Abstract
Background
Saffron (Crocus sativus L.) has been traditionally used as a spice for coloring and flavoring in some countries cuisine. One of the main components of saffron is Crocin. Recent research have shown that crocin has various pharmacological effects. The aim of this study was to assess the effects of crocin on the Pituitary-Gonadal axis and Kiss-1 gene expression in hypothalamus and ovarian tissue organization in female Wistar rats.
Materials and Methods
In this experimental study, 18 adult female Wistar rats were randomly divided into three groups. Control group received normal saline and experimental groups received two different doses of crocin (100 and 200 mg/kg) every two days for 30 days. After the treatment period, blood samples were obtained from the heart and centrifuged. Next, the serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), estrogen and progesterone hormones were measured by ELISA assay. The ovarian tissues were removed and fixed for histological investigation. The hypothalamic Kiss-1 gene expression was measured using real-time polymerase chain reaction (PCR). All data were analyzed using one-way ANOVA.
Results
A significant reduction (P=0.038) in the number of atretic graafian follicles (0.5 ± 0.31) was observed in rats treated with 200 mg/kg crocin. In addition, estrogen concentration in experimental groups (35.04 ± 0.85 and 36.18 ± 0.69 in crocin 100 and 200 mg/kg groups, respectively) compared to control group (38.35 ± 0.64) and progesterone concentration in rats treated with crocin 200 mg/kg (2.06 ± 0.07) compared to control group (2.16 ± 0.04), significantly decreased. Interestingly, relative expressions of Kiss-1 mRNA significantly decreased in experimental groups (0.00053 ± 0.00051 and 0.0011 ± 0.00066 in crocin 100 and 200 mg/kg groups, respectively) (P=0.000) compared to control group (1 ± 0).
Conclusion
Crocin, at hypothalamic level, reduces Kiss-1 gene expression and it can prevent follicular atresia and reduce serum levels of estrogen and progesterone.
Keywords: Crocin, Folliculogenesis, Gonadal Steroid Hormones, Gonadotropins, Kiss-1
Introduction
Hypothalamic-pituitary-gonadal axis (HPG axis) has an important role in hormonal regulation of reproductive system. Disruption of this axis can have unpleasant consequences on fertility (1). Kisspeptin, also known as metastin, is a hypothalamic peptide encoded by the Kiss-1 gene, which was first discovered as a metastasis inhibitor in melanoma cell lines (2). Recent studies have shown that the Kiss-1 gene is also a key regulator of female gonadotropic axis in mammals (3) and is required for follicular development and ovulation during reproduction (4).
In rodents’ central nervous system, Kisspeptin expressing neurons were found in the anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) of the hypothalamus (5). Kisspeptin neurons send projections to gonadotropin- releasing hormone (GnRH) cell bodies, regulate the secretion of GnRH (6) and thereby control the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) (7). Based on its role in sex organ development and the HPG-axis, kisspeptin neurons dysfunction can lead to abnormal fetal development and infertility (8).
Medicinal plants like Pongamia pinnata, Trachyspermum ammi and Semecarpus anacardium have shown to be able to improve fertility and resolve hormonal imbalances (9) while Trigonella foenum, Carum carvi, Achyranthes aspera, and Rivea hypocrateriformis have contradictory effects (10). So far, four types of phytochemicals including carotenoids, flavonoids, terpenoids and curcumins have been reported to be responsible for phytochemical activities of herbal drugs (11). Crocin is a carotenoid that is found in saffron (Crocus sativus L.). Saffron is traditionally has been used as a coloring or flavoring agent, as well as a herbal medicine (12). Moreover, previous studies have introduced crocin as an antioxidant (13), anticancer (14) and tumoricidal (15), anti-inflammatory (16), antinociceptive, antidepressant (17), and antianxiety agent (18). Crocin has been used as an effective treatment against Alzheimer’s disease (19), atherosclerosis (20), hyperlipidemia and hypertension (21). Considering various pharmacological properties of crocin, this study aimed to evaluate the effects of this phytochemical on female reproductive functions in Wistar rats.
Materials and Methods
All aspects of animal care complied with the ethical guidelines and technical requirements approved by the Institutional Animal Ethics Committee. In this study, after two-week adaptation period, 18 virgin adult female Wistar rats were maintained under standard laboratory conditions. Rats (160-180 g) were housed under controlled lighting (12 hours light and 12 hours dark) at 20 ± 2°C and had free access to food and water. Synchronization of estrus in rats was performed using estradiol valerate and progesterone. Estrous cycle was monitored by vaginal smears.
In this experimental study, 18 Rats were randomly divided into three groups as follows: control group received 2 ml normal saline, experimental group I received crocin 100 mg/kg body weight (BW) intraperitoneally (Pharmaceutical Research Center, BuAli Research Institute, Mashhad University of Medical Sciences, Mashhad, Iran) and experimental group II received crocin 200mg/kg BW intraperitoneally every two days for 30 days (22).
Hormonal assay
After the treatment period, animals in each group were anesthetized by ketamine/xylazine (k,80-100 mg/kg, X, 10-12.5 mg/kg) and blood samples were collected from their hearts. Blood samples were centrifuged for 10 minutes at 8000 rpm and the serum were separated and stored at -20°C. ELISA technique was used for evaluation of FSH, LH (commercial kits purchased from Pishtaz Teb Co., Iran), estrogen and progesterone hormones (commercial kits purchased from DRG Co., Germany).
Histological investigation
The left ovary of animals were removed and fixed in 10% formalin solution. The specimens were processed through routine paraffin embedding method. Subsequently, 6 µm serial paraffin sections were stained with haematoxylin and eosin (H&E). The total number of sections was counted and the middle section of the ovary was determined. The follicles were counted in 5 sections per ovary which included the middle section and 4 sections from either side of the center. Ovarian follicle counting was performed (23) and atretic graafian follicles were identified (24) and counted using a light microscope (Olympus IX71, Japan).
Evaluation of Kiss-1 gene expression by quantitative real-time polymerase chain reaction
Brains were removed immediately from the skull. The hypothalami were dissected and frozen at -80°C. These tissues were thoroughly homogenized. Total RNA was extracted using RNeasy Mini Kit (Qiagen, Germany) and RNA concentration was determined by Nano Drop ND- 1000. cDNA synthesis was performed using 2 µg of total RNA and QuantiTect Reverse Transcription Kit (Qiagen, Germany). The quality of synthesized cDNA was assessed by polymerase chain reaction (PCR) using ß-actin gene. PCR products were qualified by electrophoresis on 1% agarose gel. Primers were designed by Primer premier 5 (Premier Bio Soft International, Palo Alto, CA, USA) software for the reference gene, Kiss-1. The rat ß-actin gene was used as the reference gene for data normalization (Table 1).
Table 1.
Primers used for real-time quantitative polymerase chain reaction
| Primer | Primer sequencing (5ˊ-3ˊ) | Product length (bp) | Annealing temperature (°C) |
|---|---|---|---|
| Kiss-1 | F:TGCTGCTTCTCCTCTGTG | 106 | 59 |
| R:ACGAGTTCCTGGGGTCC | |||
| β-actin | F:CCATCTATGAGGGTTACGC | 105 | 60 |
| R:TGTAGCCACGCTCGGTC | |||
Real-time PCR was performed in a thermal cycler Rotor gene 6000 (Corbett, AUS). The PCR mixture for each reaction contained 5 µl SYBR premix Ex Taq II, 0.5 µl of each primer (5 pmol/µl) and 50 ng cDNA adjusted to a final volume of 10 µl using DEPC water. All reactions were carried out in triplicate. The real-time PCR protocol included 5 minutes at 95°C followed by 40 repetitive cycles for 10 seconds at 95°C, 30 seconds at 60°C and 61°C for Kiss-1 and 30 seconds at 72°C. The expression level of Kiss-1 mRNA was normalized against ß-actin expression level as a housekeeping gene. The relative expression of Kiss-1 gene was assessed using the ..Ct method and results were demonstrated as 2-..Ct based on previous reports (25, 26).
Statistical analysis
The results were statistically analyzed using SPSS 19 software. Mean ± SD was calculated for each parameter and differences among means were evaluated by ANOVA followed by the Tukey post-hoc test using the Excel computer-based program. P<0.05 were considered statistically significant.
Results
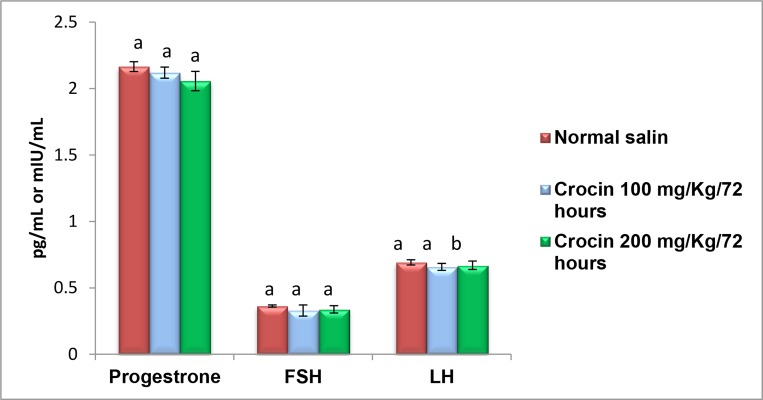
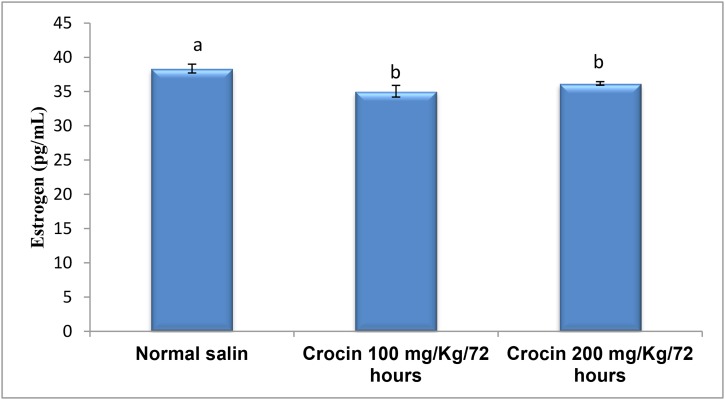
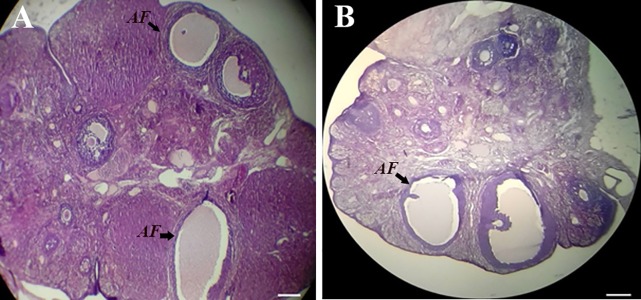
There was no significant differences in FSH hormone levels between experimental groups (0.33 ± 0.042 for crocin 100 mg/kg, P=0.158 and 0.34 ± 0.073 for crocin 200 mg/ kg, P=0.302) and control group (0.36 ± 0.008). In addition, administration of crocin 100 and 200 mg/kg doses had no effect on LH levels in female rats ((0.66 ± 0.026, P=0.120) and (0.67 ± 0.032), P=0.350, respectivly) compared to control groups (0.69 ± 0.019) (Fig .1). However, both doses of crocin significantly decreased serum estrogen levels (35.04 ± 0.85 for crocin 100 mg/kg, P=0.000) and (36.18 ± 0.69 for crocin 200 mg/kg, P=0.000) compared to the control group (38.35 ± 0.64) (Fig .2). Also, progesterone concentrations significantly reduced in rats received 200 mg/kg crocin (2.06 ± 0.07, P=0.009) compared to control group (2.16 ± 0.04) (Fig .1). The expression of Kiss-1 was significantly (P=0.000) reduced following treatment with crocin 100 and 200 mg/kg (Table 2). We also assessed the mean number of primordial, primary, growing, graafian and atretic graffian follicles and corpora lutea among rats treated with crocin 100 and 200 mg/kg compared to the control group. Apart from the number of atretic graafian follicles that was significantly lower in the rats treated with crocin 200 mg/kg (0.5 ± 0.31) compared to the control group (1.33 ± 0.45), we did not find any significant differences in the mean number of other follicles (Table 3). Figure 3 shows images of ovarian tissue in the control group and rats treated with crocin 200 mg/kg.
Fig.1.
Comparison of serum FSH, LH, and progesterone levels among the experimental and control groups. Bars labeled with different letters are significantly different from each other at P<0.05. FSH; Follicle-stimulating hormone and LH; Luteinizing hormone.
Fig.2.
Comparison of serum estrogen level among the experimental and control groups. Significant differences were observed between groups treated with Crocin and control group (P<0.05).
Table 2.
Mean hypothalamic Kiss-1 gene expression in the experimental and control groups
| Group | RF Kiss-1 (mean ± SD) | P value compared to control group |
|---|---|---|
| Control | 1 ± 0 | - |
| Crocin (100 mg/kg) | 0.00053 ± 0.0005a | 0.000 |
| Crocin (200 mg/kg) | 0.0011 ± 0.0007a | 0.000 |
All data were presented as mean ± SD.
a; Indicates a significant difference between experimental groups and control group (P<0.001).
Table 3.
Mean number of primordial, primary, growing, atretic graafian follicles, graafian follicles and corpora lutea in the ovaries of rats in the experimental and control groups
| Variable | Control | 100 mg/kg/72 hours | 200 mg/kg/72 hours | P value compared to control group |
|---|---|---|---|---|
| Primordial follicles | 8.5 ± 1.169 | 5.8 ± 0.98 | 5.8 ± 1.17 | 0.0520.056 |
| Primary follicles | 2.17 ± 1.169 | 3.17 ± 0.408 | 1.33 ± 0.516 | 0.0970.183 |
| Growing follicles | 1.67 ± 0.516 | 1.17 ± 0.408 | 1.17 ± 0.408 | 0.1630.163 |
| Graafian follicles | 1.5 ± 0.837 | 1.17 ± 0.753 | 1.83 ± 0.753 | 0.7450.745 |
| Atretic Graafian follicles | 1.33 ± 0.448 | 1.33 ± 0.448 | 0.5 ± 0.31a | 10.038 |
| Corpora lutea | 2.5 ± 0.548 | 4 ± 1.549 | 4 ± 1.265 | 0.1090.109 |
All data were presented as mean ± SD.
a; Indicates a significant difference between Crocin 200 mg/kg-treated group and control group (P<0.05).
Fig.3.
The ovarian tissue. Photomicrographs of ovary tissue (6-µm thick sections were stained with H&E; X100) in A. The control animals and B. Rats treated with 200 mg/kg crocin. AF; Atretic graafian follicle (scale bar: 100 µm).
Along with other hypothalamic factors, the Kiss-1 gene regulates the release of GnRH and thereby controls the release of FSH and LH from the pituitary gland. FSH and LH control ovarian folliculogenesis .Therefore, an alteration in this axis may affect sex-related endocrine hormones and follicular development. In this study, we evaluated the effect of crocin on these parameters. The results revealed no significant differences in serum concentrations of FSH and LH, but the levels of estrogen following treatment with both doses of crocin and the level of progesterone following administration of crocin 200 mg/kg significantly reduced.
Considering the fact that estrogen is secreted by follicular cells in the ovary, this may explain a role for crocin at the ovarian level. In this regard, previous studies have shown that carotenoids reduce the activity of cytochrome p450, thus inhibiting the transformation of cholesterol to pregnenolone, and consequently reducing the amount of estrogen. This effect of carotenoids is believed to be mediated by reduced expression of the CYP19 gene which encodes an aromatase belonging to the cytochrome P450 family (27, 28). In addition, it has been shown that crocin reduces plasma levels of total cholesterol in a dose-dependent manner (29). Therefore, reducing estrogen and progesterone levels reported in the present study could be due to a reduction in cholesterol levels.
Considering the reduction in estrogen, an increase in Kiss-1 gene expression is expected. It is well established that Kiss-1 gene expression is negatively regulated by circulatory estrogen. In contrast to this expectation, the relative expression of Kiss-1 gene was significantly down-regulated by crocin; the underlying reason(s) for this observation should be investigated in future studies. Despite reduction in Kiss-1 gene, we observed no changes in FSH and LH levels. These observations suggest that reduced expression of Kiss-1 gene is likely counterbalanced by other mechanisms controlling GnRH release and thereby pituitary FSH and LH secretion. Previous study have shown that some parameters such as NPY, GABA, Glutamate, etc. affect GnRH neurons and reproductive axis (30).
Also, differential action of estrogen on Kiss-1 neurons in the AVPV and ARC of the hypothalamus, may explain our results. Estrogen increases Kiss-1 gene expression in the AVPV while it reduces the expression of Kiss-1 gene in the ARC. Hypothetically, based on this mechanism, one might expect crocin to reduce the expression of Kiss-1 gene in ARC but not in AVPV nucleus. However, we believe that this is very unlikely and further research is required to clarify mechanisms via which crocin reduces Kiss-1 gene expression.
Following hormonal evaluation, we assessed histological sections for any alteration in folliculogenesis. The results revealed no significant changes in the mean value of the number of different follicles between the control and treated groups except for a reduction in the number of atretic graafian follicles which were significantly reduced in crocin 200 mg/kg-treated group. This observation may be related to the anti-apoptotic effect of carotenoids, like crocin. Carotenoids up-regulate the expression of Cx43 (31), which is dominantly expressed in granulosa cells and maintains the integrity of the follicle thus reducing follicular apoptosis (32). In this regard, previous studies indicated that crocin can inhibit apoptosis via increasing Bcl2/Bax expression ratio (33, 34).
Another process involved in the generation of atretic follicles is excessive production of reactive oxygen species (ROS). Assimopoulou et al. (13) showed that crocin has a marked radical-scavenging activity. In this regard, Soeda et al. (35) reported that crocin inhibits oxidative stress- induced cell death via a glutathione (GSH)-dependent mechanism. Also, Hosseinzadeh et al. (36) showed that crocin decreases malondialdehyde (MDA) generation. The role of crocin as an anti-apoptotic agent is well established in different systems (37, 38).
As ovarian follicles synthesize estrogen, one may expect that a decrease in the number of atretic follicles may result in an increase in estrogen production, which is contrary to results showing a reduction in estrogen levels. This effect might be due to a reduction in aromatase activity induced by crocin. The reduction in estrogen might be possibly due to crocin effect on the ovary rather than on the hypothalamus, as crocin had no effects on FSH and LH levels.
Based on the literature, crocin can induce the expression of genes like XBP, BiP, CHOP, F4/80, TNF-a, NOS-2, IFN-a (39), Mmp-9, Cox-1, Cox-2, Bcl-2, and Bax (40). In our study, crocin reduced the expression of the Kiss- 1gene which might be a result of crocin interaction with factors involved in regulation of gene expression.
Conclusion
Our results revealed that at the hypothalamic level, crocin reduces Kiss-1 gene expression; however, reduced Kiss-1 gene expression did not affect sex-related hormones, indicating that other mechanisms may have counterbalanced this reduction. At the ovarian level, crocin acts as an anti-apoptotic agent and reduces follicular atresia. Crocin may also indirectly reduce aromatization via regulating genes involved in this process. Overall, these data suggest that crocin may interfere with factors regulating gene expression and this hypothesis needs further investigations.
Acknowledgments
This work was financially supported by Science and Research Branch, Islamic Azad University, Tehran, Iran. There is no conflict of interest in this article.
Author’s Contributions
K.P., M.H.S., N.H.R.; Contributed to conception and design. D.Z.; Performed data collection and evaluation, drafting and statistical analysis. All authors performed editing and approving the final version of this paper for submission, also participated in the finalization of the manuscript and approved the final draft.
References
- 1.Amah CI, Yama OE, Noronha CC. Infecund evaluation of cycling female Sprague-Dawley rats: an aftermath treatment with Momordica charantia seed extract. Middle East Fertil Soc J. 2012;17(1):37–41. [Google Scholar]
- 2.Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. Kiss-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88(23):1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 3.Roa J, Tena-Sempere M. KiSS-1 system and reproduction: comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol. 2007;153(1-3):132–140. doi: 10.1016/j.ygcen.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Fernandois D, Na E, Cuevas F, Cruz G, Lara HE, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol. 2016;228(3):161–170. doi: 10.1530/JOE-15-0429. [DOI] [PubMed] [Google Scholar]
- 5.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147(12):5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.d'Anglemont de Tassigny X, Fagg LA, Carlton MB, Colledge WH. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology. 2008;149(8):3926–3932. doi: 10.1210/en.2007-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruni JF, Huang HH, Marshall S, Meites J. Effects of single and multiple injections of synthetic GnRH on serum LH, FSH and testosterone in young and old male rats. Biol Reprod. 1977;17(3):309–312. doi: 10.1095/biolreprod17.3.309. [DOI] [PubMed] [Google Scholar]
- 8.Skakkebaek NE, Jørgensen N, Main KM, Rajpert-De Meyts E, Leffers H, Andersson AM, et al. Is human fecundity declining? Int J Androl. 2006;29(1):2–11. doi: 10.1111/j.1365-2605.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 9.Kumar RV, Reddy GV, Reddy MK. Medicinal plants having fertility related and pharmacological activities. Int J Pharm Med Bio Sci. 2012;1(1):102–117. [Google Scholar]
- 10.Priya G, Saravanan K, Renuka C. Medicinal plants with potential antifertility activity-a review of sixteen years of herbal medicine research (1994-2010) Int J PharmTech Res. 2012;4(1):481–494. [Google Scholar]
- 11.Singh G, Maurya S, de Lampasona MP, Catalan C. Chemical constituents, antifungal and antioxidative potential of Foeniculum vulgare volatile oil and its acetone extract. Food Control. 2006;17(9):745–752. doi: 10.1021/jf035211c. [DOI] [PubMed] [Google Scholar]
- 12.Melnyk JP, Wang S, Marcone MF. Chemical and biological properties of the world's most expensive spice: Saffron. Food Res Int. 2010;43(8):1981–1989. [Google Scholar]
- 13.Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L.extract and its bioactive constituents. Phytother Res. 2005;19(11):997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 14.Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, et al. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol. 2007;29(3):175–180. [PMC free article] [PubMed] [Google Scholar]
- 15.Xu HJ, Zhong R, Zhao YX, Li XR, Lu Y, Song AQ, et al. Proliferative inhibition and apoptotic induction effects of crocin on human leukemia HL-60 cells and their mechanisms. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18(4):887–892. [PubMed] [Google Scholar]
- 16.Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L.stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7–7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effect of Crocus sativus L.stigma extracts and their constituents, crocin and safranal, in mice. International Symposium on Saffron Biology and Biotechnology. 2003;650:435–445. [Google Scholar]
- 18.Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis PA, Sakellaridis N. Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine. 2008;15(12):1135–1139. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Papandreou MA, Kanakis CD, Polissiou MG, Efthimiopoulos S, Cordopatis P, Margarity M, et al. Inhibitory activity on amyloid-beta aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J Agric Food Chem. 2006;54(23):8762–8768. doi: 10.1021/jf061932a. [DOI] [PubMed] [Google Scholar]
- 20.He SY, Qian ZY, Tang FT, Wen N, Xu GL, Sheng L. Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci. 2005;77(8):907–921. doi: 10.1016/j.lfs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Lee IA, Lee JH, Baek NI, Kim DH. Antihyperlipidemic effect of crocin isolated from the fructus of Gardenia jasminoides and its metabolite crocetin. Biol Pharm Bull. 2005;28(11):2106–2110. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 22.Hosseinzadeh H, Ziaee T, Sadeghi A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine. 2008;15(6-7):491–495. doi: 10.1016/j.phymed.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Talsness CE, Kuriyama SN, Sterner-Kock A, Schnitker P, Grande SW, Shakibaei M, et al. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ Health Perspect. 2008;116(3):308–314. doi: 10.1289/ehp.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard CJ, Greenwald GS. Morphological changes in atretic Graafian follicles during induced atresia in the hamster. Anat Rec. 1985;212(4):353–357. doi: 10.1002/ar.1092120405. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Vaerman J, Saussoy P, Ingargiola I. Evaluation of real-time PCR data. J Biol Regul Homeost Agents. 2004;18(2):212–214. [PubMed] [Google Scholar]
- 27.Miller WL, Geller DH, Rosen M. Ovarian and adrenal androgen biosynthesis and metabolism. In: Azziz R, Nestler JE, Dewailly D, editors. Androgen excess disorders in women. 2nd ed. New York: Humana Press; 2006. pp. 19–33. [Google Scholar]
- 28.Simpson ER, Michael MD, Agarwal VR, Hinshelwood MM, Bulun SE, Zhao Y. Cytochromes P450 11: expression of the CYP19 (aromatase) gene: an unusual case of alternative promoter usage. FASEB J. 1997;11(1):29–36. doi: 10.1096/fasebj.11.1.9034163. [DOI] [PubMed] [Google Scholar]
- 29.Mashmoul M, Azlan A, Mohd Yusof BN, Khaza'ai H, Mohtarrudin N, Boroushaki MT. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J Funct Foods. 2014;8:180–187. [Google Scholar]
- 30.Hameed S, Jayasena CN, Dhillo WS. Kisspeptin and fertility. J Endocrinol. 2011;208(2):97–105. doi: 10.1677/JOE-10-0265. [DOI] [PubMed] [Google Scholar]
- 31.Hix LM, Lockwood SF, Bertram JS. Bioactive carotenoids: potent antioxidants and regulators of gene expression. Redox Rep. 2004;9(4):181–191. doi: 10.1179/135100004225005967. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y, Inoue N, Matsuda-Minehata F, Goto Y, Maeda A, Manabe N. Changes in expression and localization of connexin 43 mRNA and protein in porcine ovary granulosa cells during follicular atresia. J Reprod Dev. 2005;51(5):627–637. doi: 10.1262/jrd.17035. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Qian Z. Effects of crocin on cholestane-3beta-5alpha-6beta-triol-induced apoptosis and related gene expression of cultured endothelial cells. J Chin Pharm Univ. 2005;36(3):254–254. [Google Scholar]
- 34.Xu G, Gong Z, Yu W, Gao L, He S, Qian Z. Increased expression ratio of Bcl-2/Bax is associated with crocin-mediated apoptosis in bovine aortic endothelial cells. Basic Clin Pharmacol Toxicol. 2007;100(1):31–35. doi: 10.1111/j.1742-7843.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 35.Soeda S, Ochiai T, Tanaka H, Shoyama Y, Shimeno H. Prevention of ischemic neuron death by a saffron’s carotenoid pigment crocin and its mechanism of action. In: Coleman RM, editor. Focus on neurochemistry research. New York: Nova Sceince Publishers, Inc; 2005. pp. 139–156. [Google Scholar]
- 36.Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L.stigma and its bioactive constituents, crocin and safranal. Phcog Mag. 2009;5(20):419–424. [Google Scholar]
- 37.Mousavi SH, Tayarani NZ, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. 2010;30(2):185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Beshbishy HA, Hassan MH, Aly HA, Doghish AS, Alghaithy AA. Crocin “saffron” protects against beryllium chloride toxicity in rats through diminution of oxidative stress and enhancing gene expression of antioxidant enzymes. Ecotoxicol Environ Saf. 2012;83:47–54. doi: 10.1016/j.ecoenv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Deslauriers AM, Afkhami-Goli A, Paul AM, Bhat RK, Acharjee S, Ellestad KK, et al. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J Immunol. 2011;187(9):4788–4799. doi: 10.4049/jimmunol.1004111. [DOI] [PubMed] [Google Scholar]
- 40.Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]