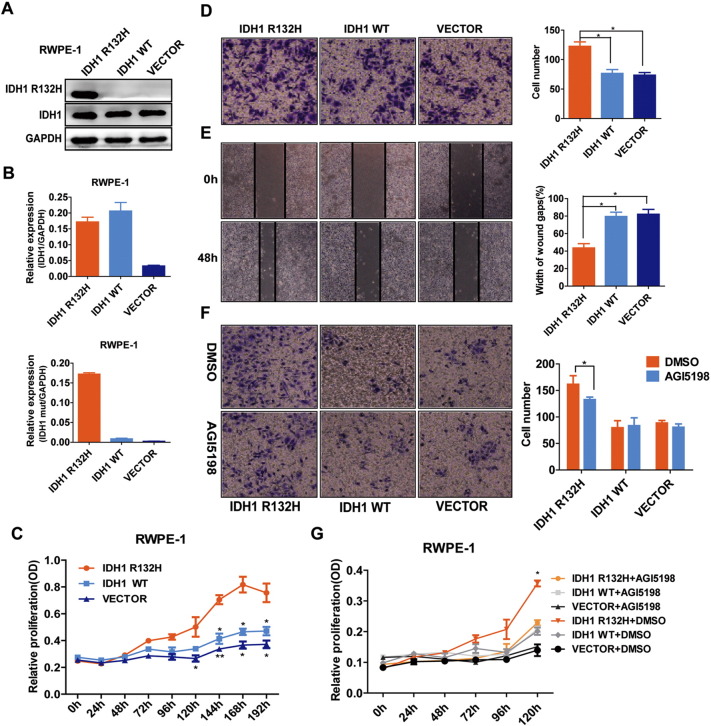
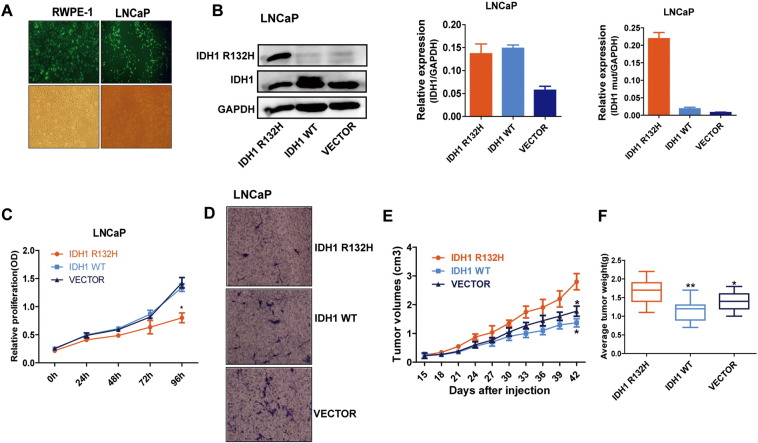
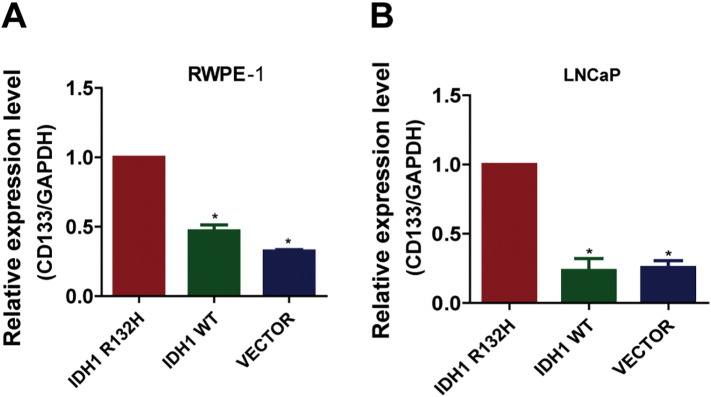
At present, there are still controversial arguments about the functions of IDH1 mutations [25], [26], [27]. To further characterize the role of IDH1R132H in PCa development, we stably transfected the nontransformed prostate benign epithelial cell RWPE-1 and PCa cell LNCaP with lentiviral vectors encoding GFP-tagged mutant IDH1 (IDH1R132H), wild-type IDH1 (IDH1WT), or empty vector (VECTOR). The mRNA and protein expression levels of IDH1R132H were verified by qRT-PCR and Western blot (Figures 2, A and B and S1, A and B). As shown in Figure 2C, in the absence of cytokines (1/4 normal-dose EGF and BPE), the growth rate of IDH1WT and VECTOR RWPE-1 cells was gradually sluggish, but RWPE-1 cells expressing IDH1R132H became cytokine independent and had significant growth advantage. In addition, wound-healing and Transwell assays demonstrated that IDH1R132H can promote migration of RWPE-1cells (Figure 2, D and E). These data suggested that IDH1R132H might have the ability to induce malignant transform of benign prostate epithelial cells. We then evaluated the function of IDH1R132H in PCa cell line. However, different from benign prostatic epithelial cells, the capacities of proliferation and migration were decreased in IDH1R132H-mutant LNCaP cells (Figure S1, C and D). By contrast, interestingly, IDH1R132H-mutant LNCaP cells grew more rapidly than IDH1WT and VECTOR LNCaP cells in vivo (Figure S1, E and F). Many studies showed that mutant IDH is associated with cell differentiation and stem cell features [28], [29]. Therefore, we next tentatively tested stem cell marker CD133 in IDH1R132H-expressed cells. qRT-PCR revealed that IDH1R132H could promote the expression of CD133 in both RWPE-1 and LNCAP cells (Figure S2, A and B).
Figure 2.
IDH1R132H promotes malignant transformation of prostate benign epithelial cell RWPE-1. (A, B) Western blot and qRT-PCR analysis of protein and mRNA expression levels of wild-type IDH1 and IDH1R132H in RWPE-1cells, following stably expressing empty vector, wild-type, or mutant IDH1R132H. (C) IDH1R132H enhances the cytokine-independent growth of benign prostatic epithelial cells. The growth of stable RWPE-1 cells expressing IDH1R132H, IDH1WT, and VECTOR was examined by MTS assay. Cells were cultured under cytokine-poor conditions (1/4 normal dose EGF and BPE). (D, E) IDH1R132H promotes migration of benign prostatic epithelial cells. The migration of IDH1R132H on RWPE-1 cells was examined by wound-healing assay and Transwell migration assay. (F, G) The growth and migration of RWPE-1 cells. Cells were treated with 20 μM AGI-5198 or DMSO and analyzed using MTS and Transwell migration assay. All data represent mean ± SD of at least three independent replicates. *P < .05, **P < .01.
Figure S1.
IDH1R132H decreased proliferation and migration in LNCAP cells. (A) The representative transfection efficiency of virus observation in RWPE-1 and LNCAP cells under fluorescence microscope. (B) The wild-type and mutant IDH1 protein and mRNA expression levels of LNCAP cells stably expressing IDH1R132H, wild-type, and empty vector were tested by Western blot and qRT-PCR. (C, D) MTS and Transwell migration assays were used to identify the abilities of proliferation and migration in IDH1R132H, IDH1WT, and VECTOR-expressing LNCAP cells. (E) Growth curves were used to test tumors formation of subcutaneous xenograft assay. (F) The weight of tumors was measured at harvest time (n = 6 per group). Data in C and D represent mean ± SD of at least three independent replicates. P values: *P < .05
Figure S2.
IDH1R132H promotes the expression of CD133. (A, B) qRT-PCR was performed to detect the expression of CD133 in RWPE-1 and LNCAP cells. All data represent mean ± SD of at least three independent replicates. P values: *P < .05
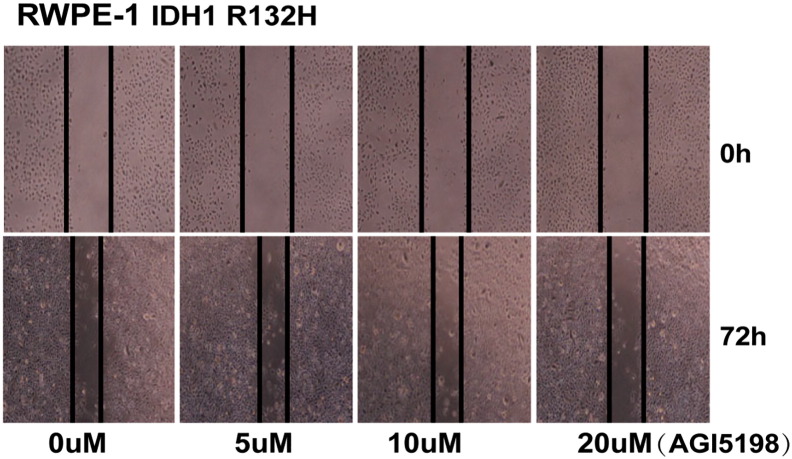
AGI-5198, a specific inhibitor of mutant IDH1R132H, attenuates D-2-hydroxyglutarate production, delays growth, and promotes differentiation of glioma [30]. We next sought to determine whether AGI-5198 would similarly inhibit carcinogenesis activity in IDH1-mutant benign prostate epithelial cells. IDH1R132H-mutant RWPE-1 cells were treated with increasing concentrations (0 to 20 μM) of AGI-5198 and analyzed using wound-healing assay after 72 hours of treatment. The result showed that 20 μM AGI-5198 significantly inhibited migration of IDH1R132H-mutant RWPE-1 cells (Figure S3). Effects of AGI-5198 on cell viability and migration capacity were assessed using MTS and Transwell assay. AGI-5198 treatment at 20 μM inhibited proliferation in the absence of cytokine and migration in IDH1R132H-expressing RWPE-1cells (Figure 2, G and F).
Figure S3.
Twenty micomolars of AGI5198 inhibits migration of IDH1R132H RWPE-1 cells. IDH1R132H-mutant RWPE-1 cells were treated with increasing concentrations of AGI-5198 and corresponding DMSO, and were analyzed by wound-healing assay after 72 hours of treatment. All data represent three independent replicates.
Downregulation of miR-141-3p/miR-7-5p/miR-223-3p by IDH1R132H in RWPE-1 Cells
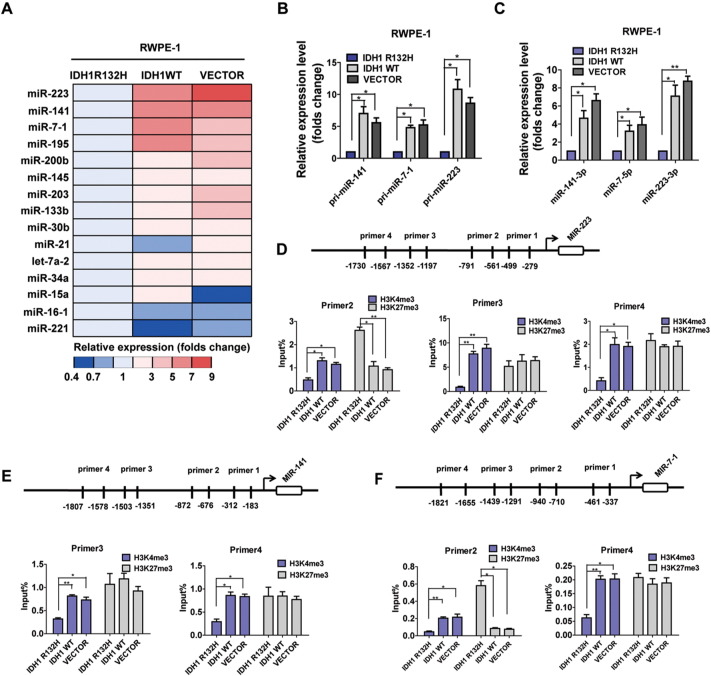
As miRNAs were increasingly recognized as important regulators of gene expression and other biological processes during the progression of PCa [11], [31], we asked whether alterations in miRNA expression were associated with IDH1R132H-induced malignant transformation in RWPE-1 cells. Based on the literature review, we chose a curated set of prostate-specific 15 miRNAs [11], [32], [33], [34], [35] and analyzed the expression of miRNAs precursors in RWPE-1 cells stably expressing IDH1R132H, IDH1WT, and VECTOR (Figure 3A). We selected miR-223, miR-141, and miR-7-1, the most evidently downregulated miRNAs with characterization of tumor suppressor, for conducting further experiment. Utilizing qRT-PCR analysis, we showed that the expressions of pri-miR-223, miR-141, and miR-7-1 and mature miR-141-3p, miR-7-5p, and miR-223-3p were all significantly decreased in IDH1R132H-mutant RWPE-1 cells (Figure 3, B and C).
Figure 3.
IDH1R132H downregulates miR-141-3p/miR-7-5p/miR-223-3p in RWPE-1 Cells. (A) qRT-PCR was used to assess the expression color heat map of miRNAs precursors in RWPE-1 cells which were transfected with IDH1R132H, IDH1WT, and VECTOR. The results are shown as folds number. (B, C) Expressions of primary precursor and mature miR-141-3p, miR-223-3p, and miR-7-5p were assessed by qRT-PCR in RWPE-1 cells. The results are shown as folds number. (D, E, F) ChIP-qPCR was performed to evaluate the enrichment of H3K4me3 and H3K27me3 in different promoter regions of miR-223, miR-141, and miR-7-1 in RWPE-1 cells. All data represent mean ± SD of at least three independent replicates. P values: *P < .05, **P < .01.
Mutations in IDH1 acquired a neomorphic activity that converts α-ketoglutarate to D-2-hydroxyglutarate, altering specific histone marks and inducing extensive DNA hypermethylation [5], [28], [36]. We hypothesized that the alternations of epigenetic modifications might contribute to the decrease of miRNAs, and the enrichments of active marker H3K4me3 and repressive marker H3K27me3 in promoters of the miRNAs were analyzed. Four pairs of primers (Table S1) were used for detecting possibly altered sites in each miRNA promoter (Figure 3, D-F). As shown in Figure 3, D-F, the enrichments of H3K4me3 were specifically reduced at the P3 and P4 promoter regions of miR-141; P2, P3, and P4 promoter regions of miR-223; and P2 and P4 promoter regions of miR-7-1 in IDH1R132H-mutant RWPE-1 cells. The enrichments of H3K27me3 were increased at P2 promoter of miR-223 and P2 promoter of miR-7-1 (Figure 3, D and F). Taken together, our data revealed that the alternations of histone modifications might contribute to the repression of miRNAs.
Increased IGF1R expression by IDH1R132H-Downregulated miRNAs
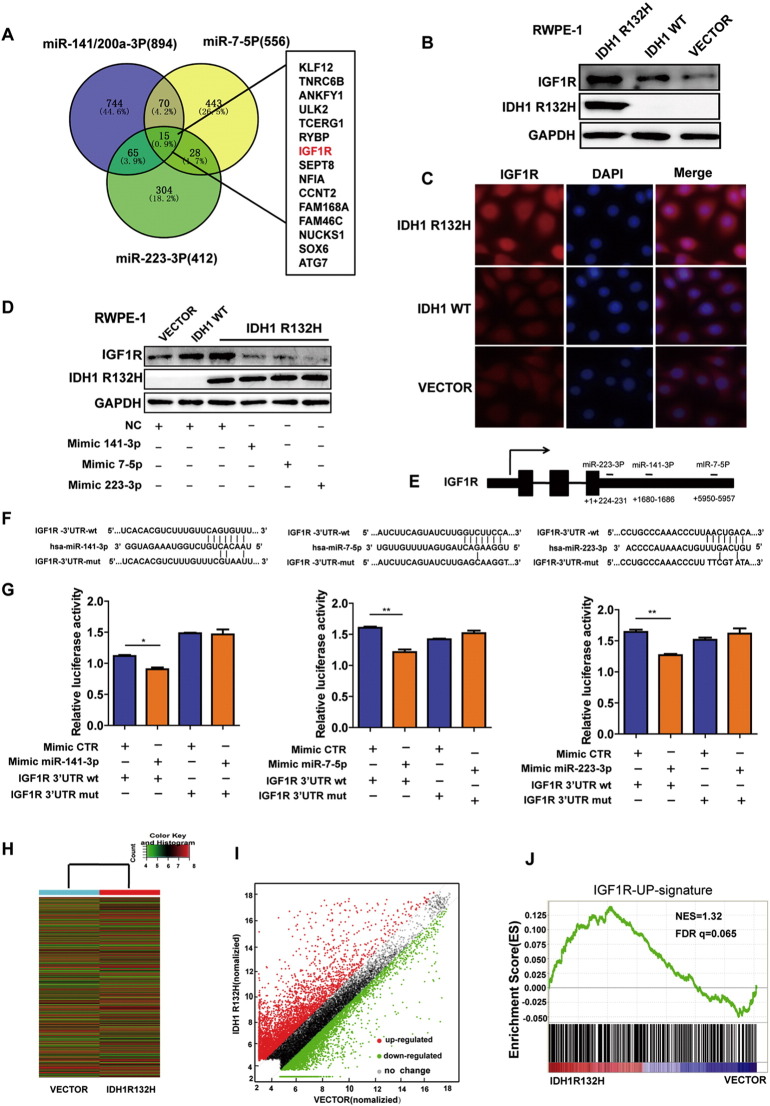
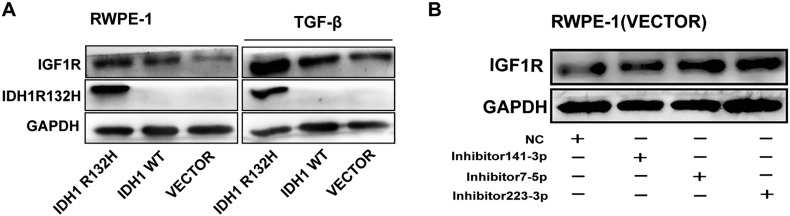
To further characterize the mechanism of miRNAs during RWPE-1 cell malignant transformation, we applied TargetScan prediction software (http://www.targetscan.org) to identify the potential targets of the miRNAs. A total of 15 genes are their common targets (Figure 4A), in which IGF1R was included. A variety of studies have elucidated that the IGF network is associated with the early stage of carcinogenesis and neoplasm growth and is a potential therapy target in PCa [8], [37]. To verify whether the expression IGF1R was actually regulated by IDH1R132H, we evaluated the protein levels of IGF1R in IDH1R132H, IDH1WT, and VECTOR RWPE-1 cells. Both Western blot and immunofluorescence showed that IDH1R132H enhanced IGF1R protein expression compared with IDH1WT and VECTOR (Figure 4, B and C), and the overexpression of IGF1R was more evident under the treatment of TGF-β (Figure S4A). To estimate whether IDH1R132H regulates IGF1R expression through inhibiting miRNAs, we screened the protein expression levels of IGF1R after inhibiting the endogenous and overexpressing exogenous miRNAs in VECTOR and IDH1R132H RWPE-1cells. Exogenous mimics of miR-141-3p, miR-7-5p, and miR-223-3p reduced IGF1R expression in protein level in IDH1R132H-mutant RWPE-1 cells (Figure 4D). By contrast, inhibitors of the miRNAs enhanced IGF1R protein level in VECTOR RWPE-1 cells (Figure S4B). To determine whether this effect was direct, we assayed luciferase (LUC) reporter gene expression in HEK293T cells co-transfected with a pGL3-promoter vector carrying the respective wild-type or mutant IGF1R 3′UTR and corresponding mimics of miR-141-3p, miR-7-5p, and miR-223-3p. The relative LUC activity was inhibited by mimics of three miRNAs, and such effects were not observed when treated with the mutant construction of IGF1R 3′-UTR (Figure 4, E-G).
Figure 4.
IDH1R132H-downregulated miRNAs lead to increased IGF1R expression. (A) The predicted target gene analysis of miR-141-3p, miR-223-3p, and miR-7-5p was performed by TargetScan software. (B, C) Western blot and immunofluorescence were used to examine IGF1R protein levels in RWPE-1 cells expressing IDH1R132H, IDH1WT, and VECTOR. (D) The protein levels of IGF1R were assessed in IDH1R132H-mutant RWPE-1 cells after treatment with miR-141-3p, miR-223-3p, and miR-7-5p mimics or negative control (Western blot). (E) Diagram indicates the recognition sites for miR-141-3p, miR-223-3p, and miR-7-5p in IGF1R 3′-UTR region, respectively. (F) WT- and mutated recognition sites of miR-141-3p, miR-223-3p, and miR-7-5p in IGF1R 3′-UTR region. (G) LUC reporter assay was performed in HEK293T cells. Cells were transiently co-transfected with wt- or mutated IGF1R 3′-UTR region together with corresponding miRNA mimics, respectively. After incubation for 48 hours, luciferase activities were measured. (H, I) Heat map and scatter diagram were used to indicate the different gene expression of IDH1R132H and VECTOR in RWPE-1 cells. (G) IGF1R-upregulated (GSE5225) gene signatures were further analyzed by GSEA in mRNA microarray of RWPE-1 cells expressing IDH1R132H and VECTOR. FDR q = 0.065. All histograms represent mean ± SD of at least three independent replicates. P values: *P < .05, **P < .01.
Figure S4.
IDH1R132H-downregulated miRNAs lead to increased IGF1R expression. (A) Western blot analysis of IGF1R protein expression in stable RWPE-1 cells treated with TGF-β. (B) The protein levels of IGF1R were assessed by Western blot analysis in RWPE-1-VECTOR cells transfected with miR-141-3p, miR-223-3p, miR-7-5p inhibitor, or negative control. WB was performed independently three times.
To characterize the molecular signature associated with IDH1R132H in RWPE-1 cells, we next analyzed differential gene expression in RWPE-1 cells transfected with IDH1R132H and VECTOR using the Agilent Human 4x44K Gene Expression Microarrays v2 (Figure 4H). A total of 8931 genes were differentially expressed between IDH1R132H and VECTOR in RWPE-1 cells, 4360 genes were upregulated, and 4571 genes were downregulated in IDH1R132H-mutant RWPE-1 cells (Figure 4I). In addition, GSEA indicated that the upregulated gene signatures of IGF1R (GSE5225) were significantly enriched in IDH1R132H-mutant RWPE-1 cells (Figure 4J). These results suggested that IGF1R signaling was activated in IDH1R132H-mutant RWPE-1 cells.
The requirment of IGF1R for IDH1R132H-Mediated Malignant Transformation
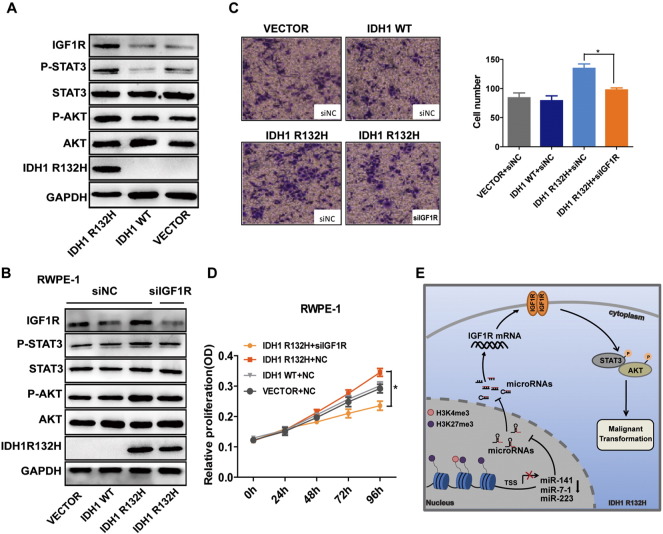
To further explore whether IGF1R overexpression was able to induce IDH1R132H-mutant RWPE-1 cells malignant transformation, we performed MTS and Transwell assay after knocking down IGF1R. The results showed that the abilities of migration and proliferation of IDH1R132H-mutant RWPE-1 cells were all decreased after knocking down IGF1R (Figure 5, C and D). We then evaluated IGF1R downstream signaling pathway genes AKT and STAT3 in RWPE-1 cells [38], [39]. As shown in Figure 5A, p-AKT and p-STAT3 were upregulated in IDH1R132H-mutant RWPE-1 cells. After knocking down IGF1R, the protein levels of P-AKT and P-STAT3 were all decreased in IDH1R132H-mutant RWPE-1 cells (Figure 5B).
Figure 5.
IGF1R is required for IDH1R132H-mediated malignant transformation. (A) Western blot analysis of indicted genes in RWPE-1 cells transfected with IDH1R132H, IDH1WT, or VECTOR. (B) Representative Western blot showed the protein levels of indicated genes in IDH1R132H-mutant RWPE-1 cells after knocking down IGF1R. (C, D) MTS and Transwell assays were used to determine the ability of proliferation and migration after silencing IGF1R in IDH1R132H-mutant RWPE-1 cells. (E) Schematic illustration of IDH1R132H-stimulated RWPE-1 cells’ malignant transformation. IDH1R132H reduces the expression of miR-141-3p, miR-223-3p, and miR-7-5p by altering histone modifications in their promoter region; the reduction of miRNAs eventually leads to the activation of the IGF1R-AKT/STAT3 signaling, which leads to the malignant transformation of RWPE-1 cells. Data are means of biological triplicate and mean ± SD. P values: *P < .05.
Discussion
PCa is clinically and molecularly heterogeneous. Approximately 15% of these men appear to have a poor prognosis, whereas many can be left untreated or minimally treated with a good outcome [40]. PCa genome and transcriptome characterization has identified molecular subtypes defined by essentially mutually exclusive genetic/transcriptomic events [41], including ETS gene fusions (most commonly involving ERG) and SPINK1 overexpression. Our group previously has characterized the genomic alterations of ERG rearrangement and SPINK1 overexpression in a subset of Chinese PCa patients [15], [20]. In a recent attempt of molecular subtyping of PCa, Tomlins et al. suggested that gene expression profiling of 1577 PCa cases supports three underlying molecularly defined groups: m-ERG+, m-ETS+, and m-SPINK1+/triple negative [42]. In other two studies, mutant IDH1 may be defined as a special subtype in PCa [2], [3]. Our current data showed that the incidence of IDH1R132H in Chinese PCa is 0.6%. In addition, IDH1R132H-mutant PCa patients possess fewer other canonical genomic lesions that are common in most other PCa. The rare incidence and clinical pathological features were consistent with previous reports [16], [43].
To the best of our knowledge, it is the first time to systematically describe the role of IDH1 mutations in PCa. Recent studies about the function of mutant IDH1 in malignancies are still contradictory. Some referred to mutant IDH1 as a driver of cellular immortalization, transformation, and tumorigenesis [44], [45]. Some pointed out that mutant IDH1 was associated with decreased proliferation in vitro and with good survival in vivo [27], [46]. Our study demonstrated that IDH1R132H promotes malignant transformation of prostatic benign epithelial cell RWPE-1, including the abilities of cytokine independence and migration. Cytokine independence property is consistent with the function of R-2HG in leukemia [45], [47]. These data suggested a critical role of IDH1R132H in prostatic malignant transformation. In addition, by contrast, IDH1R132H decreased proliferation and migration capacity of LNCaP cells in vitro. These tumor-suppressor characteristics also occurred in glioma [27], [48]. Interestingly, IDH1R132H-mutant LNCaP cells grew more rapidly than IDH1WT and VECTOR LNCaP cells in vivo. One explanation of this discrepancy may be that the cytotoxic effects of R-2HG could be eliminated timely to some extent in vivo even though R-2HG may exert toxic effect directly to the LNCaP cells in vitro. Therefore, the oncogenic role of IDH1R132H in promoting tumor cell growth could be masked by the toxic effect of R-2HG in vitro.
Additionally, our data also showed that stem cell marker CD133 was upregulated in IDH1R132H, while many studies showed that mutant IDH1 inhibited cell differentiation, which is associated with the characteristics of stem cell [28], [29], [49]. Whether IDH1R132H plays a role in stem cell property in PCa needs to be further studied in the future.
IGF1R activation is one of the most frequent events in human malignancy, including PCa [9]. Dysregulation of IGFIR expression has been found during development of more aggressive phenotype of PCa after initial transformation [50]. Our study highlighted an unrecognized mechanism of IGF1R upregulation in IDH1 mutant prostate epithelial cells, which is associated with miRNAs dysregulation. Specifically, we showed that the proliferative and migratory properties of IDH1R132H-overexpressed cells could be significantly impaired by targeting IGF1R expression. This study provided preliminary evidence suggesting that the malignant transformation caused by IDH1R132H might be affected by targeting IGF1R in vitro in PCa. In addition, AKT and STAT3, the downstream signaling pathway genes of IGF1R, often were found to be activated in PCa and other malignancies [38], [39]. Our data showed that AKT and STAT3 are all activated in IDH1R132H-mutant RWPE-1 cells. Consisted with our results, the activation of p-AKT in IDH1 mutations has also been identified in other studies [47], [51]. Many studies revealed that AKT and STAT3 are potential targeting genes for cancer therapy [52]. Thus, we inferred that AKT and STAT3 might also be the potential therapeutic targets in IDH1R132H in PCa.
MiRNA expression patterns are correlated with tumorigenesis and progression in PCa [11]. In this study, we proposed a novel mechanism that IDH1R132H promotes RWPE-1 cells malignant transformation by repressing miR-141-3p, miR-7-5p, and miR-223-3p. These three miRNAs have the abilities to suppress PCa stem cells, metastasis, or tumorigenesis in PCa [53], [54], [55], [56], [57]. Recent studies showed that mutant IDH1 changed gene expression by the alternation of epigenetics, including histone modification and DNA methylation [28], [58], [59]. Here, we focused on histone modification in IDH1R132H. Our data showed that the altered enrichments of H3k4me3 and H3K27me3 on promoter of miR-141, miR-7-1, and miR-223 might promote the reduction of miRNAs. In addition, some studies found that miR-141 can also be regulated by methylation [60]. To determine whether these miRNAs are repressed by other mechanisms, either alternation of DNA methylation or transcription factor, further studies are required in the future. As previously shown, TGF-β1 could regulate gene expression by epigenetic modification [61]. Our data showed that the upregulation of IGF1R was more obvious in IDH1R132H-mutant RWPE-1 cells under the treatment of TGF-β. This may partly prove the regulation mechanism.
Conclusions
In conclusion, our data suggested that DH1R132H may define a rare subtype (<1%) in Chinese PCa patients. We showed that IDH1R132H promotes malignant transformation in benign prostatic epithelium. Importantly, we proposed a novel model for an IDH1R132H-microRNAs-IGF1R regulatory axis, which might provide insight into the function of IDH1R132H in PCa development.
The following are the supplementary data related to this article.
Primers Used in This Study
Details of siRNA Used in This Study
Details of Mimics and Inhibitor Used in This Study
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant nos. 81330050, 81672554, and 81472417).
References
- 1.Zhao L, Yu N, Guo T, Hou Y, Zeng Z, Yang X, Hu P, Tang X, Wang J, Liu M. Tissue biomarkers for prognosis of prostate cancer: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1047–1054. doi: 10.1158/1055-9965.EPI-13-0696. [DOI] [PubMed] [Google Scholar]
- 2.Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, Tannock I, Drake CG, de Bono JS. Prostate cancer. Lancet. 2016;387:70–82. doi: 10.1016/S0140-6736(14)61947-4. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research N The molecular taxonomy of primary prostate cancer. Cell. 2015;163:1011–1025. doi: 10.1016/j.cell.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yen KE, Bittinger MA, Su SM, Fantin VR. Cancer-associated IDH mutations: biomarker and therapeutic opportunities. Oncogene. 2010;29:6409–6417. doi: 10.1038/onc.2010.444. [DOI] [PubMed] [Google Scholar]
- 5.Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3:730–741. doi: 10.1158/2159-8290.CD-13-0083. [DOI] [PubMed] [Google Scholar]
- 6.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 7.Pollak M. The insulin receptor/insulin-like growth factor receptor family as a therapeutic target in oncology. Clin Cancer Res. 2012;18:40–50. doi: 10.1158/1078-0432.CCR-11-0998. [DOI] [PubMed] [Google Scholar]
- 8.Papatsoris AG, Karamouzis MV, Papavassiliou AG. Novel insights into the implication of the IGF-1 network in prostate cancer. Trends Mol Med. 2005;11:52–55. doi: 10.1016/j.molmed.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–2950. [PubMed] [Google Scholar]
- 10.Heidegger I, Massoner P, Sampson N, Klocker H. The insulin-like growth factor (IGF) axis as an anticancer target in prostate cancer. Cancer Lett. 2015;367:113–121. doi: 10.1016/j.canlet.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Fang YX, Gao WQ. Roles of microRNAs during prostatic tumorigenesis and tumor progression. Oncogene. 2014;33:135–147. doi: 10.1038/onc.2013.54. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Sun F, Dong N, Sun Z, Diao Y, Zheng C, Sun J, Yang Y, Jiang D. MicroRNA-7 directly targets insulin-like growth factor 1 receptor to inhibit cellular growth and glucose metabolism in gliomas. Diagn Pathol. 2014;9:211–216. doi: 10.1186/s13000-014-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu YH, Zhang L, Wu DS, Zhang Z, Huang FF, Zhang J, Chen XP, Liang DS, Zeng H, Chen FP. MiR-223 regulates human embryonic stem cell differentiation by targeting the IGF-1R/Akt signaling pathway. PLoS One. 2013;8:e78769. doi: 10.1371/journal.pone.0078769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang M, Wu L, Qin Y, Li Z, Luo S, Qin H, Yang Y, Chen J. Anti-proliferative role and prognostic implication of miR-141 in gastric cancer. Am J Transl Res. 2016;8:3549–3557. [PMC free article] [PubMed] [Google Scholar]
- 15.Han B, Mehra R, Lonigro RJ, Wang L, Suleman K, Menon A, Palanisamy N, Tomlins SA, Chinnaiyan AM, Shah RB. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22:1083–1093. doi: 10.1038/modpathol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauzo SH, Lee M, Petros J, Hunter S, Chang CM, Shu HK, Bellail AC, Hao C, Cohen C. Immunohistochemical demonstration of isocitrate dehydrogenase 1 (IDH1) mutation in a small subset of prostatic carcinomas. Appl Immunohistochem Mol Morphol. 2014;22:284–287. doi: 10.1097/PAI.0b013e3182649d1c. [DOI] [PubMed] [Google Scholar]
- 17.Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan W, Wang L, Ma Q, Qi M, Lu N, Zhang L, Han B. Adiponectin as a potential tumor suppressor inhibiting epithelial-to-mesenchymal transition but frequently silenced in prostate cancer by promoter methylation. Prostate. 2015;75:1197–1205. doi: 10.1002/pros.23002. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Wang L, Su B, Lu N, Song J, Yang X, Fu W, Tan W, Han B. Serine protease inhibitor Kazal type 1 promotes epithelial-mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate. 2014;74:689–701. doi: 10.1002/pros.22787. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Zhang J, Yang X, Chang YW, Qi M, Zhou Z, Zhang J, Han B. SOX4 is associated with poor prognosis in prostate cancer and promotes epithelial-mesenchymal transition in vitro. Prostate Cancer Prostatic Dis. 2013;16:301–307. doi: 10.1038/pcan.2013.25. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Song G, Tan W, Qi M, Zhang L, Chan J, Yu J, Han J, Han B. MiR-573 inhibits prostate cancer metastasis by regulating epithelial-mesenchymal transition. Oncotarget. 2015;6:35978–35990. doi: 10.18632/oncotarget.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang MR, Kim MS, Oh JE, Kim YR, Song SY, Seo SI, Lee JY, Yoo NJ, Lee SH. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009;125:353–355. doi: 10.1002/ijc.24379. [DOI] [PubMed] [Google Scholar]
- 24.Capper D, Weissert S, Balss J, Habel A, Meyer J, Jager D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20:245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suva ML, Bernstein BE. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature. 2016;529:110–114. doi: 10.1038/nature16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boutzen H, Saland E, Larrue C, de Toni F, Gales L, Castelli FA, Cathebas M, Zaghdoudi S, Stuani L, Kaoma T. Isocitrate dehydrogenase 1 mutations prime the all-trans retinoic acid myeloid differentiation pathway in acute myeloid leukemia. J Exp Med. 2016;213:483–497. doi: 10.1084/jem.20150736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bralten LB, Kloosterhof NK, Balvers R, Sacchetti A, Lapre L, Lamfers M, Leenstra S, de Jonge H, Kros JM, Jansen EE. IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol. 2011;69:455–463. doi: 10.1002/ana.22390. [DOI] [PubMed] [Google Scholar]
- 28.Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, Gurumurthy S, Akbay EA, Sia D, Cornella H. Mutant IDH inhibits HNF-4alpha to block hepatocyte differentiation and promote biliary cancer. Nature. 2014;513:110–114. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardella C, Al-Dalahmah O, Krell D, Brazauskas P, Al-Qahtani K, Tomkova M, Adam J, Serres S, Lockstone H, Freeman-Mills L. Expression of Idh1R132H in the murine subventricular zone stem cell niche recapitulates features of early gliomagenesis. Cancer Cell. 2016;30:578–594. doi: 10.1016/j.ccell.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–630. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- 32.Hassan O, Ahmad A, Sethi S, Sarkar FH. Recent updates on the role of microRNAs in prostate cancer. J Hematol Oncol. 2012;5:9–18. doi: 10.1186/1756-8722-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim WT, Kim WJ. MicroRNAs in prostate cancer. Prostate Int. 2013;1:3–9. doi: 10.12954/PI.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrozek K, Margeson D, Holland KB, Whitman SP, Becker H, Schwind S. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:2348–2355. doi: 10.1200/JCO.2009.27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng W, Ren X, Zhang C, Han S, Wu A. Expression and prognostic value of microRNAs in lower-grade glioma depends on IDH1/2 status. J Neurooncol. 2017;132:207–218. doi: 10.1007/s11060-016-2368-6. [DOI] [PubMed] [Google Scholar]
- 36.Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–483. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y. Implications of insulin-like growth factor-I for prostate cancer therapies. Int J Urol. 2009;16:161–167. doi: 10.1111/j.1442-2042.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- 38.Zong CS, Chan J, Levy DE, Horvath C, Sadowski HB, Wang LH. Mechanism of STAT3 activation by insulin-like growth factor I receptor. J Biol Chem. 2000;275:15099–15105. doi: 10.1074/jbc.M000089200. [DOI] [PubMed] [Google Scholar]
- 39.Chapuis N, Tamburini J, Cornillet-Lefebvre P, Gillot L, Bardet V, Willems L, Park S, Green AS, Ifrah N, Dreyfus F. Autocrine IGF-1/IGF-1R signaling is responsible for constitutive PI3K/Akt activation in acute myeloid leukemia: therapeutic value of neutralizing anti-IGF-1R antibody. Haematologica. 2010;95:415–423. doi: 10.3324/haematol.2009.010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svensson MA, LaFargue CJ, MacDonald TY, Pflueger D, Kitabayashi N, Santa-Cruz AM, Garsha KE, Sathyanarayana UG, Riley JP, Yun CS. Testing mutual exclusivity of ETS rearranged prostate cancer. Lab Invest. 2011;91:404–412. doi: 10.1038/labinvest.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomlins SA, Alshalalfa M, Davicioni E, Erho N, Yousefi K, Zhao S, Haddad Z, Den RB, Dicker AP, Trock BJ. Characterization of 1577 primary prostate cancers reveals novel biological and clinicopathologic insights into molecular subtypes. Eur Urol. 2015;68:555–567. doi: 10.1016/j.eururo.2015.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghiam AF, Cairns RA, Thoms J, Dal Pra A, Ahmed O, Meng A, Mak TW, Bristow RG. IDH mutation status in prostate cancer. Oncogene. 2012;31:3826. doi: 10.1038/onc.2011.546. [DOI] [PubMed] [Google Scholar]
- 44.Ohba S, Mukherjee J, Johannessen TC, Mancini A, Chow TT, Wood M, Jones L, Mazor T, Marshall RE, Viswanath P. Mutant IDH1 expression drives TERT promoter reactivation as part of the cellular transformation process. Cancer Res. 2016;76:6680–6689. doi: 10.1158/0008-5472.CAN-16-0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Losman JA, Looper RE, Koivunen P, Lee S, Schneider RK, McMahon C, Cowley GS, Root DE, Ebert BL, Kaelin WG., Jr. (R)-2-Hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science. 2013;339:1621–1625. doi: 10.1126/science.1231677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Huang J, Huang F, Jin Q, Zhu H, Wang X, Chen M. Decreased expression of IDH1-R132H correlates with poor survival in gastrointestinal cancer. Oncotarget. 2016;7:73638–73650. doi: 10.18632/oncotarget.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu H, Zhang Y, Chen J, Qiu J, Huang K, Wu M, Xia C. IDH1 R132H mutation enhances cell migration by activating AKT-mTOR signaling pathway, but sensitizes cells to 5-FU treatment as NADPH and GSH are reduced. PLoS One. 2017;12:e0169038. doi: 10.1371/journal.pone.0169038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, El Hallani S, Boisselier B, Mokhtari K, Hoang-Xuan K. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 49.Kats LM, Reschke M, Taulli R, Pozdnyakova O, Burgess K, Bhargava P, Straley K, Karnik R, Meissner A, Small D. Proto-oncogenic role of mutant IDH2 in leukemia initiation and maintenance. Cell Stem Cell. 2014;14:329–341. doi: 10.1016/j.stem.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Yu E. Insulin-like growth factor receptor-1 (IGF-IR) as a target for prostate cancer therapy. Cancer Metastasis Rev. 2014;33:607–617. doi: 10.1007/s10555-013-9482-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carbonneau M, L MG, Lalonde ME, Germain MA, Motorina A, Guiot MC, Secco B, Vincent EE, Tumber A, Hulea L. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat Commun. 2016;7:12700–12711. doi: 10.1038/ncomms12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosch-Barrera J, Menendez JA. Silibinin and STAT3: a natural way of targeting transcription factors for cancer therapy. Cancer Treat Rev. 2015;41:540–546. doi: 10.1016/j.ctrv.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Chang YL, Zhou PJ, Wei L, Li W, Ji Z, Fang YX, Gao WQ. MicroRNA-7 inhibits the stemness of prostate cancer stem-like cells and tumorigenesis by repressing KLF4/PI3K/Akt/p21 pathway. Oncotarget. 2015;6:24017–24031. doi: 10.18632/oncotarget.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurozumi A, Goto Y, Matsushita R, Fukumoto I, Kato M, Nishikawa R, Sakamoto S, Enokida H, Nakagawa M, Ichikawa T. Tumor-suppressive microRNA-223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci. 2016;107:84–94. doi: 10.1111/cas.12842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Liu R, Zhang D, Deng Q, Liu B, Chao HP, Rycaj K, Takata Y, Lin K, Lu Y. MicroRNA-141 suppresses prostate cancer stem cells and metastasis by targeting a cohort of pro-metastasis genes. Nat Commun. 2017;8:14270–14283. doi: 10.1038/ncomms14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li JZ, Li J, Wang HQ, Li X, Wen B, Wang YJ. MiR-141-3p promotes prostate cancer cell proliferation through inhibiting Kruppel-like factor-9 expression. Biochem Biophys Res Commun. 2017;482:1381–1386. doi: 10.1016/j.bbrc.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Ma YY, Wang J, Zeng XF, Li R, Kang W, Hao XK. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco Targets Ther. 2016;9:139–148. doi: 10.2147/OTT.S95565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sasaki M, Knobbe CB, Munger JC, Lind EF, Brenner D, Brustle A, Harris IS, Holmes R, Wakeham A, Haight J. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012;488:656–659. doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duncan CG, Barwick BG, Jin G, Rago C, Kapoor-Vazirani P, Powell DR, Chi JT, Bigner DD, Vertino PM, Yan H. A heterozygous IDH1R132H/WT mutation induces genome-wide alterations in DNA methylation. Genome Res. 2012;22:2339–2355. doi: 10.1101/gr.132738.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lynch SM, O'Neill KM, McKenna MM, Walsh CP, McKenna DJ. Regulation of miR-200c and miR-141 by methylation in prostate cancer. Prostate. 2016;76:1146–1159. doi: 10.1002/pros.23201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Li Y, Yang X, Yuan H, Li X, Qi M, Chang YW, Wang C, Fu W, Yang M. ERG-SOX4 interaction promotes epithelial-mesenchymal transition in prostate cancer cells. Prostate. 2014;74:647–658. doi: 10.1002/pros.22783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers Used in This Study
Details of siRNA Used in This Study
Details of Mimics and Inhibitor Used in This Study