Abstract
Background
Failure in the endometrial receptivity may account for a significant number of infertility cases including unexplained infertility in women. Reduction in the endometrial estrogen receptor-alpha (ER-α) expression during implantation may be a critical event that coincides with the expression of specific genes and the formation of a receptive endometrium. The aim of the present study was to assess the expression of ER-α in the mid-secretory phase in the endometrium of women with unexplained infertility.
Materials and Methods
This case-control study was carried out on randomly selected fertile (n=10) and infertile (n=16) women whose source of infertility remained unexplained. We evaluated the expression of ER-α and glycode-lin-A (GdA) through mRNA level measurement with real-time polymerase chain reaction (PCR) in the endometrium of fertile women and patients suffering from unexplained infertility and fertile women. Endometrial biopsies of each subject were collected during a single menstrual cycle 7 days after the peak of luteinizing hormone (LH+7).
Results
Endometrial expression level of ER-α was significantly (P<0.05) higher in the patients with unexplained infertility compared to the control. Significantly (P<0.05) lower levels of GdA expression were seen in women with unexplained infertility. A statistically non-significant negative correlation was observed between ER-α and GdA mRNA expression.
Conclusion
Our findings demonstrate that reduction in the endometrial GdA expression is associated with elevated expression of ER-α in mid-luteal phase. Disruption in the endometrial ER-α expression, which leads to defects in uterine receptivity, may contribute to unexplained infertility.
Keywords: Estrogen Receptor-α, Glycodelin-A, Implantation
Introduction
Endometrial receptivity plays a key role in the establishment of a successful implantation and its impairment may contribute to infertility in women (1). A variety of molecules such as hormones, receptors, adhesion molecules, growth factors and cytokines mediate the embryomaternal crosstalk and facilitate the reception of a blastocyst and the establishment of implantation (2). During the menstrual cycle uterine receptivity is regulated by the secretion of the ovarian steroids. Endometrial proliferation is induced by estrogen during the preovulatory phase, whereas progesterone causes secretory changes in the estrogen- primed endometrium (3).
Ligand-specific intracellular receptors located in stro. mal and epithelial endometrial cells mediate the actions of estrogen and progesterone (4). It is thought that the pres. ence of progesterone after appropriate estrogen priming is required to stimulate key implantation-specific events in the mid-secretory phase of the menstrual cycle (5).
Estrogen receptor-alpha (ER-α) increases during the proliferative phase in response to estrogen and is downregulated during the window of implantation in response to progesterone (6). The disappearance of ER-α at the time of implantation has been reported in most mammalian species (7). The decline in ER-α coincides with endometrial gene expression in the mid-luteal phase, and is a critical event in the establishment of endometrial receptivity (8). High levels of ER-α during implantation were observed in women with polycystic ovarian syndrome (PCOS) and endometriosis. Elevated expression of ER-α in both groups of patients was associated with the reduction in beta 3 integrin expression, a marker of endometrial receptivity (9). It has been suggested that the disappearance of ER-α at the time of implantation may disturb the expression pattern of proteins that regulate the endometrial receptivity.
Glycodelin-A (GdA) is a progesterone-regulated glycoprotein with immunosuppressive properties that is highly upregulated in glandular epithelium at implantation and plays a role in the formation a receptive endometrium (10). GdA expression is concurrent with pinopode formation in the receptive endometrium (11), indicating that it can potentially be seen as a diagnostic marker of morphological differentiation of human endometrium (12). A lower glycodelin expression in secretory phase was found in eutopic endometrium of endometriosis patients and in uterine flushings from women with unexplained infertility when compared to the healthy controls (13, 14).
Assuming that unexplained infertility can be due to disturbances in the molecular and the cellular biomarkers involved in implantation (15), we hypothesized that continued ER-α expression may be detrimental to the development of endometrial receptivity. In present study expression of GdA, as a particular marker of endometrial receptivity, was assessed at the time of implantation.
Materials and Methods
This case-control study was approved by the Research Ethics Committee of Shahid Chamran University of Ahvaz, Iran. The study was performed in the Laboratory of Embryology, Department of Biology. Written informed consent was obtained from each participant.
Sample collection
Endometrial biopsy samples were collected using a Novak curette in the mid-luteal phase at day luteinizing hormone (LH)+7 from healthy volunteers women with proven fertility (n=10, age 32.5 ± 3.2 Y) and women with unexplained infertility (n=16, age 31.6 ± 3.0 Y) that showed primary infertility for more than 2 years (30.5 ± 4.7 months). The unfertile females were randomly selected from a population of such females listed in Imam Khomeini hospital medical records. Endometrial samples were divided into two parts. One sample was fixed in 10% formalin and embedded in paraffin. After tissue processing, 5-6 µm sections were stained with haematoxylin- eosin, evaluated histologically to correspond all samples to the assumed time in the cycle according to the Noyes et al. (16) criteria. The other sample was immediately stored in RNA later at -80°C for later use in real-time polymerase chain reaction (RT-PCR). Sample size was determined based on previous studies (17, 18). Sample size was smaller in the fertile group due to the low collaboration. The concentration of LH in morning urine (ACON Laboratories, Inc., USA) was used to determine the day of the surge.
All women included in this study had normal ovarian function and regular menstrual cycles, confirmed based on their menstrual histories, and none of them had used steroid hormones, (for at least 6 months prior to study), and intra-uterine contraceptives. Women with unexplained infertility showed normal ovulatory cycles and mid-luteal serum progesterone levels, normal tubal patency and no recognizable endometriosis based on symptoms and clinical examination in transvaginal ultrasonography or diagnostic laparoscopy. Moreover, unexplained infertile women had partners with normal semen according to WHO criteria. Patients with history of pelvic inflammatory diseases, pelvic surgery including cesarean section, unilateral tubal patency, ovarian hyperstimulation syndrome, diminished ovarian response, endometriosis or multiple female factor were excluded from this study.
Hormone assay
Blood samples were obtained in the fasted state on the same day as endometrial sampling and serum levels of LH, follicle stimulating hormone (FSH), estradiol (E2), and progesterone (P4) were measured using commercially available kits (Abcam plc, UK).
RNA extraction
Total RNA was extracted from the endometrial tissues (approximately 50-100 mg) using Tripure (Roche Diagnostics, Germany), according to the recommended protocol by the manufacturer. RNA integrity was analyzed via electrophoresis and total RNA concentration was obtained using a spectrophotometer at an optical density of 260 nm. The RNA was stored at -70°C for future procedures.
cDNA synthesis
Synthesis of cDNA was carried out using 1 mg of total RNA from each sample with random hexamer primers using prime Script™ RT reagent Kit (Takara Bio Inc., Japan) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain reaction analysis
Real-time PCR was performed for relative quantification of the ER-α and GdA genes expression using ABI StepOne plus™ System (Applied Biosystems, Germany). Hypoxanthine phosphoribosyltransferase (HPRT) gene was used as the housekeeping gene. Forward and reverse primer sequences for each gene are presented in Table 1. The specificity of primers for each gene was analyzed in the BLAST database. The reaction mixture consisted of 10 µl Master mix SYBR Green, 2 µl cDNA, 1 µl of each primer (10 pmol/µl), and 7 µl dH2O (Qiagen, Germany). The standard cycling protocol used for all genes consisted of DNA denaturation and enzyme activation at 95°C for 10 minutes, denaturation 95°C for 15 seconds, annealing at 62°C for 15 seconds and extension and florescence acquiring at 72°C for 15 seconds. The RT-PCR procedure was carried out 40 cycles. Melting curve analysis was performed by bringing the temperature from 95°C to 60°C for 60 seconds at the transition rate of 1 degree per second. As Livak and Schmittgen (2001) described, for sample analysis the threshold was set based on the exponential phase of products and the 2-ΔΔCT method was performed to analyze the data (19).
Table 1.
Primer sequences used in real-time polymerase chain reaction
| Gene | Primer sequencing (5´→3´) | Accession number |
|---|---|---|
| ER-α | F: TGCTTCAGGCTACCATTATGGA | NM-001122742 |
| R: TGGCTGGACACATATAGTCGTT | ||
| GdA | F: GAGATCGTTCTGCACAGATGG | NM-001018049 |
| R: CGTTCGCCACCGTATAGTTGAT | ||
| HPRT | F: TGGACAGGACTGAACGTCTTG | NM-000194 |
| R: CCAGCAGGTCAGCAAAGAATTTA | ||
ER-α; Estrogen receptor-alpha, GdA; Glycodelin-A, and HPRT; Hypoxanthine hosphoribosyltransferase.
Statistical analysis
Data was analyzed by SPSS version 16 software (SPSS Inc., USA). Independent samples t test was performed to compare characteristics and hormonal profile of the fertile and the infertile women. Results are expressed as mean ± SD. Comparison of ER-α and GdA expression in studied groups was done using Mann-Whitney U-test. Spearman correlation analysis was carried out to investigate the relationship between variables. The level of significance was set at P<0.05.
Results
Of the 54 couples with unexplained infertility, 8 couples were excluded based on their medical records. Among 25 randomly-selected eligible patients with unexplained infertility, 9 couples refused participation. As a result, 16 infertile couples were included in the study. In addition, 10 fertile women (16.1%) out of the 62 eligible couples were included in the study. The mean age, body mass index (BMI), cycle length, duration of menses and hormonal profile in women of both groups are presented in Table 2. There were no differences in age, BMI, cycle length, duration of menses and serum LH, FSH, estradiol and progestrone concentrations between the two groups. Microscopic analysis of the endometrial biopsies showed that all samples corresponded histologically to the mid- luteal phase of endometrial cycle (Fig .1).
Fig.1.
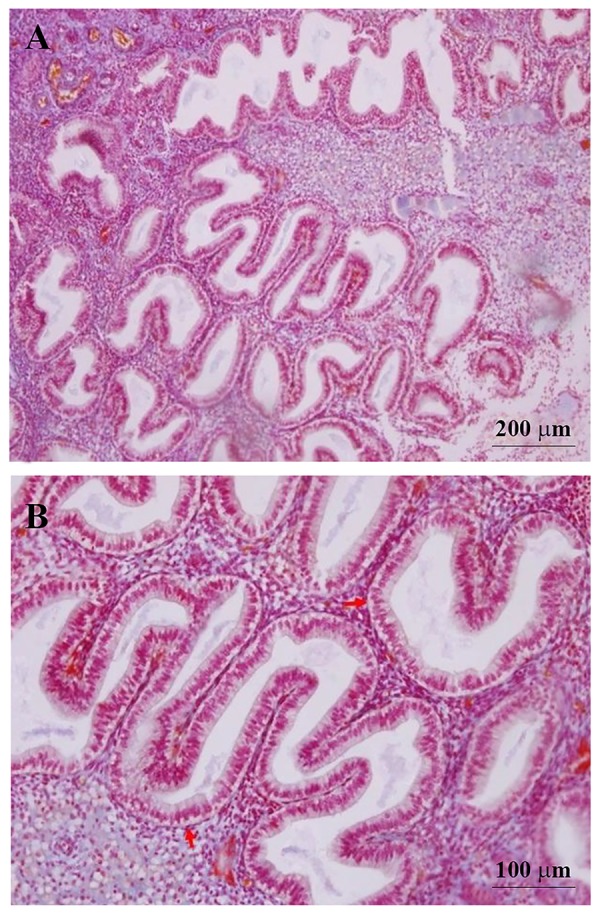
Microscopic structure of endometrium at the mid-luteal phase. A. Scale bar=200 µm and B. Scale bar=100 µm, H&E. Stromal edema and coiled endometrial glands that contain secretions with sub-nuclear vacuolization (red arrows) in their epithelium exhibit endometrium in the mid-luteal phase.
Table 2.
Characteristics and hormonal profile of the fertile and infertile women in the mid-luteal phase
| Parameter | Fertile women n=10 | Infertile women n=16 | P value |
|---|---|---|---|
| Age (Y) | 31.7 ± 5.9 | 32.2 ± 5.5 | NS |
| BMI (kg/m2) | 23.7 ± 2.8 | 23.4 ± 2.6 | NS |
| Cycle length (days) | 28.2 ± 1.3 | 28.5 ± 1.5 | NS |
| Menses duration (days) | 4.2 ± 0.5 | 4.5 ± 0.6 | NS |
| LH (mIU/mL) | 12.54 ± 6.85 | 13.27 ± 7.13 | NS |
| FSH (mIU/mL) | 5.90 ± 2.62 | 6.58 ± 2.50 | NS |
| Estradiol (pg/ml) | 139.3 ± 55.4 | 142.9 ± 61.6 | NS |
| Progestrone (ng/mL) | 10.93 ± 3.21 | 11.48 ± 4.86 | NS |
Independent samples t test was done as the test of significant. Results expressed as mean ± SD. The level of significance was set at P<0.05. BMI; Body mass index, LH; Luteinizing hormone, FSH; Follicle stimulating hormone, and NS; Non significant.
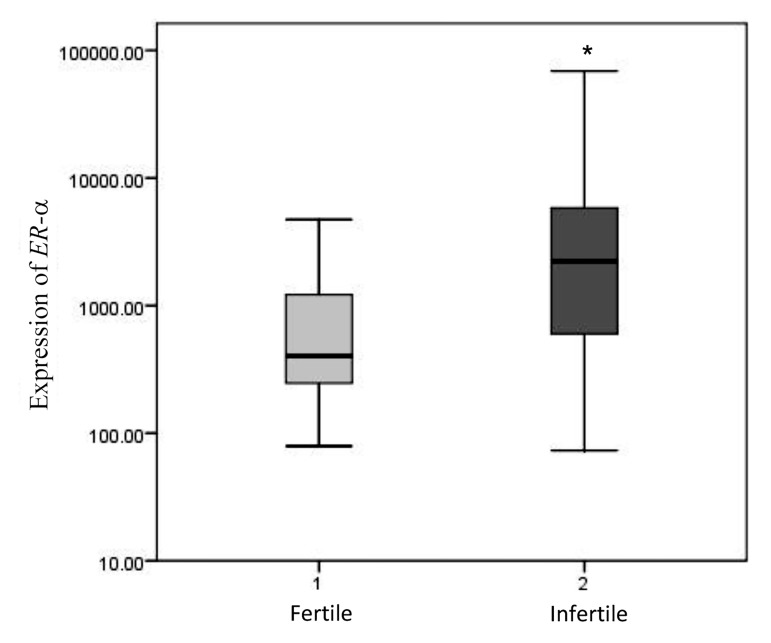
Relative expressions of ER-α and GdA in the mid-luteal endometrium of the patients with unexplained infertility and healthy fertile women are shown in Figures 2 and 3. Expression levels of ER-α and GdA mRNA are given relative to the expression levels of the reference gene, HPRT. Levels of ER-α mRNA expression in the endometrium of the patients with unexplained infertility were significantly higher than those in the fertile women (P=0.007, Mann-Whitney U-test, Fig .2).
Fig.2.
Relative expression of ER-α in the mid-luteal endometrium of patients with unexplained infertility (n=16) was significantly higher than those in healthy fertile women (n=10, P=0.007, Mann-Whitney U-test). *; P<0.05.
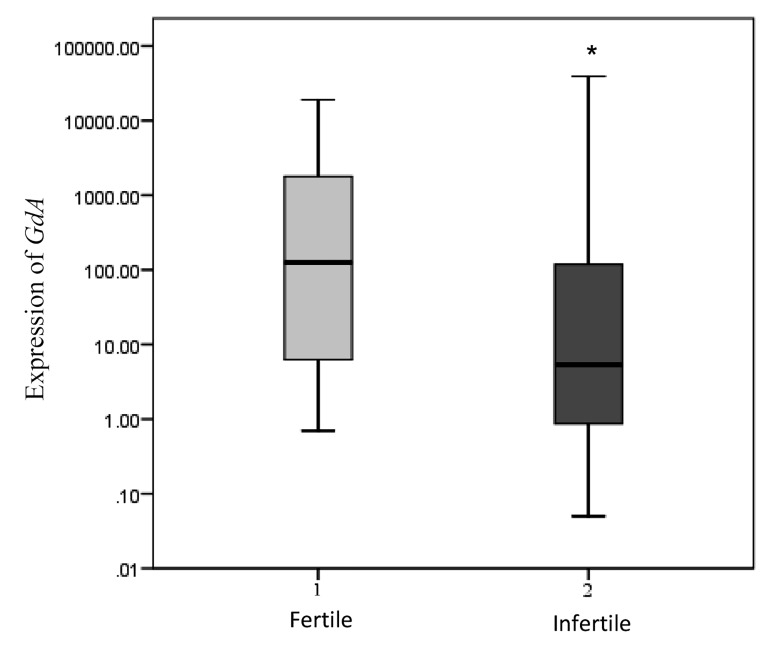
GdA mRNA levels were significantly lower in the infertile women compared to the healthy fertile group (P=0.045, Mann-Whitney U-test, Fig .3).
Fig.3.
Relative expression of GdA in the mid-luteal endometrium of patients with unexplained infertility (n=16) was significantly lower than those in healthy fertile women (n=10, P=0.045, Mann-Whitney U-test). *; P<0.05.
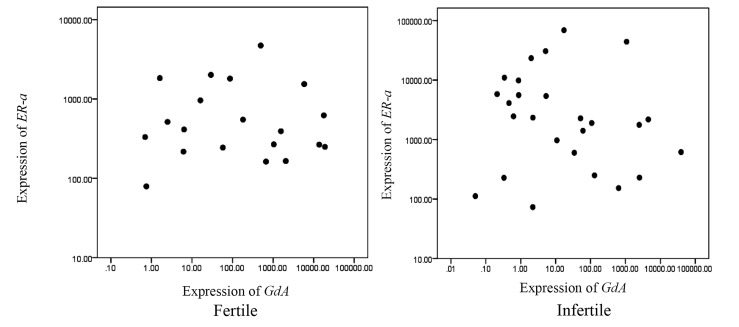
A statistically non-significant negative correlation was observed between ER-α and GdA mRNA expression levels in the fertile women (r=-0.047, P=0.845) and in the patients with unexplained infertility (r=-0.205, P=0.316, Fig .4).
Fig.4.
Correlation between ER-α and GdA mRNA expressions in the mid- luteal endometrium of the healthy fertile women (r=-0.047, P=0.845) and the patients with unexplained infertility (r=-0.205, P=0.316).
Discussion
Implantation failure is believed to be a major cause of infertility (20). Successful embryo implantation depends on the development of an endometrium that is receptive to the embryo (21). Coordinated interactions between estrogen and progesterone resulting in a series of synchronized molecular events during menstrual cycle ultimately lead to the preparation of a receptive endometrium (22).
The present study showed that a lack of appropriate levels of ER-α downregulation in the mid-luteal phase in the patients with unexplained infertility relative to the control group. During implantation ER-α is being downregulated in response to progesterone. Downregulation of ER-α during the mid-secretory phase is one of the primary actions of progesterone. The combination of estrogen withdrawal and progesterone action is required to stimulate the endometrial gene expression in the mid-luteal phase (8). Disappearance of ER-α in the mid-luteal phase provides the opportunity for progesterone to act alone specifically on the stroma (6). Paracrine activity of stroma in response to progesterone results in epithelial gene expression (7). Similar findings have been reported in patients with endometriosis and in women with PCOS (9).
Inadequate progesterone levels, defects in the progesterone receptor, hypersensitivity to estrogen, inappropriate expression of aromatase and progesterone resistance are among the reasons that can cause this failure to downregulate ER-α in the mid-luteal phase. Insufficient serum level of progesterone in the luteal phase defect (LPD) may delay the timing of ER-α downregulation during implantation (23). Resistance to progesterone due to aberrant expression or activity of receptor results in estrogenicity in endometrial tissue (24). The loss of progesterone activity caused by defect in the progesterone receptor (25) and/ or an increase in the local estrogen production due to inappropriate expression of aromatase (26) may cause the persistence of ER-α in endometriosis patients. A failure in ER-α downregulation has been reported in ovarian and peritoneal endometriosis (27). Increased production of estrogen contributes to the pathophysiology of the endometriosis as a mitogen causing aberrant proliferation (28) and inhibition of apoptosis (29). Overexpression of steroid receptor co-activators in PCOS patients which marks the hypersensitivity to estrogen may explain elevated endometrial ER-α expression (9).
Moreover, it seems that any change in the balance between estrogen and progesterone could disturb the timing of ER-α downregulation in mid-luteal phase. Endocrine disrupting chemicals (EDCs) or xenoestrogens are natural or synthetic chemicals in the diet or the environment that mimic the endogenous estrogens functions or interfere with estrogen signaling pathways (30). Lower levels of progesterone metabolite have been found during the luteal phase with higher concentration of Dichlorodiphenyldichloroethylene (DDE) (31). Impaired implantation has been reported in patients with an increase in serum 17ß-estradiol (E2) levels during the pre-implantation period, while reducing E2 levels during the pre-implantation period by a step-down protocol increases implantation and pregnancy rates (32). Accordingly, the possibility of manipulating the receptivity window with the use of different doses of E2 has been suggested (33). Aberrant uterine expression of implantation-related genes has been found at high estrogen levels (34), suggesting that in in vitro fertilization (IVF) programs estrogen levels regulation is important for improvement of women fertility.
Any inability in the ER-α downregulation may lead to failure to express essential proteins associated with uterine receptivity, in turn resulting in either infertility or pregnancy loss (35). The present study showes that ER-α overexpression is accompanied by downregulation of GdA in the mid-luteal endometrium of the patients with unexplained infertility. GdA, a potential diagnostic marker of the endometrial receptivity, is the major progesterone- regulated glycoprotein and has been demonstrated in the pinopodes of receptive-phase human endometrium (11). Lower levels of GdA has been reported in the secretory phase of the menstrual cycle in the eutopic tissue of patients with endometriosis (13). In addition, lower levels of GdA were detected in the uterine flushings on days LH+10 and LH+12 in women with unexplained infertility (14) and recurrent miscarriage (36). A negative but statistically non-significant correlation was found between ER-α and GdA in fertile women and in patients with unexplained infertility. Although transcription, synthesis, and secretion of endometrial GdA are regulated by progesterone, according to our findings one can assume that the overexpression of endometrial ER-α disturbs the expression of special genes during the implantation, which is detrimental to the development of uterine receptivity.
Inadequate uterine receptivity is responsible for approximately two-thirds of implantation failures (37). A range of cellular and molecular endometrial defects has been associated with unexplained infertility (38). Microarray analysis demonstrated that endometrial gene expression at the time of embryo implantation is considerably different in the unexplained infertile patients compared to the fertile women (39).
Therefore, the failure in ER-α downregulation and the observed disturbance in GdA expression in the patients with unexplained infertility may elucidate the causes of unexplained infertility. Our observations suggest that endometrial ER-α expression may participate in the cascade of molecular events leading to successful implantation.
The random inclusion of all cases diagnosed with unexplained infertility is the main strength of this study. Furthermore, real-time PCR based assay of endometrial markers, an extremely sensitive technique that allows the precise measurement of gene expression (40), increases the accuracy and external validity of our results. However, data was collected from a single randomized center and subjects represent only a fraction of the population, thus reducing the population validity. Moreover, unexplained infertile women with secondary infertility were excluded, so its external validity is restricted to women with primary infertility.
Conclusion
The present study shows the prognostic significance of ER-α expression in patients with unexplained infertility. Disruption in the endometrial ER-α expression, which leads to defects in the uterine receptivity may contribute to unexplained infertility. In addition, our findings demonstrate that reduction in endometrial GdA expression was associated with elevated expression of ER-α in the mid-luteal phase. However, our study has some limitations including the low number of cases of unexplained infertile women with primary infertility. Studies including more tissue samples and protein-based assays such as immunohistochemistry and western blot analysis are also needed to further determine the role of endometrial ER-α. Understanding of biomarkers involved in the implantation and the mechanisms governing their relationships in endometrial receptivity could provide new therapeutic strategies for unexplained infertility. Whether such defects of uterine receptivity could be treated by the therapeutic blockage of ER-α activity or by dealing with the related causes of ER-α overexpression, e.g., using progestins or aromatase inhibitors to normalize the expression pattern of endometrial biomarkers associated with implantation, requires further investigation.
Acknowledgments
The authors wish to thank the Vice Chancellor for Research of Shahid Chamran University of Ahvaz for providing the research grants (No. 867615). The authors declare that there is no conflict of interest.
Author’s Contributions
M.D.; Designed and directed the project. F.M.; Contributed to sample preparation. H.-o-a.G.; Planned the RT-PCR method. N.K.; Performed the experiments. All authors discussed the results and contributed to the writing and read and approved the final manuscript.
References
- 1.Cakmak H, Taylor HS. Implantation failure: molecular mechanisms and clinical treatment. Hum Reprod Update. 2011;17(2):242–253. doi: 10.1093/humupd/dmq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haouzi D, Mahmoud K, Fourar M, Bendhaou K, Dechaud H, De Vos J, et al. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod. 2009;24(1):198–205. doi: 10.1093/humrep/den360. [DOI] [PubMed] [Google Scholar]
- 3.Katzenellenbogen BS. Dynamics of steroid hormone receptor. Annu Rev Physiol. 1980;42:17–35. doi: 10.1146/annurev.ph.42.030180.000313. [DOI] [PubMed] [Google Scholar]
- 4.Healy DL, Hodgen GD. The endocrinology of human endometrium. Obstet Gynecol Surv. 1983;38(8):509–530. doi: 10.1097/00006254-198308000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Simon C, Domínguez F, Valbuena D, Pellicer A. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab. 2003;14(5):197–199. doi: 10.1016/s1043-2760(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 6.Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS Jr. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67(2):334–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- 7.Vermeirsch H, Van Den Broeck W, Coryn M, Simoens P. Immunolocalization of sex steroid hormone receptors in the canine uterine tube and their relation to sex steroid hormone concentrations. Reprod Fertil Dev. 2002;14(3-4):241–250. doi: 10.1071/rd01084. [DOI] [PubMed] [Google Scholar]
- 8.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinquishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 9.Gregory CW, Wilson EM, Apparao KB, Lininger RA, Meyer WR, Kowalik A, et al. Steroid receptor coactivator expression throughout the menstrual cycle in normal and abnormal endometrium. J Clin Endocrinol Metab. 2002;87(6):2960–2966. doi: 10.1210/jcem.87.6.8572. [DOI] [PubMed] [Google Scholar]
- 10.Mylonas I, Jeschke U, Kunert-Keil C, Shabani N, Dian D, Bauerfeind I, et al. Glycodelin A is expressed differentially in normal human endometrial tissue throughout the menstrual cycle as assessed by immunohistochemistry and in situ hybridization. Fertil Steril. 2006;86(5):1488–1497. doi: 10.1016/j.fertnstert.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 11.Stavreus-Evers A, Mandelin E, Koistinen R, Aghajanova L, Hovatta O, Seppälä M. Glycodelin is present in pinopodes of receptive-phase human endometrium and is associated with down-regulation of progesterone receptor B. Fertil Steril. 2006;85(6):1803–1811. doi: 10.1016/j.fertnstert.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Seppälä M, Taylor RN, Koistinen H, Koistinen R, Milgrom E. Glycodelin: a major lipocalin protein of the reproductive axis with diverse actions in cell recognition and differentiation. Endocr Rev. 2002;23(4):401–430. doi: 10.1210/er.2001-0026. [DOI] [PubMed] [Google Scholar]
- 13.Meola J, Dentillo DB, Rosa e Silva JC, Ferriani RA, Veiga LC, Paro de Paz CC, et al. Glycodelin expression in the endometrium of healthy women and in the eutopic and ectopic endometrium of women with endometriosis. Fertil Steril. 2009;91(5):1676–1680. doi: 10.1016/j.fertnstert.2008.02.158. [DOI] [PubMed] [Google Scholar]
- 14.Mackenna A, Li TC, Dalton C, Bolton A, Cooke I. Placental protein 14 levels in uterine flushing and plasma of women with unexplained infertility. Fertil Steril. 1993;59(3):577–582. doi: 10.1016/s0015-0282(16)55803-8. [DOI] [PubMed] [Google Scholar]
- 15.Sharkey AM, Smith SK. The endometrium as a cause of implantation failure. Best Pract Res Clin Obstet Gynaecol. 2003;17(2):289–307. doi: 10.1016/s1521-6934(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 16.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263. doi: 10.1016/s0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 17.Amjadi F, Aflatoonian R, Javanmard SH, Saifi B, Ashrafi M, Mehdizadeh M. Apolipoprotein A1 as a novel anti-implantation biomarker in polycystic ovary syndrome: a case-control study. J Res Med Sci. 2015;20(11):1039–1045. doi: 10.4103/1735-1995.172813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuQuesnay R, Wright C, Aziz AA, Stamp GW, Trew GH, Margara RA, et al. Infertile women with isolated polycystic ovaries are deficient in endometrial expression of osteopontin but not alphavbeta3 integrin during the implantation window. Fertil Steril. 2009;91(2):489–499. doi: 10.1016/j.fertnstert.2007.11.063. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207. doi: 10.1210/er.2005-0015. [DOI] [PubMed] [Google Scholar]
- 21.Revel A. Defective endometrial receptivity. Fertil Steril. 2012;97(5):1028–1032. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Douglas NC, Thornton MH 2nd, Nurudeen SK, Bucur M, Lobo RA, Sauer MV. Differential expression of serum glycodelin and insulin-like growth factor binding protein 1 in early pregnancy. Reprod Sci. 2013;20(11):1376–1381. doi: 10.1177/1933719113485290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritz MA, Lessey BA. Defective luteal function. In: Fraser IS, Jansen RPS, Lobo RA, Whitehead MI, editors. Estrogens and progestogens in clinical practice. 1st ed. London: Churchhill Livingstone; 1998. pp. 437–453. [Google Scholar]
- 24.Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28(1):51–58. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, et al. Osteen KG.Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8- tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84(1):67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 26.Kitawaki J, Kusuki I, Koshiba H, Tsukamoto K, Fushiki S, Honjo H. Detection of aromatase cytochrome P-450 in endometrial biopsy specimens as a diagnostic test for endometriosis. Fertil Steril. 1999;72(6):1100–1106. doi: 10.1016/s0015-0282(99)00424-0. [DOI] [PubMed] [Google Scholar]
- 27.Pellegrini C, Gori I, Achtari C, Hornung D, Chardonnens E, Wunder D, et al. The expression of estrogen receptors as well as GREB1, c-MYC, and Cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril. 2012;98(5):1200–1208. doi: 10.1016/j.fertnstert.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 28.Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertil Steril. 2009;92(4):1246–1249. doi: 10.1016/j.fertnstert.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Critchley HO, Tong S, Cameron ST, Drudy TA, Kelly RW, Baird DT. Regulation of bcl-2 gene family members in human endometrium by antiprogestin administration in vivo. J Reprod Fertil. 1999;115(2):389–395. doi: 10.1530/jrf.0.1150389. [DOI] [PubMed] [Google Scholar]
- 30.Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24(1):6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Windham GC, Lee D, Mitchell P, Anderson M, Petreas M, Lasley B. Exposure to organochlorine compounds and effects on ovarian function. Epidemiology. 2005;16(2):182–190. doi: 10.1097/01.ede.0000152527.24339.17. [DOI] [PubMed] [Google Scholar]
- 32.Valbuena D, Martin J, de Pablo JL, Remohí J, Pellicer A, Simón C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962–968. doi: 10.1016/s0015-0282(01)02018-0. [DOI] [PubMed] [Google Scholar]
- 33.Simon C, Domínguez F, Valbuena D, Pellicer A. The role of estrogen in uterine receptivity and blastocyst implantation. Trends Endocrinol Metab. 2003;14(5):197–199. doi: 10.1016/s1043-2760(03)00084-5. [DOI] [PubMed] [Google Scholar]
- 34.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA. 2003;100(5):2963–2968. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79(2):643–649. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 36.Dalton CF, Laird SM, Estdale SE, Saravelos HG, Li TC. Endometrial protein PP14 andCA-125 in recurrent miscarriage patients; correlation with pregnancy outcome. Hum Reprod. 1998;13(11):3197–3202. doi: 10.1093/humrep/13.11.3197. [DOI] [PubMed] [Google Scholar]
- 37.Lédée-Bataille N, Laprée-Delage G, Taupin JL, Dubanchet S, Frydman R, Chaouat G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod. 2002;17(1):213–218. doi: 10.1093/humrep/17.1.213. [DOI] [PubMed] [Google Scholar]
- 38.Edi-Osagie EC, Seif MW, Aplin JD, Jones CJ, Wilson G, Lieberman BA. Characterizing the endometrium in unexplained and tubal factor infertility: a multiparametric investigation. Fertil Steril. 2004;82(5):1379–1389. doi: 10.1016/j.fertnstert.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 39.Altmäe S, Martínez-Conejero JA, Salumets A, Simón C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16(3):178–187. doi: 10.1093/molehr/gap102. [DOI] [PubMed] [Google Scholar]
- 40.Deepak S, Kottapalli K, Rakwal R, Oros G, Rangappa K, Iwahashi H, et al. Real-time PCR: revolutionizing detection and expression analysis of genes. Curr Genomics. 2007;8(4):234–251. doi: 10.2174/138920207781386960. [DOI] [PMC free article] [PubMed] [Google Scholar]